Abstract
• Background and Aims Plants have complex mechanisms of aerial biomass exposition, which depend on bud composition, the period of the year in which shoot extension occurs, branching pattern, foliage persistence, herbivory and environmental conditions.
• Methods The influence of water availability and temperature on shoot growth, the bud composition, the leaf phenology, and the relationship between partial leaf fall and branching were evaluated over 3 years in Cerrado woody species Bauhinia rufa (BR), Leandra lacunosa (LL) and Miconia albicans (MA).
• Key Results Deciduous BR preformed organs in buds and leaves flush synchronously at the transition from the dry to the wet season. The expansion time of leaves is <1 month. Main shoots (first-order axis, A1 shoots) extended over 30 d and they did not branch. BR budding and foliage unfolds were brought about independently of inter-annual rainfall variations. By contrast, in LL and MA evergreen species, the shoot extension rate and the neoformation of aerial organs depended on rainfall. Leaf emergence was continuous for 2–6 months and lamina expansion took place over 1–4 months. The leaf life span was 5–20 months and the main A1 shoot extension happened over 122–177 d. Both evergreen species allocated biomass to shoots, leaves or flowers continuously during the year, branching in the middle of the wet season to form second-order (A2 shoots) and third-order (A3 shoots) axis in LL and A2 shoots in MA. Partial shed of A1 shoot leaves would facilitate a higher branching intensity A2 shoot production in LL than in MA. MA presented a longer leaf life span, produced a lower percentage of A2 shoots but had a higher meristem persistence on A1 and A2 shoots than LL.
• Conclusions It was possible to identify different patterns of aerial growth in Cerrado woody species defined by shoot-linked traits such as branching pattern, bud composition, meristem persistence and leaf phenology. These related traits must be considered over and above leaf deciduousness for searching functional guilds in a Cerrado woody community. For the first time a relationship between bud composition, shoot growth and leaf production pattern is found in savanna woody plants.
Keywords: Bauhinia rufa, branching, Brazil, bud composition, Cerrado, flowering, leaf phenology, Leandra lacunosa, meristem persistence, Miconia albicans, synchronic leaves production, continuous leaf production
INTRODUCTION
Plants have complex mechanisms of aerial biomass exposition, which depend on their branching patterns (Schulze et al., 1986) and are the expression of an interaction between endogenous growth processes and exogenous constraints exerted by the environment (Barthélémy et al., 1989). Water and nutrient uptake have been considered growth limiting factors, while fire and herbivory modulate the biomass exposition in neotropical savannas (Medina and Silva, 1990). However, plants have some physiological independence from seasonal environmental conditions, allowing several possibilities for adjusting phenophases during the year (Borchert, 1980; Sarmiento and Monasterio, 1983; Reich and Borchert, 1984; Reich, 1995; Williams et al., 1997; Oliveira, 1998; Eamus and Prior, 2001).
Leaf production and fall patterns are well identified for Australian savanna species (Williams et al., 1997) and for South American Neotropical savanna species (Barros and Caldas, 1980; Sarmiento et al., 1985; Mantovani and Martins, 1988; Morais et al., 1995; Franco, 1998, 2002). According to Sarmiento and Monasterio (1983), most of the woody Cerrado species produce new leaves and flowers during the dry period, indicating available water in the rhizosphere. Reich and Borchert (1984) showed that, in seasonal tropical environments, the periodicity of plant phenological events is caused both by their endogenous rhythm and by seasonal climatic changes. According to these authors, at the end of the dry season, deciduous species of Costa Rica tropical forest lose all their leaves so that transpiration is reduced and reserves of water in the soil are sufficient to allow plant re-hydration.
The Brazilian Cerrado is a Neotropical savanna where the dry season corresponds to the autumn and winter months. This biome occupies approx. 20 % of the Brazilian territory (Ratter et al., 1996), differing from other savannas by its high diversity of woody species (approx. 2000; Mendonça et al, 1998; Castro et al., 1999), wide physiognomic range from grassland to tall woodlands (Sarmiento, 1984; Eiten, 1992), and under-representation of therophytes (Batalha and Martins, 2002). Phenological studies of Cerrado plants have allowed the description of the periodicity of leaf emergence and fall (Barros and Caldas 1980; Mantovani and Martins, 1988; Morais et al., 1995; Franco, 1998) but there are no quantitative data on variation in leaf number throughout the year for species with different patterns of leaf abscission. Estimates of leaf longevity for Cerrado woody species are not based on studies that followed leaf survival from emergence to abscission. Thus, there is no information about the average leaf life span for species of different phenological groups. The time for the leaves of Cerrado woody plants to reach mature size is available for only three species (Nascimento et al., 1990; Paulilo and Felippe, 1992). Franco (1998) pointed out that shoots of the woody species Roupala montana grew only at the onset of the growing season and Damascos (in press) has shown that three deciduous Cerrado woody species, Tocoyena formosa (Bignoniaceae), Diospyros hispida (Ebenaceae) and Tabebuia ochracea (Rubiaceae) preformed organs in buds, flushed their leaves before the beginning of the wet season, and grew shoots during a short period. There is no information about the number of growth units (the growth unit is defined as a shoot resulting from one terminal or axillary bud during a continuous growth cycle; Hallé et al., 1978) produced during the wet and dry seasons by Cerrado species with different leaf phenology. Except for the previously mentioned study (Damascos, in press) there are no data about bud composition, branching patterns and plant architecture of Cerrado woody species.
The following questions about three Cerrado woody species with different leaf phenology patterns were addressed. (a) Does shoot production occur in both wet and dry seasons? (b) Do these species differ in the number of growth units produced during the year? (c) Do climatic variables influence shoot initiation, growth and branching intensity? (d) Does bud composition differ among Cerrado species with distinct leaf phenology as established by Kikuzawa (1984) for temperate forest woody species? (e) Does the pattern of leaf fall during the dry season affect branching intensity in savanna woody species?
The results obtained over a 3-year period on bud composition, leaf phenology patterns (leaf production, mean leaf expansion time, mean leaf life span), shoot production and branching patterns in three Cerrado woody species [Bauhinia rufa (Caesalpiniaceae), Leandra lacunosa (Melastomataceae) and Miconia albicans Triana (Melastomataceae)] are presented.
