Abstract
• Background and Aims Oxaziclomefone (OAC), a new herbicide, inhibits cell expansion, especially in roots and cell-cultures of gramineous monocots. OAC does not affect turgor in cultured maize cells, and must therefore inhibit wall-loosening or promote wall-tightening.
• Methods The effects of OAC in living cultured maize cells on various biochemical processes thought to influence wall extension were studied.
• Key Results OAC did not affect 14C-incorporation from d-[U-14C]glucose into the major sugar residues of the cell wall (cellulosic glucose, non-cellulosic glucose, arabinose, xylose, galactose, mannose or uronic acids). OAC had no effect on 14C-incorporation from trans-[U-14C]cinnamate into wall-bound ferulate or its oxidative coupling-products. OAC did not influence the secretion or in-vivo action of peroxidase or xyloglucan endotransglucosylase activities—proposed wall-tightening and -loosening activities, respectively. The herbicide did not affect the consumption of extracellular l-ascorbate, an apoplastic solute proposed to act as an antioxidant and/or to generate wall-loosening hydroxyl radicals.
• Conclusions OAC decreased wall extensibility without influencing the synthesis or post-synthetic modification of major architectural wall components, or the redox environment of the apoplast. The possible value of OAC as a probe to explore aspects of primary cell wall physiology is discussed.
Keywords: Oxaziclomefone, herbicide, cell expansion, wall loosening, xyloglucan endotransglucosylase/hydrolase (XTH), peroxidase, vitamin C, Gramineae
INTRODUCTION
The pectin–hemicellulose–cellulose wall and its mechanism of expansion are unique to plants, and indispensable for plant growth. Therefore, a specific inhibitor of any essential aspect of wall metabolism would be a potential herbicide. However, very few of the herbicides whose modes of action are currently known have been shown to target wall metabolism. The few such examples include 2,6-dichlorobenzonitrile (= dichlobenil), isoxaben, triazofenamide, a thiazolidinone and quinclorac, which have been reported to inhibit cellulose biosynthesis (reviewed by Vaughn, 2002). A recent addition to the list is an aminotriazine compound known as AE F150944 (Kiedaisch et al., 2003), whereas the claimed action of quinclorac on cell wall biosynthesis has recently been doubted (Tresch and Grossmann, 2003). Isoxaben and thiazolidinones appear to interact directly with a cellulose synthase (Scheible et al., 2001). In addition, a Streptomyces phytotoxin (thaxtomin A, at 100–500 nm) has been reported to inhibit cellulose biosynthesis (Scheible et al., 2003), although this is probably indirectly via effects on ion flux (Tegg et al., 2005). These herbicides have in common a characteristic effect on root swelling or ‘clubbing’ (Heim et al., 1998). Quinclorac, primarily an ‘auxin-type’ herbicide (Grossmann, 2000), may inhibit the biosynthesis both of cellulose and of some hemicelluloses (Koo et al., 1996); and since isoxaben may also inhibit callose formation, it has been speculated to inhibit the biosynthesis of UDP-glucose from sucrose (Sabba and Vaughn, 1999). In addition, Congo Red (Robinson, 1981) and CGA 325615 (an experimental thiatriazine-based herbicide; Peng et al., 2001) inhibit the formation of crystalline cellulose without preventing β-(1→4)-glucan biosynthesis.
Besides these few examples, it seems likely that there is a large untapped resource of potential novel targets for herbicide action among the numerous processes other than cellulose production that are involved in cell wall metabolism. Of particular interest is the discovery of important taxonomic variation in wall biochemistry; for example, gramineous monocots uniquely possess mixed-linkage β-(1→3),(1→4)-d-glucan (MLG) and are richer than most dicots (except the Chenopodiaceae) in their content of ferulate residues (Carpita, 1996). Furthermore, major chemical shifts in primary cell wall composition accompanied several major steps in land plant evolution (Popper and Fry, 2003, 2004). This taxonomic distribution of potential targets in wall metabolism raises the prospect of new selective herbicides.
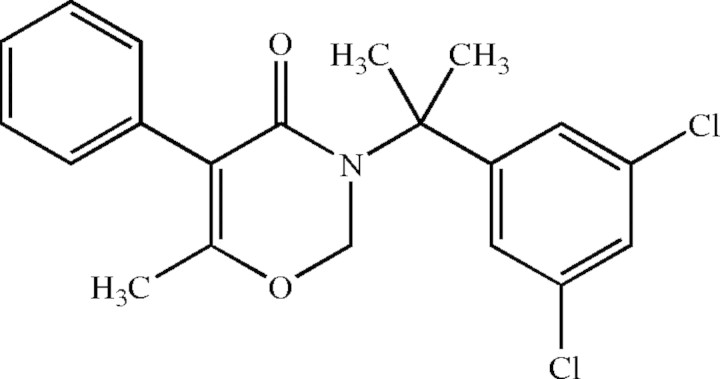
Oxaziclomefone [OAC; IUPAC name 3-(1-(3,5-dichlorophenyl)-1-methylethyl)-3,4-dihydro-6-methyl-5-phenyl-2H-1, 3-oxazin-4-one; Reg. No. 153197-14-9] is a potent new herbicide used to control cockspur (Echinochloa crus-galli) and other weeds in rice paddy fields (Suzuki et al., 2003) (Fig. 1). OAC inhibits the growth of gramineous plants more effectively than that of most dicots (Jikihara et al., 1997). OAC appeared to possess a novel mode of action clearly distinct from that of any established class of herbicide (Miller et al., 2001). Because OAC is a non-ionic, non-polar compound, it is likely to penetrate cell membranes readily and could therefore act on components in any sub-cellular compartment.
Fig. 1.
The structure of oxaziclomefone (OAC).
Cell expansion is driven by turgor pressure, and in healthy tissues is usually limited by the extensibility of the cell wall or sometimes by the wall yield threshold (Cosgrove, 1993; Kutschera, 2001). In recent work (O'Looney and Fry, 2005) it was found that OAC inhibits cell expansion in maize cell cultures, with an ID50 (dose causing 50 % inhibition) of approx. 5 nm. OAC did not affect the osmotic pressure of the cell sap and would thus not have altered the turgor pressure in cultured cells adequately supplied with water; it also did not impede the cells' ability to acidify the apoplast. If cell expansion is inhibited by OAC despite the maintenance of turgor pressure, it follows that OAC treatment must evoke a decrease in the wall's ability to expand (decreased extensibility or increased yield threshold). In the present work, possible metabolic targets for OAC in wall biochemistry were therefore considered. Increasing the wall extensibility or decreasing the wall yield threshold can collectively be described as wall ‘loosening’; the converse is wall ‘tightening’. A priori, OAC could act either by inhibiting wall loosening or by promoting wall tightening.
