Abstract
• Background and Aims Marcgraviaceae are a rather small family of seven genera and approx. 130 neotropical species. This study aims to present a detailed palynological survey of the family in order to comment on the intrafamily relationships and possible correlations with pollinators.
• Methods In total, 119 specimens representing 67 species and all genera are observed using light microscopy and scanning electron microscopy. Furthermore, eight species from five genera are studied with transmission electron microscopy.
• Key Results Our results show that pollen grains of Marcgraviaceae are small (20–35 µm), have three equatorial apertures, granules on the colpus membrane, oblate spheroidal to prolate spheroidal shapes, mainly psilate to perforate ornamentations, and lalongate colpus-shaped thinnings at the inner layer of the exine, and show the presence of orbicules. Based on our fragmentary knowledge of the pollination biology of the family, there are no clear correlations between pollinators and pollen features.
• Conclusions The genus Marcgravia has a high percentage of reticulate sexine patterns and a relatively thin nexine. Sarcopera can be defined by the presence of an oblate spheroidal to even suboblate shape, while Ruyschia and Souroubea typically show prolate spheroidal to subprolate pollen grains. The presence of a thick foot layer in the pollen wall is characteristic of the genera Norantea, Sarcopera and Schwartzia. Pollen features that are taxonomically useful within the family are the shape, sexine sculpturing, and ultrastructure of the pollen wall.
Keywords: Balsaminoids, Ericales, Marcgraviaceae, neotropics, orbicules, palynology, SEM, TEM
INTRODUCTION
Marcgraviaceae is a neotropical family of lianas, climbing shrubs and treelets, and comprises seven genera and approx. 130 species ranging from northern Bolivia and southern Brazil to southern Mexico including the West Indies. The family is characterized by various kinds of sclereids, hypophyllous glands and specific nectary bracts (de Roon, 1967; Dressler, 2004). Several insects, birds and mammals have been suggested or proven as likely pollinators (Sazima and Sazima, 1980; Sazima et al., 1993; Tschapka and von Helversen, 1999; Machado and Lopes, 2000; Dressler and Tschapka, 2002; Dressler, 2004), but some species appear to be self-pollinated or even cleistogamous (Bailey, 1922).
Marcgraviaceae were traditionally placed in the order Theales of the subclass Dilleniidae (Cronquist, 1988; Takhtajan, 1997), but molecular data place a large part of Theales, including Marcgraviaceae, into the newly circumscribed Ericales at the base of the asterids (APG II, 2003). Besides members of the former Theales and Ericales, the Ericales sensu APG mainly consists of the former Ebenales, Primulales, Lecythidales and Diapensiales (Soltis et al., 2000; Savolainen et al., 2000; APG II, 2003). Within Ericales, Marcgraviaceae are placed together with Balsaminaceae, Pellicieraceae and Tetrameristaceae in the so-called balsaminoid clade, which is sister to all other ericalean families (Anderberg et al., 2002; Bremer et al., 2002; Geuten et al., 2004). This clade represents one of the few suprafamilial groups within Ericales that are well supported by molecular sequence data, although morphological coherence is poor.
Major morphological differences in the family are found in the type of inflorescences, and the shape and position of the nectary bracts (de Roon, 1975; Bedell, 1985). In 1997, de Roon and Dressler used these characters to elevate three new genera from the Norantea s.l. complex, i.e. Marcgraviastrum, Sarcopera and Schwartzia. Recent phylogenetic analyses based on chloroplast sequences support the established recognition of two subfamilies, i.e. Marcgravioideae including Marcgravia and Noranteoideae comprising all other genera (Ward and Price, 2002). The difference between Marcgravia and the other genera is also supported by morphological features, such as the special type of umbellate inflorescences, the presence of tetramerous flowers, the occurrence of leaf dimorphism and the presence of wide multiseriate rays in the wood (Dressler, 2004; Lens et al., 2005). Despite the strong support for the monophyly of the two subfamilies, deeper phylogenetic relationships remain poorly resolved (Ward and Price, 2002).
The first description of marcgraviaceaous pollen dates back to Mohl (1834). Pollen grains of all genera (except the largest genus Marcgravia) were studied using light microscopy (LM) observations by Punt (1971). His study revealed 12 pollen types that mainly differed in the shape of the grains, the ornamentation of the tectum, the length of the colpi and the thickness of the pollen wall. The same features were used by Dressler (1994) in his palynological study of Marcgravia to define five pollen types based on LM and scanning electron microscopy (SEM) observations. Other palynological papers on the family mainly describe the pollen morphology of a few species only, such as Erdtman (1952) (LM), Barth (1963) (LM), Alvarado and Palacios-Chavez (1987) (LM + SEM), Roubik and Moreno (1991) (LM), da Silva Correa and Esteves (1997) (LM) and Colinvaux et al. (1999) (LM).
The present study aims to present a detailed pollen morphological description of the entire family using LM, SEM and transmission electron microscopy (TEM). Pollen grains investigated by LM were all acetolysed; a part of the SEM material was critical point dried and another part acetolysed in order to investigate the impact of the acetolysis on the pollen grains within one specimen, and TEM material was treated with a special protocol (see MATERIALS AND METHODS). Special attention is paid to some poorly known features in Marcgraviaceae, such as the inside wall ornamentation of the pollen grains and the ultrastructure of the pollen wall. Furthermore, this study wants to trace the presence of orbicules (Kosmath, 1927; Erdtman et al., 1961), a character that has not been reported before in the family. The palynological variation observed will be used to comment on the intrafamily relationships within Marcgraviaceae. A pollen morphological comparison with the other balsaminoid families is treated in this issue (Janssens et al., 2005).
MATERIALS AND METHODS
Flowering buds in the stage just before anthesis from 119 specimens representing 67 species of all seven genera were removed from herbarium specimens at BM, BR, C, FR, HAC, HAL, K, M, MO, NY, P, S, U, US and W (see Table 1; Appendix).
Table 1.
Overview of palynological diversity within Marcgraviaceae
| Species studied |
P (μm) |
E (μm) |
Oblate spheroidal |
Spheroidal |
Prolate spheroidal |
Subprolate |
Apocolpium index |
Ectocolpus lentgh (μm) |
Ectocolpus width (μm) |
Granules on foot layer |
Psilate AC |
Perforate AC |
Fossulate AC |
Foveolate AC |
Microreticulate AC |
Reticulate AC |
Psilate MC |
Perforate MC |
Fossulate MC |
Foveolate MC |
Microreticulate MC |
Reticulate MC |
Thickness nexine (μm) |
Thickness exine (μm) |
Sexine/nexine |
Figures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marcgraviabrachysepala | 15–18.6–22 | 15–17.9–21 | − | ± | + | − | 0·29 | 11·5 | 0·6 | − | − | + | − | − | − | − | − | − | − | − | + | + | ||||
| M. brownei | 20–26·0–31 | 22–26·4–30 | + | ± | ± | − | 0·67 | 7·1 | 0·4 | − | − | + | + | − | − | − | − | + | + | − | − | − | 1B | |||
| M. caudata | 18–19·6–20 | 19–20·9–23 | + | ± | − | − | 0·61 | 11·7 | 1·2 | − | − | + | − | − | − | − | − | + | − | + | − | − | ||||
