Abstract
• Background and Aims The objective of this study is to examine the palynological diversity of Balsaminaceae (two genera/±1000 species), Tetrameristaceae (two genera/two species) and Pellicieraceae (one genus/one species). The diversity found will be used to infer the systematic value of pollen features within the balsaminoid clade.
• Methods Pollen morphology and ultrastructure of 29 species, representing all families of the balsaminoid clade except Marcgraviaceae, are investigated by means of light microscopy, scanning electron microscopy and transmission electron microscopy.
• Key Results Balsaminaceae pollen is small to medium sized with three to four apertures, which can be either colpate or porate, and a sexine sculpturing varying from coarsely reticulate to almost microreticulate. Tetrameristaceae pollen is small sized, 3-colporate, with a heterobrochate reticulate sculpturing and granules present in the lumina. Pellicieraceae pollen is large sized, 3-colporate with long ectocolpi and a perforate sexine sculpturing with large verrucae. Furthermore, Pelliciera is characterized by the occurrence of aggregated orbicules, while orbicules are completely absent in both Balsaminaceae and Tetrameristaceae. Balsaminaceae pollen differs from the other balsaminoid families due to the occurrence of colpate or porate grains with an oblate to peroblate shape, a very thin foot layer and a lamellated endexine.
• Conclusions From a pollen morphological point of view, Balsaminaceae are completely different from the other balsaminoid families. Therefore, no pollen morphological synapomorphies could be defined for the balsaminoid clade. However, various pollen features were observed that could indicate a possible relationship between Tetrameristaceae, Pellicieraceae and Marcgraviaceae. Despite the palynological similarities in the latter three families, it remains unclear to what extent they are related to each other.
Keywords: Balsaminaceae, Ericales, orbicules, Pellicieraceae, pollen, SEM, TEM, Tetrameristaceae
INTRODUCTION
According to recent molecular analyses, balsaminoid Ericales form a well-supported clade comprising Balsaminaceae, Marcgraviaceae, Pellicieraceae and Tetrameristaceae (Anderberg et al., 2002; Bremer et al., 2002). The four families include 12 genera and >1130 species (Dressler, 2004; Fischer, 2004; Kubitzki, 2004a, b). Balsaminaceae, consisting of the monotypic genus Hydrocera and the large genus Impatiens, is by far the largest family with ±1000 species and occurs mainly in the montane regions in the Old World tropics and subtropics (Grey-Wilson, 1980b; Fischer, 2004). It is a family of predominantly annual and perennial herbs with a semi-succulent habitus, which can be easily distinguished from other balsaminoids by its zygomorphic flowers with a spurred petaloid sepal (Grey-Wilson, 1980b). Marcgraviaceae are a small neotropical family comprising approx. 130 species and seven genera (Bedell, 1985; Dressler, 2004). Representatives of the family are confined to an area ranging from northern Bolivia to southern Mexico including the West Indian islands. Most representatives are lianas, while climbing shrubs and small trees can also be found. Marcgraviaceae are easy to recognize by their often brightly coloured nectariferous bracts (Dressler, 2004) and they are characterized by their various kinds of sclereids (de Roon, 1967). Pelliciera rhizophorae, which is the only representative of the Pellicieraceae, is a mangrove tree growing in the tidal swamps along the Pacific Coast from Costa Rica to Ecuador (Kobuski, 1951), and more sparsely on the Atlantic coast of Nicaragua, Panama and Columbia (Roth and Grijalva, 1991). Pelliciera has solitary flowers that are accompanied by two large coloured prophylls. These prophylls might refer to the coloured bracts of Marcgraviaceae and the petaloid sepals of Balsaminaceae (Albert et al., 1998; Geuten et al., 2003). Only two monotypic genera are present in the Tetrameristaceae. Both Pentamerista and Tetramerista are woody: the former shrubby or forming small trees, the latter moderate to large-sized trees. They show a disjunct distribution pattern. Tetramerista glabra occurs only in Malaysia and parts of Sumatra and Borneo, while P. neotropica is endemic to the Guyana Highlands of Venezuela (Maguire et al., 1972; Kubitzki, 2004b). Recently, Pellicieraceae and Tetrameristaceae have been united in Tetrameristaceae s.l. (Bremer et al., 2002; APG II, 2003).
The balsaminoid clade, which is sister to all other ericalean families, is one of the few groups in Ericales that is well supported by molecular sequence data (Savolainen et al., 2000; Soltis et al., 2000; Anderberg et al., 2002; Bremer et al., 2002; APG II, 2003; Geuten et al., 2004). In the past, several botanists considered there to be a close relationship between Marcgraviaceae, Pellicieraceae and Tetrameristaceae (Cronquist, 1988; Takhtajan, 1997), and Hallier (1916, 1921) even treated them as one family. On the other hand, the inclusion of Balsaminaceae is somewhat surprising considering the aberrant systematic position of this family in previous morphological classifications. Traditionally, Balsaminaceae were included in Geraniales (Cronquist, 1988; Thorne, 2000), while some important taxonomists treated Balsaminaceae as an order; the Balsaminales (Dahlgren, 1989; Takhtajan, 1997). Although there is strong molecular evidence for the monophyly of the balsaminoids (Yuan et al., 2004), the interfamilial relationships remain obscure. Based on molecular data, it is obvious that Pelliciera is closely related to Tetrameristaceae, and should probably be regarded as one family Tetrameristaceae s.l. (Bremer et al., 2002; APG II, 2003). Both families are characterized by the presence of unusual glandular pits on the inner surface of the sepals and one single ovule per locule (Cronquist, 1981), and they share a similar wood structure (Lens et al., 2005a). However, the question of whether Tetrameristaceae s.l. are more related to Marcgraviaceae than to Balsaminaceae is still a matter of dispute. According to Anderberg et al. (2002), Balsaminaceae are the most basal family and are sister to the Marcgraviaceae–Tetrameristaceae group. Bremer et al. (2002) suggest a basal position for Marcgraviaceae, while Geuten et al. (2004) found that Balsaminaceae and Marcgraviaceae share the most common ancestor.
The intrafamilial relationships in Balsaminaceae and Marcgraviaceae remain unclear. Within Balsaminaceae, Hydrocera is sister to Impatiens (Yuan et al., 2004; Janssens et al., in press). Due to hybridization, high species radiation and the extremely high diversity of floral morphological features, the relationships within the large Impatiens complex are extremely difficult to resolve (Warburg and Reiche, 1895; Hooker, 1905; Grey-Wilson, 1980b). Recently, Yuan et al. (2004) carried out a molecular study based on ITS sequences in order to understand the phylogenetic insights of the family. Although the results showed some strongly supported lineages, the overall relationships within Impatiens could not be resolved. Also intrafamilial relationships within Marcgraviaceae remain unclear. Based on differences in shape and position of the nectariferous bracts and the large variation in inflorescences, Marcgraviastrum, Sarcopera and Schwartzia were elevated from the Norantea s.l. complex (de Roon and Dressler, 1997). A preliminary molecular study based on chloroplast sequences corroborated two subfamilies, i.e. Marcgravioideae (including Marcgravia) and Noranteoideae (including all other genera), but the generic relationships within the Noranteoideae were poorly resolved (Ward and Price, 2002).