One class of proteins thought to be involved in wall loosening is the xyloglucan endotransglucosylase/hydrolases (XTHs), which exhibit one or both of two enzymic activities: XET (xyloglucan endotransglucosylase) and XEH (xyloglucan endohydrolase) (Rose et al., 2002). Wall loosening has been proposed to involve some or all of the following processes: (a) partial digestion of wall polymers by hydrolytic enzymes [e.g. endo-β-glucanase (Hatfield and Nevins, 1987; Nicol et al., 1998), XTHs with XEH activity (Kaku et al., 2002) or proteinases (Grobe et al., 1999)]; (b) transglycosylation of xyloglucans by XTHs with XET activity (Fry et al., 1992; Rose et al., 2002); (c) the breaking of hydrogen-bonds between wall polysaccharides by α- or β-expansins (McQueen-Mason et al., 1992; Cosgrove, 2000); (d) decreasing the yield threshold through unknown mechanisms by yieldins (Okamoto-Nakazato et al., 2001); and (e) the non-enzymic scission of polysaccharides by apoplastic hydroxyl radicals (•OH; Fry, 1998; Schopfer, 2001). In principle, OAC could inhibit any of the enzymes mentioned or could interfere with the provision of the apoplastic substrates (e.g. ascorbate; Fry, 1998; Green and Fry, 2005a) required for •OH production.
Probable wall-tightening mechanisms include the oxidative cross-linking of polymer-bound phenolics (ferulate, p-coumarate or tyrosine residues), catalysed by peroxidase in the presence of H2O2, to form dimers and higher oligomers, e.g. di- and triferulates (Markwalder and Neukom, 1976; Fry et al., 2000; Grabber et al., 2000; Rouau et al., 2003; Baydoun et al., 2004; Kerr and Fry, 2004). Therefore these processes in response to OAC have also been investigated.
In addition, prolonged wall expansion requires continued wall synthesis, which has also been investigated.
MATERIALS AND METHODS
Maintenance of cell-suspension cultures
Cell-suspension cultures of maize (Zea mays L. ‘Black Mexican’) were maintained (at 25 °C under constant dim fluorescent lighting, with shaking at approx. 110 rpm) at 220 ml per 500-ml flask in a standard medium [Murashige and Skoog inorganic salts (Sigma Chemical Co.), 4·7 g L−1; sucrose, 20 g L−1; 2,4-D, 4·4 µm; pH before autoclaving, 5·6–5·8 with NaOH] and sub-cultured fortnightly by 11-fold dilution into fresh medium.
Application of OAC
OAC [kept as a 10 mm stock solution in dimethylsulphoxide (DMSO)] was added to aqueous media to give a final concentration of 480 nm, unless otherwise stated. All control cultures received the same concentration of DMSO (usually approx. 0·005 %, v/v) as accompanied the OAC.
Radioisotope assay
Aqueous solutions were assayed for 3H and 14C after addition of ten volumes of OptiPhase HiSafe scintillation fluid (Wallac Oy, Turku, Finland). Dry samples, e.g. strips of chromatography paper, were assayed by liquid scintillation counting in OptiScint HiSafe (Wallac).
Peroxidase measurements
The cells of a 4-d-old maize culture were collected on muslin, washed in fresh medium, dispensed at 2·4 g f. wt per flask and suspended in 25 mL of fresh medium. OAC was added to three replicate cultures to 333 nm. After 11 h with or without OAC, a 20-µL aliquot of the medium was added to 2·0 mL 50 mm tartrate buffer (Na+, pH 4·5), containing 0·8-mm o-dianisidine, followed by 0·6 ml 0·8-mm H2O2. The increase in A430 was recorded at 20 °C.
In-vivo oxidation of a p -[14C]coumaroyl ester
For the preparation of 14C-labelled phenolic esters, trans-[U-14C]cinnamic acid was fed to a 4-d-old maize cell-suspension culture for 8 h and the low-Mr 14C-labelled compounds, extracted with ethanol, were fractionated by preparative paper chromatography. A radioactive compound identified as p-coumaroyl β-glucosyl ester (Harborne and Corner, 1961) was eluted and stored frozen.
In an experiment (based on the method of Encina and Fry, 2005) to determine the effect of OAC on the oxidation of exogenous p-coumaroyl esters, 4-d-old cultures, pre-treated with or without 480-nm OAC for 24 h, were dispensed at 10 mL per 60-mL beaker and then fed 50 or 75 Bq mL−1 of the p-[14C]coumaroyl ester. At intervals, the cells were allowed to sediment for 1 min and 500 µL of cell-free medium was removed: of this, 200 µL was assayed for total 14C and 300 µL was mixed with 1·3 ml of ethanol, held at 4 °C for 18 h to precipitate the extracellular polysaccharides plus any covalently linked 14C-phenolic groups, then centrifuged and the supernatant was assayed for 14C.
Effect of OAC in vitro on XET activity extracted from maize roots
Roots of 7-d-old maize seedlings were homogenized in ice-cold buffer A [350 mm succinate (Na+, pH 5·5) containing 16·7 mm CaCl2 and 1·7 mm dithiothreitol], then centrifuged for 10 min at approx. 850 g. The supernatant (crude enzyme extract) was incubated for 1 h at 20 °C with or without 480 nm OAC. A portion (10 µL) of the supernatant (with or without OAC) was added to 20 µL of 0·3 % xyloglucan (from tamarind seed) containing a radioactive xyloglucan oligosaccharide ([3H]XLLGol; 80 Bq µL−1; 100 MBq µmol−1) and incubated for 0–60 min. The reaction was stopped by addition of 20 µL of 50 % formic acid. The yield of reaction product ([3H]polysaccharide) was determined by the filter paper binding assay (Fry et al., 1992).
Effect of OAC in vivo on secretion of XET activity
Maize cell cultures (4 d old) were treated with or without 480 nm OAC for 24 h, then the cells were collected on ‘Miracloth’ (Calbiochem) and the culture filtrate was stored at 0 °C. From each flask, 3 g (f. wt) of cells was homogenized in 4·5 mL ice-cold buffer A. The extract and the culture filtrate were separately assayed for XET activity as before except that 20 µL of sample was added to 10 µL of substrate mixture.
Effect of OAC on ascorbate consumption
Triplicate 4-d-old maize cultures were treated with or without 480 nM OAC. Ascorbic acid was added to each flask (to 200 µm) at 0 and 320 min after the OAC and normal incubation was continued. At intervals, the cells were allowed to sediment for 1 min and 0·5 mL of medium was removed, added to 0·5 mL of 10 % (w/v) HPO3 and immediately titrated against 0·1 % 2,6-dichlorophenolindophenol (DCPIP; Jones and Hughes, 1983) until it turned colourless and remained so for at least 3 s.
