Abstract
• Background and Aims Broomrapes (Orobanche spp.) are holoparasitic weeds that cause devastating losses in many economically important crops. The molecular mechanisms that control early stages of host infection in Orobanche are poorly understood, partly due to the lack of experimentally tractable in vitro systems that allow the efficient application of molecular tools. Here an improved axenic system for the analysis of pre-infection stages in O. ramosa in the absence of the host plant is described.
• Methods An optimized protocol for seed disinfection, based on formaldehyde, was developed. Orobanche ramosa seeds were conditioned in Petri dishes with filter paper, stimulated by addition of the synthetic strigol analogue GR24, and the percentage of germination as well as attachment-organ formation was determined.
• Key Results Treatment of O. ramosa seeds with tobacco-root exudate or with GR24 resulted in highly reproducible germination rates around 70 %. A conditioning period of 8 d was both necessary and sufficient to allow optimal germination in response to GR24. Conditioned seeds that were dehydrated for several months remained fully responsive to GR24 without the need of a new conditioning period. Treatments as short as 5 min with GR24 were sufficient to fully and irreversibly induce the seed germination response. Approximately half of the germinated seeds initiated attachment-organ development. Similar rates of attachment organ induction were also detected in the rare cases of seeds that had germinated spontaneously on water.
• Conclusions The results suggest that the conditioning period produces persistent changes in the seeds required for responsiveness to external stimulants. The rapid action of GR24 suggests that it may act via a receptor-mediated signalling mechanism. While germination in O. ramosa is induced by exogenous stimuli, attachment organ differentiation appears to be triggered by unknown endogenous signals. The new in vitro culture system will have useful applications for the molecular analysis of early stages of parasitic development in Orobanche.
Keywords: Attachment-organ formation, conditioning, germination, GR24, in vitro culture, Nicotiana tabacum, Orobanche ramosa, parasitic, seed disinfection, signal
INTRODUCTION
The ability of plants to fulfil their nutritional needs by parasitizing other plants has originated several times during the evolution of angiosperms, and about 4000 species in 22 dicot families are currently recognized as parasitic (Nickrent et al., 1998). Species of the genus Orobanche (broomrapes) are holoparasitic weeds that cause devastating losses in many economically important crops (Parker and Riches, 1993). Among these, O. ramosa attacks a wide range of host plants including tobacco, tomato and potato (Kreutz, 1995), as well as the model plant Arabidopsis thaliana (Goldwasser et al., 2000).
Many crucial developmental processes in parasitic plants, such as seed germination or differentiation of infection organs, are regulated by signals from the host plant (Bouwmeester et al., 2003). One of the keys to advance our understanding of the molecular mechanisms underlying these early infection processes is the availability of experimentally tractable in vitro systems that take full advantage of the increasing number of molecular tools at hand (Yoder, 1999). The advantages of in vitro culture systems include the ability to obtain axenic cultures in the absence of contamination by micro-organisms, the optimization and standardization of environmental and nutritional conditions, and, most importantly, the development of pre-infection stages of the parasite in the absence of the host. The latter point not only facilitates study of the developmental effects of specific metabolites and substrates (Okonkwo, 1994), but provides biological material exclusively from the parasitic plant, without interference of the host tissue. This aspect is particularly relevant for applications that are based on DNA, RNA or protein extraction, during developmental stages where the parasite is in intimate contact with the host, and physical separation of the two organisms is difficult to achieve.
In the present study, an efficient and reproducible in vitro experimental system has been developed that allows the study of molecular factors controlling germination and pre-infection development of O. ramosa in the absence of the host plant. The system has been employed to study the role of endogenous and exogenous signals during the early developmental stages of O. ramosa. The results suggest that in this parasitic plant, similarly to Striga, seed germination and attachment organ differentiation are independent processes that are controlled by distinct signals. Evidence for the presence of an endogenous, host-independent signal that triggers attachment-organ formation of O. ramosa is provided.
MATERIALS AND METHODS
Viability test
Viability of Orobanche ramosa seeds was assessed by detecting respiratory activity through staining with 2,3,5-triphenyl tetrazolium chloride (TTC) (Sigma) following a previously reported protocol (López Granados and García Torres, 1999) with the following modifications. Seventy milligrams of seeds were placed in small tubes containing a 1 % solution of TTC. Tubes were kept for 72 h at 37 °C in the dark. To facilitate detection of the red colour, the testa of the seeds was bleached with 10 % sodium hypochlorite. Red or orange seeds were considered viable, while white seeds were considered dead. Changes in metabolic activity during O. ramosa germination were also detected by TTC staining as described previously (Aalders and Pieters, 1985).