MATERIALS AND METHODS
Study site
Measurements were carried out under natural conditions in a reserve of Cerrado vegetation at the São Carlos Federal University, São Carlos, Brazil (21°58′S, 47°52′W) 850 m above sea level. The regional climate is tropical with a dry period during winter and part of spring (June–September) followed by a wet period during summer and part of autumn (October–March). Following the Koeppen climatic classification, this region is between Aw and Cwa (Tolentino, 1967). The average ± standard deviation values for São Carlos city from 1939 to 1993 of annual rainfall (1506 ± 26 mm), relative air humidity (71 ± 5 %), air temperature (21·0 ± 0·5 °C) and vapour pressure deficit (0·72 ± 0·13 kPa) were determined by the Ministério da Agricultura and by Tolentino (1967). Station number 83726 of the Brazilian national meteorological service, located 1·0 km from the area where the woody species were growing, provided the monthly rainfall and air temperature values within the study period. The soil of the study site is a distrophic oxisol on a flat topography. The soil water content from 0 to 3 m depth follows the seasonal pattern of rainfall (Kano, 1998) and the water-table was reached 10 m below the soil surface. In the study area the trees rarely touch each other above well-defined shrub and herb strata, which characterizes a Cerrado physiognomy sensu stricto (Ribeiro and Walter, 1998).
Plant species
Adult plants of Bauhinia rufa (Bongard) Stendel (BR), Leandra lacunosa Cogn. (LL) and Miconia albicans (Sw.) Triana (MA) were studied under natural conditions. BR and MA grow as shrubs or small trees reaching up to 3·0 m tall and adult LL is a 1·0–1·8 m tall shrub. Leaf insertion is alternate in BR and opposite and decussate in MA and LL. BR and MA grow in sunny areas while LL shrubs grow in sunny sites and in sites partially shaded by tall shrubs and trees.
The three species were selected because they belong to two of the principal families listed for Cerrado (Mendonça et al., 1998) where especially BR and MA are frequent (Ratter et al., 1996). LL is not as frequent as MA and BR in Cerrado physiognomies, but it also occurs in gallery forest (Mendonça et al., 1998), and its leaves are conspicuously pubescent, showing papery (chartaceous) instead of leathery (coriaceous) texture as in BR or MA. The BR is free of leaves during a short period (20–30 d in the dry season) but MA and LL show foliage persistence during the year. These traits permit comparisons of different morphological and phenological leaf groups.
Bud composition and the number of leaves on shoots
Bud sampling was carried out during the dry season (July 2002). Before bud opening, six buds were collected in each one of five individuals per species on the most distal node of the greatest previous-year shoots. Buds were dissected under a stereomicroscope Nikon, ×40, and the number of cataphylls, stipules, lamina and flower primordia present in each bud were counted.
The number of leaf primordia in buds was compared with the number of expanding leaves on small (1·0–7·5 cm long) young shoots and with the final leaf number of mature shoots (shoots that had completed linear extension). Ten small shoots without fully expanded leaves were sampled in each one of three individuals of each species 1 week after budding (September or October 2002) and the leaf number on each shoot was registered. The final leaf number was also determined on mature shoots studied from August 2001 to March 2002 in BR (n = 90), LL (n = 50) and MA (n = 70).
Shoot growth
Shoot and leaf sampling was carried out from July 1999 to September 2002. Data from two independent groups of adult individuals growing in open sites of a homogeneous vegetation area were obtained from July in two sequential 1-year intervals (1999–2000 and 2000–2001).
In July 1999, before the end of the dry season and bud break, ten individuals of BR and five individuals of MA and LL up to 2 m tall were selected. Random points were determined along a 150 m straight transect to select the individuals. The individuals of each species nearest to the transect points were chosen. The greatest parent shoots (shoots produced in the previous year) in BR (n = 10) and in LL and MA (n = 20) were marked for each individual. The axillary bud at the most distal node of each shoot was labelled using plastic markers (100 buds per species).
The study of shoots emerging from these 100 buds per species marked in July 1999 (first-shoot group) was carried out until October 2000. Before the end of the dry season (July 2000) another group of individuals (second-shoot group) was selected along another 150 m transect and buds were marked as in the first group of shoots. The time of bud break was identified when small leaves were seen emerging from the bud. Marked buds and the organs derived from them were observed at 7-d intervals during the wet period and at 15- to 20-d intervals during the dry season. Leaf survival rate was observed until September 2002.
Shoot and leaf data
According to Barthélémy et al. (1991), and throughout this text, shoots that emerged in the beginning of the wet season from labelled buds are the first-order axis, A1 shoots; the lateral shoots or branches formed from axillary leaf buds of A1 shoots will be referred to as the second-order axis, the A2 shoots; and the branches of A2 shoots are the third-order axis, A3 shoots. At each sampling date, the linear length, the time of flowering and the branching were registered for each shoot emerging from labelled buds (A1 shoots). The length reached by A2 and A3 shoots was measured only at the end of the experiment and the time of flowering was registered. Apex persistence was studied between October 2001 and February 2002 following the new apical growth of LL and MA of previous-year A1, A2 and A3 shoots that did not flower.
At each sampling date the new leaves presented in each shoot were registered. The leaf emergence period of each shoot was defined as the time interval between the formation of its first and last leaf. Each leaf was numbered according to its position, starting from the shoot base so that P1 was the first leaf formed and P2, P3 … Pn, followed in succession. Leaf survival on marked shoots was recorded until total leaf fall. Leaves were considered senescent when they exhibited yellowing of 80 % or more of their area or when they were abscised. The maximum length and width of each leaf blade, excluding the petiole, were measured at each sampling date, as well as the shoot length from the base to the apex, using a calliper.
Five mature leaves were detached from each one of ten A1 shoots belonging to five different individuals of each species. One leaf disc with known area was detached from each leaf, avoiding major veins and the midrib. The discs were dried at 65 °C in a stove until constant mass. The leaf area and the dry mass values of the discs were used for specific leaf mass (SLM, g m−2) determinations (Prado and Moraes, 1997).
Data analysis
The leaf production pattern of each species was defined considering the time interval between the production of the first and the last leaf of each shoot. Leaf emergence pattern (according to Kikuzawa, 1984) is synchronic when leaves emerge as a flush during a short period, or continuous when leaves emerge gradually during the growth period. The leaf life span of each species was analysed (leaves consumed by herbivores were excluded) and it was calculated by averaging (in days) the survival of all leaves on marked branches of BR or the different cohorts of leaves (formed from the end of September or October to March–April) of LL and MA, and they were compared using the one-way analysis of variance (Zar, 1999).
The inter-year variation in the time of shoot extension for each species was compared with Student's t-test (Zar, 1999). The differences among species with respect to the shoot extension period, the duration of leaf production by shoot, leaf expansion time, leaf life span, and specific leaf mass were analysed by one-way analyses of variance and the Student–Neuman–Keuls multiple comparison test (Zar, 1999).
Pearson's correlation coefficient (Zar, 1999) was used to analyse the relationship between rainfall and monthly air temperature with the shoot extension rate (cm 15 d−1) or with the mean monthly leaf number. At the beginning of the second growth season (September 2000), there were both old leaves belonging to the first group of labelled plants and the new emerging leaves in the second group of labelled plants. Therefore, the correlations between monthly values of the mean number of leaves and rainfall were obtained considering the average value of old and new leaves.