Effect of OAC and two other herbicides on [14C]cinnamate metabolism
Aliquots of a 2-d-old maize cell-suspension culture (SCV 7·5 %) were dispensed at 4 ml per 60-mL beaker and shaken gently. After 90 min, herbicides were added (OAC, isoxaben or diclofop methyl; each as 16 µL of a DMSO solution) to various final concentrations and the cultures were shaken gently at 25 °C. [Note that 1090 nm, the highest concentration of OAC tested, is greater than the solubility of OAC in water; therefore a fine suspension of solid OAC may have been present in addition to a saturated solution of 480 nm.] At 2 h after addition of the herbicides, 44 kBq trans-[U-14C]cinnamate (specific radioactivity 17 MBq µmol−1) was added to each pot and incubation was continued for a further 3 h. Metabolism was then stopped by the addition of 20 mL of freshly prepared 80 % ethanol/5 % formic acid to each pot, and the suspension was shaken overnight to extract low-Mr metabolites. The cells were then washed successively with 15 mL each of ethanol/formic acid/water (5 : 1 : 4, v/v/v), 96 % ethanol and 100 % acetone. The alcohol-insoluble residues (AIRs) were dried, then saponified in 500 µL of 0·1 m NaOH in the dark at 25 °C for 24 h. Saponification was stopped by addition of 100 µL of 4 m trifluoroacetic acid (TFA) and the released phenolic acids were partitioned into 2 × 1 mL ethyl acetate. The organic phases were dried in vacuo, and the solutes re-dissolved in 20 µL propan-1-ol, of which 6 µL was analysed by TLC on silica-gel, either in benzene/acetic acid (9 : 1, v/v) or in toluene/ethyl acetate/acetic acid (7 : 2 : 1, v/v/v); in both cases the plate was exposed to a UV lamp (wavelength 360 nm) throughout development to maintain each hydroxycinnamate compound as a cis/trans equilibrium mixture (Fry, 2000). The TLC plates were autoradiographed.
Effect of OAC on wall polysaccharide synthesis
Four-day-old maize cell-suspension cultures which (0·1, 4, 8 or 30 h earlier) had been supplied with OAC (final concentration 480 nm) or with DMSO only (for controls) were washed and resuspended (to a settled cell volume of 18 %, v/v) in fresh medium with or without OAC (as before the wash) and containing 1 % glycerol (a non-favoured carbon-source, in place of the routinely used sucrose). Replicate 100-µL aliquots of each culture were then pipetted into round-bottomed glass tubes (12 mm diameter) which contained dry d-[U-14C]glucose (0·93 MBq; giving a final glucose concentration of 0·0145 % w/v), and incubation was continued with shaking for a further 2 h. By this time, >90 % of the 14C had been removed from the medium, regardless of the presence of OAC. The cells were then killed by addition of 5 ml 75 % (v/v) ethanol containing 2 % (w/v) glucose. Ethanol-soluble material was extracted with shaking overnight, and the AIR was collected, washed in acetone and dried.
One AIR sample from each treatment was incubated with 500 µL 0·1 m TFA at 100 °C for 1 h to hydrolyse furanosyl linkages (Kerr and Fry, 2003), then the hydrolysate plus remaining AIR were dried together (to remove TFA) and the residue was treated with 200 µL of 2 % Driselase [purified according to Fry (2000) and dissolved in pyridine/acetic acid/water (1 : 1 : 98) containing 0·5 % chlorobutanol as a volatile anti-microbial agent] at 37 °C for 3 d. Formic acid (20 µL) was then added to denature the Driselase, and 50 µL of the solution was subjected to paper chromatography to resolve the main mono- and disaccharides of the cell wall polysaccharides.
A duplicate sample of AIR from each treatment was incubated with 1 ml of 2 m TFA at 120 °C for 1 h to hydrolyse non-cellulosic polysaccharides. The TFA-insoluble residue (mainly cellulose) was washed repeatedly with water, dried, Driselase-digested and chromatographed to reveal cellulosic glucose.
Chromatography was conducted on Whatman 3MM paper in butan-1-ol/acetic acid/water (12 : 3 : 5) for 18 h followed in the same dimension by ethyl acetate/pyridine/water (8 : 2 : 1) for 40 h. External markers were stained with aniline hydrogen-phthalate (Fry, 2000) and the radioactive tracks were autoradiographed.
Effect of RPA800298 (an OAC analogue) on mixed-linkage glucan synthesis
Aliquots of freshly sub-cultured maize cells were dispensed as above and treated with various concentrations of the experimental herbicide, RPA800298, which is chemically and herbicidally similar to OAC. At 3 h after addition of the herbicide, 100 µL of d-[U-14C]glucose (950 kBq) was fed to each beaker, and incubation was continued for a further 3 h. By this time, 0·30 % of the 14C had been incorporated into the cell wall matrix (released as monosaccharides by hydrolysis in 2 m TFA; data not shown). Formic acid was added to each beaker to a final concentration of 9 % (w/v), and left stirring overnight. The cells were washed as above to yield AIR (yield approx. 9 mg per beaker).
The AIR (approx. 9 mg) was suspended in 1 mL of 0·1 m collidine buffer (acetate, pH 7·0, containing 0·5 % chlorobutanol) and heated at 120 °C for 2 h to solubilize and/or gelatinize MLG and starch; then 100 U of licheninase (Megazyme, Dublin) was added to the cooled suspension and digestion was allowed to proceed for 3 d at 25 °C. A portion (150 µL) of the digest was analysed by paper chromatography in ethyl acetate/acetic acid/water (10 : 5 : 6) and the profile of radioactivity monitored by scintillation counting. The zone of the chromatogram containing the trisaccharide, β-d-Glcp-(1→4)-β-d-Glcp-(1→3)-d-Glc (G4G3G; marker prepared as described by Popper and Fry, 2003), plus a short length of paper on either side thereof, was then washed with water by the method of Eshdat and Mirelman (1972) and the eluted oligosaccharides were re-run by paper electrophoresis in borate buffer, pH 9·4 (Fry, 2000), at 1·75 kV for 2 h. The distribution of radioactivity was again determined.
RESULTS AND DISCUSSION
Effect of OAC on peroxidase
Wall tightening could occur via the peroxidase-catalysed coupling of polysaccharide-bound phenolic residues. Therefore, tests were done to find out if OAC enhanced the secretion, activity or action of peroxidase. ‘Action’ describes a catalysed reaction (Fry, 2004): in vivo it depends not only on the presence of [potentially] active enzyme but also on the presence of both substrates (a phenol and H2O2) in the same sub-cellular compartment, a physical proximity between the peroxidase and its polysaccharide-bound phenolic substrate, and the absence of inhibitors (Encina and Fry, 2005) or competing substrates (e.g. ascorbate or glutathione). ‘Activity’ describes the rate at which the enzyme catalyses its reaction in vitro under optimized conditions of H2O2 and aromatic substrate concentration, pH, etc.
OAC was neither an inhibitor nor activator of peroxidase. The spent medium of cultured maize cells contained peroxidase activity that was readily assayed with o-dianisidine + H2O2 as substrates. When OAC was added to cell-free spent medium (to 480 nm, its maximum solubility in water), there was no effect on the activity of the peroxidase (data not shown).
