Abstract
• Background and Aims Macadamia integrifolia, M. tetraphylla and their hybrids are cultivated for their edible kernels. Whole kernels, i.e. intact mature embryos with cotyledons fused together, are highly valued and breakage of embryos into halves results in loss of value for the commercial macadamia industry. The morphology and ultrastructure of the mature macadamia embryo, with particular emphasis on the break zone between cotyledons, were investigated. Differences in breakage between different macadamia cultivars were also examined.
• Methods Manual cracking was used to compare breakage in five cultivars and the ultrastructure of the break zone between the cotyledons was examined using light and transmission electron microscopy.
• Key Results Breakage of macadamia embryos was strongly dependent on genotype of the female parent, with cultivars ‘HAES 344’ and ‘HAES 741’ much more likely to break than ‘HV A16’ and ‘HAES 835’. Cotyledons were surrounded by a layer of cuticle resulting in a double cuticle in the break zone between the cotyledons. Three major differences have been found in the ultrastructure of the double cuticle between cultivars: a thicker cuticle in the low-whole cultivar; convolutions in the cuticle of a low-whole cultivar, and the presence of more electron-dense objects in the high-whole cultivar.
• Conclusions Breakage of macadamia embryos depends on the cultivar, with clear ultrastructural differences in the break zone between cultivars. To ensure commercial benefits, macadamia breeding programs should identify germplasm with structural characteristics that ensure high percentages of whole kernel.
Keywords: Macadamia integrifolia, Macadamia tetraphylla, embryo, cuticle, whole kernel
INTRODUCTION
Macadamia (Proteaceae) is cultivated for its edible kernels. Nine Macadamia species have been described, with seven occurring in Australia and two in Sulawesi (Douglas, 1995; McDonald and Ismail, 1995). The two commercial Macadamia species, M. integrifolia and M. tetraphylla, are both indigenous to coastal rainforests of the east coast of Australia (Gross, 1995). Both species and their hybrids are grown in Australia, Hawaii, California and South Africa. The embryo of macadamia comprises the edible kernel (Stroschen, 1986). Macadamia is known to have a very high oil content, sometimes as high as 79–82 % and high oil content is considered an indicator of quality (Ako et al., 1994; Trueman et al., 2000). Botanically, the macadamia fruit is a follicle (Stroschen, 1986), but the term ‘nut’ is commonly used to describe both the complete seed (commercially termed ‘nut-in-shell’) and the kernel.
The ovary of macadamia contains two orthotropous ovules at anthesis (Stroschen, 1986). Both ovules increase in size following anthesis, but one more than the other. In almost all cases only the larger of the two ovules is fertilized (Sedgley, 1981) and the smaller one aborts during early fruit development (Stroschen, 1986). The embryo develops into a globular shape with a short suspensor by about 10–11 weeks after anthesis (Stroschen, 1986). The embryo is subglobose, while the cotyledons are semiglobose and large. The radicle and plumule form a small subglobose unit, the embryonic axis, whose acuminate lower end extends into the micropyle (Francis, 1928). At maturity at 33 weeks, large quantities of oil are stored in the expanded cotyledons (Jones and Shaw, 1943). Many mature embryos break at the junction of the cotyledons during processing, resulting in significant loss of value, as half kernels are of less value commercially than whole kernels. Previous studies have focused on cultural and genetic influences on embryo size and quality (e.g. Trueman and Turnbull, 1994; Wallace et al., 1996; Stephenson and Gallager, 2000). The ultrastructure of the mature macadamia embryo has not been described.
There were three main aims of this study. (1) The morphology and ultrastructure of mature macadamia embryos was investigated. (2) Genetic influences on breakage of embryos were examined by comparing the percentage of whole kernels from five cultivars of macadamia. Macadamia cultivars are primarily propagated by grafting, and all scions within a cultivar have the same genotype. Therefore, all embryos of the same cultivar have the same female parent, but may have different male parents. (3) The ultrastructure of the break zone between the cotyledons of different cultivars was compared to help elucidate the causes of breakage of whole kernels into halves.