The pollen morphological diversity within the balsaminoid clade is partially known. In 1968, Huynh carried out a palynological study on Balsaminaceae using light microscopy (LM) only. Later, Bhaskar and Razi (1973) described a new sexine sculpturing (granulate sexine) in Impatiens based on the LM observations of two species. The use of scanning electron microscopy (SEM) made it possible for Grey-Wilson (1980a) and Lu (1991) to examine Balsaminaceae pollen more accurately. Unfortunately, due to the limited number of species in both surveys, the pollen morphology could only be briefly discussed. Marcgraviaceae pollen has already been examined intensively using LM observations (Punt, 1971). In addition to our results, Lens et al. (2005b) present a detailed pollen morphological description of the family based on LM, SEM and transmission electron microscopy (TEM). Maguire et al. (1972) studied the pollen morphology of Tetrameristaceae. Pollen grains of Pellicieraceae have been examined light microscopically by Wijmstra (1968) and Graham (1977), and were illustrated by one SEM micrograph (Maguire et al., 1972).
This study aims to present detailed pollen morphological descriptions of Balsaminaceae, Tetrameristaceae and Pellicieraceae based on LM, SEM and TEM, including observations of the poorly known pollen wall and the inside ornamentation of the grains. In addition, this survey also traces the presence of orbicules (Erdtman et al., 1961) or Ubisch bodies (Kosmath, 1927), which are small sporopollenin particles that develop simultaneously with the growing pollen exine and occur on the wall of tapetum cells. Together with the pollen data of Lens et al. (2005b), our palynological observations will be used to comment on the interfamilial relationships within the balsaminoid clade.
MATERIALS AND METHODS
Flowering buds from 24 species of Impatiens, and one species of Hydrocera, Pelliciera, Tetramerista and Pentamerista were removed from herbarium specimens at BR, NY, U and L (Appendix). Species of Impatiens were carefully chosen based on previous palynological studies in order to reflect the palynological variety in Balsaminaceae (Huynh, 1968; Bashkar and Razi, 1973; Lu, 1991). Moreover, our sampling represents a large part of the geographic and taxonomic diversity in the family.
For SEM of orbicules and pollen, dried flowers or buds were rehydrated in the wetting agent Agepon® (Agfa Gevaert, Leverkusen, Germany) overnight. The anthers were picked out from the flowers, and the tips of the anthers were removed with a razor blade to facilitate rehydration of the locules. After dissection, the anthers remained overnight in the wetting agent. Following dehydration in a graded acetone series, all the material was critical point dried (CPD 030, Balzers) and mounted on stubs with double-sided adhesive tape before further preparation. The pollen grains were removed from the opened locules with a fine cactus needle to facilitate observation of the locule wall. The removed pollen grains were collected on the same stubs for further observations. In addition, pollen grains of Tetramerista, Pentamerista and Pelliciera were acetolysed, following the method of Reitsma (1969). Acetolysis of Balsaminaceae grains was not successful due to the very thin pollen wall, which caused the grains to collapse. However, some selected species were acetolysed to observe the inner pollen wall. In order to compare Balsaminaceae pollen with other balsaminoids, only critical point-dried material was taken into account. LM preparations were made in glycerine jelly.
The stubs were sputter coated with gold (SPI-MODULE™ Sputter Coater, SPI Supplies, West Chester, PA, USA). We used a Jeol JSM-6400 microscope, at 25 kV, for morphological observations. Comparative size measurements of pollen and orbicules were ascertained from SEM micrographs of critical point-dried material using Carnoy 2.0 (Schols et al., 2002).
For TEM observations, anthers of selected flowers were fixed with 2 % glutaraldehyde at pH 7·4, buffered with 0·05 m sodium cacodylate. Selected anthers are embedded in LR-White Resin (Polysciences Inc., Warrington, PA, USA). Prior to embedding, the material was dehydrated in a graded ethanol series, and block stained with 1 % phosphotungstic acid (PTA) in 100 % ethanol. Semi-thin (±1 µm) sections were cut with a Reichert Jung Ultracut E microtome and stained with 0·1 % thionin–0·1 % methylene blue. The semi-thin sections were observed with a Leica DM LB light microscope. The ultra-thin (±70 nm) sections were stained with uranyl acetate and lead citrate in an LKB 2168 Ultrostainer, and were observed in a Zeiss EM 900 transmission electron microscope at 50 kV.
Terminology follows the Glossary of Pollen and Spore Terminology (Punt et al., 1994; http://www.bio.uu.nl/~palaeo/glossary/glos-int.htm). The terminology of pollen shape in polar view follows Reitsma (1970). Terms for shape classes in equatorial view are adopted from Erdtman (1971).
RESULTS
For each family investigated, LM, SEM and TEM results are combined to present a complete pollen description. For each genus examined, the nominator gives the number of studied species and the denominator includes the total number of species. A summary of the results is presented in Table 1.
Table 1.