Pearson's correlation coefficient was also used to analyse the relationship between the mean monthly number of leaves and the monthly percentage of A2 branches produced by A1 shoots. The correlation between the latter variable and the mean rainfall values was also determined. The monthly percentage of branching was calculated as the number of A2 or A3 shoots produced during the month divided by the total number of A2 or A3 shoots produced during the study period. Flowering intensity was calculated as a percentage of A1, A2 or A3 shoots flowering in relation to the total number of A1, A2 or A3 shoots.
RESULTS
Shoot emergence and growth
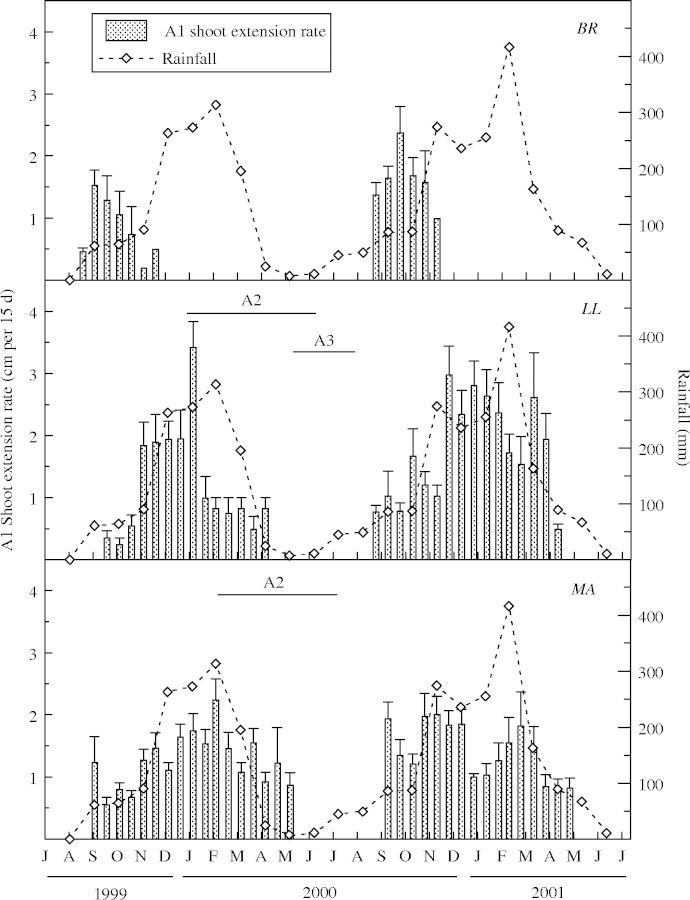
The pattern of shoot emergence from marked buds differed among species. The major A1 shoot flush in BR took place at the end of the dry season, independently of the inter-annual rainfall variation, and they grew during the first months of the wet season (November) when rainfall had not yet reached its highest intensity (Fig. 1). Shoots of MA and LL extended during the whole wet season (Fig. 1). In both species, the A1 shoot extension rate was correlated with rainfall (LL: r = 0·7812; MA: r = 0·7634, P < 0·01) and mean monthly air temperature (LL: r = 0·8302; MA: r = 0·5709, P < 0·01) while in BR it was not correlated with rainfall (r = 0·0362, P = 0·8630) or mean monthly air temperature (r = 0·0798, P = 0·8054). The maximum shoot extension rate was higher in LL (3·8 cm 15 d−1) than in the other two species (2·5 and 2·2 cm 15 d−1 in BR and MA, respectively; Fig. 1). The mean duration of the A1 shoot extension period was shorter (P < 0·05) in BR (25 ± 11 d) than in LL (130 ± 26 d) and MA (122 ± 47 d) in 1999–2000 or in 2000–2001 (37 ± 11, 166 ± 19 and 160 ± 70 d in BR, LL and MA, respectively). Besides, the same (P < 0·05) was verified comparing the A1 shoot mean final length of BR (7·95 ± 5·35 cm, n = 90), LL (20·89 ± 10·87 cm, n = 51) and MA (16·82 ± 10·25 cm, n = 71).
Fig. 1.
Columns represent the extension rate (cm per 15 d, mean ± s.e.) of A1 shoots emerging from marked buds in Bauhinia rufa (BR), Leandra lacunosa (LL) and Miconia albicans (MA) measured from 1999 to 2001 in two independent groups of individuals of each species. The monthly rainfall (open symbols), the period (horizontal bars) of A2 branch formation from axillary buds of A1 shoots, and the A3 branch formation from axillary buds of A2 shoots during 1999–2000 are shown.
A1 shoots of BR did not develop new branches until the wet season of the next year. In LL and MA, A1 shoots grew during the wet season (Fig. 1) and developed a second-order axis (A2 shoots) from axillary leaf buds since the middle of the wet season or during the dry season (Figs. 2 and 3).LL produced a third-order axis (A3 shoots) during the dry season (Figs 2 and 3). The A2 shoots in LL emerged first than in MA (Figs 2 and 3). The largest percentage of A3 shoots in LL was formed when some plants were still producing A2 shoots (Fig. 3). Both axis orders concluded linear extension and flowered before the onset of the next wet season (Fig. 2).
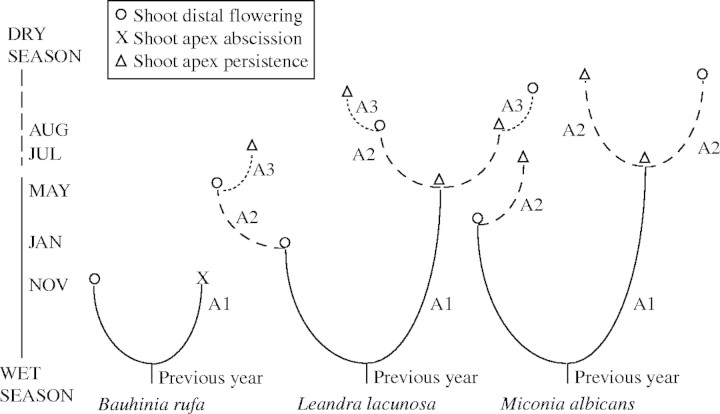
Fig. 2.
Branching diagrammatic representation of Bauhinia rufa, Leandra lacunosa and Miconia albicans during the wet and dry seasons. A1, First-order axis (continuous lines) emerging at the beginning of the wet season; A2, second-order axis (dashed lines) emerged from axillary buds of A1 shoots; A3, third-order axis (dotted lines) emerged from axillary buds of A2 shoots. Previous-year shoots are shown as thick lines at the base.