| M. coriacea | 20–22·5–25 | 21–23·4–25 | + | ± | ± | − | 0·45 | 11·8 | 0·5 | + | − | + | − | − | − | − | − | − | − | − | − | + | 0·46 | 0·98 | 1·13 | 1H and 2F |
| M. domingensis | 17–18·4–21 | 17–18·5–21 | ± | + | ± | − | 0·44 | 9·2 | 0·7 | − | − | + | + | − | − | − | − | + | + | − | − | − | ||||
| M. elegans | 17–17·4–19 | 17–17·5–18 | + | ± | ± | − | 0·41 | 12·0 | 1·5 | + | − | + | − | − | − | − | − | + | − | − | + | − | ||||
| M. evenia | 18–18·5–19 | 18–18·7–21 | + | ± | ± | − | 0·53 | 12·2 | 0·8 | − | + | + | − | − | − | − | − | + | − | − | − | − | ||||
| M. evenia subsp. calcicola | 20–20·6–21 | 19–20·1–21 | ± | ± | + | − | 0·38 | 12·0 | 0·9 | − | + | + | − | − | − | − | − | + | − | − | − | − | ||||
| M. flagellaris | 19–19·7–21 | 20–20·1–22 | + | − | − | − | 0·40 | 11·9 | 1·0 | + | − | − | − | − | + | + | − | − | − | − | + | + | ||||
| M. gentlei | 20–22·2–25 | 20–22·2–24 | ± | + | ± | − | 0·45 | 12·8 | 1·1 | + | − | + | − | − | − | − | − | − | − | − | + | + | 1I | |||
| M. hartii | 21–24·4–27 | 21–23·7–25 | − | ± | + | − | 0·40 | 10·0 | 1·5 | + | − | + | − | − | + | − | − | − | − | − | − | + | 0·36 | 1·00 | 1·75 | |
| M. lineolata | 19–20·5–22 | 20–20·5–21 | ± | + | ± | − | 0·48 | 16·0 | 1·4 | + | − | + | − | − | + | − | − | − | − | − | + | + | ||||
| M. macrophylla | 23–24·9–26 | 23–24·4–26 | − | ± | + | − | 0·69 | 7·8 | 1·2 | − | − | − | − | − | + | + | − | − | − | − | + | + | 0·34 | 0·95 | 1·79 | 1L and 4A |
| M. magnibracteata | 23–23·7–25 | 23–24·0–26 | + | ± | − | − | 0·61 | 14·5 | 1·4 | + | − | − | − | − | + | + | − | − | − | − | + | + | ||||
| M. mexicana | 19–21·1–23 | 19–21·7–24 | + | ± | ± | − | 0·60 | 13·7 | 0·9 | + | − | + | − | − | + | − | − | + | − | − | + | − | ||||
| M. nepenthoides | 18–18·5–19 | 18–18·4–19 | ± | + | ± | − | 0·42 | 12·1 | 0·6 | − | + | + | − | − | − | − | − | + | − | − | − | − | 0·38 | 0·86 | 1·26 | |
| M. nervosa | 20–22·3–25 | 20–22·8–25 | + | ± | ± | − | 0·56 | 16·0 | 1·1 | − | − | + | − | − | + | − | − | + | − | − | + | + | 0·65 | 1·25 | 0·95 | 1J and 4C–E |
| M. neurophylla | 32–33·8–36 | 30–31·6–36 | − | ± | + | − | 0·70 | 9·1 | 0·8 | + | − | − | − | − | + | − | − | − | − | − | + | − | ||||
| M. nubicola | 21–22·4–25 | 20–21·6–23 | − | ± | + | − | 0·43 | 12·9 | 0·9 | + | − | + | − | − | − | − | − | − | − | − | − | + | ||||
| M. oligandra | 22–23·3–25 | 21–22·0–23 | − | ± | + | − | 0·40 | 16·0 | 0·7 | − | + | + | − | − | − | − | − | + | − | − | − | − | ||||
| M. panamensis | 18–19·6–23 | 18–20·4–22 | + | ± | ± | − | 0·71 | 8·7 | 1·3 | − | − | + | − | − | − | − | − | + | − | − | − | − | ||||
| M. pedunculosa | 22–24·0–25 | 21–23·0–25 | − | ± | + | − | 0·47 | 8·5 | 0·7 | + | − | + | − | − | − | − | − | − | − | − | − | + | ||||
| M. pittieri | 19–20·3–23 | 19–19·9–21 | ± | ± | + | − | 0·42 | 12·2 | 1·3 | + | − | + | − | − | − | − | − | − | − | − | − | + | ||||
| M. polyantha | 17–19·1–22 | 17–18·9–22 | ± | ± | + | − | 0·45 | 15·3 | 0·8 | − | − | + | − | − | − | − | − | + | − | − | + | − | ||||
| M. purpurea | 20–22·4–24 | 20–22·2–24 | ± | + | − | − | 0·55 | 10·6 | 0·9 | + | − | + | − | − | − | − | − | − | − | − | − | + | ||||
| M. rectiflora | 27–31·3–36 | 28–31·3–35 | ± | ± | + | − | 0·51 | 10·4 | 0·7 | + | − | + | − | − | − | − | − | + | − | − | + | + | 0·75 | 1·50 | 1·00 | 4B and 6D |
| M. schippii | 18–19·5–20 | 20–20·1–21 | + | ± | − | − | 0·53 | 11·5 | 1·2 | + | − | − | − | − | + | + | − | − | − | − | − | + | 0·40 | 0·92 | 1·30 | |
| M. sintenisii | 18–23·0–27 | 19–22·8–25 | ± | ± | + | − | 0·55 | 11·6 | 1·0 | − | − | + | − | − | − | − | − | + | + | − | − | − | 0·38 | 1·08 | 1·84 | 1G, 2D and 3C |
| M. stonei | 17–18·8–20 | 17–18·5–20 | ± | ± | + | − | 0·40 | 14·8 | 0·9 | + | − | + | − | + | − | − | − | + | − | + | − | − | 0·28 | 0·97 | 2·46 | 3E |
| M. tobagensis | 20–21·0–22 | 21–22·3–23 | + | ± | − | − | 0·40 | 12·0 | 1·5 | + | − | + | − | − | − | − | − | − | − | − | − | + | ||||
| M. trianae | 18–18·5–20 | 16–16·9–18 | − | ± | + | ± | 0·44 | 10·8 | 0·6 | − | + | + | − | − | − | − | − | + | + | − | − | − | 0·32 | 0·79 | 1·47 | |
| M. trinitatis | 21–22·3–24 | 22–24·4–26 | + | ± | − | − | 0·53 | 11·0 | 1·2 | + | − | − | − | − | + | + | − | − | − | − | + | + | ||||
| M. umbellata | 21–25·0–28 | 20–24·3–27 | ± | + | ± | − | 0·50 | 16·0 | 1·4 | + | − | + | − | + | − | − | − | + | − | − | + | + | ||||
| Marcgraviastrum cuneifolium | 28–29·0–32 | 25–26·6–28 | − | − | + | ± | 0·45 | 15·2 | 1·3 | + | − | − | − | − | + | − | − | + | − | + | + | − | 0·81 | 1·48 | 0·83 | 1K, 2E, 3A, 5G and 6F |
| M. gigantophyllum | 25–27·0–29 | 23–24·8–26 | − | − | + | ± | 0·56 | 13·9 | 1·0 | − | − | + | − | − | − | − | − | + | − | − | − | − | 0·60 | 1·14 | 0·90 | 6A |
| M. mixtum | 35–36·9–38 | 34–35·1–37 | − | − | + | − | 0·56 | 15·9 | 1·6 | − | − | + | − | − | − | − | − | + | − | − | − | − | 1C | |||
| M. pauciflorum | 33–35·0–37 | 34–35·3–37 | + | ± | − | − | 0·69 | 17·5 | 1·5 | − | − | + | − | − | − | − | − | + | − | − | − | − | ||||
| M. pendulum | 39–41·7–46 | 40–41·0–45 | ± | ± | + | − | 0·59 | 16·9 | 1·2 | − | − | + | − | − | − | − | − | + | − | − | − | − | 0·56 | 1·21 | 1·16 | 5F |
| M. subsessile | 25–27·6–29 | 26–28·3–29 | ± | ± | − | − | 0·61 | 12·0 | 1·5 | − | − | + | − | − | − | − | − | + | − | − | − | − | 0·70 | 1·34 | 0·91 | |
| Norantea goyasensis | 30–32·4–36 | 30–33·0–36 | − | ± | + | − | 0·56 | 14·7 | 2·4 | − | + | − | − | − | − | − | − | + | − | − | − | − | 2·50 | 3·80 | 0·50 | 1E, 5C and 6B |
| N. guianensis subsp. guianensis | 25–26·7–28 | 27–27·7–34 | + | ± | − | − | 0·59 | 15·8 | 0·8 | − | + | − | − | − | − | − | − | + | − | − | − | − | 1·59 | 2·27 | 0·43 | |
| N. guianensis subsp. japurensis | 32–37·0–39 | 29–36·0–39 | − | ± | + | − | 0·63 | 14·6 | 0·8 | − | + | + | − | − | − | − | − | + | − | − | − | − | 1·24 | 2·25 | 0·81 | 5D and 6H |
| Ruyschia andina | 23–26·0–28 | 20–24·2–26 | − | ± | + | ± | 0·45 | 13·5 | 1·0 | − | + | + | − | − | − | − | − | + | − | − | − | − | 0·58 | 1·23 | 1·12 | |
| R. clusiaefolia | 24–24·5–25 | 22–22·7–23 | − | − | + | − | 0·48 | 14·3 | 0·7 | − | + | + | − | − | − | − | + | + | − | − | − | − | ||||
| R. pavonii | 20–20·2–21 | 17–18·0–19 | − | − | + | ± | 0·55 | 12·5 | 1·0 | − | + | + | − | − | − | − | + | + | − | − | − | − | ||||
| R. phylladenia | 23–25·5–28 | 22–23·8–25 | − | − | + | ± | 0·40 | 18·9 | 1·8 | − | + | − | − | − | − | − | + | + | − | − | − | − | ||||
| R. pilophora | 22–23·9–25 | 20–21·1–22 | − | − | + | ± | 0·52 | 17·5 | 0·8 | − | + | + | − | − | − | − | + | + | − | − | − | − | 0·67 | 1·37 | 1·04 | |
| R. tremadena | 29–30·8–34 | 22–25·0–28 | − | − | ± | + | 0·14 | 20·5 | 2·0 | − | + | + | − | − | − | − | + | + | − | − | − | − | 0·66 | 1·25 | 0·89 | 2C |
| Sarcopera anomala | 18–21·0–23 | 21–23·3–25 | + | − | − | − | 0·47 | 9·6 | 0·8 | − | + | + | − | − | − | − | + | + | − | − | − | − | 1·54 | 2·26 | 0·46 | |
| S. flammifera | 35–39·0–42 | 31–36·5–40 | − | − | + | − | 0·73 | 14·2 | 1·2 | − | + | + | − | − | − | − | − | + | − | − | − | − | 1·29 | 1·99 | 0·54 | 1A and 3F |
| S. oxystylis | 21–22·0–23 | 21–23·2–24 | + | ± | − | − | 0·34 | 11·2 | 1·7 | − | + | + | − | − | − | − | − | + | − | − | − | − | 1·22 | 1·90 | 0·55 | |
| S. rosulata | 24–25·1–26 | 26–27·1–28 | + | − | − | − | 0·64 | 10·5 | 1·7 | − | − | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. sessiliflora | 20–21·6–22 | 22–22·4–24 | + | ± | − | − | 0·59 | 10·1 | 2·4 | − | − | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. tepuiensis subsp. coccinea | 23–25·0–28 | 25–27·4–29 | + | ± | − | − | 0·38 | 12·5 | 1·8 | − | − | + | − | − | − | − | − | + | − | − | − | − | 2·50 | 3·20 | 0·30 | 3B and 5A |