Summary of palynological characters within Balsaminaceae, Pellicieraceae and Tetrameristaceae
| Family |
Species |
P (µm) |
E (µm) |
P/E |
Shape |
No. of apertures |
Aperture shape |
Margo present |
Granules in lumina |
Granules on the ectocolpus |
Sexine pattern |
Orbicules present |
Figures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balsaminaceae | Hydrocera triflora | 12·76–(13·36)–13·86 | 19·2–(20·89)–22·12 | 0·64 | Oblate | 3 | Colpus | − | + | − | Reticulate | − | 1A and B, 3A and B, and 5F |
| Impatiens acaulis | 12·65–(13·26)–14·01 | 23·33–(24·38)–25·56 | 0·544 | Oblate | 3 | Porus | − | − | − | Reticulate | − | − | |
| Impatiens amphorata | 12·28–(14·17)–14·93 | 30·46–(31·83)–33·1 | 0·445 | Peroblate | 4 | Colpus | − | + | − | − | − | − | |
| Impatiens arguta | 14·08–(14·84)–15·53 | 39·53–(40·11)–40·98 | 0·37 | Peroblate | 4 | Colpus | − | + | − | Reticulate | − | 3E and F | |
| Impatiens balfourii | ? | 31·01–(31·94)–33·88 | ? | ? | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Impatiens balsamina | ? | 28·55–(29·33)–31·4 | ? | ? | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Impatiens biflora | ? | 27·98–(28·71)–29·73 | ? | ? | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Impatiens briartii | 20·79–(21·38)–21·87 | 33·63–(34·45)–34·96 | 0·621 | Oblate | 4 | Porus | − | + | − | Reticulate | − | 1G and H | |
| Impatiens burtoni | 12·91–(13·41)–14·07 | 34·71–(35·25)–36·32 | 0·381 | Peroblate | 4 | Colpus | − | + | − | Reticulate– rugulate | − | 2A and B | |
| Impatiens capensis | 10·22–(11·13)–12·05 | 24·5–2(5·39)–26·08 | 0·438 | Peroblate | 4 | Colpus | − | + | − | Reticulate | − | 2D | |
| Impatiens fischeri | 18·91–(19·37)–19·93 | 28·08–(28·91)–29·74 | 0·67 | Oblate | ? | Porus | − | − | − | Reticulate | − | − | |
| Impatiens gesneroidea | 29·56–(30·19)–30·76 | 39·02–(41·41)–43·03 | 0·729 | Oblate | ? | Colpus | + | − | − | Reticulate | − | − | |
| Impatiens glandulifera | 10·82–(11·38)–12·11 | 24·68–(25·74)–26·61 | 0·442 | Peroblate | 4 | Colpus | − | + | − | Reticulate | − | 2C and 5B | |
| Impatiens hawkeri | 31·45–(32·19)–32·85 | 40·81–(41·01)–42·28 | 0·785 | Suboblate | ? | Porus | − | − | − | Reticulate | − | − | |
| Impatiens irvingii | 28·04–(28·95)–29·62 | 33·74–(34·57)–35·87 | 0·875 | Suboblate | 3 | Porus | − | + | − | Reticulate | − | 1E and F | |
| Impatiens kamerunensis | 22·03–(23·72)–24·9 | 28·39–(29·04)–29·93 | 0·817 | Suboblate | 3 | Colpus | − | + | − | Reticulate | − | 1C and D, and 3C and D | |
| Impatiens keilii | 12·2–(13·64)–14·51 | 40·48–(41·94)–43·43 | 0·325 | Peroblate | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Impatiens kilimanjari | ? | 30·21–(31·44)–32·7 | ? | ? | 3 | Colpus | − | + | − | Reticulate | − | 4A and B, and 5E | |
| Impatiens noli–tangere | ? | 22·66–(23·08)–23·65 | ? | ? | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Impatiens piufanensis | 14·98–(15·57)–16·32 | 31·98–(32·76)–34·47 | 0·475 | Peroblate | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Impatiens racemosa | 16·2–(17·03)–17·94 | 32·27–(33·62)–34·09 | 0·505 | Oblate | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Impatiens rubromaculata | 22·54–(23·13)–23·7 | 31·54–(32·14)–33·27 | 0·719 | Oblate | 3 | Porus | + | − | − | Reticulate | − | 3G and H, and 5C | |
| Impatiens tinctoria | 24·23–(24·84)–25·54 | 47·81–(49·05)–52·21 | 0·506 | Oblate | 4 | Colpus | − | + | − | Reticulate | − | 5D | |
| Impatiens textori | 10·11–(10·72)–11·26 | 26·83–(27·59)–28·25 | 0·388 | Peroblate | 4 | Colpus | − | + | − | Reticulate | − | − | |
| Pellicieraceae | Pelliciera rhizophorae | 50·87–(52·32)–54·06 | 54·10–(55·84)–57·93 | 0·937 | Spheroidal | 3 | Colpus | − | − | + | Perforate– verrucate | + | 2E and F, 4C and D, 5A, G and H |
| Tetrameristaceae | Pentamerista neotropica | 24·59–(25·96)–27·67 | 15·54–(16·33)–16·90 | 1·589 | Prolate | 3 | Colpus | + | + | + | Reticulate | − | − |
| Tetramerista glabra | 23·99–(24·98)–25·88 | 24·56–(25·26)–26·73 | 0·989 | Spheroidal | 3 | Colpus | + | + | + | Reticulate | − | 2G and H, and 4E and F |
P = minimum-(mean)-maximum polar axis; E = minimum-(mean)-maximum equatorial axis; + = present; − = absent; ? = unknown.
Balsaminaceae (Impatiens 24/±1000, Hydrocera 1/1; Figs 1, 2A–D, 3, 4A and B, and 5B–F)
Fig. 1.
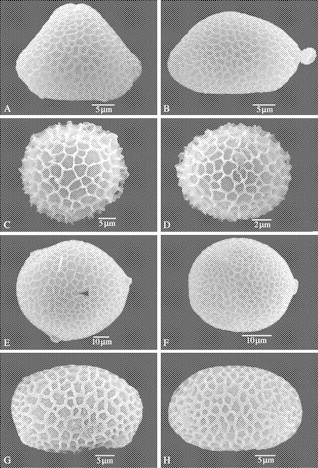
SEM of Balsaminaceae pollen grains. (A and B) Hydrocera triflora. (A) Polar view of a reticulate 3-colpate pollen grain with triangular shape. (B) Equatorial view. (C and D) Impatiens kamerunensis. (C) Polar view of a reticulate pollen grain. (D) Equatorial view. (E and F) Impatiens irvingii. (E) Polar view of a 3-porate pollen grain with a circular outline. Coalescence between the adjacent lumina caused by an incomplete surrounding by the muri (black arrowhead). (F) Equatorial view. (G and H), Impatiens briartii. (G) Polar view of a reticulate 4-colpate pollen grain with ellipsoid–rectangular shape. (H) Equatorial view.
Fig. 2.
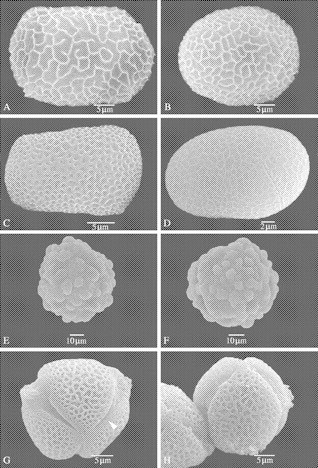
SEM of pollen of Balsaminaceae (A–D), Pellicieraceae (E and F) and Tetrameristaceae (G and H). (A and B) Impatiens burtoni. (A) Polar view of a reticulate–rugulate 4-colpate pollen. (B) General view. (C) Impatiens capensis: polar view of a reticulate 4-colpate pollen grain. (D) Impatiens glandulifera: equatorial view of a reticulate 4-aperturate pollen grain with elongated colpi. (E and F) Pelliciera rhizophorae. (E) Polar view of a large sized, 3-colporate perforate–verrucate pollen grain. (F) Equatorial view of a pollen grain with elongated ectocolpi. (G and H) Tetramerista glabra. (G) Polar view of a 3-colporate pollen grain with large elongated ectocolpi. Note the granules in the lumina and on the aperture membrane. A margo is present (see white arrowhead). (H) Equatorial view.
Fig. 3.