Seed sterilization
Orobanche ramosa seeds were collected from plants parasitizing tobacco grown in fields located in Granada (Spain), and surface-sterilized using two alternative methods. The first method was that described previously by Batchvarova et al. (1999) with some modifications. This disinfection protocol was used to obtain tubercles, and consisted of treating seeds for 10 min with 5 % sodium hypochlorite containing 0·1 % Tween 20 (both from Panreac Quimica SA, Barcelona, Spain). The second method was used for the germination assays: seeds were treated for 2 h with a solution of 0·5 % formaldehyde (37–38 % w/w, Panreac) and 0·1 % Tween 20, followed by a 20 min incubation at 50 °C. Nicotiana tabacum seeds were surface-sterilized by immersion in 70 % ethanol for 2 min and 3·5 % sodium hypochlorite containing 0·1 % Tween 20 for 10 min. In all cases, the seeds were rinsed three times with sterile distilled water.
Antibiotics and fungicides
The following antibiotics and fungicides were tested for their ability to prevent contamination by micro-organisms: 100× antibiotic antimycotic solution (Sigma A-5955) used at a 1 : 100 dilution; ciprofloxacine (Cetraxal) used at 1, 5, 10, 20 or 50 mg L−1; amphotericin B (Sigma A-6804) used at 0·25, 0·5 or 1 mg L−1; Nystatin (Sigma N-3603) used at 5000, 10000 and 20000 U L−1; benzimidazol (Merck 821956) used at 125 mg L−1; benlate 50 % (Dupont) used at 1 g L−1.
Seed conditioning, germination and development
Approximately 1000 O. ramosa seeds were sown on a 6-cm Petri dish containing a previously autoclaved moist glass-fibre filter paper (Whatman). Seeds were maintained at 24 °C in the dark for a conditioning period (usually 11 d, unless otherwise stated), before adding 0·5 mL of a solution of 0·034 mm GR24 (van Hezewijk et al., 1993), or of tobacco-root exudates (obtained as described below). GR24 was routinely purchased from University of Nijmegen, The Netherlands. Water was used as a negative control. Percentages of seed germination and differentiation of attachment organ-like structures were determined using a Leica DMR microscope, by counting four times 100 seeds per plate on each of four independent plates per experiment and by calculating the mean values and standard deviations. Photographs were recorded with a Leica DC 300F digital camera. All experiments were performed at least three times with similar results.
Tobacco-root exudate
Tobacco plants were obtained from surface-sterilized seeds (see above) and maintained in transparent boxes with autoclaved vermiculite under growth chamber conditions (24 °C, 16 h light). Plants were watered with Hoagland nutrient solution (Hoagland and Arnon, 1950). After 1 month, the roots were washed with autoclaved distilled water, submerged in a flask with autoclaved distilled water at a proportion of 0·1 g roots (f. wt) per millilitre and maintained at 24 °C for 4 h with gentle shaking. The liquid containing the root exudate was collected and sterilized through a Millipore filter (0·22 µm) before use.
In vivo germination assays
In vivo germination and attachment-organ development of O. ramosa were performed as described previously (Pérez de Luque et al., 2004). Briefly, tobacco seedlings and disinfected O. ramosa seeds were simultaneously sown in 9-cm Petri dishes with Perlite and glass-fibre filter paper, and watered with Hoagland nutrient solution. Infected roots were observed microscopically and photographs were recorded as described above.
RESULTS AND DISCUSSION
Optimization of seed disinfection protocols for studying in vitro development of O. ramosa
The seeds of Orobanche are usually severely contaminated with different soil micro-organisms (Ben-Hod et al., 1991; Batchvarova et al., 1999). For this reason it is impossible to conduct controlled experiments in vitro, unless totally axenic conditions can be obtained. Previously reported methods for surface sterilization of Orobanche seeds include the use of sodium hypochlorite (Joel and Losner-Goshen, 1994; Westwood, 2000; Goldwasser and Yoder, 2001; Zhou et al., 2004) or the addition of antibiotics (Ben-Hod et al., 1991; Losner-Goshen et al., 1996) or fungicides (Batchvarova et al., 1999). These reported protocols were tested with seeds of O. ramosa, but none of them proved entirely effective under the present experimental conditions. Application of different antibiotics and/or fungicides (antibiotic antimycotic solution, ciprofloxacine, amphotericin B, Nystatin, benzimidazol and benlate) failed to eliminate fungal contaminations, and higher concentrations of these compounds severely affected seed germination (data not shown). Therefore, two alternative methods were tested for their effectiveness in obtaining axenic cultures. The first protocol, based on the use of 5 % sodium hypochlorite, produced totally axenic in vitro cultures of O. ramosa. However, the viability of the disinfected seeds was only 31 % and the rate of GR24-induced germination was <1 % when seeds were maintained on glass-fibre filter paper (Table 1). Lower concentrations of sodium hypochlorite failed to completely eliminate the microbial contaminations. The low germination rate precluded the use of this protocol in seed germination experiments, although it might be useful for different applications. Indeed, under the culture conditions required for in vitro tubercle formation (MS medium supplemented with sucrose), seed germination rates of up to 20 % were obtained (results not shown).