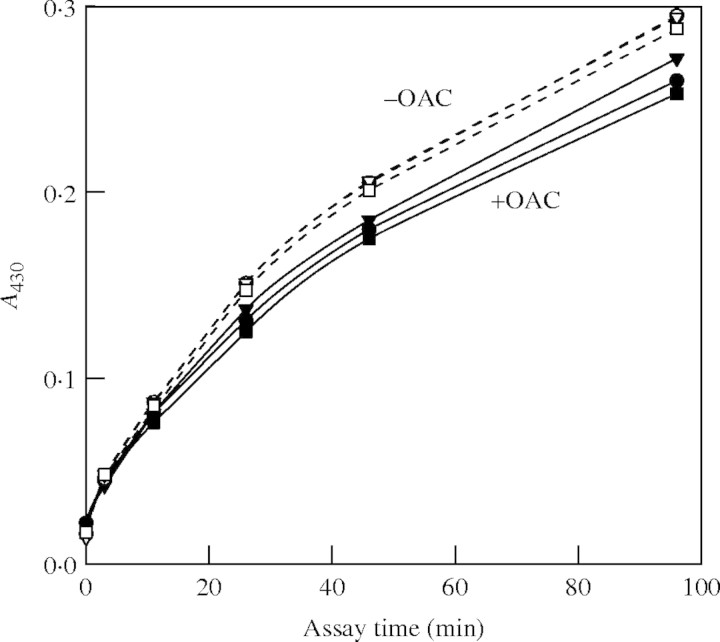
In addition, OAC had little effect on the secretion of peroxidase activity. Maize cell cultures were washed with fresh medium and then treated with or without OAC; samples of the medium were harvested, greatly diluted (to abolish any effect of endogenous anti-oxidants) and assayed for peroxidase activity with o-dianisidine + H2O2 as substrates. Peroxidase activity was gradually released into the medium. In most experiments, there was no significant effect of OAC on the release of peroxidase activity, but on one occasion there was a small inhibitory effect (Fig. 2). Inhibition of peroxidase secretion is not a plausible mechanism of growth inhibition; on the contrary, it could represent an attempted compensatory mechanism, initiated by the cells after perception of the early stages of growth inhibition.
Fig. 2.
Peroxidase activity released into the medium of maize cell cultures after 11 h in the presence or absence of OAC. Cells from a 4-d-old maize culture were suspended in fresh medium at 96 mg f. wt mL−1. OAC was added to three replicate cultures to 333 nm (closed symbols); control cultures received no OAC (open symbols). After 11 h, 20 µL of the spent medium was added to 2 mL of a peroxidase assay mixture and the increase in A430 was recorded.
OAC also did not affect the action of endogenous apoplastic peroxidase on exogenous substrates in vivo. For observations of peroxidase action in vivo, an electron-donor substrate (0·1 mm o-dianisidine) was added to maize cell-suspension cultures at various intervals after they had been treated with 480 nm OAC. This substrate yielded a brown product in the cell walls (but not the medium) approx. 1 h after the addition of o-dianisidine, regardless of the duration of OAC pre-treatment (data not shown). This is a test both for the co-occurrence of peroxidase + H2O2 in the apoplast and for the absence of antioxidants. Thus, OAC did not promote peroxidase action on a model aromatic substrate in the apoplast in vivo.
The cultures exhibited some degree of physiological variation. In certain experiments similar to the above, no colour was formed after the addition of o-dianisidine. If H2O2 was also added in such experiments, the characteristic brown product was formed but only transiently, suggesting the presence of an antioxidant capable of re-reducing any o-dianisidine oxidation products (comparable with that reported by Encina and Fry, 2005). However, in these experiments, OAC still had no effect.
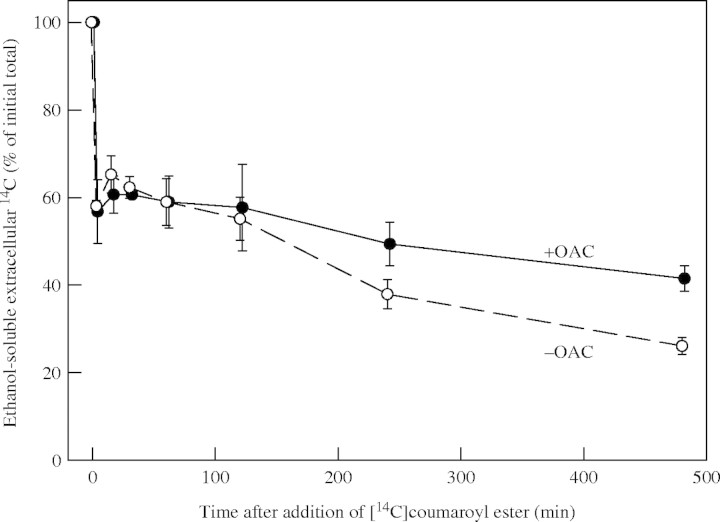
Further peroxidase substrates, p-[14C]coumaroyl esters, were also tested in a similar way in place of o-dianisidine. The p-[14C]coumaroyl esters remained water-soluble in the culture medium but were gradually incorporated into ethanol-insoluble products, presumably by peroxidase/H2O2-dependent oxidative coupling reactions with soluble extracellular polysaccharides (Kerr and Fry, 2004; Encina and Fry, 2005). This oxidative coupling, again thought to represent the in-vivo action of apoplastic peroxidase using endogenous H2O2, was not promoted by pre-treatment of the cells with OAC (Fig. 3). If anything, the coupling was retarded by OAC treatment—an effect considered unlikely to be responsible for the herbicide's ability to restrict cell expansion.
Fig. 3.
Effect of OAC on the oxidative coupling of a soluble extracellular [14C]coumaroyl ester. Cultured maize cells were treated with 480 nm OAC for 24 h, then supplied with a low concentration of p-[14C]coumaroyl β-glucosyl ester. At intervals (x-axis), samples of spent medium were assayed for remaining (ethanol-soluble) low-Mr 14C-labelled material. Three replicate cultures were tested and the s.e. is shown.
Effect of OAC on [14C]cinnamate metabolism
It remained possible that OAC could tighten the cell wall by promoting the action of endogenous peroxidase + endogenous H2O2 on endogenous wall-bound feruloyl-polysaccharides. This could in principle be achieved by any of various mechanisms that would not have influenced the tests so far described; for example, OAC treatment could evoke an alteration in the physical proximity of peroxidase to wall-bound feruloyl-polysaccharides, or it could alter the feruloylation of wall polysaccharides. In an investigation of these possibilities, the metabolic fate in vivo of endogenous feruloyl-polysaccharides were radiolabelled and studied.
Cultured maize cells were treated with OAC or DMSO for 2 h, and then fed [14C]cinnamate for a further 3 h. The cell walls (AIR) were by the end of this time heavily radiolabelled in their ester-bonded [14C]ferulate residues (Fig. 4). At least two different [14C]diferulates appeared to be present; one of these co-migrated with the 5,5′-dimer; the other, more abundant one, approximately co-migrated with caffeate in the toluene solvent (Fig. 4B), although it was not caffeate as shown in Fig. 4A. In addition, several slower-migrating spots were radiolabelled, which are trimers and higher oligomers of ferulate (Fry et al., 2000; Rouau, 2003). However, there was no evidence for any effect of OAC on the radiolabelling pattern. Thus, OAC did not affect either the feruloylation of wall polysaccharides or the subsequent peroxidase-catalysed oxidative coupling of polysaccharide-bound feruloyl groups.
Fig. 4.