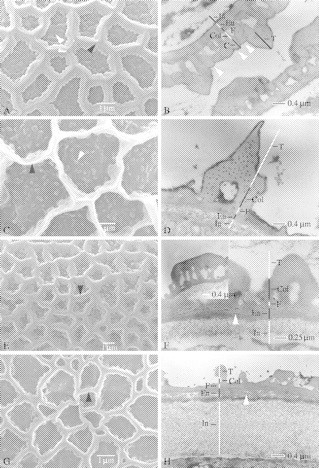
SEM and TEM of pollen of Balsaminaceae. (A and B) Hydrocera triflora. (A) Detailed view of the reticulate ornamentation. The muri have a characteristic triangular–obtuse shape (black arrowhead). The numerous, large granules present merge together (white arrowhead). (B) TEM observation of the pollen wall. The granules in the lumina (white arrowhead) represent caputs (C) that merge to form a continuous tectum supported by columellae (col). En, endexine; In, intine; F, footlayer; T, tectum. (C and D) Impatiens camerunensis. (C) Detailed view of the reticulate sexine. The muri are sharply crested (black arrowhead). The granules are fused in clusters of two or three (white arrowhead). (D) TEM observation of the pollen wall. (E and F) Impatiens arguta. (E) Detailed view of the heterobrochate reticulate sexine. The muri have an obtuse shape (black arrowhead). (F) TEM observation of the pollen wall. The endexine (en) has a lamellated appearance, and remnants of the white line (white arrowhead) can be observed. (G and H) Impatiens rubromaculata. (G) Observation of the reticulate sexine consisting of rectangular muri (black arrowhead). (H) Detailed TEM observation of the pollen wall. The prominent intine and lamellated endexine are striking. Remnants of the white line can be observed in the endexine (white arrowhead).
Fig. 4.
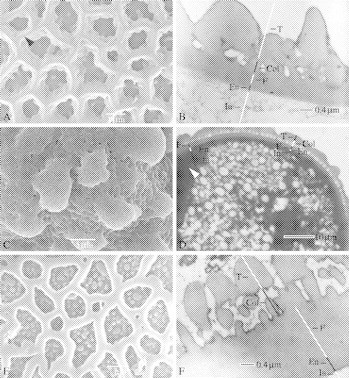
SEM, TEM and LM of Balsaminaceae (A and B), Pellicieraceae (C and D) and Tetrameristaceae (E and F). (A and B) Impatiens kilimanjari. (A) Detailed observation of the reticulate sexine with sharply crested muri (black arrowhead). (B) Detailed TEM observation of the pollen wall. (C and D) Pelliciera rhizophorae. (C) Observation of the perforate–verrucate sexine. (D) Detailed LM observation of the pollen wall. (E and F) Tetramerista glabra. (E), Many granules occur in the lumina of the heterobrochate reticulate exine. (F) Detailed TEM observation of the pollen wall. Abbreviations are the same as in Fig. 3.
Fig. 5.
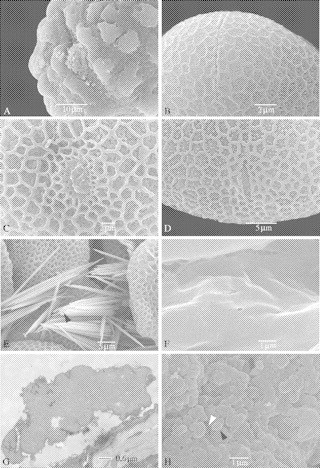
SEM and TEM pictures illustrating the apertures (A–D) and the anther locule (E–H) of Balsaminaceae (B–F) and Pellicieraceae (A and G–H). (A) Pelliciera rhizophorae: detailed observation of an ectocolpus. (B) Impatiens glandulifera: detailed observation of the narrow elongated colpus. (C) Impatiens rubromaculata: detailed view of a porus and the margo. (D) Impatiens tinctoria: detailed observation of a small colpus. (E) Impatiens kilimanjari: calcium oxalate raphides (black arrowhead) are present in the locule between the pollen grains. (F) Hydrocera triflora: smooth locule wall surface. (G and H) Pelliciera rhizophorae. (G) Detailed TEM observation of an aggregated orbicule. (H) Orbicules (black arrowhead) have an irregular shape and cover the whole locule wall surface. Interconnecting threads occur between individual orbicules (white arrowhead).
Size
Pollen grains are generally small to medium sized [P, 10·7–(18·5)–32·2 µm; and E, 20·9–(32·3)–49·0 µm (mean measurements); Table 1]. The largest pollen grains are observed in Impatiens tinctoria (P, 25 µm; E, 49 µm) and I. hawkeri (P, 32 µm; E, 41 µm), whereas the smallest grains are present in I. textori (P, 11 µm; E, 28 µm) and Hydrocera triflora (P, 13 µm; E, approx. 21 µm; Table 1).
Shape
The pollen grain shape is generally oblate to peroblate (Figs 1B and H, and 2B and D), while suboblate pollen is only observed in I. kamerunensis (Fig. 1C and D), I. hawkeri and I. irvingii (Fig. 1E and F). The outline of Balsaminaceae pollen in polar view is rectangular in most 4-aperturate grains (Figs 1G, and 2A and C). The polar view of the 3-aperturate grains is usually subcircular (Fig. 1C and E). Only in H. triflora is the amb view triangular with straight sides (Fig. 1A).
Apertures
Pollen grains are 3-aperturate in Hydrocera (Fig. 1A and B), and 3- to 4-aperturate in Impatiens (Figs 1C–H and 2A–D; Table 1). Apertures are simple and consist only of an ectocolpus or an ectoporus (Fig. 5B–D).
Ectoapertures
Apertures consist only of an ectocolpus (Fig. 5B and D) or an ectoporus (Fig. 5C) with margins that are always obviously demarcated. The largest ectoapertures are observed in H. triflora (length 14·5 µm; width 0·4 µm; Fig. 1A and B) and I. piufanenesis (length 10·9 µm; width 0·2 µm); and the smallest ones are observed in I. fischeri (length 3·4 µm; width 0·5 µm) and I. rubromaculata (length 3·9 µm; width 2·1 µm; Fig. 5C). The percentage of ectocolpi in the species examined is significantly higher than ectopori (71 vs. 29 %, respectively). The ends of the ectocolpi are acute or obtuse. In Balsaminaceae, granules are always absent on the ectoaperture membranes (Fig. 5B–D).
Sexine
The sexine is reticulate (Figs 1, and 2C and D), except in I. burtoni, which is reticulate–rugulate (Fig. 2A and B). Several differences in the reticulate pattern are observed. Most of the species are reticulate homobrochate, but heterobrochate pollen (Figs 3G and 5C) is also present within the family. The longest axis of the lumina ranges from 1·07 mm in I. keilii to 4·60 mm in I. hawkeri. The density of the lumina fluctuates from 0·06 mm−2 in I. hawkeri to 0·66 mm−2 in I. noli-tangere. In some species, the lumina are not completely surrounded by the muri, causing coalescence between the adjacent lumina (I. briartii, I. gesneroidea and I. irvingii; Fig. 1E, see black arrowhead). The thickness and the shape of the muri differ between the species. The shape of the muri varies from sharply crested (Fig. 3A and C, see black arrowhead), to rectangular (Fig. 3G, see black arrowhead) to obtuse (Figs 3E and 4A, see black arrowhead). Most of the pollen grains have granules on the inside of the lumina. These granules can be solitary (e.g. I. tinctoria) to fused in twos or threes (Fig. 3C, see white arrowhead), or even completely merged together (e.g. H. triflora; Fig. 3A, see white arrowhead). A small margo is only present in I. rubromaculata (Fig. 5C) and I. gesneroidea.