Table 1.
Effect of various disinfection protocols on germination and viability, as determined by staining with TTC, of Orobanche ramosa seeds
| Disinfection treatment |
% seed germination |
% seed viability |
|---|---|---|
| None | Mc* | 84 ± 3 |
| 3·5 % NaClO | Mc | 49 ± 6 |
| 5 % NaClO | 0 ± 0 | 31 ± 6 |
| 0·5 % formaldehyde | 70 ± 3 | 73 ± 3 |
The mean values and standard deviations are shown (for details see Materials and Methods).
Microbial contamination.
To establish an axenic system that allows the analysis of germination and early infection stages in Orobanche on the glass-fibre filter paper, an alternative sterilization protocol, based on the use of 0·5 % formaldehyde, was developed. This protocol resulted in germination rates around 70 % in O. ramosa while effectively eliminating bacterial or fungal contaminations (Table 1). It was concluded that this disinfection protocol was appropriate for germination assays in O. ramosa, where the support was glass-fibre filter paper moistened with water.
Effect of the conditioning period on seed germination of O. ramosa
Among the most critical steps during the life cycle of Orobanche are those that precede physical contact and penetration of the host. The molecular mechanisms that control these early developmental stages of the parasite remain largely unknown (Westwood, 2001). To validate the utility of the host-free in vitro system for the analysis of pre-infection development of Orobanche, it was decided to focus on three crucial pre-infection steps: seed conditioning, seed germination, and attachment-organ formation.
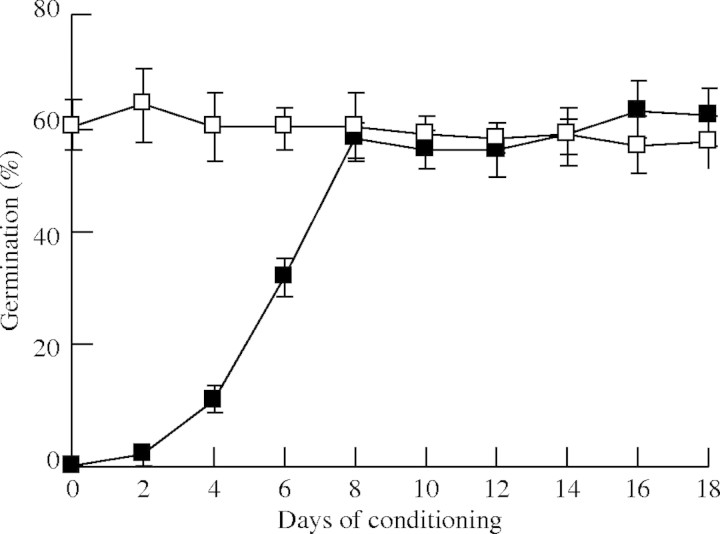
The first of these steps is seed conditioning. Previous studies have shown that a period of several days of moist environment at permissive temperatures is required in order to render the ripe seeds of Orobanche responsive to germination stimuli (Joel et al., 1995). The exact physiological role of this preparatory phase, which has been termed conditioning or pre-conditioning, are poorly understood. In this respect, three distinct but not mutually exclusive hypotheses could be considered. (1) Conditioning induces metabolic changes that are necessary for the response to GR24. During conditioning gibberellin, which is required for seed germination, is synthesized but is not yet present in the seeds. Indeed, inhibitors of gibberellin synthesis were shown to prevent germination after GR24 treatment and this effect could be reversed by exogenous GA3 (Zehhar et al., 2002). (2) During the conditioning period the receptor proteins for GR24 or other natural stimulants are synthesized. (3) Structural modifications occurring in the seeds allow the stimulant to access its putative cellular target. In any case, the effects of conditioning would be maintained during a subsequent dry period. The effect of the conditioning period on germination of O. ramosa in the host-free system was determined. Surface-sterilized seeds were subjected to different time periods of conditioning before addition of the germination stimulant GR24. As shown in Fig. 1, conditioning periods of 8 d or longer (studied up to 18 d) produced an optimal germination response to GR24, whereas shorter conditioning periods resulted in significantly lower rates of germination. These results show that a minimum conditioning period of 8 d is required to induce optimal rates of germination in O. ramosa. Similar periods of conditioning (7 d) were recently applied by Zhou et al. (2004).
Fig. 1.