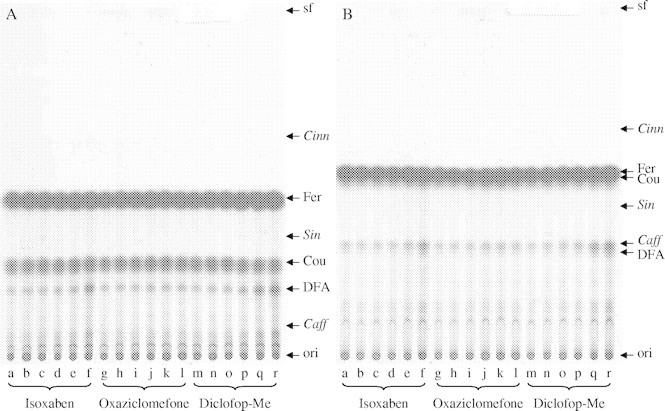
Effect of three herbicides on the production and cross-linking of polysaccharide-bound hydroxycinnamic acids. Aliquots of 2-d-old maize culture were pre-treated with the herbicides for 2 h, then fed [14C]cinnamate for 3 h, after which the cells were assayed for polysaccharide-esterified phenolics by TLC in either (A) benzene/acetic acid or (B) toluene/acetic acid/water, and detected by autoradiography. The herbicide doses were (final concentrations in nm): isoxaben—a, 0; b, 360; c, 1200; d, 3600; e, 12000; f, 36000; oxaziclomefone—g, 0; h, 11; i, 33; j, 109; k, 330; l, 1090; diclofop methyl—m, 0; n, 117; o, 350; p, 1170; q, 3500; r, 11700. The positions of authentic markers are indicated (Cinn, cinnamate; Cou, p-coumarate; Fer, ferulate; Sin, sinapate; Caff, caffeate; DFA, 5,5′-diferulate); those in italics were not detectable in radioactive form. sf, Solvent front; ori, origin.
Two other herbicides were tested at the same time for comparison: isoxaben, which inhibits cellulose synthesis (Heim et al., 1991; Sabba and Vaughn, 1999), and diclofop methyl, which permeabilizes the plasma membrane and inhibits acetyl-CoA carboxylase, leading to membrane damage and causing oxidative stress (Shimabukuro and Hoffer, 1994). These two herbicides also had little effect except at relatively high concentrations (Fig. 4), which caused an increase in production of dimers and higher oligomers, indicating some oxidative stress.
Effect of OAC on xyloglucan endotransglucosylase activity
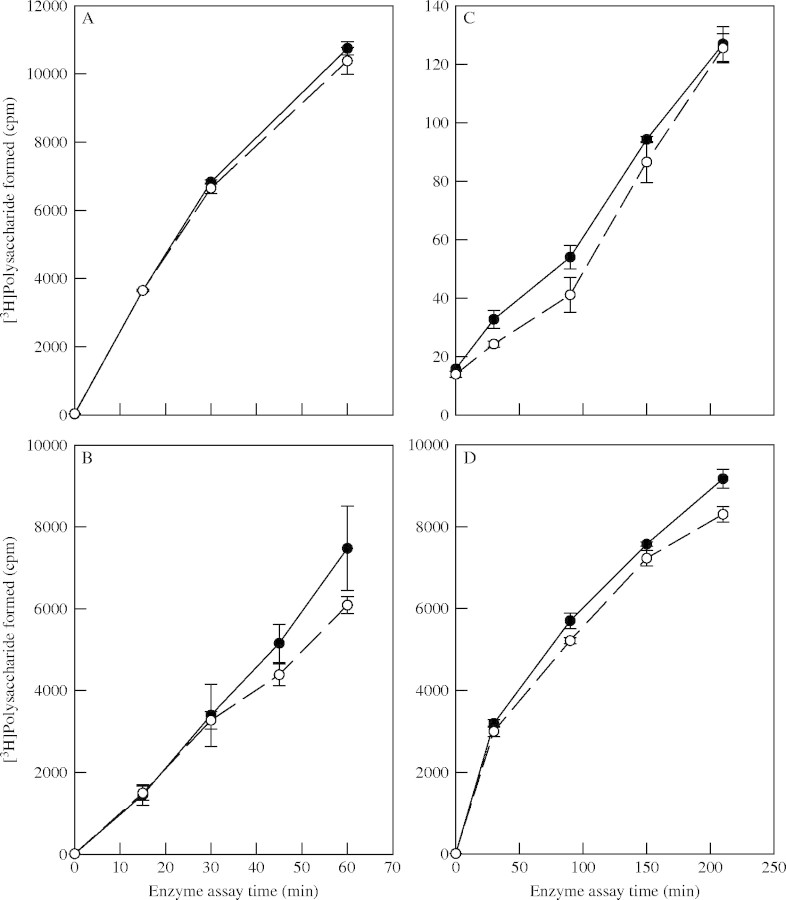
A proposed wall-loosening enzyme activity, whose inhibition by OAC could in principle account for the inhibition of cell expansion, is XET. However, OAC had no effect on XET activity when added in vitro to an XTH-rich extract previously prepared from maize roots (Fig. 5A). The same conclusion was also reached if the OAC was added to the enzyme 1 h before addition of the substrates in order to allow time for any non-competitive inhibition to take effect (Fig. 5B). Thus, OAC did not directly inhibit XET activity. Also, the amounts of XET activity present in the culture medium (Fig. 5C) and extractable from cultured maize cells (Fig. 5D) were unaffected by a 24-h pre-treatment with OAC. Thus, OAC did not appear to inhibit XET production, secretion or catalytic activity.
Fig. 5.
Effects of OAC on XET. (A and B) The in-vitro effects of OAC on previously extracted XET. The enzyme was extracted from maize roots and then OAC was added to the enzyme preparation either (A) simultaneously with or (B) 1 h before the radiolabelled substrate mixture. (C and D) Effects of OAC on the in-vivo accumulation and secretion of XET activity. Cultured maize cells were treated for 24 h with or without OAC, and then XET activity was assayed in (C) the spent medium and (D) the cell homogenates. Closed circles, + 480 nM OAC; open circles, control (DMSO only).
Effect of OAC on ascorbate consumption
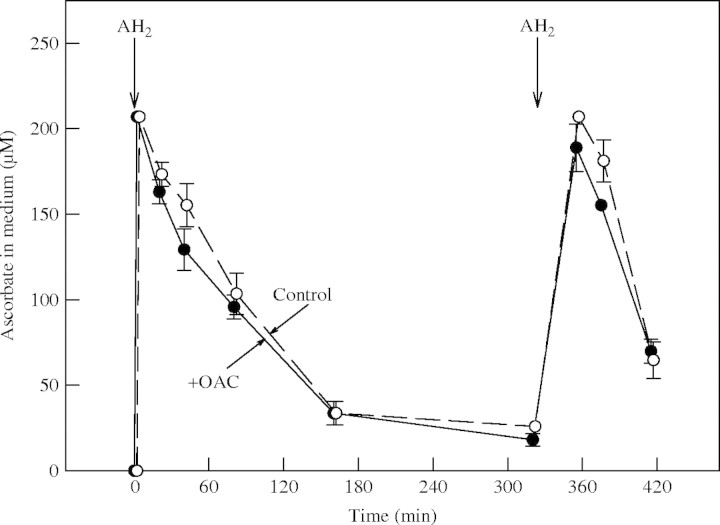
Other potential wall-loosening agents are apoplastic ascorbate and its oxidation products (Hidalgo et al., 1991; Lin and Varner, 1991; Green and Fry, 2005a). When added to cell cultures, ascorbate is gradually consumed (oxidized and/or taken up; Green and Fry, 2005b). A change in ascorbate consumption rate could be diagnostic of any of several changes in apoplastic redox metabolism including the generation (Fry, 1998) or dissipation (Smirnoff, 1996) of active oxygen species, inhibition of peroxidase action (Takahama and Oniki, 1994), the action of b cytochromes in the plasma membrane (Horemans et al., 1994), the action of apoplastic ascorbate oxidase (Lin and Varner, 1991), or the action of an ascorbate carrier (Rautenkranz et al., 1994; Heber et al., 2003). However, no effect of OAC on ascorbate consumption by cultured maize cells was observed (Fig. 6). Thus, OAC did not appear to have any strong effect on the apoplastic redox processes mentioned.