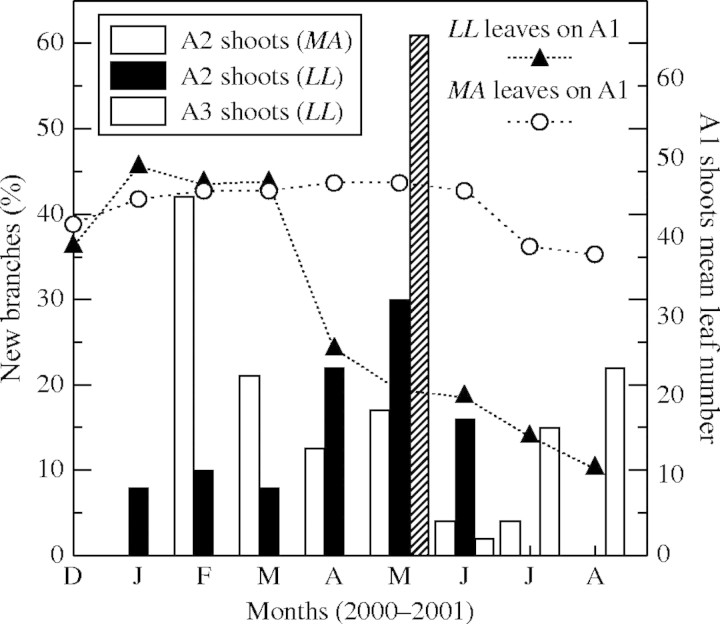
Fig. 3.
Columns indicate the new branches (A2 shoots) formed per month from axillary buds on A1 shoots, and the new branches (A3 shoots) formed per month from axillary buds on A2 shoots calculated as the percentage of the total number of A2 and A3 shoots, respectively. The monthly mean leaf numbers on A1 shoots (symbols) during the branching period (from December 2000 to August 2001) in Leandra lacunosa (LL) and in Miconia albicans (MA) are shown.
Branching intensity was higher at the end of the wet season in LL, and near the peak of the wet season in MA (Fig. 3). In LL the percentage of A2 shoots (Fig. 3) was not affected by rainfall (r = 0·5388, P = 0·2699) but it was correlated with the mean number of leaves in A1 shoots (r = −0·8168, P = 0·0249). Conversely, the A2 branching percentage of MA (Fig. 3) depended on rainfall (r = 0·9679, P = 0·0015) but it was not correlated with the monthly mean number of leaves on A1 shoots (r = −0·5937, P = 0·4063). The length of A2 shoots was similar (P < 0·05) between LL (6·84 ± 4·78 cm, n = 30) and MA (8·28 ± 4·78 cm, n = 20). The A3 shoots of LL had a mean final length of 4·15 ± 2·47 cm (n = 20).
Leaf phenology and morphology
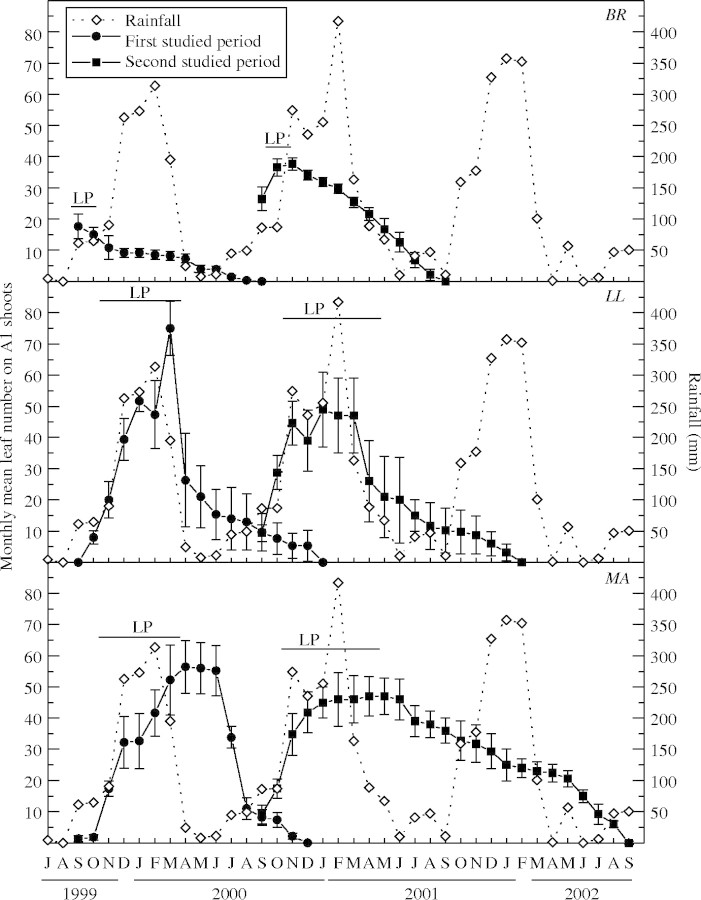
The A1 shoots of BR unfolded all their leaves at the beginning of the wet season (September–October) over <30 d, while leaves production extended over 2–6 months in LL and MA (Fig. 4). Insects consumed 43 % of the total leaves (n = 227 leaves) produced by marked A1 shoots of BR during 1999–2000, and 25 % of leaves (n = 471 leaves) during 2000–2001. For LL, leaf herbivory was 73 % (n = 217 leaves) and 48 % (n = 132 leaves) during the first and the second studied periods, respectively. All leaves that were not consumed by herbivores were lost at the end of the dry season in BR, while in LL the leaves of A1 shoots fell during the dry season or in the following wet season (Fig. 4). The monthly mean number of leaves on A1 shoots was correlated with rainfall (BR, r = 0·4420, P = 0·0306; LL, r = 0·6727, P < 0·01) and with air temperature (BR, r = 0·6177, P < 0·01; LL, r = 0·6727, P < 0·01). By contrast, leaves of the MA A1 shoots did not suffer herbivory and survived during the wet and dry seasons, abscising at the end of the dry period (1999–2000) or during the wet and dry seasons of the next year (2001–2002).
Fig. 4.
Monthly mean leaf number (solid symbols ± s.e.) on A1 shoots (emerged from previously marked buds) and the time of the leaf production (LP, horizontal bars) in Bauhinia rufa (BR), Leandra lacunosa (LL) and Miconia albicans (MA) from two independent groups of individuals studied. The data of the monthly mean rainfall (open symbols) are shown.