Percentage germination of Orobanche ramosa seeds conditioned for different periods of time (filled squares), or conditioned for 11 d, dehydrated for 2 months and resubmitted to different time periods of conditioning (open squares). The mean values and standard deviations are shown.
Next a test was carried out to see if the conditioned seeds maintained their responsiveness to GR24 even after drying. This is of high biological relevance because, under field conditions, Orobanche seeds in the soil are submitted to periods of high humidity, followed by dry periods, during which seeds are dehydrated without having found an appropriate host plant. To experimentally simulate this process, O. ramosa seeds were conditioned for 11 d, and then dried completely in a sterile hood. The dehydrated seeds were stored at room temperature for time periods up to 2 months, and stimulated by adding GR24 after an additional conditioning period ranging from 0 to 18 d. As shown in Fig. 1, seeds that had been conditioned and then dried exhibited germination rates comparable with those of freshly conditioned seeds, without the need for a new conditioning period. These results indicate that the conditioning effect is fully conserved throughout a prolonged dehydration period, and thus supports the hypothesis that conditioning produces structural changes that are required for seed responsiveness to external stimulants.
Effect of exogenous stimulants on in vitro germination and development of O. ramosa
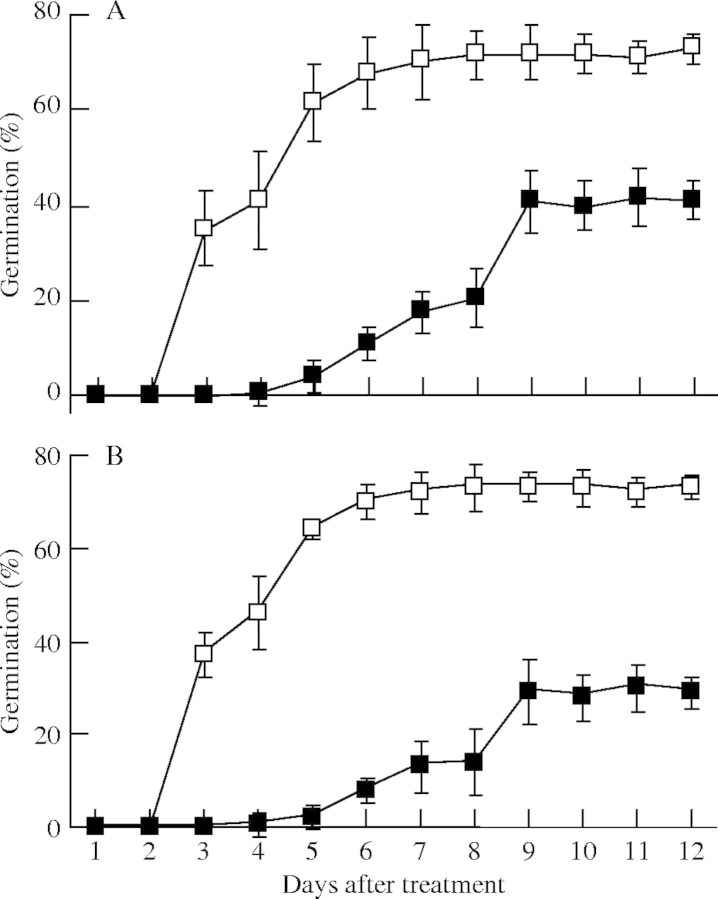
Seed germination in Orobanche was shown previously to be stimulated by host root exudate or by synthetic strigol analogues such as GR24 (Joel and Losner-Goshen, 1994; van Woerden et al., 1994). To study germination and early development in the host-free in vitro system, conditioned seeds of O. ramosa were stimulated by adding either tobacco-root exudate (TRE) or GR24, and seed germination and development were followed daily by microscopic observation. A high percentage of the stimulated seeds germinated in the presence of TRE (Fig. 2A). Germination was first visible 3 d after stimulation, when the radicle started to emerge from the testa. However, microscopic examination of seeds where the testa had been removed showed that radicle emergence was visible as early as 40 h after addition of the stimulant (results not shown). The germination rate reached a maximum of 72 % after 9 d. Treatment of O. ramosa seeds with the synthetic strigol analogue GR24 produced a highly similar pattern of germination (Fig. 2B), whereas in the absence of stimulation (water controls), the germination rate was consistently below 0·1 % (data not shown). These results show that specific exogenous compounds are required to trigger seed germination of O. ramosa under host-free in vitro conditions.
Fig. 2.
Percentage Orobanche ramosa seed germination (open squares) and attachment-organ formation (filled squares) at different time points after addition of tobacco-root exudate (A) or 0.034 mm GR24 (B). The mean values and standard deviations are shown.