Fig. 6.
Effect of OAC on consumption of ascorbate by cultured maize cells. Ascorbate (AH2) was added to a final concentration of 200 µm at 0 and 320 min after treatment with 480 nm OAC (closed circles) or without herbicide (open circles). At intervals, the medium was assayed for ascorbate by the DCPIP method.
Effect of OAC on wall polysaccharide synthesis
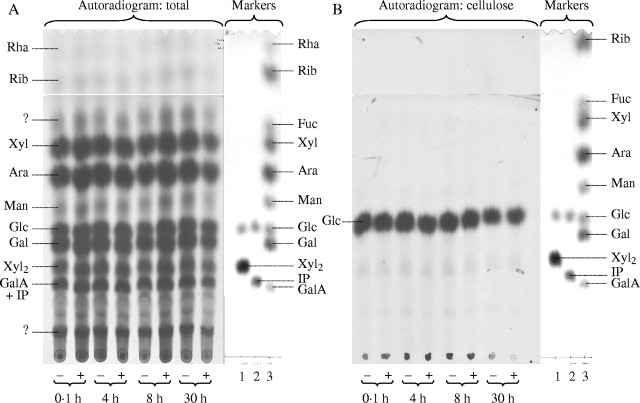
Maize cell cultures were pre-treated with or without 80 nm OAC for 0·1, 4, 8 or 30 h and then fed [U-14C]glucose for a further 2 h. Incorporation of radiolabel into the major sugar residues of the wall polysaccharides was unaffected by OAC (Fig. 7A). The identified, 14C-labelled products released from AIR by mild TFA hydrolysis plus Driselase digestion were Glc (which would include both cellulosic and wall matrix-derived Glc); Gal; Ara; Xyl and xylobiose (diagnostic of xylans); GalA and Rha (diagnostic of pectins); isoprimeverose (diagnostic of xyloglucan); Man and Rib. GalA was not resolved from isoprimeverose in the chromatogram shown (Fig. 7A); however, further studies by chromatography in other solvent systems and by electrophoresis at pH 3·5 (Fry, 2000) showed that both isoprimeverose and GalA residues were radiolabelled but that neither was affected by OAC (data not shown). Ribose is not known to be a cell wall sugar, and the polymeric component of AIR from which it was released by hydrolysis was probably RNA; the herbicide did not affect the synthesis of this polymer.
Fig. 7.
Effect of OAC on the biosynthesis of (A) total wall polysaccharides and (B) cellulose. Maize cell cultures were pre-treated either with (+) or without (−) 480 nM OAC for 0·1–30 h and then fed [U-14C]glucose for a further 2 h. One sample of AIR (A) was digested with mild TFA followed by Driselase and the total 14C-labelled digestion products were chromatographed and autoradiographed. A second sample (B) was freed of matrix polysaccharides and the cellulose-rich residue was Driselase-digested, chromatographed and autoradiographed. The marker mixtures were: 1, Xyl2 + Glc; 2, IP + Glc; 3, nine monosaccharides. Identified 14C-sugars are indicated to the left of each autoradiogram; the corresponding external markers, stained with aniline hydrogen-phthalate, are indicated to the right. Abbreviations used: Ara, arabinose; Fuc, fucose; Gal, galactose; GalA, galacturonic acid; Glc, glucose; IP, isoprimeverose; Man, mannose; Rha, rhamnose; Rib, ribose; Xyl, xylose; Xyl2, xylobiose.
After removal of the wall matrix polysaccharides by relatively severe acid hydrolysis in 2 m TFA, Driselase digestion of the insoluble residue yielded [14C]glucose as almost the sole radioactive product (Fig. 7B), indicating the radiolabelling of cellulose. However, OAC had no consistent effect on the incorporation of 14C into cellulosic Glc.
Effect of an OAC analogue on MLG and starch biosynthesis
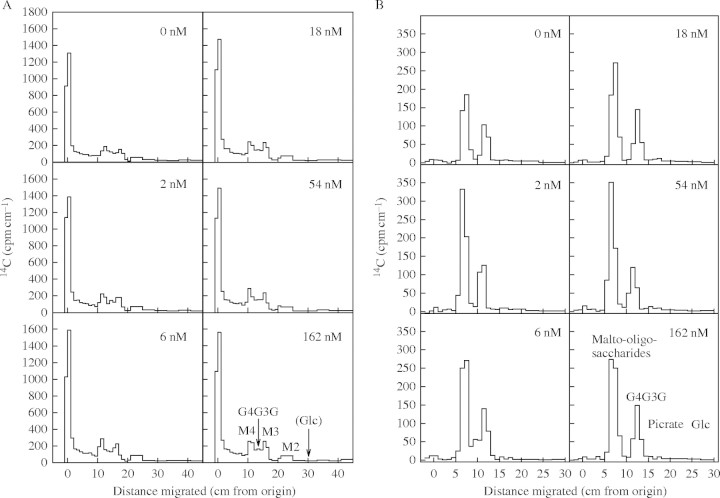
In a related experiment, radioactivity from [U-14C]glucose was incorporated into maize AIR (the fraction rich in starch and cell walls). RPA800298, an analogue of OAC, had no effect on total 14C incorporation into the non-cellulosic polysaccharides of AIR, which within 3 h amounted to 0·30 % of the 14C dose fed. When the radiolabelled AIR was digested with licheninase, the yield of the MLG trisaccharide, G4G3G, was 1·3 % of the non-cellulosic 14C-polysaccharides. It is expected that a roughly equal amount of the tetrasaccharide, G4G4G3G, would also be generated, implying that MLG constituted <3 % of the total newly synthesized non-cellulosic polysaccharides. This is a smaller percentage than in some gramineous tissues (Carpita, 1996), and suggests that MLG did not play a major role in the walls of these cultured maize cells. The herbicide had no effect on the radio-labelling of G4G3G (Fig. 8A). The peak of G4G3G was not completely resolved from neighbouring peaks of maltotriose and maltotetraose, generated from [14C]starch by the action of traces of amylase activity present in the licheninase. The radiolabelling of the malto-oligosaccharides was also unaffected by the herbicide (Fig. 8A).
Fig. 8.
Effect of an OAC analogue (RPA800298) on mixed-linkage β-glucan (MLG) and starch biosynthesis. Maize cell cultures were treated with 0–162 nm of RPA800298 for 3 h and then with [14C]glucose for a further 3 h. The radiolabelled AIR was heated, then digested with licheninase and the oligosaccharides produced were paper chromatographed (A). Identities of peaks of radioactivity: G4G3G, the major MLG-derived trisaccharide; M2–M4, maltose to maltotetraose. No free [14C]glucose (Glc) was detectable. The MLG-derived tetrasaccharide, G4G4G3G, remained very near the origin (RF = 0), not resolved from undigested polysaccharides. The partially purified G4G3G was eluted from the appropriate strips of chromatography paper in (A) and re-run by electrophoresis in borate buffer (B). In this system, the M3 and M4 approximately co-migrate but are well resolved from G4G3G.
