Abstract
• Aims To develop an in-situ, non-destructive method for observation and monitoring of the underground developmental stages of the root parasite Orobanche cumana.
• Scope The parasitic weed Orobanche causes severe damage to vegetables and field crops. Most of the damage caused to the crops occurs during the underground, unobservable parasitism stage. Sunflower (Helianthus annuus ‘Adi’) plants were planted in soil that was artificially inoculated with O. cumana seeds. Clear Plexiglas mini-rhizotron plastic observation tubes were inserted into the soil. Seed germination, early stage of penetration, and formation of tubercles and spikes were observed non-destructively and were monitored throughout the growing season by mean of a mini-rhizotron camera. Use of this technology enabled the complete individual parasite life cycle from the very early development (including germination) to Orobanche shoot to be monitored. In addition, the effect of the systemic herbicide Cadre (imazapic) on the development of O. cumana was inspected and quantified.
• Conclusions This novel methodology facilitates the in-situ study of major aspects of the host–parasite interaction and of parasite suppression, such as parasitism dynamics, parasite growth rate, and the effect of chemical treatments on the parasite.
Keywords: Orobanche cumana, broomrape, mini-rhizotron, chemical control, image analysis
INTRODUCTION
Broomrapes (Orobanche spp.) are obligate, chlorophyll-lacking root parasites that parasitize many dicotyledonous species and cause severe damage to vegetable and field crops worldwide (Parker and Riches, 1993). Broomrapes are annual plants that germinate only after being stimulated by the host. After germination, the parasite seedling directs itself toward the host roots and attaches to the external tissues. The parasite radicle then thickens to form a vascular connection—a haustorium—between the parasite and the host. Tubercles or underground vegetative tissue outside the host plant develop and accumulate resources from the host. Broomrape flower stalk emergence from the tubercle occurs about 1–4 months after the initial parasitic attachment to the host root.
Understanding of the host–parasite dynamics requires a methodology for monitoring the initial underground stages of parasitism. Several methodologies for detecting the underground stages of the parasite life cycle have been reported. Cultivation of the parasitic weed Striga spp. on fiberglass paper in polyethylene bags was first reported by Parker and Dixon (1983), who sterilized seeds and spread them on a fiberglass paper, on top of which the host was located. This method allowed the host roots to be observed and the various stages of the root–parasite development to be monitored during the parasitism process. This method was modified and used for studying the interaction between O. cumana and sunflower (Eizenberg et al., 2003b). An agar germination bioassay, which was a modification of the procedure published earlier for Striga (Hess et al., 1992), allowed monitoring of the Orobanche seed germination and attachment in the presence of host root fractions; seed germination was evaluated with a binocular microscope (Serghini et al., 2001).
It is worthwhile to grow the host and the parasite in pots that had been artificially infested with Orobanche seeds when the underground stages of the Orobanche parasitism are to be monitored in the concluding stage of the experiment (Eizenberg et al., 2003a, 2004a, 2005). When periodical examination of the parasitism process is needed, the necessary destructive observations require the use of a considerable number of pots (Eizenberg et al., 2005). In vitro infection of host roots by differentiated calli of the parasitic plant Orobanche was also achieved in O. ramosa (Zhou et al., 2004). However, none of these methods enabled repeated non-destructive observations of in situ development and parasitism of Orobanche in the soil under conditions resembling those of natural infection.
Monitoring root development with a mini-rhizotron has been reported for several annual and perennial crops during the last two decades. The method was developed in order to allow repeated, direct, non-destructive observations of the root zone (Box, 1996; Ephrath et al., 1999). The method uses clear Plexiglas tubes inserted into the root zone, and a rod with a camera that moves along the tube and takes pictures at pre-set locations. The method has been used hitherto for studying roots. The mini-rhizotron system provides a non-destructive in situ method for frequent monitoring (from appearance to death) of specific root segments without significantly impacting fine root processes. This system can be used to characterize fine root production, phenology, growth and mortality, and to observe the effects of the various injuries caused to the roots by insects, diseases and pathogens.
The objective of the present study was to evaluate the feasibility of using a mini-rhizotron for in-situ monitoring of the underground stage of Orobanche development and for detecting herbicide damage during the underground stages of parasitism.