Microscopic examination of the O. ramosa seeds that had germinated in vitro after stimulation with TRE or GR24, revealed the presence of attachment organ-like structures starting at 4 d after addition of the stimulant (Fig. 3). The conical tip of the radicle gradually became enlarged and differentiated into a structure reminiscent of the attachment organ formed during root infection (Joel and Losner-Goshen, 1994). Nine days after stimulation, the percentage of the germinated seeds that had formed an attachment organ reached a maximum of 50 % (corresponding to 37 % of the total seeds), and remained constant thereafter (Fig. 2). The attachment organ-like structures were produced without the necessity of physical contact with the substrate and exhibited a considerable variability in size and morphology, including simple, lateral, bilobed and trilobed structures (see Fig. 3A–C). To check whether the morphology and the developmental pattern of these attachment organ-like structures was comparable with that of the organ produced during plant infection, seeds of O. ramosa were germinated in the presence of tobacco roots in Petri dishes containing glass-fibre filter paper on top of Perlite (Pérez de Luque et al., 2004). After 12–14 d of contact with the host roots, 80 % of O. ramosa seeds had germinated and a significant fraction thereof had differentiated attachment organs that were morphologically indistinguishable from those produced in the in vitro system (Fig. 3D–F).
Fig. 3.
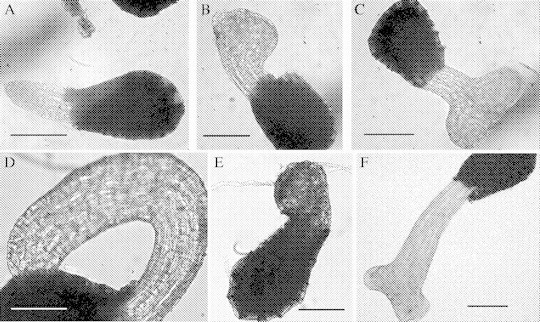
Light microscopy analysis of Orobanche ramosa seeds germinated in vitro after stimulation with GR24 (A–C) or in vivo in the presence of tobacco roots (D–F). (A, D) Radicle; (B, E) attachment organ single lobed; (C, F) bilobed. Scale bars = 100 μm.
Taken together, these results indicate that seed germination and pre-infection development of O. ramosa in the host-free in vitro system are comparable with those observed under in vitro conditions. Radicles and attachment organ were induced in vitro either by TRE or by GR24, suggesting that they simulate the presence of the host plant. For subsequent in vitro germination assays, GR24 was used as it is a commercially available product.
Evidence for a rapid, receptor-mediated mode of action of GR24
The cellular mechanisms that mediate the activity of exogenous germination stimulants, such as GR24, in parasitic plants remain largely unknown. According to Reizelman et al. (2003) structure–activity studies of natural germination stimulants or their synthetic analogues GR7 and GR24 have shown that they are active at concentrations as low as 10−7–10−15M (Wigchert and Zwanenburg, 1999), that the bioactiphore resides in the CD-part of the molecules (Magnus et al., 1992; Bergmann et al., 1993; Wigchert and Zwanenburg, 1999), and that the absolute configuration of this CD-moiety is crucial for germination activity (Thuring et al., 1997a; Sugimoto et al., 1998). Taken together, these results suggest that these compounds may induce germination via a receptor-mediated signalling mechanism (Zwanenburg and Reizelman, 2001). It was reasoned that if such a mechanism was indeed operating in Orobanche, it should display an extremely rapid mode of action and, once activated, result in an irreversible commitment to the germination programme, even after withdrawal of the initial signal. To test this hypothesis, the minimum time required for the stimulatory effect of GR24 was determined. Conditioned seeds of O. ramosa were treated with GR24 for different time periods ranging from 5 min to 36 h, and then thoroughly washed with water plus 0·1 % Tween 20, followed by five successive washes with water to remove all traces of GR24. Seeds were then transferred to new Petri dishes with glass-fibre filter paper containing only water. As positive controls, seeds subjected to the same treatments were transferred to plates with GR24 after washing. As shown in Fig. 4, even the shortest time period tested (5 min of exposure to GR24) was sufficient to trigger seed germination levels comparable with those obtained by continuous treatment with the stimulant. To exclude the possibility that traces of GR24 remained trapped in the seeds, the testa of 100 seeds was carefully removed before subjecting them to a 5-min treatment with GR24 and the series of washes described above. Again, levels of germination in these seeds were highly similar to those of the positive control (Fig. 4). TTC staining of the seeds stimulated with GR24 for 5 min detected a significant increase in metabolic activity 30 h after the treatment, before germination was visible at the seed level (data not shown). These results show that GR24 acts extremely rapidly to induce seed germination in O. ramosa and that, once activated, the germination process is irreversible even if GR24 is removed from the medium. Both lines of evidence support the hypothesis that GR24 binds to a hypothetical receptor present in conditioned seeds, and that this ligand-receptor binding triggers a signal transduction cascade that results in the activation of multiple genes involved in seed germination.