Stratification of the pollen wall
In all Balsaminaceae species studied, a one-layered intine is present, which is thick in I. rubromaculata (Fig. 3H). The endexine is sometimes lamellated (Fig. 3F and H, see white arrowhead). The foot layer is thin (0·16 mm, mean; data not shown) in comparison with other ektexine layers (Fig. 3B, D, F and H). The supratectal muri are always supported by two to three collumellae (Fig. 3B, D and H). In Hydrocera, the granules present in the lumina as seen with SEM (Fig. 3A, see white arrowhead) represent columellae supporting caputs (Fig. 3B, see white arrowheads).
Orbicules
Orbicules are absent (Fig. 5F)
Pellicieraceae (Pelliciera 1/1; Figs 2E and F, 4C and D, and 5A, G and H)
Size
Pollen grains of Pelliciera rhizophorae are medium sized [P, 50·9–(52·3)–54·1 µm; and E, 54·1–(55·8)–57·9 µm (mean measurements); Table 1].
Shape
Pellicieraceae pollen is oblate spheroidal (Fig. 2F). The outline of the grains is circular in equatorial view and triangular in polar view (Fig. 2E and F).
Apertures
Pollen grains are 3-colporate. Long ectocolpi (33·7 µm) with acute ends are always present in P. rhizophorae (Fig. 5A). Ectoaperture membranes are covered with granules (Fig. 5A).
Sexine
Pollen grains in Pellicieraceae are tectate. Pelliciera rhizophorae has a perforate sexine with large (>5 mm diameter) verrucae (Figs 2E and F, 4C and 5A). Perforations have a circular shape (on average 0·3 mm diameter), and are completely absent on the verrucae (Figs 4C and 5A). The large verrucae are psilate and have an average diameter of 7·2 mm.
Stratification of the pollen wall
Ultrastructurally, the endexine can be distinguished from the foot layer by differences in staining properties between both layers (Fig. 4D). A thick foot layer (1·32 mm, mean; data not shown) and tectum (0·84 mm, mean; data not shown) are present. The infratectum is columellar. At the apertural region, the endexine and intine are thicker (Fig. 4D, see white arrowhead).
Orbicules
The aggregated orbicules have an irregular shape and cover the whole locule wall surface (Fig. 5G and H, see black arrowhead). They have a psilate surface and are often aggregated in compound orbicules. Thin interconnecting threads of sporopollenin are present between the orbicules (Fig. 5H, see white arrowhead). The orbicules can be embedded within the tapetal remnants.
Tetrameristaceae (Pentamerista 1/1, Tetramerista 1/1; Figs 2G and H, and 4E and F)
Size
Pollen grains are small sized [P, 24·98–(25·5)–25·96 µm; and E, 25·26–(20·8)–16·33 µm (mean measurements)], with both genera almost having equally sized pollen grains (Table 1).
Shape
Tetramerista pollen is oblate spheroidal (Fig. 2H), whereas Pentamerista has prolate pollen. The outline in polar view is circular in both genera (Fig. 2G). The equatorial view is elliptic in Pentamerista grains and circular in Tetramerista pollen (Fig. 2H).
Apertures
Tetrameristaceae pollen grains are 3-colporate (Fig. 2G and H). Long ectocolpi with acute ends are present in both genera. Ectoaperture membranes are always covered with small granules (Fig. 2G and H).
Sexine
Both Tetramerista (Fig. 2G and H) and Pentamerista have pollen grains with a heterobrochate reticulate sexine. However, lumina are smaller at the poles, forming a perforate sexine. The reticulate pollen of Pentamerista and Tetramerista has large granules within the lumina (Fig. 4E). A margo is present (Fig. 2G, see white arrowhead).
Stratification of the pollen wall
The endexine is generally smooth to finely scabrate. A thick foot layer (0·9 mm, mean; data not shown) and tectum (1·13 mm, mean; data not shown) are present. The infratectum possesses a columellar–granular appearance. At the apertural region, the endexine and intine are enlarged in thickness. A thin very electron-dense peripheral layer lines the tectum and infratectum (Fig. 4F).
Orbicules
Orbicules are absent.
DISCUSSION
Balsaminaceae
Balsaminaceae have small oblate to peroblate pollen shapes, colpate or porate grains, reticulate sexine ornamentation, a thin foot layer, a one-layered intine, a lamellated endexine and lack of orbicules (Table 1). The number of apertures varies from three to four between the species (Table 1). The presence of 5-aperturate pollen in Impatiens, which is typical for hybrids according to Grey-Wilson (1980a), could not be corroborated by us. Also, the type of apertures varies from colpi to pori, with several species showing intermediate apertures (Fig. 5B–D). Several different reticulate patterns can be recognized. Furthermore, the reticulate–rugulate pollen of I. burtoni can be considered as an additional kind of sexine pattern in Balsaminaceae (Fig. 2A and B). Previous LM observations in Balsaminaceae described the occurrence of spinulate (Huynh, 1968) and granulate (Bhaskar and Razi, 1973) ornamentation in some species of Impatiens. However, our observations of I. acaulis clearly demonstrate a reticulate sexine, and not a granulate ornamentation as mentioned by Bhaskar and Razi (1973). Likewise, other aberrant sexine ornamentations observed should be re-examined. Another pollen character within Balsaminaceae that shows a distinct variation is the granule density in the lumina. Granules can be either very scarce within the lumina (Fig. 3G), or they can be fused with each other forming a dense mass (Fig. 3A). Many intermediate states are present among the species.
According to Grey-Wilson (1980a), hybridization within Impatiens probably gave rise to many convergent pollen characters, which makes pollen morphological features difficult to use taxonomically. In the present study, however, we were able to find some support for a few closely associated taxa established by Grey-Wilson (1980b) and Yuan et al. (2004). For instance, Grey-Wilson (1980b) considered I. irvingii and I. rubromaculata to be closely related within the African Impatiens. This is confirmed by our palynological data, which show that both species are 3-porate and have a very similar heterobrochate reticulate sexine with rectangular shaped muri (Figs 1E and F, and 3G). Furthermore, the ITS data of Yuan et al. (2004) and chloroplast spacer data of Janssens et al. (in press), have demonstrated that 3-colpate pollen grains with a triangular, oblate shape and elongated colpi are the basic pollen type in Balsaminaceae. This type of pollen is observed in Hydrocera triflora and some Impatiens on mount Omei in China (Lu, 1991). Lu suggested that these basal Chinese species consisting of I. omeiana and its 3-colpate, triangular shaped allies might be closely related to Hydrocera triflora.