MATERIALS AND METHODS
Plant material
Sunflower (Helianthus annuus ‘Adi’) seeds of the local cultivar (provided by Dr Baruch Retig of the ARO, Israel) were planted. This cultivar is highly sensitive to O. cumana (Eizenberg et al., 2004a).
O. cumana seeds were collected from inflorescences of O. cumana that was parasitizing sunflowers in the Jezreel Valley in northern Israel. The inflorescences were dried in a greenhouse for 60 d at temperatures ranging from 20 to 34°C, after which the seeds were separated with 300-µm sieves and were stored in darkness at 4°C. After the seeds had been preconditioned for 12 d at 24°C their germination potential was tested in the presence of GR24 (a synthetic strigol analogue germination stimulant) at 10 ppm, and 88% of the seeds germinated.
Growth conditions
Sunflower plants were planted on 28 Apr. 2004, in three 1-m3 containers, in a greenhouse at the J. Blaustein Institute for Desert Research at Sede-Boqer, Israel (30°51'N 34°46'E). The plants were grown at night/day temperatures ranging from 18 to 34°C. A detailed description of the growth container was presented by Ephrath et al. (1999); the following is a brief summary: the containers had two facing walls of 8-mm-thick glass and two of 10-mm-thick black PVC. The bottom of the container was covered with a layer of gravel overlaid with rock-wool, and an opening in the bottom, filled with rock-wool, allowed the drainage of excess water. The containers were filled with coarse dune sand, taken from the coastal plane of Israel.
Mini-rhizotron
The Model BTC-2 mini-rhizotron system (Bartz Technology Crop., Santa Barbara, CA, USA) was used. Clear acrylic observation tubes having inner and outer diameters of 52 and 60 mm, respectively, were placed in the containers during soil filling and were fitted with protective collars to exclude light (Levan et al., 1987). The tubes were placed horizontally at depths of 20 and 40 cm below the soil surface. Approximately 200 O. cumana seeds per 10-mm length of tube were spread along a10-mm-wide strip adjacent to both tubes. Root growth and parasite development were monitored with a miniature video camera inserted into the observation tubes. Video images were taken at 7 d intervals, at 80 pre-set positions along the tubes, 12·5 mm apart and indicated with a marked rod. As the camera was aimed at the same points on the tube wall in successive sets of measurements, changes in the lengths or numbers of roots, or in the parasite development, at each location could be inferred by comparing homologous video frames in the laboratory. These video frames were digitized and the numbers of broomrape seedlings or attachments in each frame was counted in the laboratory. Orobanche phenological development was classified into key stages of seed germination, attachment, penetration into the host tissues, and size and number of tubercles.
The dynamics of O. cumana parasitism development over time was quantified by using a logistic equation (Brown and Mayer, 1988; Eizenberg et al., 2005):
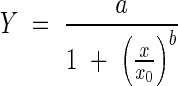 |
where Y represents the number of attachments; a the upper asymptotic limit (maximum) of the number of attachments; x0 the median time from planting to 50 % of the maximum number of attachments; and b the slope at x0. Non-linear regressions were conducted with the Sigma Plot software, version 8·02 (SPSS Inc., USA).
To test the possibility of using a mini-rhizotron to detect the effect of herbicides on O. cumana in situ, plants in one of the containers were sprayed with herbicide and the parasite development was followed as indicated above. Three tubes, each serving as a replica, were sprayed, while three other non-treated tubes served as controls. A dose equivalent to 10 ml ha−1 of the herbicide Cadre (imazapic, 240 g active ingredient L−1), was applied to sunflower foliage when six to eight true leaves had developed (28 d after planting), using a backpack sprayer (Echo SHR210, Echo Ltd). The size of the Orobanche tubercles at herbicide application was 1–4 mm. The sprayer was calibrated to deliver 200 L ha−1 through T-jet 11015 nozzles when operating at 300 kPa.
RESULTS AND DISCUSSION
As a similar potential was achieved by the mini-rhizotron technique at a depth of both 20 and 40 cm, only results of the 20-cm depth are presented. Parasitism of O. cumana could be detected in situ and visualized by means of the a mini-rhizotron system, which visually distinguished between the various phases of the infection. Figure 1 presents repeated measurements over time at the same selected location. Non-germinated O. cumana seeds ranging in size from 0·1 to 0·3 mm are presented in Fig. 1A. The O. cumana seeds did not germinate within the first 14 d from sunflower planting. Between 14 and 21 d after planting (DAP) the seeds germinated (Fig. 1B). The time required for O. cumana seeds to germinate in the present study was similar to that reported by Eizenberg et al. (2003b) for the same temperature regimes.