Fig. 4.
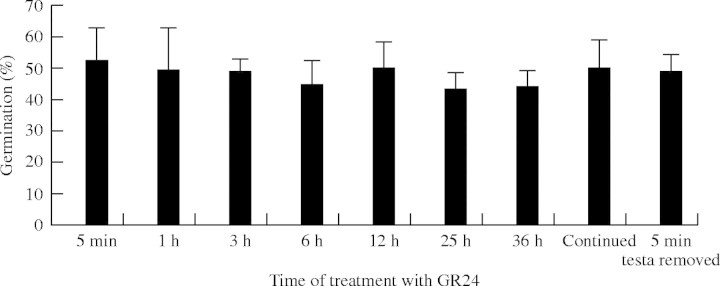
Percentage germination of Orobanche ramosa seeds treated with GR24 for the time periods indicated, washed and transferred to plates with water. In ‘continued’ seeds were directly transferred to plates containing GR24. The mean values and standard deviations are shown.
Evidence for the presence of endogenous signals for attachment-organ formation in O. ramosa
Once the seeds of parasitic angiosperms have germinated, they need chemical or physical signals in order to initiate development of the attachment organ. This process has been studied in detail in Striga and Triphysaria, where at least two steps of chemical host signal recognition have been proposed (Chang and Lynn, 1986; Albrecht et al., 1999; Keyes et al., 2000). The first acts at the level of germination and is mediated by strigol analogues such as GR24, whereas the second requires a distinct class of signalling compounds, usually benzoquinones, which act as attachment organ initiators (Press et al., 1990). It has been suggested that the benzoquinone signal may be generated through oxidation of phenols of host origin by peroxidases present in parasitic plants (Frick et al., 1996; Kim et al., 1998; Albrecht et al., 1999). Whether a similar two-step signalling mechanism is also operating in Orobanche remains unknown.
It was noted that, although GR24 in Orobanche has been described mainly as a germination stimulant (Thuring et al., 1997b), the application of this compound to O. ramosa seeds consistently resulted both in germination and in formation of the attachment organ (see Figs 2 and 3). It was reasoned that GR24 might either be necessary and sufficient to trigger both of these developmental processes, or alternatively, induce only germination, whereas a distinct signal would trigger attachment-organ development, similar to that in Striga. To discriminate between these two hypotheses, advantage was taken of the fact that a very low percentage (<0·1 %) of spontaneous germination was consistently detected on the control plates of O. ramosa seeds, which had been treated only with water. These spontaneously germinated seeds were transferred to new plates supplemented either with GR24 or water (50 seeds each), and attachment-organ formation was monitored. Interestingly, the percentage of spontaneously germinated seeds that developed an attachment organ on water (48 %) did not differ significantly from that of seeds transferred to GR24 (51 %), and both of these rates were highly similar to those previously observed in seeds germinated after stimulation with GR24 (see Fig. 2B). This result suggests that GR24 specifically acts as a stimulant for seed germination in O. ramosa, but is not required to trigger attachment-organ formation. Thus, both in O. ramosa and in Striga, germination and attachment-organ development are mediated by different types of signals. However, whereas in Striga the attachment organ-inducing signals are thought to be of host origin (Press et al., 1990), the present results suggest that these signals in O. ramosa are endogenously generated and, therefore, host plant-independent. However, it cannot be excluded that the low percentage of seeds that germinate spontaneously could be mutants in which GR24 response is constitutively activated. In this case, other potential GR24-dependent processes such as attachment-organ formation could also be constitutively activated in these seedlings.
In summary, an axenic, host-free system for O. ramosa based on a new seed disinfection protocol has been developed, which provides reproducible germination rates around 70 %, and results in developmental events such as attachment organ differentiation that are highly similar to those observed in vivo. The system has been applied to the examination of the three key pre-infection stages in O. ramosa: conditioning, germination and attachment-organ differentiation. It was found that a conditioning period of 8 d was both necessary and sufficient to allow optimal germination, and that conditioned seeds could be dehydrated and stored for several months without losing their responsiveness to the stimulant. Evidence is provided that GR24 uses an extremely rapid mechanism to fully and irreversibly induce the seed germination response, suggesting that it acts via a receptor-mediated signalling mechanism. Finally, similar rates of attachment-organ differentiation were found in seeds treated with different exogenous stimulants and those that had germinated spontaneously on water, suggesting that attachment-organ differentiation is triggered by unknown endogenous signals. It is predicted that the new in vitro culture system will have useful applications for the molecular analysis of early stages of parasitic development in Orobanche.