MATERIALS AND METHODS
Study site and plant materials
Samples of M. integrifolia Maiden and Betche and M. tetraphylla L.A.S. Johnson were collected from mature, bearing trees in a commercial orchard in south-east Queensland, Australia (26°9·63′S, 152°48·65′E). Mature fruits were collected manually from the ground and the fibrous pericarps (husks) were removed using a mechanical dehusker immediately following harvest. Fruits were then dried in a fan-forced laboratory oven to a moisture content of 1·5 % with a drying regime of 2 d at 38 °C, 2 d at 45 °C and 2 d at 58 °C (Meyers et al., 1999). Moisture content (as a percentage of wet weight) was determined by drying samples at 105 °C for 24 h. All nuts were cracked manually using a TJ's Nutcracker™. All possible care was taken to minimize stresses on nuts during cracking; for example, care was taken to align the axis of the hilum and micropyle with the jaws of the cracker (see Braga et al., 1999).
Five commercially grown cultivars were selected to determine percentage whole kernel: ‘HAES 842’, ‘HAES 835’, ‘HAES 741’, ‘HAES 344’ and ‘HV A16’ (HAES = Hawaii Agricultural Experiment Station; HV = Hidden Valley). Three trees of each cultivar were selected, and four replicate samples, each of 50 nuts, were harvested from each tree on 19 May 2000, making a total of 12 replicates per cultivar. An exception was ‘HAES 741’, where one tree had only three replicates due to scarcity of nuts. Whole kernel percentage for the cultivars was calculated as weight of whole kernel/weight of total kernel. Means for whole kernel were analysed using an analysis of variance and subsequent Tukey's HSD test.
Light microscopy
Transverse hand sections of fresh ‘HAES 344’ kernels at 1·5 % moisture content were cut in the region of the cotyledon junction with stainless-steel razor blades. Sections were stained with safranin O (Johansen, 1940; O'Brien and McCully, 1981) to test for the presence of cuticle. Samples of the cotyledon junction zone were also cut from kernels of ‘HAES 835’ and ‘HAES 344’, and fixed in an aqueous solution of 2·5 % glutaraldehyde and 2·5 % formaldehyde for 24 h. They were then washed in water, dehydrated in an ethanol series and embedded in Spurr's resin. Thin sections (1·5 µm) were cut on a Sorvall Porter Blum microtome and stained with safranin O (Johansen, 1940) or toluidine blue O (pH 4·4). Samples were cut from the embryonic axis region of cultivars ‘HAES 835’ and ‘HAES 741’ for Spurr's resin embedding. Sections were examined using a Leitz Diaplan microscope fitted with a Wild MP-552 light camera.
Transmission electron microscopy
Samples of the cotyledon junction were cut from whole kernels of ‘HAES 741’ and ‘HAES 835’ cultivars. Samples were fixed in 3 % glutaraldehyde in 0·1 mol L−1 phosphate buffer at pH 7·0 for 48 h. They were washed twice in 0·1 mol L−1 phosphate buffer for two 24-h periods, fixed in 1 % osmium tetroxide in 0·1 mol L−1 cacodylate buffer for 24 h and washed twice in 0·1 mol L−1 phosphate buffer for 3 h. Samples were then dehydrated in an acetone series and infiltrated in a Spurr's resin series. Transverse sections of the cotyledon junction 50–60 nm thick were cut parallel to the axis with a Leica Ultracut T ultramicrotome and mounted on pioloform-coated copper slot grids. Sections were stained with uranyl acetate and Reynold's lead citrate and viewed in a JEOL 1010 transmission electron microscope operated at 80 kV.
RESULTS
Comparison of whole kernel percentage of cultivars
Whole kernel percentage was significantly different between some of the cultivars (P < 0·001; Fig. 1). The highest mean whole kernel percentage was for cultivar ‘HV A16’, significantly higher than cultivars ‘HAES 842, ‘HAES 741’ and ‘HAES 344’ (Fig. 1; Tukey's HSD, P < 0·05). Whole kernel percentage for the widely planted cultivar ‘HAES 344’ was significantly lower than ‘HV A16’ (P < 0·01). The cultivar ‘HAES 741’ yielded significantly lower whole kernel percentage than all other cultivars (P < 0·001; Fig. 1).
Fig. 1.
Whole kernel percentage for five cultivars of Macadamia: ‘HV A16’, ‘HAES 835’, ‘HAES 842, ‘HAES 344’ and ‘HAES 741’. Means and standard errors are presented. Different letters indicate significant differences between means (Tukey's test, P < 0.05).
Light microscopy
The macadamia kernel consists primarily of two large cotyledons, and two half kernels are produced if the cotyledon separates during processing. Each cotyledon is surrounded by an epidermis consisting of a single cell layer. This produces a double epidermis at the adaxial surfaces between the cotyledons, a consistent and striking feature of all sections (Fig. 2A and B). Hand sections from fresh macadamia kernels stained with safranin O revealed red-staining cuticle between the cotyledons (Fig. 2A).