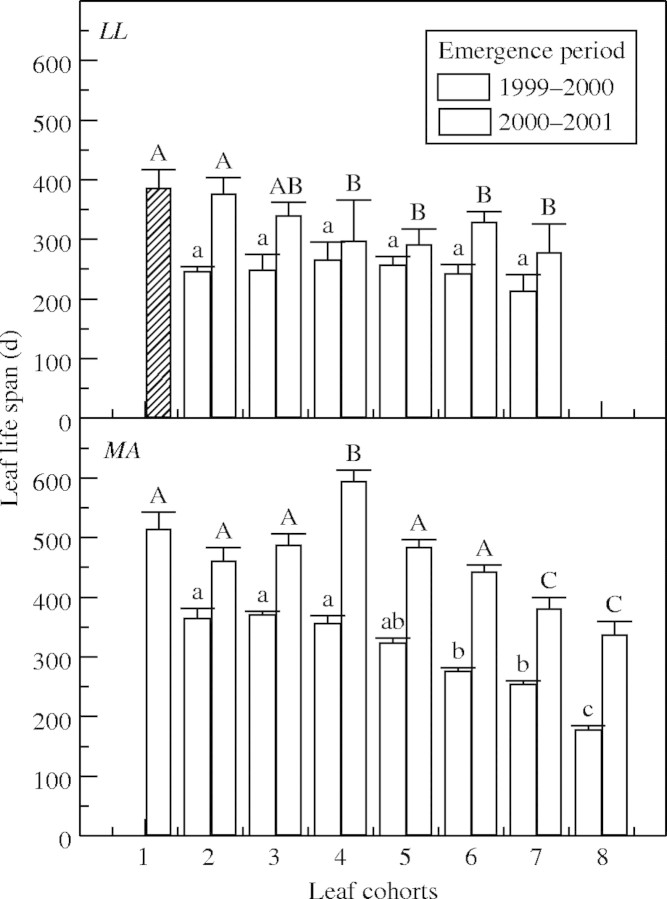
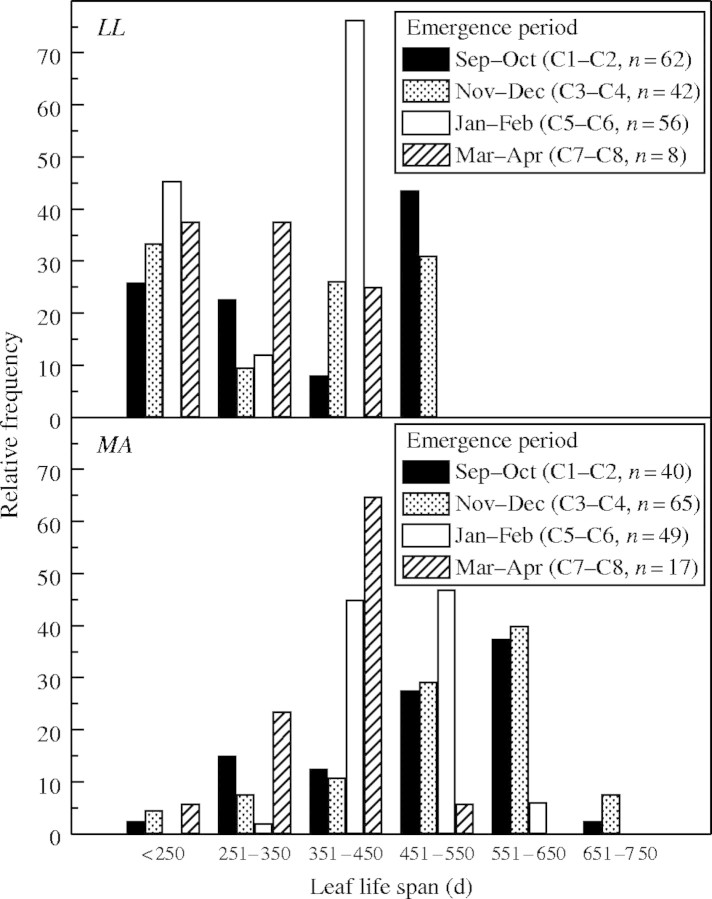
The expansion time of LL and MA leaves was higher than that of BR during the first and second study periods and during the second period it was greater in MA than in LL (Table 1). BR and MA exhibited higher SLM values than LL (Table 1). The leaf life span of the first leaf cohort differed among species only in the second period studied (Table 1) when the total annual rainfall was greater and the rains started earlier (Fig. 4). In LL and MA the mean leaf life span of A1 shoot leaf cohorts was lower in the first period studied (Fig. 5). In both species the first leaf cohorts of A1 shoots (formed between September and December) had a longer leaf life span than those (from the fifth to eight cohorts) formed near the end of the wet season (February–April; Fig. 6). Leaf longevity on A2 shoots was longer in MA than in LL (data not shown). Leaves of A2 and A3 shoots formed during the dry period fell during the following wet and dry seasons (data not shown).
Table 1.
Mean values ( ± s.e.) of the interval of leaf production per shoot, leaf expansion time, leaf life span and specific leaf mass (SLM) of Bauhinia rufa (BR), Leandra lacunosa (LL) and Miconia albicans (MA)
| Variables |
Bahuinia rufa |
Leandra lacunosa |
Miconia albicans |
|||
|---|---|---|---|---|---|---|
| Time period of leaf production per shoot (days) | ||||||
| (n = number of shoots) | ||||||
| Leaves emerged during 1999 | 14·49 ± 1·46 a | 141·34 ± 9·27 b | 119·43 ± 7·90 b | |||
| n = 93 | n = 29 | n = 39 | ||||
| Leaves emerged during 2000 | 9·93 ± 0·32 a | 125·15 ± 2·78 b | 133·51 ± 6·60 b | |||
| n = 90 | n = 50 | n = 71 | ||||
| Leaf expansion time (days) | ||||||
| (n = number of leaves) | ||||||
| Leaves emerged during 1999 | 28·46 ± 1·17 a A | 61·87 ± 2·42 b A | 58·10 ± 1·08 b A | |||
| n = 56 | n = 63 | n = 274 | ||||
| Leaves emerged during 2000 | 29·66 ± 0·93 a A | 76·33 ± 2·53 b B | 92·05 ± 2·98 c B | |||
| n = 216 | n = 123 | n = 144 | ||||
| Leaf life span (days)* | ||||||
| (n = number of leaves) | ||||||
| Leaves emerged during 1999 | 258·55 ± 9·00 a A | 247·75 ± 19·67 a A | 364·28 ± 17·53 a A | |||
| n = 49 | n = 20 | n = 69 | ||||
| Leaves emerged during 2000 | 272·39 ± 5·30 a A | 371·79 ± 13·73 b B | 516·00 ± 27·41 c B | |||
| n = 210 | n = 99 | n = 40 | ||||
| SLM (g m−2) | 147·00 ± 4·52 a | 105·11 ± 4·40 b | 162·32 ± 9·64 a | |||
| n = 50 leaves | ||||||
Different lower-case letters in the rows indicate significant differences among species (ANOVA, P < 0·05) while upper-case letters (in the columns) indicate significant differences between the two periods studied for each species (Student's t-test, P < 0·05).
Leaves emerged in September (BR) or in October–November (LL and MA).
Fig. 5.