Supplementary Material
Acknowledgments
The authors are grateful to Alejandro Pérez de Luque for helpful advice with the in vivo germination protocol. This work was supported by grants AGL2001–2018-C02-01 from the Spanish Ministerio de Ciencia y Tecnología and the European Community Project EUFABA QLRT-2001-02307 to J.I.C. C.I.G.-V. was supported by a PhD fellowship from the Ministerio de Ciencia y Tecnología. A.D.P. is a recipient of a Ramón y Cajal grant from the Ministerio de Ciencia y Tecnología.
LITERATURE CITED
- Aalders AJG, Pieters R. 1995.In vitro testing with 2,3,5 triphenyl tetrazolium chloride (TTC) of Orobanche crenata seed metabolism. FABIS Newsletter 13: 35–37. [Google Scholar]
- Albrecht H, Yoder JI, Phillips DA. 1999. Flavonoids promote haustoria formation in the root parasite Triphysaria versicolor Plant Physiology 119: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchvarova RB, Slavov SB, Bossolova SN. 1999.In vitro culture of Orobanche ramosa Weed Research 39: 191–197. [Google Scholar]
- Ben-Hod G, Losner D, Joel DM, Mayer AM. 1991.In vitro culture of Orobanche aegyptiaca Annals of Botany 68: 413–416. [Google Scholar]
- Bergmann C, Wegmann K, Frischmuth K, Samson E, Kranz A, et al. 1993. Stimulation of Orobanche crenata seed germination by (+)strigol and structural analogues: dependence on constitution and configuration of the germination stimulants. Journal of Plant Physiology 142: 338–342. [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. 2003. Secondary metabolite signalling in host–parasitic plant interactions. Current Opinion in Plant Biology 6: 358–364. [DOI] [PubMed] [Google Scholar]
- Chang M, Lynn DG. 1986. The haustorium and the chemistry of host recognition in parasitic angiosperms. Journal of Chemical Ecology 12: 561–579. [DOI] [PubMed] [Google Scholar]
- Frick E, Frahne D, Wegmann K. 1996. Biochemical synthesis of 2,6-dimethoxy-para-benzoquinone, a haustorial stimulant of Striga asiatica (L.) Kuntze. Natural Products Letters 9: 153–159. [Google Scholar]
- Goldwasser Y, Yoder JI. 2001. Differential induction of Orobanche seed germination by Arabidopsis thaliana Plant Science 160: 951–959. [DOI] [PubMed] [Google Scholar]
- Goldwasser Y, Plakhine D, Yoder JI. 2000.Arabidopsis thaliana susceptibility to Orobanche spp. Weed Science 48: 342–346. [Google Scholar]
- van Hezewijk MJ, van Beem AP, Verkleij JAC, Pieterse AH. 1993. Germination of Orobanche crenata seeds, as influenced by conditioning temperature and period. Canadian Journal of Botany 71: 786–792. [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agriculture Experiment Station Circular No. 347: 1–39. [Google Scholar]
- Joel DM, Losner-Goshen D. 1994. The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Canadian Journal of Botany 72: 564–574. [Google Scholar]
- Joel DM, Steffens JC, Matthews DE. 1995. Germination of weedy root parasites. In: Kigel J, Negbi M, Galili G, eds. Seed development and germination. New York: Marcel Dekker, 567–598. [Google Scholar]
- Keyes WY, O'Malley RC, Kim D, Lynn DG. 2000. Signaling organogenesis in parasitic Angiosperms: xenognosin generation, perception, and response. Journal of Plant Growth Regulation 19: 217–231. [DOI] [PubMed] [Google Scholar]
- Kim D, Kocz R, Boone L, Keyes WJ, Lynn DG. 1998. On becoming a parasite: evaluating the role of wall oxidases in parasitic plant development. Chemistry and Biology 5: 103–117. [DOI] [PubMed] [Google Scholar]
- Kreutz CAJ. 1995. Orobanche: the European broomrape species. Maastricht: Stichting Natuurpublicaties, Limburg. [Google Scholar]
- López Granados F, García Torres L. 1999. Longevity of crenata broomrape (Orobanche crenata) seed under soil and laboratory conditions. Weed Science 47: 161–166. [Google Scholar]
- Losner-Goshen D, Ben-Hod G, Mayer A.M, Joel DM. 1996. Aseptic broomrape infection of tomato root culture. Israel Journal of Plant Sciences 44: 89–94. [Google Scholar]
- Magnus EM, van Vliet LA, Vandenput DAL, Zwanenburg B. 1992. Structural modifications of strigol analogues: influence of the B and C rings on the bioactivity of the germination stimulant GR24. Journal of Agricultural and Food Chemistry 40: 1222–1229. [Google Scholar]