In future studies, a more extensive pollen study will be carried out within Balsaminaceae in order to find palynological support for additional subclades that are established by molecular data.
Tetrameristaceae
Tetrameristaceae are a stenopalynous family with small sized, 3-colporate pollen, and a heterobrochate reticulate sculpturing (cf. Maguire et al., 1972). Ectoaperture membranes and lumina are always covered with granules (Fig. 2G and H). Pollen grains in Pentamerista are prolate, whereas Tetramerista has oblate spheroidal pollen (Table 1). Both genera lack orbicules. It can be concluded that the grains of Tetramerista and Pentamerista strongly resemble each other.
Pellicieraceae
Pelliciera rhizophorae has larger pollen than found in the other genera. It is 3-colporate with long ectocolpi and granules present on the ectoaperture membrane (Table 1; Figs 2E and F, and 5A). The sexine is clearly perforate with large verrucae (Fig. 5A), and orbicules are present (Fig. 5G and H). The observations of Graham (1977), on the other hand, showed a variable sexine ornamentation in Pelliciera, ranging from reticulate to microreticulate with mound-like verrucae. He also observed a fluctuation in grain size from 40 to 90 mm.
Interfamily relationships within the balsaminoid clade
From a palynological point of view, it is noticeable that Balsaminaceae are very different from the other balsaminoid families due to the occurrence of colpate or porate grains with an oblate to peroblate shape (Table 1), a very thin foot layer and a lamellated endexine (Fig. 3F and H). Consequently, it is possible to divide the balsaminoid Ericales into two major groups, i.e. Balsaminaceae and Marcgraviaceae–Pellicieraceae–Tetrameristaceae. Based on the molecular results of S. Janssens et al. (unpubl. res.), we conclude that the 3-aperturate pollen grains with a reticulate sculpturing represent the primitive pollen type in Balsaminaceae. This type is also present in Tetrameristaceae (Table 1) and Marcgraviaceae (Lens et al., 2005b), and might be the basal condition within balsaminoids as a whole. Nevertheless, it remains very difficult to identify the sister group relationship between the latter three families based on pollen morphological features only.
The strong molecular and morphological arguments that unite Tetrameristaceae and Pellicieraceae into one family could not be corroborated by our palynological results (Bremer et al., 2002; APG II, 2003). Both Tetramerista and Pentamerista have small sized pollen with a heterobrochate reticulate sculpturing, whereas pollen grains in Pelliciera show a characteristic perforate sexine with large verrucae (Table 1). Moreover, orbicules are absent in Tetrameristaceae, while aggregated types occur in Pellicieraceae (Fig. 5G and H). Despite these dissimilarities, both families are characterized by colporate apertures, and a pollen wall including a well developed nexine and sexine.
Features that could indicate a possible relationship between Tetrameristaceae and Marcgraviaceae are the prolate spheroidal to oblate spheroidal pollen shapes, the small grain size, the presence of colporate apertures and a pollen wall including a well developed nexine and sexine. Moreover, the presence of a coarse reticulate sexine pattern with granules in the lumina of several Marcgravia species could contribute to this (Lens et al., 2005b). On the other hand, it is interesting to note that orbicules are completely absent within Tetrameristaceae, while they are present in every species of Marcgraviaceae studied.
Pellicieraceae pollen is distinct within the balsaminoid clade because of its large size and its perforate sexine with large verrucae. A relationship with Marcgraviaceae is favoured because of the peculiar sexine pattern, which is also found in M. nervosa (Lens et al., 2005b). In addition, both Marcgraviaceae and Pellicieraceae have 3-colporate pollen with elongated ectocolpi and granules on the ectoaperture membrane. Furthermore, aggregated orbicules are present in both species (Fig. 5G and H).
Balsaminoids vs. other Ericales
Although balsaminoids are a strongly supported subclade within Ericales based on molecular data (Anderberg et al., 2002; Bremer et al., 2002; Geuten et al., 2004), there are no pollen morphological synapomorphies that could define this basal group. The only morphological synapomorphy that is still valid is the presence of raphides (Fig. 5E, see black arrowhead; Kubitzki, 2004a, b; Lens et al., 2005a).
Within balsaminoid families, there are some palynological features that are rare in the order as a whole. This is especially the case for Balsaminaceae that could easily be distinguished from other Ericales due to a combination of single apertures, oblate pollen shapes and a distinctive pollen wall. Single apertures are also found in Asteranthos and Grias (Lecythidaceae; Muller, 1979), Lissocarpa (Ebenaceae; Harley, 1991) and Afrostyrax (Styraceae; Harley, 1991), but they are never unique to a family. Another peculiar pollen feature is the perforate–verrucate ornamentation of Pelliciera (Figs 2E and F, and 5A) and M. nervosa, which is also present in species of Polemoniaceae (Taylor and Levin, 1975), Sapotaceae (Harley, 1986), Symplocaceae (van der Meijden, 1970) and Sarraceniaceae (Thanikaimoni and Vasanthy, 1972). Pollen features that are more common, such as 3-colporate grains, are typical of most ericalean families except for Polemoniaceae (Taylor and Levin, 1975) and Sarraceniaceae (Thanikaimoni and Vasanthy, 1972). Likewise, reticulate sexine patterns are found in several families, i.e. Polemoniaceae (Taylor and Levin, 1975), Fouquieriaceae (Henrickson, 1967), Theaceae (Morton and Dickison, 1992), Symplocaceae (van der Meijden, 1970) and Lecythidaceae (Muller, 1979).
APPENDIX
Specimens examined
Balsaminaceae A. Rich.