Average ( ± s.e.) values of leaf life span of different leaf cohorts of Leandra lacunosa (LL) and Miconia albicans (MA) during two periods (1999–2000 and 2000–2002). Only leaves not attacked by herbivores were considered. Different lower-case letters indicate significant differences among leaf cohorts emerged during 1999–2000 while upper-case letters indicate significant differences among leaf cohorts emerged during 2000–2001 (ANOVA, P < 0·05).
Fig. 6.
Relative frequency of leaf life span (d) of Leandra lacunosa (LL) and Miconia albicans (MA) of A1 shoots leaves emerged at different months. Only leaves not attacked by herbivores were considered. C, Leaf cohort.
Shoot flowering and apex persistence
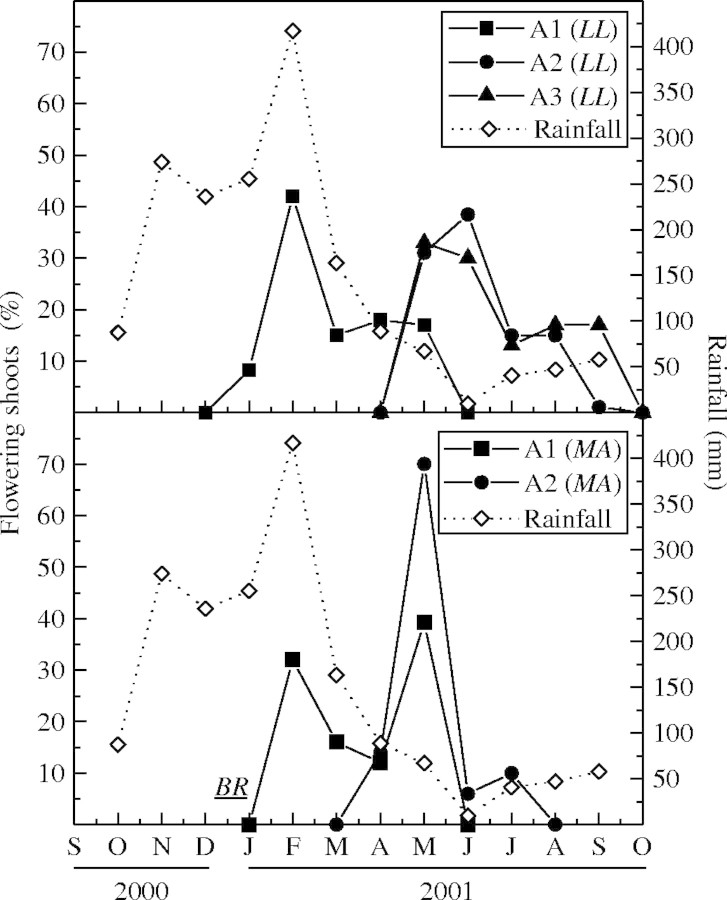
Final A1, A2 and A3 shoot extension was determined by (a) the development of a distal inflorescence, (b) apex death without flowering or (c) apical meristem dormancy (Fig. 2). Only about 5 % of the marked A1 shoots of BR flowered during the wet season (December, Fig. 7) while in LL and MA the flowering of A1 shoots showed a higher frequency than in BR and took place from December (LL) or January (MA) to June (Fig. 7). Flowering intensity was similar in both study periods in LL (2000 = 83 %, 2001 = 74 %) and in MA (2000 = 68 %, 2001 = 67 %). Plants of LL and MA flowered from the middle of the wet season to the end of the dry season but on shoots of different categories (A1, A2, A3 in LL, A1 and A2 in MA) and at different periods (Fig. 7). All BR shoots that did not flower (95 %) suffered apical meristem abscission early in the wet season, starting in December. Apical meristem persistence was 11 % in A1 and 46 % in A2 shoots of MA. In LL, the apical meristem was observed to persist only in A2 (30 %) and A3 (20 %) shoots.
Fig. 7.
Frequency of distal flowering (solid symbols) on A1 (shoots emerged from previously marked buds), A2 (shoots formed from axillary buds of A1 shoots) and A3 shoots (formed from axillary buds of A2 shoots) in Leandra lacunosa (LL) and in Miconia albicans (MA) from December 2000 to August 2001. The duration of the A1 shoot flowering period of Bauhinia rufa (BR, flowering intensity = 5 %) and the monthly rainfall values (open symbols) are shown.
Bud composition
Buds of the deciduous species BR were formed by cataphylls, stipules and lamina primordia. Each lamina primordium present in BR buds contained two free lateral stipules. The number of leaf primordia in buds (mean ± standard error = 5·57 ± 0·1) was similar (P < 0·05) to the number of expanding leaves present on young, 1-week-old shoots (5·67 ± 0·29), and to expanded leaves (5·19 ± 0·15) present on mature shoots (those which had concluded linear extension). Leaf axillary buds and young (1-week-old) shoots of MA and LL exhibited only one pair of leaf primordia or small expanding leaves, respectively, while mature shoots showed a larger number of leaves (LL = 4·96 ± 0·25, MA = 3·24 ± 0·15).
DISCUSSION
The absence of differences between the number of mature leaves on the shoots and the number of leaf primordia present in BR buds indicates organ preformation, meaning that embryonic organs are enclosed within a bud before unfolding (Caraglio and Barthélémy, 1997; Puntieri et al., 2000; Souza et al., 2000). Organ preformation and early budding were also observed by Damascos (in press) in three other deciduous Cerrado woody species: Tocoyena formosa (TF), Diospyros hispida (DH) and Tabebuia ochracea (TO). The total numbers of leaves on current-year-shoots (A1 shoots) were 6·54 ± 0·14, 11·41 ± 0·48 and 4·91 ± 0·19 in TF, DH and TO, respectively, which did not differ from the number of leaf primordia presented in buds. BR, TF, DH and TO remained leafless for 1 month before the wet season and their leaf life spans were between 6 and 11 months.
BR expanded all new photosynthetic areas in a synchronic pattern before the beginning of the wet period and during the shortest period among the species studied. As indicated by Reich and Borchert (1984) in subtropical forest and by Williams et al. (1997) in Australian savannas, the reduction of the leaf transpiration area of deciduous species would improve plant water status and allow plants to flower or produce leaves during the dry season. Thus, the elimination of water deficit within the woody plant initiates the leaf flush (Eamus and Prior, 2001). Deciduous species usually have deeper root systems than species with persistent foliage in Cerrado (Jackson et al., 1999), which would make deciduous species less dependent on rainfall than evergreen species for expanding leaves. It was possible to observe by excavation in the field a dimorphic root system (deep main root plus shallow lateral roots) in BR, where the main root was followed to a depth of 1·2 m (C. H. B. A. Prado, unpubl. res.). Scholz et al. (2002) suggested that the presence of dimorphic root systems in Cerrado deciduous species might play a facilitating role for leaf expansion near the end of the dry season, when the soil surrounding lateral roots is dry. The Cerrado soil water potential at a depth of 1·5 m does not reach −1·5 MPa at the peak of the dry season even after 4 months of virtually no rain (Franco, 2002).