For better resolution of MLG- and starch-derived oligosaccharides, the zone of the chromatogram containing G4G3G, plus a proportion of the malto-oligosaccharides on either side was eluted, and the eluted mixture re-run by high-voltage electrophoresis in borate buffer. This gave excellent resolution of G4G3G from the malto-oligosaccharides, and confirmed that the herbicide had no effect on the radiolabelling of the MLG-derived oligosaccharide (Fig. 8B).
CONCLUSIONS
OAC had previously been shown to inhibit cell expansion in maize cell cultures without decreasing the cells' turgor pressure (O'Looney and Fry, 2005). Any of a wide range of herbicides that disrupt steps in basic cell metabolism would have been expected to cause damage to the cells, leading to loss of turgor. The fact that turgor was maintained for at least 2 d in the presence of relatively high concentrations of OAC (a saturated aqueous solution; i.e. approx. 100 × the ID50) points to OAC exerting a more direct effect on the susceptibility of the cell wall to turgor-driven expansion. Therefore when this work was started, it was with the assumption that the herbicide would either promote the tightening or inhibit the loosening of the primary cell wall.
None of the metabolic processes tested as potential targets for such an action turned out to be affected by OAC treatments. This conclusion also applied to those aspects of cell wall metabolism that might be predicted to play a particularly important role in the cell expansion of gramineous monocots and that that might therefore account for the greater efficacy of OAC on maize than on dicots. Such aspects include polysaccharide feruloylation, feruloyl cross-linking and MLG biosynthesis. The metabolism of ferulate had seemed a particularly likely candidate in view of the fact that this phenolic compound is a substituent of wall polysaccharides in both the Gramineae and the Chenopodiaceae, which includes spinach—a species shown to be more OAC-susceptible than other dicots (O'Looney and Fry, 2005).
It is concluded that none of the processes in cell wall metabolism studied here is responsible for imposing the growth inhibition characteristic of OAC. These processes include: cellulose biosynthesis; wall matrix polysaccharide biosynthesis, including MLG; production, secretion and action of XET activity; production, secretion and action of peroxidase activity; polysaccharide feruloylation; oxidative coupling of feruloyl or p-coumaroyl residues; and consumption of apoplastic ascorbate, a potential source of ⋅OH radicals. In addition, it had been shown previously (O'Looney and Fry, 2005) that OAC does not interfere with the cells' ability to acidify their medium (which may be necessary for ‘acid growth’).
Thus the results point to an action of OAC on a highly specific aspect of wall biochemistry, not one of those tested here. This strongly suggests that OAC is a valuable tool for probing certain specific physiological functions of the primary cell wall.
Supplementary Material
Acknowledgments
We thank Dr David Cole (Aventis CropScience UK Ltd, Ongar, Essex) for supplying OAC and RPA800298, and for valuable discussions. N.O'L. thanks the BBSRC and Aventis CropScience UK Ltd for funding a studentship. We thank Dr Antonio Encina for preparing the 14C-labelled phenolic esters.
Footnotes
Present address: Scottish Centre for Genomic Technology and Informatics, University of Edinburgh Medical School, The Chancellor's Building, Little France Crescent, Edinburgh EH16 4SB, UK
LITERATURE CITED
- Baydoun EA-H, Pavlencheva N, Cumming CM, Waldron KW, Brett CT. 2004. Control of dehydrodiferulate cross-linking in pectins from sugar-beet tissues. Phytochemistry 65: 1107–1115. [DOI] [PubMed] [Google Scholar]
- Carpita NC. 1996. Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Physiology and Plant Molecular Biology 47: 445–476. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 1993. Wall extensibility: its nature, measurement and relationship to plant cell growth. New Phytologist 124: 1–23. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 1996. Plant cell enlargement and the action of expansins. BioEssays 18: 533–540. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 2000. Loosening of plant cell walls by expansins. Nature 407: 321–326. [DOI] [PubMed] [Google Scholar]
- Encina A, Fry SC. 2005. Oxidative coupling of a feruloyl-arabinoxylan trisaccharide (FAXX) in the walls of living maize cells requires endogenous hydrogen peroxide and is controlled by a low-Mr apoplastic inhibitor. Planta (in press; 10.1007/00425-005-0033-y). [DOI] [PubMed] [Google Scholar]
- Eshdat Y, Mirelman D. 1972. An improved method for the recovery of compounds from paper chromatograms. Journal of Chromatography A 65: 458–459. [Google Scholar]
- Fry SC. 1998. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochemical Journal 332: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. 2000.The growing plant cell wall: chemical and metabolic analysis, reprint edn. Caldwell, NJ: The Blackburn Press. [Google Scholar]
- Fry SC. 2004. Tansley Review: Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist 161: 641–675. [DOI] [PubMed] [Google Scholar]
- Fry, SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. 1992. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochemical Journal 357: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Willis SC, Paterson AEJ. 2000. Intraprotoplasmic and wall-localised formation of arabinoxylan-bound diferulates and larger ferulate coupling-products in maize cell-suspension cultures. Planta 211: 679–692. [DOI] [PubMed] [Google Scholar]
- Grabber JH, Ralph R, Hatfield RD. 2000. Cross-linking of maize walls by ferulate dimerization and incorporation into lignin. Journal of Agricultural and Food Chemistry 48: 6106–6113. [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC. 2005. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 433: 83–88. [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC. 2005. Apoplastic degradation of ascorbate: novel enzymes and metabolites permeating the plant cell wall. Plant Biosystems 139: 2–7. [Google Scholar]
- Grobe K, Becker W-M, Sclaak M, Petersen A. 1999. Grass group I allergens (β-expansins) are novel, papain-related proteinases. European Journal of Biochemistry 263: 33–40. [DOI] [PubMed] [Google Scholar]
- Grossmann K. 2000. The mode of action of quinclorac: a case study of a new auxin-type herbicide. In: Cobb AH, Kirkwood RC, eds. Herbicides and their mechanisms of action. Sheffield: Sheffield Academic Press, 181–214. [Google Scholar]
- Harborne JB, Corner JJ. 1961. Plant polyphenols. 4. Hydroxycinnamic acid–sugar derivatives. Biochemical Journal 81: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield RD, Nevins DJ. 1987. Hydrolytic activity and substrate-specificity of an endoglucanase from Zea mays seedling cell-walls. Plant Physiology 83: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U, Bukhov NG, Wiese C, Hedrich R. 2003. Energized uptake of ascorbate and dehydroascorbate from the apoplast of intact leaves in relation to apoplastic steady state concentrations of ascorbate. Plant Biology 5: 151–158. [Google Scholar]