Hydrocera triflora Wight & Arn.: Malaysia (Botanic Garden Singapore), Md. Shah 27; Sri Lanka, A.G. Robyns 7260 (BR),
Impatiens acaulis Arn.: Sri Lanka, Kostermans 26871 (L),
Impatiens amphorata Edgew.: France, B. de Ketz 5566 (BR),
Impatiens arguta Hook.f. & Thoms.: China, R.R. Stewart 1962 (L),
Impatiens balfourii Hook.f.: United Kingdom, J.R. Palmer 0835392 (BR-S.P.),
Impatiens balsamina L.: India, F. Billiet & J. Leonard 6485 (BR),
Impatiens biflora Walter: Canada, M. Pils (BR),
Impatiens briartii Wildem. & T. Durand.: Democratic Republic of Congo, Detilleux 260 (BR),
Impatiens burtoni Hook.f.: Democratic Republic of Congo, Deru 204 (BR),
Impatiens capensis Meerb.: Canada, V. Blais, G. Deshaies & P. Forest 10831 (BR),
Impatiens fischeri Warb.: Kenya, P. Bamps 6717 (BR),
Impatiens gesneroidea Gilg: Democratic Republic of Congo, F. Hendrickx 5375 (BR),
Impatiens glandulifera Royle: Canada, A. Legault & P. Forest 9081; Russia, S. Kharkevich & T. Buch 585 (BR),
Impatiens hawkeri W.Bull: Papua New Guinea (eastern highlands), V. Demoulin 7046 (BR),
Impatiens irvingii Hook.f.: tropical Africa, W.J.J.O. de Wilde & B.E.E. de Wilde-Duyfjes (BR),
Impatiens kamerunensis Warb.: Republic of Equatorial Guinea (Bioko), Carvalho 2778; Cameroon, R. Letouzey 14292 (BR),
Impatiens keilii Gilg: Burundi (Muramrya), J. Lewalle 1451 (BR),
Impatiens kilimanjari Oliver: Tanzania, R.B. Drummond & J.H. Hemsley 1287 (BR),
Impatiens macroptera Hook.f.: Cameroon, R. Letouyez 13582 (BR),
Impatiens piufanensis Hook.f.: China (Guizhou), Sino-American Guizhou Botanical Expedition 1309 (BR),
Impatiens rubromaculata Warb.: Kenya, C.H.S. Kabuye 69 (BR),
Impatiens tinctoria A. Rich.: Ethiopia (Kaffa), I. Friis, G. Aweke, F. Rasmussen & K. Vollesen 1605 (BR),
Impatiens textori Miq.: Japan, H. Kubata 1022 (BR).
Tetrameristaceae Hutch.
Pentamerista neotropica Maguire: Venezuela (Amazonas, Cerro Yapacana base), B. Maguire 30480A (NY), Venezuela (Amazonas, Rio Atabapo), J.J. Wurdack & L. Adderley 42865 (NY),
Tetramerista glabra Miq.: Borneo (Kalimantan Barat), T.G. Laman 984; Borneo (West Koetai), van Steenis 29577 (NY); Brunei (Belait), K.M. Wong 133 (L).
Pellicieraceae (Triana & Planch.) L. Beauvis. ex Bullock
Pelliciera rhizophorae Planch. & Triana: Panama, S. Dressler s.n. (FR).
Acknowledgments
The directors of the National Botanic Garden of Belgium (BR), the New York Botanical Garden (NY) and the herbaria of Leiden (L) and Utrecht (U) are greatly acknowledged for their supply of flowering buds. We thank Koen Collart, An Vandoren (TEM, K.U.Leuven), Natascha Steffanie (TEM, L.U.C), Anja Vandeperre (K.U.Leuven) and Marcel Verhaegen (SEM, BR) for technical assistance. This work has been financially supported by research grants from the K.U.Leuven (OT/01/25, PDM/03/145) and the Fund for Scientific Research-Flanders (Belgium) (F.W.O.-Vlaanderen) (G.0268.04). S.V. is a postdoctoral fellow of the Fund for Scientific Research-Flanders (Belgium) (F.W.O.-Vlaanderen). K.G. is a PhD bursary of the K.U.Leuven (OT/01/25).
LITERATURE CITED
- Anderberg AA, Rydin C, Källersjö M. 2002. Phylogenetic relationships in the order Ericales s.l.: analyses of molecular data from five genes from the plastid and mitochondrial genomes. American Journal of Botany 89: 677–687. [DOI] [PubMed] [Google Scholar]
- Albert AA, Gustafsson MHG, DiLaurenzio L. 1998. Ontogenetic systematics, molecular developmental genetics, and the angiosperm petal. In: Soltis, DE, Soltis, PS, Doyle JJ, eds. Molecular systematics of plants II, DNA sequencing. Norwell, MA: Kluwer Academic Publishers, 349–374. [Google Scholar]
- APG II. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436. [Google Scholar]
- Bedell HG. 1985.A generic revision of Marcgraviaceae. PhD Thesis, Department of Botany, University of Maryland, USA. [Google Scholar]
- Bhaskar V, Razi BA. 1973. A new kind of exine sculpturing in Impatiens L. (Balsaminaceae) from South India. Current Science 42: 510–512. [Google Scholar]
- Bremer B, Bremer K, Heidari N, Erixon P, Olmstead RG, Anderberg AA, Källersjö M, Barkhordarian E. 2002. Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Molecular Phylogenetics and Evolution 24: 274–301. [DOI] [PubMed] [Google Scholar]
- Cronquist A. 1981. An integrated system of classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- Cronquist A. 1988.The evolution and classification of flowering plants, 2nd edn. New York: The New York Botanical Garden. [Google Scholar]
- Dahlgren G. 1989. An updated angiosperm classification. Botanical Journal of the Linnean Society 100: 197–203. [Google Scholar]
- Dressler S. 2004. Marcgraviaceae. In: Kubitzki K, ed. Families and genera of vascular plants, vol. 6. Berlin: Springer-Verlag, 258–265. [Google Scholar]
- Erdtman G. 1971.Pollen morphology and plant taxonomy. Angiosperms. Corrected reprint of 1952 edition. New York: Hafner Publishing Company. [Google Scholar]
- Erdtman G, Berglund B, Praglowski J. 1961.An introduction to a Scandinavian pollen flora. Stockholm: Almqvist and Wiksell. [Google Scholar]
- Fischer E. 2004. Balsaminaceae. In: Kubitzki K, ed. Families and genera of vascular plants, vol. 6. Berlin: Springer-Verlag, 20–25. [Google Scholar]
- Geuten K, Becker A, Yuan YM, Theissen G, Smets E. 2003. MADS-box genes in flowers of Balsaminaceae and Marcgraviaceae. In: Bayer C, Dressler S, Schneider J, Zizka G, eds. 16th International Symposium Biodiversity and Evolutionary Biology, 17th International Senckenberg Conference, 21–27 September 2003. Abstracts: 157. Palmarum Hortus Francofurtensis 7. Frankfurt am Main: Palmgarten der Stadt Frankfurt am Main, 264. [Google Scholar]
- Geuten K, Smets E, Schols P, Yuan YM, Janssens S, Küpfer P, Pyck N. 2004. Conflicting phylogenies of balsaminoid families and the polytomy in Ericales: combing data in a Bayesian framework. Molecular Phylogenetics and Evolution 31: 711–729. [DOI] [PubMed] [Google Scholar]
- Graham A. 1977. New records of Pelliciera (Theaceae/Pelliceriaceae) in the Tertiary of the Caribbean. Biotropica 9: 48–52. [Google Scholar]
- Grey-Wilson C. 1980. Hybridisation in African Impatiens Studies in Balsaminaceae. Kew Bulletin 34: 689–722. [Google Scholar]