| S. tepuiensis subsp. tepuiensis | 30–32·0–35 | 31–32·4–35 | + | ± | ± | − | 0·54 | 13·7 | 1·6 | − | + | + | − | − | − | − | − | + | − | − | − | − | 2·20 | 3·00 | 0·36 | 5B |
| Schwartzia adamantium | 30–31·4–34 | 26–27·6–29 | ± | ± | + | − | 0·38 | 14·9 | 2·5 | − | − | + | − | − | − | − | − | + | + | − | − | − | 0·97 | 1·47 | 0·52 | 1F, 2B and 6C |
| S. brasiliensis | 25–26·3–29 | 25–25·4–27 | ± | ± | + | − | 0·41 | 16·0 | 1·8 | − | + | + | − | − | − | − | − | + | + | − | − | − | 0·90 | 1·47 | 0·63 | |
| S. brenesii | 27–28·9–30 | 28–28·6–30 | ± | + | ± | − | 0·36 | 17·5 | 2·0 | − | − | + | − | − | − | − | − | + | − | − | − | − | ||||
| S. costaricensis | 28–28·9–30 | 29–29·6–30 | + | ± | − | − | 0·32 | 17·0 | 2·5 | − | − | + | − | − | − | − | − | + | − | − | − | − | ||||
| S. magnifica | 30–31·3–33 | 30–30·8–33 | ± | ± | + | − | 0·19 | 19·5 | 1·8 | − | + | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. parrae | 28–31·9–34 | 28–31·8–35 | ± | + | ± | − | 0·55 | 6·3 | 1·3 | − | + | + | − | − | − | − | + | + | − | − | − | − | 1·22 | 1·95 | 0·60 | |
| S. spiciflora | 33–34·3–35 | 33–34·8–37 | + | ± | ± | − | 0·45 | 15·8 | 1·5 | − | − | + | + | − | − | − | − | + | + | − | − | − | 1·66 | 2·31 | 0·39 | 5E |
| Souroubea corallina | 20–21·0–23 | 18–19·2–21 | − | − | + | ± | 0·47 | 10·4 | 0·5 | − | − | + | − | − | − | − | − | + | − | − | − | − | 0·39 | 0·86 | 1·21 | |
| S. exauriculata* | 23–24·4–25 | 23–24·5–25 | ± | + | ± | − | 0·43 | 13·4 | 1·0 | − | + | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. gilgii* | 20–22·4–24 | 18–20·0–21 | − | − | + | ± | 0·35 | 17·5 | 1·5 | − | + | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. loczyi subsp. loczyi* | 20–23·0–24 | 20–20·6–21 | − | ± | + | ± | 0·45 | 17·2 | 1·2 | − | − | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. loczyi subsp. minima* | 19–21·0–23 | 18–19·3–21 | − | − | + | ± | 0·43 | 17·0 | 1·5 | − | − | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. guianensis subsp. amazonica | 23–24·5–26 | 23–23·5–25 | − | ± | + | − | 0·39 | 15·1 | 0·9 | − | + | + | − | − | − | − | + | + | + | − | − | − | 0·50 | 1·00 | 1·00 | |
| S. guianensis subsp. cylindrica | 25–26·6–28 | 23–25·9–28 | ± | ± | + | − | 0·38 | 14·7 | 1·5 | − | + | + | − | − | − | − | + | + | − | − | − | − | 0·77 | 1·61 | 1·09 | 3D, 4F and 6G |
| S. guianensis subsp. guianensis | 26–29·4–33 | 25–29·2–32 | ± | ± | + | − | 0·40 | 14·5 | 1·6 | − | + | + | − | − | − | − | + | + | − | − | − | − | 0·64 | 1·30 | 1·03 | 6E and 4H |
| S. sympetala | 23–27·9–34 | 22–25·4–29 | ± | ± | ± | + | 0·46 | 17·1 | 0·7 | − | − | + | − | − | − | − | − | + | − | − | − | − | 0·45 | 1·11 | 1·60 | 1D, 2A and 4G |
| S. vallicola* | 20–22·9–25 | 20–23·3–25 | ± | + | ± | − | 0·45 | 16·2 | 1·2 | − | + | + | − | − | − | − | + | + | − | − | − | − | ||||
| S. venosa | 21–23·8–28 | 20–21·3–27 | − | − | ± | + | 0·41 | 12·8 | 0·5 | − | + | + | − | − | − | − | + | + | − | − | − | − |
Size measurements are based on acetolysed pollen grains. A blank cell means that no measurements were available. An asterisk indicates that the species were only observed by LM. Numbers refer to mean values, except for P (polar axis length) and E (equatorial axis length) values that correspond to minimum-mean-maximum. AC = apocolpium; MC = mesocolpium; + = present; ± = sometimes present; – = absent.
Pollen grains of all species investigated were acetolysed for 10 min using the method of Reitsma (1969), and studied using a light microscope. LM preparations were made in a glycerine jelly. For SEM observations of pollen and orbicules, dried flowers or buds of all species were hydrated in the wetting agent Agepon® (Agfa Gevaert, Leverkusen, Germany) overnight. The anthers were picked out from the flowers and the tips of the anthers were removed with a razor blade to facilitate hydration of the locules. After dissection, the anthers remained for another hour in the wetting agent. Following dehydration in a graded acetone series, the material was critical point dried (BAL-TEC CPD 030, Balzers, Liechtenstein) and mounted on stubs with double-sided adhesive tape. The pollen grains were gently removed from the opened locules with a fine cactus needle to facilitate observation of the locule surface. The removed pollen grains were collected on the same stubs for further observations. Other pollen grains from the same specimens were also acetolysed, following the method of Reitsma (1969), in order to compare acetolysed grains with critical point-dried grains. The acetolysed pollen grains (suspended in 70 % ethanol) were mounted on stubs and air dried. Pollen grains of about half of the species studied were broken using glass beads (Huysmans et al., 1994).
The stubs were sputter coated with gold (SPI-MODULE™ Sputter Coater, SPI Supplies, West Chester, PA, USA). A Jeol JSM-6360 microscope was used at 10–25 kV for morphological observations. Comparative size measurements of acetolysed and critical point-dried pollen, and orbicules were ascertained from SEM photographs using Carnoy 2·0 (Schols et al., 2002), while the polar axis and equatorial diameter were measured on 10 grains in equatorial view with LM using a camera lucida.
The TEM results of this study are based on Marcgravia nervosa (Maas and Dressler 746; Stevens 23729), M. rectiflora (Fuertes 587), Marcgraviastrum cuneifolium (Irwin et al. 28513), Norantea guianensis subsp. japurensis (Krukoff 8915), Sarcopera flammifera (Agostini 402), S. tepuiensis subsp. tepuiensis (Steyermark 93267), Souroubea guianensis subsp. cylindrica (Krukoff 8702), S. guianensis subsp. guianensis (Gillespie 1200) and S. sympetala (Smith 1555). For TEM observations, anthers of selected flowers were fixed with 2 % glutaraldehyde at pH 7·4 and buffered with 0·05 m sodium cacodylate. Prior to embedding in LR-White Resin (Polysciences Inc., Warrington, PA, USA), the anthers were dehydrated in a graded ethanol series, and block-stained with 1 % phosphotungstic acid (PTA) in 100 % ethanol. Semi-thin (±1 µm) sections were cut with a Reichert Jung Ultracut E microtome, and stained with 0·1 % thionin–0·1 % methylene blue. The semi-thin sections were observed with a Leica DM LB light microscope. The ultra-thin (±70 nm) sections were stained with uranyl acetate and lead citrate in a LKB 2168 Ultrostainer, and observed in a Zeiss EM 900 transmission electron microscope at 50 kV.
Terminology follows the Glossary of Pollen and Spore Terminology (Punt et al., 1994; http://www.bio.uu.nl/~palaeo/glossary/glos-int.htm). Terms for shape classes in equatorial view are adopted from Erdtman (1971).
RESULTS
The palynological description of Marcgraviaceae is arranged according to the following pollen features: size; dispersal unit and shape; apertures; sexine; stratification of the pollen wall; and presence of orbicules. A summary of the results is presented in Table 1.
Size
Acetolysed pollen grains usually range between 20 and 35 µm (Figs 1–3; Table 1). The largest pollen grains are observed in Marcgraviastrum pendulum (on average P, 41·7 µm and E, 41·0 µm), while several Marcgravia species are characterized by grains with a mean value of <20 µm. The size of critical point-dried pollen grains compared with acetolysed grains is always much smaller, generally between 10 and 25 µm.
Fig. 1.