Fig. 1.

Detection of timing of Orobanche cumana development stages: (A) ungerminated seeds, 14 d after planting (DAP); (B) O. cumana seedlings, 21 DAP; (C) seed penetration into the host tissue, 21 DAP; (D) O. cumana attachments, 28 DAP; (E) production of tubercles, 35 DAP.
The next parasitism stage, the penetration of the parasite into the host tissue, occurred 21 DAP (Fig. 1C). The penetration and parasite establishment stages are crucial for certain studies related to host–parasite interactions such as resistance to Orobanche, and for modelling the dynamics and chemical control of the parasitism. The parasite continued to develop, and formed tubercles (Fig. 1D and E).
Use of this technology enabled changes in parasitism over time to be quantified in an undisturbed system. The parasitism dynamics are described by a sigmoid equation (Fig. 2):
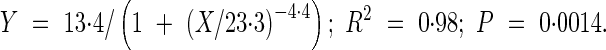 |
The same equation was first applied in the model of the parasitism of O. minor on red clover (Eizenberg et al., 2005). One of the important advantages of the mini-rhizotron technology is the ability to monitor changes in individual attachments over time. In the present study an uninfected sunflower root was observed 14 DAP (Fig. 3A). On 21 DAP a 1-mm broomrape attachment was observed on this root (Fig. 3B); it had enlarged by 28 DAP (Fig. 3C) and enlarged further by 35 DAP (Fig. 3D).
Fig. 2.
Time course of Orobanche cumana parasitism in sunflower, quantified by means of mini-rhizotron. Measurements were taken at 20 cm depth. Bars represent the standard error of ten measurements.
Fig. 3.

Changes in individual Orobanche cumana parasitism over time: (A) 14 d after planting (DAP); (B) 21 DAP; (C) 28 DAP; (D) 35 DAP.
The herbicide imazapic selectively controls O. cumana on sunflower, but the precise timing for optimal effectiveness of the application is not properly defined because of the inadequacy of the means for detection of the infestation stage (Aly et al., 2001). In the present study application of imazapic on 1-mm O. cumana attachments at 28 DAP controlled the parasite without causing any damage to the host (Fig. 4). Several methodologies have been reported for evaluating the efficacy of chemical control. Eizenberg et al. (2004b) washed tomato roots to determine the efficacy of chemical control of O. aegyptiaca, but this kind of evaluation does not allow repeated measurements on the sample plant. The use of a polyethylene bag system enabled the time course of chemical control efficacy to be evaluated, but under hydroponic conditions (Plakhine et al., 2001). The disadvantage of this system is the unfavourable conditions for the host.
Fig. 4.

Visualized demonstration of the effect of imazapic on Orobanche cumana: (A) at time of application, 28 d after planting (DAP); (B) the same attachments, 35 DAP.
To date there have been no reports in the literature on the use of a mini-rhizotron for in situ measurements of Orobanche seed germination and its subsequent parasitism. Since Orobanche seedlings are very small, the very early stages of parasitism, i.e. seed germination and penetration into the host tissue, are not detectable in situ. However, the use of a mini-rhizotron enabled repeated observations to be made on the host roots and Orobanche development, without disturbance, under natural conditions. These conditions are essential for studying major aspects of the host–parasite relationship to gain knowledge for use in modelling the parasitism process, optimizing chemical control and studying the resistance mechanism of resistant cultivars. Using the mini-rhizotron enables detection of Orobanche seeds, observation of the parasitism stages of germination, penetration into the host tissue, establishment, tubercle production and apex production. The mini-rhizotron system used in the present study magnifies the 12 × 18-mm frame of an image by 15 times, enabling the accuracy of the measurements to be increased significantly.