- Grey-Wilson C. 1980. Impatiens of Africa. Rotterdam: Balkema. [Google Scholar]
- Hallier H. 1916. Beiträge zur Flora von Borneo. Marcgraviaceae. Beihefte zum Botanischen Centralblatt 34: 35–40. [Google Scholar]
- Hallier H. 1921. Beiträge zur Kenntnis der Linaceae (DC. 1819) Dumort. Beihefte zum Botanischen Centralblatt 39: 1–178. [Google Scholar]
- Harley M. 1986. Distinguishing pollen characters for the Sapotaceae. Canadian Journal of Botany 64: 3091–3100. [Google Scholar]
- Harley M. 1991. Pollen morphology of the Sapotaceae. In: Peddington TD, ed. The genera of Sapotaceae. Kew: Royal Botanical Gardens, 23–50. [Google Scholar]
- Henrickson J. 1967. Pollen morphology of the Fouquieriaceae. Aliso 6: 137–160. [Google Scholar]
- Hooker JD. 1905. An epitome of the British Indian species of Impatiens Records of the Botanical Survey of India 4: 1–58. [Google Scholar]
- Huynh KL. 1968. Morphologie du pollen des Tropaeolacées et des Balsaminacées I & II. Grana Palynologica 8: 88–184; 277–516. [Google Scholar]
- Janssens SB, Geuten K, Yuan YM, Song Y, Küpfer P, Smets E. In press Phylogenetics of Impatiens and Hydrocera (Balsaminaceae) using chloroplast atpB-rbcL spacer sequences. Systematic Botany. [Google Scholar]
- Kobuski CE. 1951. Studies in the Theaceae, XXIII. The genus Pelliciera Journal of the Arnold Arboretum 32: 256–262. [Google Scholar]
- Kosmath L. 1927. Studie über das Antherentapetum. Österreichische Botanische Zeitschrift 76: 235–241. [Google Scholar]
- Kubitzki K. 2004. Pellicieraceae. In: Kubitzki K, ed. Families and genera of vascular plants, vol. 6. Berlin: Springer-Verlag, 297–299. [Google Scholar]
- Kubitzki K. 2004. Tetrameristaceae. In: Kubitzki K, ed. Families and genera of vascular plants, vol. 6. Berlin: Springer-Verlag, 461–462. [Google Scholar]
- Lens F, Dressler S, Jansen S, Van Evelghem L, Smets E. 2005. Relationships within balsaminoid Ericales: a wood anatomical approach. American Journal of Botany 92: 941–953. [DOI] [PubMed] [Google Scholar]
- Lens F, Dressler S, Vinckier S, Janssens S, Smets E. 2005. Palynological variation in balsaminoid Ericales. I Marcgraviaceae. Annals of Botany 96: 1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YQ. 1991. Pollen morphology of Impatiens L. (Balsaminaceae) and its taxonomic implications. Acta Phytotaxonomica Sinica 29: 352–357. [Google Scholar]
- Maguire B, de Zeeuw C, Huang YC, Clare CC. 1972. Tetrameristaceae. In: Maguire B. The botany of the Guyana Highland–Part IX. Memories of the New York Botanical Garden 23: 165–192. [Google Scholar]
- van der Meijden R. 1970. A survey of the pollenmorphology of the Indo-Pacific species of Symplocos (Symplocaceae). Pollen and Spores 12: 513–551. [Google Scholar]
- Morton CM, Dickison WC. 1992. Comparative pollen morphology of the Styracaceae. Grana 31: 1–15. [Google Scholar]
- Muller J. 1979. V. Pollen. Lecythidaceae. Part I. In: Prance GT, Mori SA,eds. The actinomorphic-flowered New World Lecythidaceae (Asteranthos, Gustavia, Grias, Allantoma, and Cariniana). Flora Neotropica, Monograph, 72–76. [Google Scholar]
- Punt W. 1971. Pollen morphology of the genera Norantea, Souroubea, and Ruyschia (Marcgraviaceae). Pollen and Spores 13: 199–232. [Google Scholar]
- Punt W, Blackmore S, Nilsson S, Le Thomas A. 1994.Glossary of pollen and spores terminology. LPP Contributions Series nr 1. Utrecht: Laboratory of Palaeobotany and Palynology Foundation, [Google Scholar]
- Reitsma T. 1969. Size modifications of recent pollen under treatments. Review of Palaeobotany and Palynology 9: 175–202. [Google Scholar]
- Reitsma T. 1970. Suggestions towards a unifications of descriptive terminology of angiosperm pollen grains. Review of Palaeobotany and Palynology 10: 39–60. [Google Scholar]
- de Roon AC. 1967. Foliar sclereids in the Marcgraviaceae. Acta Botanica Neerlandica 15: 585–628. [Google Scholar]
- de Roon AC, Dressler S. 1997. New taxa of Norantea Aubl. s.l. (Marcgraviaceae) from Central America and adjacent South America. Botanische Jahrbücher für Systematik 119: 327–335. [Google Scholar]
- Roth LC, Grijalva A. 1991. New record of the mangrove Pelliciera rhizophorae (Theaceae) on the Caribbean coast of Nicaragua. Rhodora 93: 183–186. [Google Scholar]
- Savolainen V, Chase MW, Hoot SB, Morton CM, Soltis DE, Bayer C, Fay MF, de Bruijn AY, Sullivan S, Qui YL. 2000. Phylogenetics of flowering plants based on combined analyses of plastid atpB and rbcL gene sequences. Systematic Biology 49: 306–362. [DOI] [PubMed] [Google Scholar]
- Schols P, Dessein S, D'Hondt C, Huysmans S, Smets E. 2002. Carnoy: a new digital measurement tool for palynology. Grana 41: 124–126. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, et al. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381–461. [Google Scholar]
- Takhtajan A. 1997.Diversity and classification of flowering plants. New York: Cambridge University Press. [Google Scholar]
- Taylor T, Levin D. 1975. Pollen morphology of Polemoniaceae in relation to systematics and pollination systems: scanning electron microscopy. Grana 15: 91–112. [Google Scholar]
- Thanikaimoni G, Vasanthy G. 1972. Sarraceniaceae: palynology and systematics. Pollen and Spores 14: 143–155. [Google Scholar]
- Thorne RF. 2000. The classification and geography of the flowering plants: dicotyledons of the class angiospermae: (subclasses Magnoliidae, Ranunculidae, Caryophyllidae, Dilleniidae, Rosidae, Asteridae, and Lamiidae). Botanical Review 66: 441–647. [Google Scholar]
- Warburg O, Reiche K. 1895. Balsaminaceae. In: Engler A, Prantl K, eds. Die natürlichen Planzenfamilien. T. 3, Abt. 5. Leipzig: W. Engelmann, 383–392. [Google Scholar]
- Ward MN, Price RA. 2002. Phylogenetic relationships of Marcgraviaceae: insights from three chloroplast genes. Systematic Botany 27: 149–160. [Google Scholar]
- Wijmstra TA. 1968. The identity of Psilatricolporites and Pelliciera. Acta Botanica Neerlandica 17: 114–116. [Google Scholar]
- Yuan YM, Song Y, Geuten K, Rahelivololona E, Wohlhauser S, Fischer E, Smets E, Küpfer P. 2004. Phylogeny and biogeography of Balsaminaceae inferred from ITS sequence data. Taxon 53: 391–403. [Google Scholar]