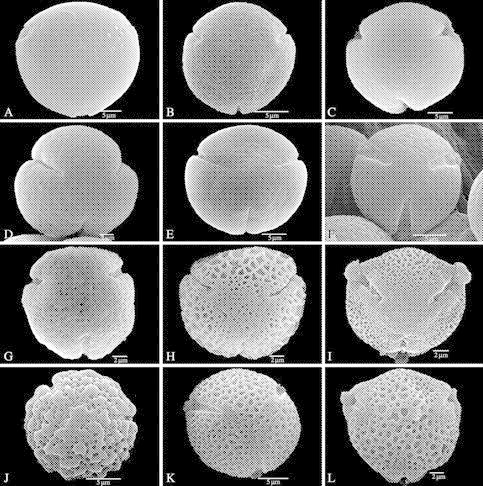
Polar views of Marcgraviaceae pollen grains (SEM) showing a more or less circular amb, a difference in ectocolpus length, and psilate to reticulate sexine patterns on the apocolpium. (A–B, D–E, G–H) treated with acetolysis; (C, F, I–L) critical point dried. (A–F) Psilate apocolpium with few perforations. (A) Sarcopera flammifera (Steyermark 93162). (B) Marcgravia brownei (Burger 8198). (C) Marcgraviastrum mixtum. (D) Souroubea sympetala (Gentle 9254). (E) Norantea goyasensis (Hatschbach 32389). (F) Schwartzia adamantium (Irwin et al. 9718). (G) Marcgravia sintenisii (Webster and Miller 8710), perforate apocolpium. (H) Marcgravia coriacea (Veth and Manou 241), perforate apocolpium that is distinctive from reticulate mesocolpium. (I) Marcgravia gentlei, perforate apocolpium that is distinctive from micro-reticulate mesocolpium. (J) Marcgravia nervosa (Maas and Dressler 746), perforate–verrucate apocolpium. (K) Marcgraviastrum cuneifolium (Glaziou 2937), microreticulate apocolpium. (L) Marcgravia macrophylla, reticulate heterobrochate apocolpium.
Fig. 2.
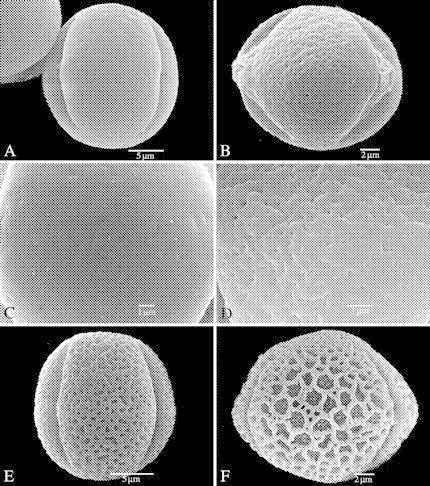
Equatorial views of Marcgraviaceae pollen grains (SEM) showing an oblate spheroidal to prolate spheroidal shape, and a psilate to reticulate sexine pattern on the mesocolpium. (A, C–F) treated with acetolysis; (B) critical point dried. (A) Souroubea sympetala (Gentle 9254), prolate spheroidal shape, psilate mesocolpium. (B) Schwartzia adamantium (Irwin et al. 9718), oblate spheroidal shape, perforate mesocolpium. (C) Ruyschia tremadena (Diederichs 13), detail of psilate mesocolpium. (D) Marcgravia sintenisii (Webster and Miller 8710), detail of fossulate mesocolpium. (E) Marcgraviastrum cuneifolium (Glaziou 2937), prolate spheroidal shape, microreticulate mesocolpium. (F) Marcgravia coriacea (Veth and Manou 241), suboblate spheroidal shape, reticulate heterobrochate mesocolpium, granules on foot layer.
Fig. 3.
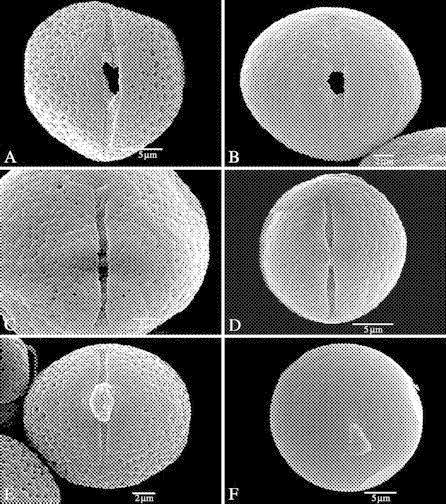
Equatorial views of Marcgraviaceae pollen showing the aperture (SEM). (A–D) treated with acetolysis; (E and F) critical point dried. (A) Marcgraviastrum cuneifolium (Glaziou 2937), ectoaperture with obtuse ends showing an irregularly shaped mesoporus and granules on the colpus membrane; indistinct margo present. (B) Sarcopera tepuiensis subsp. coccinea, ectoaperture with obtuse ends showing a circular mesoporus and granules on the colpus membrane; indistinct margo present. (C) Marcgravia sintenisii (Webster and Miller 8710), ectoaperture showing sharp and fish tail-like ends, granules on colpus membrane. (D) Souroubea guianensis subsp. cylindrica (Cowan and Wurdack 31458), ectoaperture with sharp and obtuse ends. (E) Marcgravia stonei (Reko 4140), protruding intine through porus clearly visible, ectoaperture with obtuse to sharp ends and granules on ectocolpus. (F) Sarcopera flammifera (Steyermark 93162), protruding intine through porus clearly visible, short aperture with sharp ends.
Dispersal unit and shape
Monads occur in all species studied. Spheroidal pollen shapes or P/E values that are close to 1·00 are characteristic of Marcgravia, Norantea and Schwartzia (Fig. 2B), and some grains of Sarcopera (Fig. 3F). In Souroubea and especially Ruyschia, prolate spheroidal to subprolate pollen is typical (Figs 2A and 3D), but prolate spheroidal grains may also occur sometimes in all other genera (Fig. 2E; Table 1). Oblate spheroidal grains are the main type in Sarcopera (Fig. 3B), although this shape is also observed in Marcgravia (Figs 2F and 3E) and Schwartzia (Fig. 2B). Pollen shapes in Marcgraviastrum are variable. The polar view of most grains is circular with sunken colpi (Fig. 1). The shape of acetolysed grains is not different from that of the critical point-dried material.
Apertures
Pollen is 3-zonoaperturate (Fig. 1), but rarely 4-aperturate grains are found in species of Marcgravia, Sarcopera and Schwartzia. The amount of 4-aperturate grains is usually only 5 %, but in a few Marcgravia species this can reach up to 40 %. The apertures are compound, consisting of three units.
Ectocolpi are always present in the family (Figs 1 and 3). The length of the ectocolpus is short to relatively long (Fig. 1; Table 1), which is indicated by a mean apocolpium index of 0·5. The shortest colpi are observed in Schwartzia parrae, and the longest in Ruyschia tremadena (on average 6·3 and 20·5 µm, respectively). Ectocolpi are mostly narrow (Fig. 3C–E), often between 0·5 and 1·5 µm in width (Table 1), but some species of Norantea, Sarcopera and Schwarzia have relatively wide apertures (>2 µm in width; Fig. 3A and B). The ends of the ectocolpi show a lot of variation within the family (Fig. 3) and even within a single grain (Fig. 3C–E). The ends are usually acute or obtuse, but sometimes also fish tail-like, truncate or vague. The margins of the ectoapertures are often obviously demarcated. In all species studied, granules are found on the colpus membranes (Fig. 3A–C and E).
A mesoporus is present in all specimens observed, although it is often not clearly visible in SEM images due to the narrow apertures (Fig. 3). The mesoporus is the region where ecto- and endoaperture overlap, and has a lalongate, circular or rectangular shape. The mesoporus seen from the outside of the grain can be irregularly shaped (Fig. 3A), more or less circular (Fig. 3B) or rectangular (Fig. 3C).
The endoaperture, which is incongruent with the ectoaperture, can be circumscribed as a lalongate colpus-shaped thinning at the inner layer of the exine (Fig. 5E and F). This thinning is always shorter (on average 8·2 µm) and wider (on average 3·1 µm) than the ectocolpus; the ends are obtuse, rectangular, sharp, fish tail-like or diffuse (Fig. 5E–G).
Sexine
Pollen grains in Marcgraviaceae are tectate. The surface of the tectum is generally smooth, but it is bumpy in Marcgravia nervosa (Fig. 1 J).
A perforate sexine type is characteristic of Norantea, Ruyschia, Sarcopera (Fig. 3B), Schwartzia (Figs 1F and 2B)and Souroubea (Figs 1D and 3D). Some of the perforated species have a psilate tectum on the poles, especially in Norantea (1E), Souroubea (Fig. 1D) and Ruyschia, while Ruyschia characteristically shows additionally an almost psilate mesocolpium (Fig. 2C), which is sometimes also clearly present in Sarcopera (Fig. 3F). Perforations are equally distributed in the pollen wall (Fig. 1G, J and K), or they are clearly more abundant on the equator than on the poles (Fig. 1D and F). In some species of Marcgravia, foveolate sexine patterns are observed on the poles, while this sexine pattern is also present on the mesocolpium of Marcgravia and Marcgraviastrum (Fig. 2E). A perforate–verrucate pattern is only observed in Marcgravia nervosa (Fig. 1 J). Fossulate patterns are present on the polar region of Marcgravia brownei and Schwartzia spiciflora, and on the equatorial region of Marcgravia brownei, M. domingensis, M. sintenisii (Fig. 2D), Schwartzia adamantium, S. brasiliensis, S. spiciflora and Souroubea guianensis. About 50 % of the Marcgravia species studied show (micro)reticulate and heterobrochate reticulate sexine sculptures on the equator (Fig. 2F), while the other half show perforate patterns (Figs 1B and G, and 3C and E).
Within Marcgravia, the shape of the lumina is elongated or irregular. Most of the reticulate species have granules on the foot layer in the lumina (Fig. 2F). Within the family, a distinct margo is absent. However, perforations near the apertures become smaller and less frequent, giving the appearance of an indistinct margo (Fig. 3A, B, D and E), except for Norantea and Ruyschia.