As was observed in BR, other deciduous Cerrado species studied (Damascos, in press) had a short shoot extension period (32 ± 11, 23 ± 9 and 9 ± 9 d in TF, DH and TO, respectively) and A1 shoots did not branch (DH and TO) or produce new branches simultaneously with shoot extension during the wet season (TF). Apex abscission, as exhibited by BR shoots during the wet period, is often linked to low shoot vigour (Puntieri et al., 1998). In DH and TO, apical meristem death was observed, and in TF early distal flowering occurred (M. A. Damascos, unpubl. res.). Deciduous Cerrado species seem to have a reduced shoot growth pattern in time and space during the wet season and could be full of stored carbon in roots and shoots during the dry season as in temperate deciduous species in winter (Larcher and Thomaser-Thin, 1988). These reserves accumulated during the favourable period, and could be mobilized at the beginning of the next wet season starting the leaf flush with the organ preformed in the buds. On the other hand, LL and MA produced buds, new shoots and leaves throughout the year, flowering during the dry season. It would impose high demand of photosynthetic assimilates in dry and wet seasons. Aerial biomass production in LL and MA is probably more dependent than in BR on short-term assimilation. Therefore, LL and MA should maintain a high photosynthetic area (foliage persistence) during the year.
Evergreen LL and MA present organ neo-formation. Neoformed organs are never included in a bud as primordia, and extend as they are differentiated by the meristem (Hallé et al., 1978; Caraglio and Barthélémy, 1997). Both species formed their leaves gradually on A1 shoots for 7–8 months, producing one pair of leaves per month. The transpiration area reduction by partial leaf loss on A1 shoots during the dry season, from April to June, would facilitate higher branching intensity in LL (A2 shoot production) than in MA. Afterwards, LL produced new leaves on A2 and A3 shoots, quickly offsetting the drop in leaf transpiration and photosynthetic area.
MA had the longest leaf life span and the greatest percentage of leaves on A1 and A2 shoots during the year. It produced fewer orders of shoots than LL but had higher apical meristem persistence on A1 and A2 shoots. Budding in LL and MA is linked to leaf phenology and probably to short vertical growth of the root system of Cerrado evergreen and semi-deciduous species (Jackson et al., 1999). There are no data on LL root systems but adult MA individuals about 1·2 m tall had root systems reaching only 0·50 m beneath the soil surface (Monteiro and Prado, in press). Possibly a short root system and foliage persistence during the dry season determine higher rainfall dependence for bud opening in LL and in MA than in deciduous BR species.
In both evergreen species, leaves survived until the wet (LL) or dry (MA) seasons of the next year. However, the mean leaf life span of different cohorts of both species was reduced by senescence during the drier year (the first study period). In addition, the leaves that emerged during the end of the wet season exhibited lower leaf longevity than those formed earlier. It suggests that, irrespective of season, water stress reduces the longevity of leaves formed during the drier period in evergreen Cerrado species. LL showed the lowest value of SLM and the highest number of leaves consumed by herbivory in both periods studied. These events could be linked since greater SLM promotes mechanical protection against tissue herbivory (Coley and Barone, 1996). Similar values of SLM between BR and MA and the absence of herbivory only in MA would indicate that MA has an anti-herbivory chemical defence more efficient than BR.
CONCLUSIONS
Organ preformation in buds, early budding and reduced shoot growth pattern (in time and space) are linked traits in BR as in deciduous species from seasonal climates. Evergreen LL and MA continuously consumed resources by leaf, shoot and flower formation during wet and dry seasons. Both evergreen species showed higher water demand during the whole year on account of their foliage persistence and branching. Therefore, MA and LL depended on water availability from rainfall to maintain the shoot extension rate, branching (in MA) and leaf longevity. In LL the partial leaf fall probably reduces water stress, transitionally allowing higher branching intensity than in MA. Bud composition, branching pattern and leaf phenology are linked in Cerrado woody species. Variations among them result in contrasting behaviour of aerial space acquisition and different dependence on ambient factors during the year. Functional guilds of Cerrado woody species must be analysed in relation to these linked attributes over and above the plant deciduousness. The relationship of branching intensity and leaf fall should be studied experimentally according to periodic plant water status throughout the year.
Acknowledgments
This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil. We acknowledge the support provided by the Universidad Nacional del Comahue (Argentina) and by Pronex-CNPq (Brazil) during 1999. We are grateful to Carlos A. Casali and Nadia F. de Paula for their invaluable field assistance and to Javier Puntieri, Linda S. Caldas, and Marco A. Batalha for the critical manuscript revision.
LITERATURE CITED
- Barros MAG, Caldas, LS. 1980. Acompanhamento de eventos fenológicos apresentados por cinco gêneros nativos do cerrado (Brasília, DF). Brasil Florestal 42: 7–14. [Google Scholar]
- Barthélémy D, Edelin C, Hallé F. 1989. Architectural concepts for tropical trees. In: Holm-Nielsen LB, Nielsen I, Balslei H, eds. Tropical forest: botanical dynamics, speciation and diversity. London: Academic Press, 89–100. [Google Scholar]
- Barthélémy D, Edelin C, Hallé F. 1991. Canopy architecture. In: Raghavendra, AS, ed. Physiology of trees. New York: John Wiley & Sons, 1–5. [Google Scholar]
- Batalha MA, Martins FR. 2002. Life-form spectra of Brazilian cerrado sites. Flora 197: 452–460. [DOI] [PubMed] [Google Scholar]
- Borchert R. 1980. Phenology and ecophysiology of tropical trees: Erythrina poeppigiana O. F. Cook. Ecology 61: 1065–1074. [Google Scholar]
- Caraglio Y, Barthélémy D. 1997. Revue critique des termes relatifs á la croissance et a la ramification des tiges des végétaux vasculaires. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l'architecture des végétaux. Paris: INRA editions, Science update, 11–87. [Google Scholar]
- Castro AAJF, Martins FR, Tomashiro JY, Shepherd GJ. 1999. How rich is the flora of Brazilian cerrados? Annals of the Missouri Botanical Garden 86:192–224. [Google Scholar]