- Heim DR, Skomp JR, Waldron C, Larrinua IM. 1991. Differential response to isoxaben of cellulose biosynthesis by wild type and resistant strains of Arabidopsis thaliana Pesticide Biochemistry and Physiology 39: 93–99. [Google Scholar]
- Heim DR, Larrinua IM, Murdoch MG, Roberts JL. 1998. Triazofenamide is a cellulose biosynthesis inhibitor. Pesticide Biochemistry and Physiology 59: 163–168. [Google Scholar]
- Hidalgo A, García-Herdugo G, González-Reyes JA, Morré D J, Navas P. 1991. Ascorbate free radical stimulates onion root growth by increasing cell elongation. Botanical Gazette 152: 282–288. [Google Scholar]
- Horemans N, Asard H, Caubergs RJ. 1994. The role of ascorbate free-radical as an electron-acceptor to cytochrome b-mediated trans-plasma membrane electron-transport in higher-plants. Plant Physiology 104: 1455–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jikihara K, Maruyama T, Morishige J, Suzuki H, Ikeda K, Takagi A, Usui Y, Go A. 1997. MY-100 a new herbicide for pre- and early post-emergence barnyard grass control in rice. Brighton Crop Protection Conference 1: 73–80. Farnham, Surrey: British Crop Protection Council. [Google Scholar]
- Jones E, Hughes RE. 1983. Foliar ascorbic acid in some angiosperms. Phytochemistry 22: 2493–2499. [Google Scholar]
- Kaku T, Tabuchi A, Wakabayashi K, Kamisaka S, Hoson T. 2002. Action of xyloglucan hydrolase within the native cell wall architecture and its effect on cell wall extensibility in azuki bean epicotyls. Plant Cell Physiology 43: 21–26. [DOI] [PubMed] [Google Scholar]
- Kerr EM, Fry SC. 2003. Pre-formed xyloglucans and xylans increase in molecular weight in three distinct compartments of a maize cell-suspension culture. Planta 217: 327–339. [DOI] [PubMed] [Google Scholar]
- Kerr EM, Fry SC. 2004. Extracellular cross-linking of xylan and xyloglucan in maize cell-suspension cultures: the role of oxidative phenolic coupling. Planta 219: 73–83. [DOI] [PubMed] [Google Scholar]
- Kiedaisch BM, Blanton RL, Haigler CH. 2003. Characterization of a novel cellulose synthesis inhibitor. Planta 217: 922–930. [DOI] [PubMed] [Google Scholar]
- Koo SJ, Neal JC, DiTomaso JM. 1996. 3,7-Dichloroquinolinecarboxylic acid inhibits cell-wall biosynthesis in maize roots. Plant Physiology 112: 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U. 2001. Stem elongation and cell wall proteins in flowering plants. Plant Biology 3: 466–480. [Google Scholar]
- Lin LS, Varner JE. 1991. Expression of ascorbic acid oxidase in zucchini squash (Cucurbita pepo L). Plant Physiology 96: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove, DJ. 1992. Two endogenous proteins that induce cell wall extension in plants. The Plant Cell 4: 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwalder HU, Neukom H. 1976. Diferulic acid as a possible cross-link in hemicelluloses from wheat-germ. Phytochemistry 15: 836–837. [Google Scholar]
- Miller SK, Pallett KE, Cole DJ. 2001. Histological investigations into the mode of action of the novel grass herbicide oxaziclomefone. In: Proceedings of the British Crop Protection Council (BCPC) Conference ‘Weeds 2001’ (12–15 Nov. 2001, Brighton), 2: 569–574. Farnham, Surrey: British Crop Protection Council. [Google Scholar]
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Höfte H. 1998. A plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis EMBO Journal 17: 5563–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto-Nakazato A, Takahashi K, Katoh-Semba R, Katou K. 2001. Distribution of yieldin, a regulatory protein of the cell wall yield threshold, in etiolated cowpea seedlings. Plant Cell Physiology 42: 952–958. [DOI] [PubMed] [Google Scholar]
- O'Looney N, Fry SC. 2005. The novel herbicide oxaziclomefone inhibits cell expansion in maize cell cultures without affecting turgor pressure or wall acidification. New Phytologist (in press; 10.1111/j.1469-8137.2005.01501.x). [DOI] [PubMed] [Google Scholar]
- Peng L, Xiang F, Roberts E, Kawagoe Y, Greve LC, Kreuz K, et al. 2001. The experimental herbicide CGA 325'615 inhibits synthesis of crystalline cellulose and causes accumulation of non-crystalline β-1,4-glucan associated with CesA protein. Plant Physiology 126: 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. 2003. Primary cell wall composition of bryophytes and charophytes. Annals of Botany 91: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. 2004. Primary cell wall composition of pteridophytes and spermatophytes. New Phytologist 164: 165–174. [DOI] [PubMed] [Google Scholar]
- Rautenkrantz A, Liantje L, Mächler F, Martinola E, Oertli J. 1994. Transport of ascorbic and dehydroascorbate acids across protoplasts and vacuole membranes isolated from barley (Hordeum vulgare L. cv. Gerbel) leaves. Plant Physiology 106: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG. 1981. The assembly of polysaccharide fibrils. In: Tanner W, Loewus FA, eds. Plant Carbohydrates II (Encyclopedia of Plant Physiology New Series, Vol. 13B). Berlin: Springer, 25–28. [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. 2002. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiology 43: 1421–1435. [DOI] [PubMed] [Google Scholar]
- Rouau X, Cheynier V, Surget A, Gloux D, Barron C, Meudec E, Louis-Montero J, Criton M. 2003. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 63: 899–903. [DOI] [PubMed] [Google Scholar]
- Sabba RP, Vaughn KC. 1999. Herbicides that inhibit cellulose biosynthesis. Weed Science 47: 757–763. [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C. 2001. Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proceedings of the National Academy of Sciences of the USA 98: 10079–10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Fry B, Kochevenko A, Schindelasch D, Zimmerli L, Somerville S, et al. 2003. An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. The Plant Cell 15: 1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P. 2001. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. The Plant Journal 28: 679–688. [DOI] [PubMed] [Google Scholar]
- Shimabukuro RH, Hoffer BL. 1994. Effects on transmembrane proton gradient and lipid biosynthesis in the mode of action of diclofop methyl. Pesticide Biochemistry and Physiology 48: 85–97. [Google Scholar]
- Smirnoff N. 1996. The function and metabolism of ascorbic acid in plants. Annals of Botany 78: 661–669. [Google Scholar]
- Suzuki H, Jikihara K, Sonoda M, Usui Y. 2003. Development of a new herbicide, oxaziclomefone. Journal of Pesticide Science 28: 241–248. [Google Scholar]
- Takahama U, Oniki T. 1994. The association of ascorbate and ascorbate oxidase in the apoplast with IAA-enhanced elongation of epicotyls from Vigna angularis Plant Cell Physiology 35: 257–266. [Google Scholar]
- Tegg RS, Melian L, Wilson CR, Shabala S. 2005. Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: calcium and hydrogen flux patterns revealed by the non-invasive MIFE technique. Plant and Cell Physiology 46: 638–648. [DOI] [PubMed] [Google Scholar]
- Tresch S, Grossmann K. 2003. Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots. Pesticide Biochemistry and Physiology 75: 73–78. [Google Scholar]
- Vaughn KC. 2002. Cellulose biosynthesis inhibitors. In: Böger P, Wakabayashi K, Hirai K, eds. Herbicide classes in development. Modes of action, targets, genetic engineering, chemistry. Springer: Berlin, 139–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.