Stratification of pollen wall
The inner surface of the nexine is generally scabrate (Fig. 5A–C), which appears granular at the ultrastructural level (Fig. 4B). Clear costae are always absent, but the lamellated endexine may be thickened at the apertural region (Fig. 4H). TEM observations show that the endexine could be distinguished from the foot layer by the differences in electron density between both layers (Figs 4B, D and G, and 5B and D). Sometimes the central portion of the endoaperture is thinned, which is called costae endocolpus by Punt (1971). A one-layered intine is present in all species studied. Non-acetolysed species show protruding intine at the apertures through the porus, resulting in the formation of papillae (Figs 3E and F, and 4E and H).
Fig. 4.
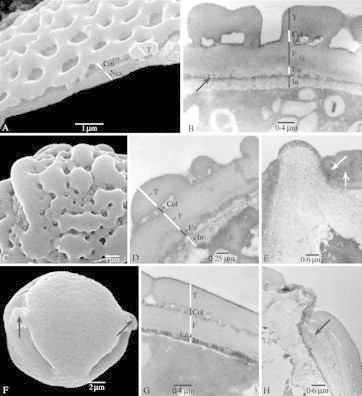
SEM and TEM pictures showing the surfaces and sections of the pollen wall in Marcgravia and Souroubea. (A and C) treated with acetolysis; (B and D–H) critical point dried. (A) Marcgravia macrophylla, SEM, thin pollen wall showing nexine (Nex), columellae (Col) and tectum (T). (B) M. rectiflora (Fuertes 587), TEM, thin pollen wall showing intine (In), endexine (En), foot layer (F), columellae (Col) and tectum (T); the arrow points to granular endexine. (C) M. nervosa (Maas and Dressler 746), SEM, detail of perforate–verrucate sexine ornamentation. (D) M. nervosa (Stevens 23729), TEM, ultrastructure of pollen wall showing intine (In), endexine (En), foot layer (F), columellae (Col) and tectum (T). (E) M. nervosa (Stevens 23729), TEM, protruding intine at the apertural region, endexine lamellated near aperture (arrows). (F) Souroubea guianensis subsp. cylindrica, SEM, equatorial overview showing papillae (arrows). (G) S. sympetala (Smith 1555), TEM, detail of pollen wall showing intine (In), endexine (En), foot layer (F), columellae (Col) and tectum (T). (H) S. guianensis subsp. guianensis (Gillespie 1200), TEM, papillae consisting of protruding intine at the apertural region, endexine lamellated near apertures (arrow).
Fig. 5.
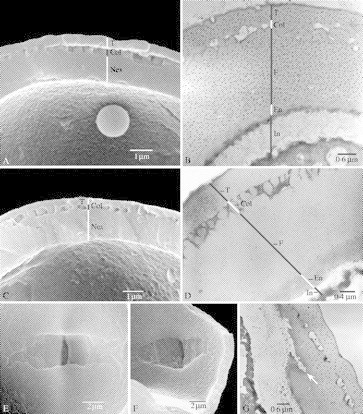
SEM and TEM pictures illustrating the structure of the pollen wall (A–D) and the endoapertures (E–G) in Marcgraviastrum, Norantea, Sarcopera and Schwartzia. (A, C, E and F) treated with acetolysis; (B, D and G) critical point dried. (A) Sarcopera tepuiensis subsp. coccinea, SEM, thick pollen wall showing nexine (Nex), columellae (Col) and tectum (T). (B) S. tepuiensis subsp. tepuiensis (Steyermark 93267), TEM, detail of pollen wall showing intine (In), endexine (En), foot layer (F), columellae (Col) and tectum (T). (C) Norantea goyasensis (Maguire et al. 56587), SEM, thick pollen wall showing nexine (Nex), columellae (Col) and tectum (T). (D) N. guianensis subsp. japurensis (Krukoff 8915), TEM, detail of pollen wall showing intine (In), endexine (E), foot layer (F), columellae (Col) and tectum (T). (E) Schwartzia spiciflora (Steyermark 94954), SEM, inside surface of a pollen grain showing a lalongate endoaperture with fish tail-like ends. (F) Marcgraviastrum pendulum, SEM, inside surface of a pollen grain showing an lalongate endoaperture with obtuse to rectangular ends. (G) M. cuneifolium (Irwin et al. 28513); TEM, endoaperture with sharp ends (arrow).
The thickness of the pollen exine is <1·5 µm in Marcgravia (Fig. 4A, B and D), between 1 and 1·5 µm in Marcgraviastrum, Ruyschia and Souroubea (Fig. 4G and H), and between 1·5 and 4 µm in Norantea, Sarcopera and Schwartzia (Fig. 5A–D). The sexine has a rather homogeneous thickness within the family, and ranges from 0·4 to 0·8 µm. The thickness of the tectum ranges between 0·2 and 0·5 µm, and the columellae vary in length between 0·1 and 0·3 µm. In general, the infratectum is columellar (Fig. 4B, D and G), but in Norantea and Sarcopera granular structures are present (Fig. 5B and D). The nexine shows much more variation in thickness than the sexine: the nexine is thick in Norantea, Sarcopera and Schwartzia, and ranges between 0·9 and 2·5 µm, especially due to a thick foot layer (Fig. 5A–D), while the genera Marcgravia, Marcgraviastrum, Souroubea and Ruyschia show an nexine thickness of 0·4–1 µm, (Fig. 4A, B, D, G and H). As a result, the sexine/nexine ratio is 1·00 or higher in Marcgravia, Souroubea and Ruyschia, while Norantea, Sarcopera and Schwartzia often show low values, often between 0·3 and 0·6. The sexine is more or less equal in thickness to the nexine in Marcgravia, Marcgraviastrum, Ruyschia and Souroubea (Fig. 4A, B, D, G and H).
Orbicules
All taxa examined possess orbicules. The mean length of the longest axis ranges between 0·2 and 2·1 µm. The variation of the size within species and even within specimens is sometimes remarkably high (Fig. 6A). The shape of the orbicules is mostly round or elliptic, sometimes irregular. Orbicules are mostly irregularly scattered throughout the locule wall, and they may have an aggregated distribution in species with a high orbicule density (Fig. 6D–F). Orbicules embedded in the tapetal remnants are found in Norantea guianensis subsp. guianensis, Marcgraviastrum cuneifolium (Fig. 6F) and Souroubea guianensis subsp. amazonica. The wall of the orbicules is always smooth, and lacks any ornamentation (Fig. 6A–F).
Fig. 6.
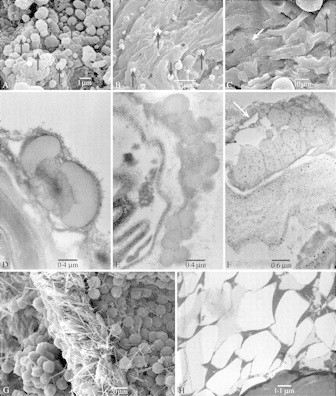
SEM and TEM photographs showing the presence of orbicules (A–F) and raphides (G and H). All critical point dried. (A) Marcgraviastrum gigantophyllum, SEM, irregularly shaped orbicules without ornamentation, densely covering the locule wall (arrows). (B) Norantea goyasensis (Maguire et al. 56587), SEM, sparsely distributed orbicules (arrows). (C) Schwartzia adamantium (Glaziou 20698), SEM, large orbicules (arrows). (D) Marcgravia rectiflora (Fuertes 587), TEM, aggregated orbicules. (E) Souroubea guianensis subsp. guianensis (Gillespie 1200), TEM, densely crowded, aggregated orbicules on the tapetal remnants. (F) Marcgraviastrum cuneifolium (Irwin et al. 28513), TEM, idem, arrow points to remnants of tapetal cells. (G) Souroubea guianensis subsp. cylindrica, SEM, raphides located in the septa of the locule wall. (H) Norantea guianensis subsp. japurensis (Krukoff 8915), TEM, transverse section of raphides.
Mineral inclusions in locule wall
As in most species of balsaminoid Ericales, raphides are found in the septa of the locule wall (Fig. 6G and H).
DISCUSSION
Palynological diversity within Marcgraviaceae
Marcgraviaceae have small (20–35 µm) 3-zonocolporate pollen with oblate spheroidal to prolate spheroidal shapes, psilate, perforate or reticulate ornamentation patterns, granules on the colpus membrane, compound apertures comprising ectocolpi, mesopori and lalongate endoapertures, and orbicules.
Most of our observations are in agreement with previous LM studies (Erdtman, 1952; Barth, 1963; Punt, 1971; Alvarado and Palacios-Chavez, 1987; Roubik and Moreno, 1991; da Silva Correa and Esteves, 1997; Colinvaux et al., 1999), although some differences can be noted. Punt (1971) found mostly oblate spheroidal shapes and reticulate sexine patterns within the Norantea s.l. complex. However, most grains in our study have a spheroidal or prolate spheroidal shape (except for Sarcopera) with a psilate, perforate or foveolate tectum. Additionally, Punt (1971) stated that the colpus membrane does not possess granules, a feature that we observed in every species. Furthermore, the low sexine/nexine ratios of Sarcopera and Schwartzia are not always supported by Punt (1971). This is especially the case for species in his Norantea oxystylis pollen type, of which the S/N ratios of three species are available in this study, i.e. Schwartzia adamantium, S. brasiliensis and Sarcopera oxystilis. On the other hand, the low sexine/nexine ratio of Punt's Norantea anomala type, comprising mostly species of the modern Sarcopera, is supported by our observations.