LITERATURE CITED
- Aly R, Goldwasser Y, Eizenberg H, Hershenhorn J, Golan S, Kleifeld Y. 2001. Broomrape (Orobanche cumana) control in sunflower (Helianthus annuus) in fields. Weed Technology 15: 306–309. [Google Scholar]
- Box JE. 1996. Modern methods for root investigations. In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots: the hidden half, 2nd edn. New York: Marcel Dekker, 193–327. [Google Scholar]
- Brown RF, Mayer DG. 1988. Representing cumulative germination: the use of the Weibull function and other empirically derived curves. Annals of Botany 61: 127–138. [Google Scholar]
- Eizenberg H, Colquhoun JB, Mallory-Smith CA. 2005. A predictive degree-days model for small broomrape (Orobanche minor) parasitism in red clover (Trifolium pratense) in Oregon. Weed Science 53: 37–40. [Google Scholar]
- Eizenberg H, Goldwasser Y, Golan S, Plakhine D, Hershenhorn J. 2004. Egyptian broomrape (Orobanche aegyptiaca) control in tomato with sulfonylurea herbicides—greenhouse studies. Weed Technology 18: 490–496. [Google Scholar]
- Eizenberg H, Hershenhorn J, Plakhine D, Kleifeld Y, Rubin B. 2004. Variation in resistant and sensitive sunflower (Helianthus annuus L.) response to the parasitic weed broomrape (Orobanche spp.). Plant Disease 88: 479–484. [DOI] [PubMed] [Google Scholar]
- Eizenberg H, Hershenhorn J, Plakhine D, Shtienberg D, Kleifeld Y, Rubin B. 2003. Effect of temperature on susceptibility of sunflower varieties to Orobanche cumana and O. aegyptiaca Weed Science 51: 279–286. [Google Scholar]
- Eizenberg H, Plakhine D, Hershenhorn J, Kleifeld Y, Rubin R. 2003. Resistance to broomrape (Orobanche spp.) in sunflower (Helianthus annuus L.) is temperature-dependent. Journal of Experimental Botany 54: 1305–1311. [DOI] [PubMed] [Google Scholar]
- Ephrath JE, Silberbush M, Berliner PR. 1999. Calibration of minirhizotron readings against root length density data obtained from soil cores. Plant and Soil 209: 201–208. [Google Scholar]
- Hess DE, Ejeta G, Butler LG. 1992. Selecting sorghum genotypes expressing a quantitative biosynthetic trait that confers resistance to Striga Phytochemistry 31: 493–497. [Google Scholar]
- Levan MA, Ycas JW, Hummel JW. 1987. Light leak effects on near surface soybean rooting observed in minirhizotrons. In: Taylor HM, ed. Minirhizotron observation tubes: methods and applications for measuring rhizosphere dynamics. Special Publication No. 50, Madison: American Society of Agronomy, 1–23. [Google Scholar]
- Parker C, Dixon N. 1983. The use of polyethylene bags in the culture study of Striga spp. and other organisms on crop roots. Annals of Applied Biology 103: 485–488. [Google Scholar]
- Parker C, Riches CR. 1993. In: Parasitic weeds of the world: biology and control. Wallingford, UK: CAB International, 111–164. [Google Scholar]
- Plakhine D, Eizenberg H, Hershenhorn J, Goldwasser Y, Kleifeld Y. 2001. Control of Orobanche aegyptiaca with sulfonylurea herbicides in tomato—polyethylene bag studies. In: Fer A, Thalouran P, Joel DM, Musselman LJ, Parker C, Verkleij JAC, eds. Proceedings of the 7th International Parasitic Weed Symposium, Nantes, France: Imprimerie Parentheses 44300, 294–295. [Google Scholar]
- Serghini K, de Luque AP, Munoz MC, Torres LG, Jorrin JV. 2001. Sunflower (Helianthus annuus L.) response to broomrape (Orobanche cernua Loefi.) parasitism: induced synthesis and excretion of 7-hydroxylated simple coumarins. Journal of Experimental Botany 52: 2227–2234. [DOI] [PubMed] [Google Scholar]
- Zhou WJ, Yoneyama K, Takeuchi Y, Iso S, Rungmekarat S, Chae SH, Sato D, Joel DM. 2004.In vitro infection of host roots by differentiated calli of the parasitic plant Orobanche. Journal of Experimental Botany 55: 899–907. [DOI] [PubMed] [Google Scholar]