- Nickrent DL, Duff RJ, Colwell AE, Wolfe AD, Young ND, Steiner KE, et al. 1998. Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis DE, Soltis PS, Doyle JJ, eds. Molecular systematics of plants. Vol. II DNA sequencing, Boston: Kluwer Academic Publishers, 211–241. [Google Scholar]
- Okonkwo SNC. 1994. Aseptic culture of parasitic weeds—an overview. Biology and management of Orobanche In: Pieterse AH, Verkleij JAC ter Borg SJ, eds. Third International Workshop on Orobanche and Related Striga Research. Amsterdam, The Netherlands: Royal Tropical Institute. [Google Scholar]
- Parker C, Riches CR. 1993.Parasitic weeds of the world: biology and control. Wallingford, UK: CABI, 114–116. [Google Scholar]
- Pérez de Luque A, Jorrín JV, Rubiales D. 2004.Crenate broomrape control in pea by foliar application of Benzothiadiazole (BTH). Phytoparasitica 32: 21–29. [Google Scholar]
- Press MC, Graves JD, Stewart GR. 1990. Physiology of the interaction of angiosperm parasites and their higher plant hosts. Plant, Cell and Environment 13: 91–104. [Google Scholar]
- Reizelman A, Wigchert SCM, del-Bianco C, Zwanenburg B. 2003. Synthesis and bioactivity of labelled germination stimulants for the isolation and identification of the strigolactone receptor. Organic and Biomolecular Chemistry 1: 950–959. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Wigchert SCM, Thuring JWJF, Zwanenburg B. 1998. Synthesis of all eight stereoisomers of the germination stimulant sorgolactone. Journal of Organic Chemistry 64: 1259–1267. [Google Scholar]
- Thuring JWJF, Heinsman NWJT, Jacobs RWAWM, Nefkens GHL, Zwanenburg B. 1997. Asymmetric synthesis of all stereoisomers of demethylsorgolactone. Dependence of the stimulatory activity of Striga hermonthica and Orobanche creneta seed germination on the absolute configuration. Journal of Agricultural and Food Chemistry 45: 507–513. [Google Scholar]
- Thuring JWJF, Nefkens GHL, Zwanenburg B. 1997. Asymmetric synthesis of all stereoisomers of the strigol analog GR24. Dependence of the absolute configuration on the stimulatory activity of Striga hermonthica and Orobanche crenata seed germination. Journal of Agricultural and Food Chemistry 45: 2278–2283. [Google Scholar]
- Westwood JH. 2000. Characterization of the Orobanche–Arabidopsis system for studying parasite–host interactions. Weed Science 48: 742–748. [Google Scholar]
- Westwood JH. 2001. Parasitic plant research in the era of genomics. In: Fer A, Thalouarn P, Joel DM, Musselman LJ, Parker C, Verkleij JAC, eds. Proceedings of the 7th International Parasitic Weed Symposium. Nantes, France: Faculte des Sciences, University of Nantes, 82–87. [Google Scholar]
- Wigchert SCM, Zwanenburg B. 1999. A critical account on the inception of Striga seed germination. Journal of Agricultural and Food Chemistry 47: 1320–1325. [DOI] [PubMed] [Google Scholar]
- van Woerden IC, van Ast A, Zaitoun FMF, Ter Borg SJ. 1994. Root exudates of resistant faba bean cultivars are strong stimulants of broomrape germination. In: Pieterse AH, Verkleij JAC, ter Borg SJ, eds. Third International Workshop on Orobanche and Related Striga Research. Amsterdam, The Netherlands: Royal Tropical Institute. [Google Scholar]
- Yoder JI. 1999. Parasitic plant responses to host plant signals: a model for subterranean plant–plant interactions. Plant Biology 2: 65–70. [DOI] [PubMed] [Google Scholar]
- Zehhar N, Ingouuj M, Bouya D, Fer A. 2002. Possible involvement of gibberellins and ethylene in Orobanche ramosa germination. Weed Research 42: 464–469. [Google Scholar]
- Zhou WJ, Yoneyama K, Takeuchi Y, Iso S, Rungmekarat S, Chae SH, Sato D, Joel DM. 2004.In vitro infection of host roots by differentiated calli of the parasitic plant Orobanche Journal of Experimental Botany 55: 899–907. [DOI] [PubMed] [Google Scholar]
- Zwanenburg B, Reizelman A. 2001. En route to the isolation and characterization of the strigolactone receptor using biotin labelled strigolactone analogues. In: Fer A, Thalouarn P, Joel DM, Musselman LJ, Parker C, Verkleij JAC, eds. Proceedings of the 7th International Parasitic Weed Symposium. Nantes, France: Faculte des Sciences, University of Nantes, 102–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.