All previous pollen studies in Marcgraviaceae are based on acetolysed material, and the size measurements of acetolysed pollen in this study agree with previous literature data. However, critical point-dried pollen grains are significantly smaller, usually only 50–70 % of their acetolysed counterparts, but the shape remains the same. The increase of pollen size was discussed intensively in Reitsma (1969), who stated that the pollen grains increase in size exponentially when they make contact with the hot acetolysis mixture, and that the size decreased during the process of acetolysis. However, ‘in most instances acetolysing for three minutes or more does not affect the size significantly’ (Reitsma, 1969, p. 194). More recent studies also corroborate the increase of pollen size after acetolysis, although differences are usually smaller, i.e. between 6 and 30 % (e.g. Schols et al., 2004).
Taxonomic usefulness and intrafamily relationships
This study demonstrates that several palynological data prove to have taxonomic value within the family. Besides the already known morphological differences between Marcgravioideae and Noranteoideae (Dressler, 2004), the presence of a reticulate sexine ornamentation in approx. 50 % of the Marcgravia species observed could provide an additional feature to separate the two subfamilies (Table 1; Dressler, 1994). The absence of reticulate patterns outside Marcgravia is in contradiction to the observations of Punt (1971) who found coarsely reticulate sexine sculpturing with lumina distinctly larger than 1 µm in his Norantea delpiniana (Marcgraviastrum delpinianum) and N. cuneifolia (Marcgraviastrum cuneifolium) types. According to our observations, the sexine pattern of M. cuneifolium varies largely between perforate, microreticulate and foveolate, although there are some small portions of the pollen wall that could be called reticulate (Figs 1K and 2E; Table 1). We cannot comment on the M. delpinianum pattern because of lack of material. Punt (1971) also found ‘finely reticulate’ patterns with lumina usually 1 µm or less in his N. grandiflora type, which includes several Marcgraviastrum species that are not represented in this study, as well as Marcgraviastrum mixtum and Schwartzia spiciflora, which have an exclusively perforate or perforate to fossulate sexine according to our observations. Larger perforations occur in several species of Marcgraviastrum, and the presence of granules on the foot layer could indicate a closer relationship with Marcgravia, as already suggested by de Roon based on the morphology of sclereids (de Roon, 1967). However, this relationship is very vague, since our samples (except for M. cuneifolium) are all perforate and do not show granules on the foot layer (see Table 1).
Within the genus Marcgravia, at least a group with small spheroidal pollen with tectate–perforate sexine, and narrow colpi could be discerned that corresponds to the Galeatae species group, represented here by M. domingensis, M. evenia, M. oligandra, M. sintenisii, M. stonei and M. trianae. This group is morphologically characterized by boat-shaped extrafloral nectar cups and a long inflorescence axis exhibiting a more racemose arrangement of the flowers. However, several other species studied also closely resemble the Galeatae species group, and these include M. caudata, M. hartii, M. mexicana, M. nepenthoides, M. panamensis and M. polyantha. Likewise, the reticulate Marcgravia species are not characteristic for a specific subgenus or species grouping. In order to comment on the systematic value of pollen morphological characters within Marcgravia, we need a more natural intrageneric classification of the genus than available at present.
Within the subfamily Noranteoideae, it is possible to define two groups based on the thickness of the exine and nexine, which is predominantly determined by the thickness of the foot layer (Figs 4 and 5). The first group includes the genera Marcgraviastrum, Ruyschia and Souroubea, and can be identified by a relatively thin exine (approx. 1·5 µm or less). Pollen grains of Souroubea and Ruyschia are similar due to the shared occurrence of prolate spheroidal to subprolate shapes, which is rare in other Marcgraviaceae, and the presence of a psilate to perforate sexine (cf. Punt, 1971). Other morphological characters, such as the low number of stamens or ovary locules, and the sometimes rather unspecialized nectarial bracts, could also point towards a close relationship between these two genera (Dressler, 2004). There are even arguments for reconsidering generic separation of Souroubea and Ruyschia (de Roon, 2005). A possible relationship with Marcgraviastrum could be futher corroborated by the presence of prolate spheroidal to subprolate pollen grains in M. cuneifolium and M. gigantophyllum (Table 1). The second group, consisting of Norantea, Sarcopera and Schwartzia, has pollen grains with a thick exine (between 1·5 and 4 µm), which is mainly caused by a thick nexine layer (between 1 and 2·5 µm). As a result, the second group can be defined by a low sexine/nexine ratio, often ranging from 0·3 to 0·6. Only the presence of a clear oblate spheroidal or suboblate shape might be used to distinguish Sarcopera from the rest, although S. flammifera shows exclusively prolate spheroidal grains. Other species that are oblate spheroidal have a mean P/E value close to 1·00, and could also be considered to be more or less spheroidal.
According to our observations, other palynological characters are phylogenetically less important, and probably have arisen several times. Examples are the length and the width of the ectocolpi, the presence of foveolate sexine ornamentations, the thickness of the sexine layer, and the presence of ‘costae endocolpi’. When we compare our results with the six evolutionary trends postulated by Punt et al. (1994) in Norantea s.l. and applied by Dressler (1994) in Marcgravia, only the P/E ratio, the thickness of the exine and the sexine/nexine ratio seem to have a value for phylogenetic interpretation. However, any palynological trend must be treated with caution, because generic relationships within the family are still not resolved (Ward and Price, 2002; Dressler, 2004).
Relationship of pollen grains to pollinators
Although detailed flower ecological studies are lacking in the family, it seems as if quite a range of pollinators interact with the approx. 130 species. Vogel (1990) proposed five pollination syndromes. According to the literature and our own field observations, one could assume: flies or bees in Ruyschia, butterflies and hawk moths in Souroubea, birds in Marcgravia, Norantea, Sarcopera and Schwartzia brasiliensis, and bats (or also hawk moths) in most Schwartzia species, Marcgraviastrum, and Marcgravia (Sazima and Sazima, 1980; Sazima et al., 1993; Tschapka and von Helversen, 1999; Machado and Lopes, 2000; Dressler and Tschapka, 2002; Dressler, 2004; Tschapka et al., in press). Thus, it seems appropriate to search for a correlation between the palynological diversity and different pollinators.
The palynological data presented here do not demonstrate a clear relationship with the different kinds of pollen vectors. Marcgravia, a genus with a proven high number of bat-pollinated species (Tschapka and von Helversen, 1999; Tschapka et al., in press), is the only taxon in which reticulate sexine patterns are observed, but chiropterophily is certainly abundant in the genera Marcgraviastrum and Schwartzia as well. The only palynological trend in chiropterophilous flowers found by Stroo (2000) was pollen size enlargement (see also Stuchlik, 1967), but our data show that the smallest pollen in the family are found in the genus Marcgravia. The thin exine in Ruyschia and Souroubea could be interpreted as an adaptation to ‘gentle’, non-destructive pollen vectors, as mentioned above, but the thin-walled grains in the chiropterophilous Marcgraviastrum species question this hypothesis.
We can conclude that, based on the fragmentary knowledge of pollination ecology in Marcgraviaceae so far, no correlations between palynological features and pollen dispersal mechanisms are found, which is in agreement with earlier studies (e.g. Taylor and Levin, 1975; Dobat and Peikert-Holle, 1985). It can be assumed that the pollinator–plant relationship finds more reflection in inflorescence morphology and possibly nectar composition (Baker and Baker, 1990; Galetto et al., 1998; Dressler, 2004).
Taxonomic value of orbicules
The phylogenetic usefulness of orbicules has already been proven in several angiosperm taxa (e.g. Hesse, 1986; Raj and El-Ghazaly, 1987; Huysmans et al., 1997; Vinckier and Smets, 2002). However, within Marcgraviaceae, orbicules do not seem to be taxonomically important due to lack of variation.