- Coley PD, Barone J. 1996. Herbivory and plant defences in tropical forest. Anual Review of Ecology and Systematics 27: 305–335. [Google Scholar]
- Damascos MA. . Conteúdo das gemas, momento da brotação, padrão e produção de folhas em espécies lenhosas do cerrado. In: Larcher W, ed. Ecofisiologia vegetal, 2nd edn. São Carlos: Editora Rima Artes e Textos (in press). [Google Scholar]
- Eamus D, Prior L. 2001. Ecophysiology of trees in seasonally dry tropics: comparisons among phenologies. Advances in Ecological Research 32: 113–197. [Google Scholar]
- Eiten G. 1992. Natural Brazilian vegetation types and their causes. Academia Brasileira de Ciências 64: 35–65. [Google Scholar]
- Franco AC. 1998. Seasonal patterns of gas exchange, water relations and growth of Roupala montana, an evergreen savannah species. Plant Ecology 136: 69–76. [Google Scholar]
- Franco AC. 2002. Ecophysiology of woody plants. In: Oliveira OS, Marquis RJ, eds. The cerrados of Brazil: ecology and natural history of a neotropical savannah. Irvington: Columbia University Press, 178–197. [Google Scholar]
- Hallé F, Oldeman R, Tomlinson P. 1978.Tropical trees and forest. An architectural analysis. New York: Springer-Verlag. [Google Scholar]
- Jackson PC, Meinzer FC, Bustamante M, Goldstein G, Franco A, Rundel PW, et al. 1999. Partitioning of soil water among tree species in a Brazilian Cerrado ecosystem. Tree Physiology 19: 717–724. [DOI] [PubMed] [Google Scholar]
- Kano SS. 1998.Estudo ecofisiológico de duas espécies nativas do cerrado: Kielmeyera coriaceae e Kielmeyera variabilis. A disponibilidade hídrica e sua relação com as trocas gasosas, o potencial hídrico foliar e a fenologia. PhD Thesis, Universidade Federal de São Carlos, São Carlos, Brazil. [Google Scholar]
- Kikuzawa K. 1984. Leaf survival of woody plants in deciduous broad-leaved forest. II. Small trees and shrubs. Canadian Journal of Botany 62: 2551–2556. [Google Scholar]
- Larcher W, Thomaser-Thin W. 1988. Seasonal changes in energy content and storage patterns of mediterranean sclerophylls in the Northernmost. Acta Oecologia 4: 347–376. [Google Scholar]
- Mantovani W, Martins FR. 1988. Variações fenológicas das espécies de cerrado da reserva biológica de Moji-Guaçu, Estado de São Paulo. Revista Brasileira de Botânica 11: 101–112. [Google Scholar]
- Medina E, Silva JF. 1990. Savannahs of northern South America: a steady state regulated by water-fire interactions on a background of low nutrient availability. Journal of Biogeography 17: 403–413. [Google Scholar]
- Mendonça RC, Felfili JM, Walter BMT, Silva JRMC, Rezende AB, Filgueiras TS, et al. 1998. Flora vascular do cerrado. In: Sano SM, Almeida SP, eds. Cerrado, ambiente e flora. Planaltina, Brasil: EMBRAPA, 289–556. [Google Scholar]
- Monteiro JA, Prado CHBA. . Apparent carboxylation efficiency and relative stomatal and mesophyll limitations of photosynthesis in an evergreen cerrado species during water stress. Photosynthetica (in press). [Google Scholar]
- Morais HC, Diniz IR, Baumgarten L. 1995. Padrões de produção de folhas e sua utilização por larvas de Lepidoptera em um cerrado de Brasília. Revista Brasileira de Botânica 18: 163–170. [Google Scholar]
- Nascimento MT, Villela DM, Lacerna LD. 1990. Foliar growth, longevity and herbivory in two ‘cerrado’ species near Cuiabá, MT, Brazil. Revista Brasileira de Botânica 13: 27–32. [Google Scholar]
- Oliveira PE. 1998. Fenologia e biologia reprodutiva das espécies do cerrado. In: Sano SM, Almeida SP, eds. Cerrado, ambiente e flora. Planaltina: Embrapa, 169–192. [Google Scholar]
- Paulilo MTS, Felippe GM. 1992. Crescimento de folhas de árvores de Qualea grandiflora Mart. Revista Brasileira de Botânica 15: 85–93. [Google Scholar]
- Prado CHBA, Moraes JAPV. 1997. Photosynthetic capacity and specific leaf mass in twenty woody species of Cerrado vegetation under field conditions. Photosynthetica 33: 103–112. [Google Scholar]
- Puntieri JG, Barthélémy D, Martinez P, Raffaele E, Brion C. 1998. Annual-shoot growth and branching patterns in Nothofagus dombeyi (Fagaceae). Canadian Journal of Botany 76: 673–685. [Google Scholar]
- Puntieri JG, Souza MS, Barthélémy D, Brion C, Nuñez M, Mazzini C. 2000. Preformation, neoformation, and shoot structure in Nothofagus dombeyi (Nothofagaceae). Canadian Journal of Botany 78: 1044–1054. [Google Scholar]
- Ratter JA, Bridgewater S, Atkinson R, Ribeiro JF. 1996. Analysis of the floristic composition of the Brazilian cerrado vegetation. II. Comparison of the woody vegetation of 98 areas. Edinburgh Journal of Botany 53: 153–180. [Google Scholar]
- Reich PB. 1995. Phenology of tropical forest: patterns, causes, and consequences. Canadian Journal of Botany 73: 164–174. [Google Scholar]
- Reich PB, Borchert R. 1984. Water stress and tree phenology in a tropical dry forest in the Lowlands of Costa Rica. Journal of Ecology 72: 61–74. [Google Scholar]
- Ribeiro JP, Walter BMT. 1998. Fitofisionomias do Bioma Cerrado. In: Sano SM, Almeida SP, eds. Cerrado, ambiente e flora. Planaltina: EMBRAPA, 89–166. [Google Scholar]
- Sarmiento G. 1984.The ecology of neotropical savannas. Cambridge, MA: Harvard University Press. [Google Scholar]
- Sarmiento G, Monasterio M. 1983. Life forms and phenology. In: Bourlière F, ed. Tropical Savannas. Elsevier, Amsterdan, 79–108. [Google Scholar]
- Sarmiento G, Goldstein G, Meinzer F. 1985. Adaptive strategies of woody species in Neotropical savannas. Biological Review 60: 315–355. [Google Scholar]
- Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC. 2002. Hydraulic redistribution of soil water by neotropical savanna trees. Tree Physiology 22: 603–612. [DOI] [PubMed] [Google Scholar]
- Schulze ED, Kuppers M, Matysser R. 1986. The role of carbon balance and branching pattern in the growth of woody species. In: Givinish T, ed. On the economy of plant form and function. New York: Cambridge University Press, 585–601. [Google Scholar]
- Souza MS, Puntieri JG, Barthélemy D, Brion C. 2000. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae). Annals of Botany 85: 547–555. [Google Scholar]
- Tolentino M. 1967.Estudo crítico sobre o clima da região de São Carlos. São Carlos: Prefeitura Municipal de São Carlos. [Google Scholar]
- Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D. 1997. Leaf phenology of woody species in a north Australian tropical savanna. Ecology 78: 2542–2558. [Google Scholar]
- Zar JH. 1999.Biostatistical analysis, 4th edn. Upper Saddle River, New Jersey: Prentice Hall. [Google Scholar]