APPENDIX
Marcgravia brachysepala Urb.: Jamaica, H. R. Wullschlaegel 834 (M); M. brownei (Triana & Planch.) Krug & Urb.: Costa Rica, P. H. Allen 1488 (MO); Costa Rica, W. C. Burger 8198 (U); Jamaica, T. G. Yuncker 18790 (NY); M. caudata Triana & Planch.: Panama, G. D. McPherson 9686 (MO); M. coriacea Vahl: Brazil, P. v. Luetzelburg 21572 (M); French Guyana, B. Veth and Manou 241 (U); French Guyana, J. J. de Granville B-5161 (P); M. domingensis Urb.: Hispaniola, M. D. Fuertes 1503 (W); Hispaniola, A. H. Liogier 13331 (NY); M. elegans Krug & Urb.: Trinidad, W. E. Broadway 5644 (K); M. evenia Krug & Urb.: Cuba, H. F. A. v. Eggers 5722 (K); M. evenia Krug & Urb. subsp. calcicola (Britton) S. Dressler: Cuba, J. T. Roig 2936 (HAC); M. flagellaris Poeppig: Peru, B. Berlin 248 (MO); M. gentlei Lundell: British Honduras, P. H. Gentle 3176 (U); M. hartii Krug & Urb.: Trinidad, A. Fendler 214 (NY); Trinidad, N. L. Britton et al. 2579 (US); Trinidad, J. H. Hart 6587 (K); M. lineolata Krug & Urb.: Dominica, C. Whitefoord 3491 (US); M. macrophylla (Wittm.) Gilg: Peru, E. P. Killip and A. C. Smith 29032 (P); M. magnibracteata Lanj. & Heerdt: Colombia, T.C. Plowman et al. 2321 (P); M. mexicana Gilg: Mexico, H. Wawra 1132 (W); M. nepenthoides Seem.: British Honduras, W. A. Schipp 476 (S); Costa Rica, A. Tonduz 8280 (BR); Panama, T. B. Croat 7033 (NY); M. nervosa Triana & Planch.: Costa Rica, W. D. Stevens 23729 (MO); Costa Rica, P. Maas and R. L. Dressler 746 (NY); Colombia, J. J. Triana s.n. (P); Panama, K. J. Sytsma 1919 (MO); M. neurophylla Gilg: Brazil, A. F. M. Glaziou 13581 (BR); M. nubicola de Roon: Venezuela, J. A. Steyermark et al. 100301 (M); M. oligandra Griseb.: E. Ekman 2514 (S); M. panamensis S.Dressler: Panama, J. F. McDonagh et al. 305 (BM); M. pedunculosa Triana & Planch.: Colombia, J. J. Triana s.n. (P); M. pittieri Gilg: Panama, S. A. Mori and J. Kallunki 4542 (MO); M. polyantha Delpino: Brazil, Magalhaes-Gomez 1311 (P); Brazil, H. Mosen 2893 (P); M. purpurea I.W.Bailey: British Guyana, H. Lang and A. C. Persaud 230 (FR); British Guyana, J. S. de la Cruz 3498 (US); Surinam, J. C. Lindeman et al. 448 (C); M. rectiflora Triana & Planch.: Hispaniola, M. D. Fuertes 587 (U); Puerto Rico, P. Sintensis 269 (S); Puerto Rico, R. J. Wagner 488 (S); M. schippii Standl.: Panama, B. Hammel et al. 4919 (NY); M. sintenisii Urb.: Puerto Rico, G. L. Webster and K. Miller 8710 (U); Puerto Rico, P. Sintensis 5321 (S); Puerto Rico, R. J. Wagner 712 (S); Puerto Rico, R. A. Howard 16813 (BR, S); M. stonei Utley: Mexico, B. P. Reko 4140 (US); Mexico, D. E. Stone and C. R. Broome 2877 (MO); Mexico, S. Dressler s.n., 17·03·2004, (FR); M. tobagensis Urb.: Trinidad, W. E. Broadway 6289 (S); M. trianae Baill.: Venezuela, J. A. Steyermark 91956 (U); M. trinitatis C. Presl: Dominica, D. H. Nicolson 4083 (US); Guadeloupe, H. Stehlé 1998 (US); Trinidad[?], F. W. Sieber 342 (HAL); M. umbellata L.: Dominica, W. H. Hodge 1048 (US); Germany, Munich, Botanical Garden 1/1969 (M); Marcgraviastrum cuneifolium (Gardner) Bedell: Brazil, H. S. Irwin et al. 28513 (U); Brazil, A. F. M. Glaziou 2937 (BR); M. gigantophyllum (Gilg) S. Dressler: Ecuador, M. T. Madison et al. 4554 (U); M. mixtum (Triana & Planch.) Bedell: Venezuela, B. Maguire and J. J. Wurdack 34972 (U); M. pauciflorum de Roon & Bedell: Panama, G. D. McPherson 7803 (MO); M. pendulum (Lanj. & Heerdt) Bedell: Guyana, M. Jansen-Jacobs et al. 3577 (U); M. subsessile (Benth.) Bedell: Panama, J. J. Pipoly 7051 (FR); Norantea goyasensis Cambess.: Brazil, G. Hatschbach 32389 (BR); Brazil, B. Maguire et al. 56587 (U); N. guianensis Aubl. subsp. guianensis: French Guyana, J. J. de Granville et al. 5852 (BR); French Guyana, F. Billiet and B. Jadin 1858 (BR); Suriname, J. G. Wessels Boer 607 (U); N. guianensis subsp. japurensis (Mart.) Bedell: Brazil, B. A. Krukoff 8915 (BR); Brazil, G. T. Prance et al. 24797 (U); Ruyschia andina de Roon [2005]: Ecuador, S. Matezki 453 (FR); R. clusiaefolia Jacq.: Dominica, H. F. A. v. Eggers 781 (FR); R. pavonii G. Don: Peru, M. Weigend et al. 5768 (FR); R. phylladenia Sandwith: Panama, G. D. McPherson 15327 (FR); R. pilophora Triana & Planch.: Colombia, R. Callejas and F. J. Roldan 10690 (FR); R. tremadena (Ernst) Lundell: Venezuela, L. Aristeguieta 4636 (U); Venezuela, E. Diederichs 13 (U); Sarcopera anomala (Kunth) Bedell: Bolivia, M. Thiv s.n. [Bol 60] (FR); Ecuador, B. Klitgaard et al. 652 (FR); S. flammifera de Roon & Bedell: Venezuela, J. A. Steyermark 93162 (U); Venezuela, G. Agostini 402 (U); S. oxystilis (Baill.) Bedell ex Gir.-Cañas: Peru, R. A. Ferreyra 16651 (FR); S. rosulata de Roon & Bedell: Colombia, R. Callejas et al. 8732 (FR); Panama, J. D. Dwyer and M. Nee 11918 (MO); S. sessiliflora (Triana & Planch.) Bedell: Costa Rica, C. Kernan 932 (MO); Nicaragua, W. D. Stevens 12364 (FR); S. tepuiensis (de Roon) Bedell subsp. coccinea de Roon & S. Dressler: Venezuela, J. A. Steyermark and G. S. Bunting 103167 (U); S. tepuiensis (de Roon) Bedell subsp. tepuiensis: Venezuela, J. A. Steyermark 93267 (U); Schwartzia adamantium (Cambess.) Bedell ex Gir.-Cañas: Brazil, A. F. M. Glaziou 20698 (BR); Brazil, H. S. Irwin et al. 9718 (U); S. brasiliensis (Choisy) Bedell ex Gir.-Cañas: Brazil, A. F. M. Glaziou 1356 (BR); Brazil, F.N. 6666 (U); S. brenesii (Standl.) Bedell: Costa Rica, R. B. Primack et al. 445 (MO); S. costaricensis (Gilg) Bedell: Panama, S. D. Knapp 4972 (MO); S. magnifica (Gilg) Bedell: Peru, R. Vasquez and J. Campos 25367 (FR); S. parrae Gir.-Cañas: Colombia, R. Callejas et al. 7957 (FR); S. spiciflora (A.L.Juss.) Bedell: Guadeloupe, F. Billiet and B. Jadin 7351 (BR); Venezuela, J. A. Steyermark 94954 (U); Souroubea corallina (Mart.) de Roon: Peru, J. Torres 123 (U); Peru, M. Rimachi Y. 6425 (BR); S. exauriculata Delpino: Honduras, G. Davidse and R. W. Pohl 2122 (MO); S. gilgii V. A. Richt.: Costa Rica, F. Almeda et al. 3190 (MO); Costa Rica, B. Jacobs 2747 (MO); S. loczyi (V.A.Richt.) de Roon subsp. loczyi: British Honduras, P. H. Gentle 4602 (MO); S. loczyi (V.A.Richt.) de Roon subsp. minima de Roon: Costa Rica, J. Utley and K. Utley 5414 (MO); S. guianensis Aubl. subsp. amazonica (Mart.) de Roon: Brazil, J. Falcao et al. 1220 (U); Brazil, B. A. Krukoff 6222 (BR); S. guianensis Aubl. subsp. cylindrica (Wittm.) de Roon: Brazil, B. A. Krukoff 8702 (BR); Venezuela, R. S. Cowan and J. J. Wurdack 31458 (U); S. guianensis Aubl. subsp. guianensis: French Guyana, P. A. Sagot 75 (BR); Guyana, L. J. Gillespie 1200 (U); S. sympetala Gilg: Belize, P. H. Gentle 2895 (U), Belize, P. H. Gentle 9254 (U); Colombia, H. H. Smith 1555 (BR); Costa Rica, R. W. Lent 2655 (MO); S. vallicola de Roon: Costa Rica, T. B. Croat and M. H. Grayum 59931 (MO); S. venosa Schery: Panama, P. H. Allen 3537 (MO).
Acknowledgments
We thank the directors of the herbaria BM, BR, C, FR, HAC, HAL, K, M, MO, NY, P, S, U, US and W for the supply of flowering buds, and Koen Collart and Anja Vandeperre for technical assistance. This work has been financially supported by research grants of the K.U.Leuven (OT/01/25; PDM/03/145) and the Fund for Scientific Research-Flanders (Belgium) (F.W.O.-Vlaanderen) (G.0104·01, 1·5·069·02, G.0268·04). S.V. and Steven Dessein are postdoctoral fellows of the Fund for Scientific Research-Flanders (Belgium) (F.W.O.-Vlaanderen).
LITERATURE CITED
- Alvarado JL, Palacios-Chávez R. 1987. Catálogo palinólogico para la Flora de Veracruz. No. 35. Familia Marcgraviaceae. Biotica 12: 67–77. [Google Scholar]
- Anderberg AA, Rydin C, Källersjö M. 2002. Phylogenetic relationships in the order Ericales s.l.: analyses of molecular data from five genes from the plastid and mitochondrial genomes. American Journal of Botany 89: 677–687. [DOI] [PubMed] [Google Scholar]
- APG II. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Sociey 141: 399–436. [Google Scholar]
- Bailey IW. 1922. The pollination of Marcgravia: a classical case of ornithophily? American Journal of Botany 9: 370–384. [Google Scholar]
- Baker HG, Baker I. 1990. The predictive value of nectar chemistry to the recognition of pollinator types. Israelian Journal of Botany 39: 157–166. [Google Scholar]
- Barth OM. 1963. Catalago sistematico dos pólens das plantas arbóreas do Brasil Meridional, III—Theaceae, Marcgraviaceae, Ochnaceae, Guttiferae e Quiinaceae. Memorias do Instituto Oswaldo Cruz Rio de Janeiro 61: 89–109. [DOI] [PubMed] [Google Scholar]
- Bedell HG. 1985.A generic revision of Marcgraviaceae. PhD thesis, Department of Botany, University of Maryland. [Google Scholar]
- Bremer B, Bremer K, Heidari N, Erixon P, Olmstead RG, Anderberg AA, Källersjö M, Barkhordarian E. 2002. Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Molecular Phylogenetics and Evolution 24: 274–301. [DOI] [PubMed] [Google Scholar]
- Colinvaux P, De Oliveira PE, Moreno PJE. 1999.Amazon pollen manual and atlas. Amsterdam: Harwood. [Google Scholar]
- Cronquist A. 1988.The evolution and classification of flowering plants, 2nd edn. New York: The New York Botanical Garden. [Google Scholar]
- Dobat K, Peikert-Holle T. 1985.Blüten und Fledermäuse (Chiropterophilie). Senckenberg-Buch 60, Frankfurt/M., Germany. [Google Scholar]
- Dressler S. 1994.Marcgravia L. in der Karibik—Studien zur Evolution und Systematik der Gattung. PhD thesis, Humboldt University, Berlin. [Google Scholar]
- Dressler S. 2004. Marcgraviaceae. In: Kubitzki K, ed. Families and genera of vascular plants, vol. 6. Berlin: Springer-Verlag, 258–265. [Google Scholar]
- Dressler S, Tschapka M. 2002. Bird versus bat pollination in the genus Marcgravia and the description of a new species. [Curtis's] Botanical Magazine, Ser. 6, 19: 104–114. [Google Scholar]
- Erdtman G. 1952.Pollen morphology and plant taxonomy; angiosperms. Stockholm: Almqvist and Wiksell. [Google Scholar]
- Erdtman G. 1971.Pollen morphology and plant taxonomy. Angiosperms. Corrected reprint of 1952 edition. New York: Hafner Publishing. Co. [Google Scholar]
- Erdtman G, Berglund B, Praglowski J. 1961.An introduction to a Scandinavian pollen flora. Stockholm: Almqvist and Wiksell. [Google Scholar]
- Galetto L, Bernardello G, Sosa CA. 1998. The relationship between floral nectar composition and visitors in Lycium (Solanaceae) from Argentina and Chile: what does it reflect? Flora 193: 303–314. [Google Scholar]
- Geuten K, Smets E, Schols P, Yuan Y-M, Janssens S, Küpfer P, Pyck N. 2004. Conflicting phylogenies of balsaminoid families and the polytomy in Ericales: combining data in a Bayesian framework. Molecular Phylogenetics and Evolution 31: 711–729. [DOI] [PubMed] [Google Scholar]
- Hesse M. 1986. Orbicules and the ektexine are homologous sporopollenin concretions in Spermatophyta. Plant Systematics and Evolution 153: 37–48. [Google Scholar]
- Huysmans S, Robbrecht E, Smets E. 1994. Are the genera Hallea and Mitragyna (Rubiaceae-Coptosapelteae) pollen morphologically distinct? Blumea 39: 321–340. [Google Scholar]
- Huysmans S, El-Ghazaly G, Nilsson S, Smets E. 1997. Systematic value of tapetal orbicules: a preliminary survey of the Cinchonoideae (Rubiaceae). Canadian Journal of Botany 75: 815–826. [Google Scholar]
- Janssens S, Vinckier S, Lens F, Dressler S, Smets E. 2005. Palynological variation in balsaminoid Ericales. II. Balsaminaceae, Tetrameristaceae, Pellicieraceae and general conclusions. Annals of Botany 96: 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmath L. 1927. Studie über das Antherentapetum. Oesterreichische Botanische Zeitschrift 76: 235–241. [Google Scholar]
- Lens F, Dressler S, Jansen S, Van Evelghem L, Smets E. 2005. Relationships within balsaminoid Ericales: a wood anatomical approach. American Journal of Botany 92: 941–953. [DOI] [PubMed] [Google Scholar]
- Machado IC, Lopes AV. 2000.Souroubea guianensis Aubl.: quest for its legitimate pollinator and the first record of tapetal oil in the Marcgraviaceae. Annals of Botany 85: 705–711. [Google Scholar]
- Mohl H. 1834.Beiträge zur Anatomie und Physiologie der Gewächse. Erstes Heft, Über den Bau und die Form der Pollenkörner. Bern: Fischer. [Google Scholar]
- Punt W. 1971. Pollen morphology of the genera Norantea, Souroubea, and Ruyschia (Marcgraviaceae). Pollen et Spores 13: 199–232. [Google Scholar]
- Punt W, Blackmore S, Nilsson S, Le Thomas A. 1994.Glossary of pollen and spores terminology. LPP Contributions Series nr 1. Utrecht: Laboratory of Palaeobotany and Palynology Foundation. [Google Scholar]
- Raj B, El-Ghazaly G. 1987. Morphology and taxonomic application of orbicules (Ubisch bodies) in Chloranthaceae. Pollen et Spores 29: 151–166. [Google Scholar]
- Reitsma T. 1969. Size modifications of recent pollen under treatments. Review of Palaeobotany and Palynology 9: 175–202. [Google Scholar]
- de Roon AC. 1967. Foliar sclereids in the Marcgraviaceae. Acta Botanica Neerlandica 15: 585–628. [Google Scholar]
- de Roon AC. 1975.Contributions towards a monograph of the Marcgraviaceae. PhD thesis, University of Utrecht, The Netherlands. [Google Scholar]
- de Roon AC. 2005. A new species of Ruyschia (Marcgraviaceae) from the South American Andes. Novon 15: 414–417. [Google Scholar]
- de Roon AC, Dressler S. 1997. New taxa of Norantea Aubl. s.l. (Marcgraviaceae) from Central America and adjacent South America. Botanische Jahrbücher für Systematik 119: 327–335. [Google Scholar]
- Roubik DW, Moreno PJE. 1991. Pollen and spores of Barro Colorado Island. Monographs in Systematic Botany from the Missouri Botanical Garden 36. [Google Scholar]
- Savolainen V, Chase MW, Hoot SB, Morton CM, Soltis DS, Bayer C, Fay MF, de Bruijn AY, Sullivan S, Qui Y-L. 2000. Phylogenetics of flowering plants based on combined analysis of plastid atpB and rbcL gene sequences. Systematic Biology 49: 306–362. [DOI] [PubMed] [Google Scholar]
- Sazima M, Sazima I. 1980. Bats vistis to Marcgravia myriostigma Tr. et Planch. (Marcgraviaceae) in southeastern Brazil. Flora 169: 84–88. [Google Scholar]
- Sazima I, Buzato S, Sazima M. 1993. The bizarre inflorescence of Norantea brasiliensis (Marcgraviaceae): visits of hovering and perching birds. Botanica Acta 106: 507–513. [Google Scholar]
- Schols P, Dessein S, D'Hondt C, Huysmans S, Smets E. 2002. Carnoy: a new digital measurement tool for palynology. Grana 41: 124–126. [Google Scholar]
- Schols P, Es K, D'Hondt C, Merckx V, Smets E. 2004. A new enzyme-based method for the treatment of fragile pollen grains collected from herbarium material. Taxon 53: 777–782. [Google Scholar]
- da Silva Correa AM, Esteves LM. 1997. Flora polínica da reserva do parque estadual das Fontes do Ipiranga (São Paulo, Brasil). Hoehnea 24: 171–174. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, et al. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381–461. [Google Scholar]
- Stroo A. 2000. Pollen morphological evolution in bat pollinated plants. Plant Systematics and Evolution 222: 225–242. [Google Scholar]
- Stuchlik L. 1967. Pollen morphology and taxonomy of the family Polemoniaceae. Review of Palaeobotany and Palynology 4: 325–333. [Google Scholar]
- Takhtajan AL. 1997.Diversity and classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- Taylor TN, Levin DA. 1975. Pollen morphology of Polemoniaceae in relation to systematics and pollination systems: scanning electron microscopy. Grana 15: 91–112. [Google Scholar]
- Tschapka M, von Helversen O. 1999. Pollinators of syntopic Marcgravia species in Costa Rican lowland rain forest: bats and opossums. Plant Biology 1: 382–388. [Google Scholar]
- Tschapka M, Dressler S, von Helversen O. 2005. Bat visits to Marcgravia pittieri Gilg and notes on inflorescence diversity within the genus Marcgravia Flora 201: in press. [Google Scholar]
- Vinckier S, Smets E. 2002. Systematic importance of orbicule diversity in Gentianales. Grana 41: 158–182. [Google Scholar]
- Vogel S. 1990. Radiacion adaptativa del sindrome floral en las familias neotropicales. Boletin de la Academia Nacional de Ciencias 59: 5–30. [Google Scholar]
- Ward MN, Price RA. 2002. Phylogenetic relationships of Marcgraviaceae: insights from three chloroplast genes. Systematic Botany 27: 149–160. [Google Scholar]