Fig. 2.

Light microscope images of mature macadamia embryos. (A) Fresh hand section of junction of cotyledons of cultivar ‘HAES 344’, stained with Safranin O. c, cuticle; o, oil globule; ec, epidermal cell. Scale bar = 20 μm. (B) Spurr's resin section of junction of cotyledons of cultivar ‘HAES 344’, stained with Safranin O. de, double epidermis. Scale bar = 50 μm. (C) Spurr's resin section of abaxial epidermis of cultivar ‘HAES 835’, stained with toluidine blue O. e, epidermis; ob, oil bodies. Scale bar = 20 μm. (D) Embryonic axis of cultivar ‘HAES 835’, stained with toluidine blue, showing attachment to cotyledons and provascular tissue (procambium). ea, embryonic axis; a, apex; pv, provascular tissue; c, cotyledon. Scale bar = 200 μm. (E) Embryonic axis of cultivar ‘HAES 741’, stained with toluidine blue, showing radical and calyptra. ea, embryonic axis; ca, calyptra; r, radicle. Scale bar = 100 μm. (F) Root–shoot axis of cultivar ‘HAES 741’, stained with toluidine blue. ea, embryonic axis; ram, root apical meristem. Scale bar = 100 μm.
The cells of the adaxial epidermis of the cotyledons appear cuboidal or columnar adjacent to the embryonic axis (Fig. 2D) and rectangular parallel to the break zone away from the axis (Fig. 2A and B). By contrast, the abaxial epidermis of the cotyledons has mainly cuboidal cells (Fig. 2C). Parenchyma storage cells are the predominant cell type comprising the macadamia embryo beneath the epidermal layer. These cells are generally globose and larger than epidermal cells, and contain many large globules of oil, a consistent feature of all sections examined (Fig. 2A and C).
The embryonic axis is located adjacent to the apex of the kernel (Fig. 2D). A typical embryonic axis structure was observed and some features of the embryonic axis were clearly identified. For example, a well-defined root cap (calyptra) is evident with thickened cell walls covering the radicle (Fig. 2E and F; Stroschen, 1986), and behind the calyptra an apical meristem can be distinguished (Fig. 2E and F). Provascular tissue extends from both sides of the embryonic axis into the cotyledons (Fig. 2D).
Transmission electron microscopy
Oil bodies (oleosomes) are abundant in parenchymatous cells of the macadamia embryo. Oleosomes appear as grey or white globose bodies (Fig. 3A), approx. 1 µm in diameter. Many of the cells contain numerous storage protein bodies, which appear as electron-dense objects (EDOs). Most of these contain a single globoid crystal. These protein bodies are sometimes surrounded by oil bodies, giving a daisy-like appearance (Fig. 3A). The parenchyma cells had many more protein bodies than the epidermal cells. Laminated epidermal cell walls were 4–5 µm thick (Figs 3B and 4A).
Fig. 3.
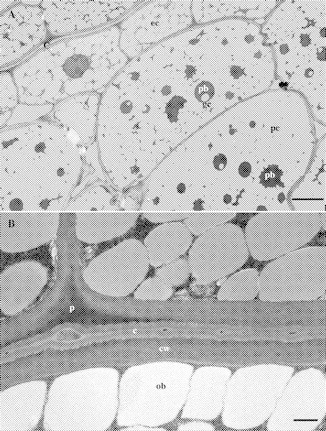
TEM micrographs of macadamia kernel, cultivar ‘HAES 835’. Cells are densely packed with oil bodies (grey, globose objects). (A) c, cuticle; ec, epidermal cell; pc, parenchyma cell; pb, protein bodies; gc, globoid crystals. Scale bar = 5 μm. (B) Transverse section of the cotyledon junction of a whole macadamia kernel. Note well-developed, bilamellate cuticle and small, electron-dense objects in the cuticle. c, cuticle; p, cuticular peg; cw, cell wall; ob, oil body. Scale bar = 500 nm.
Fig. 4.
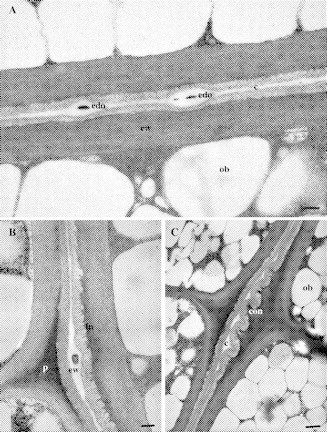
TEM images of cotyledon junction of macadamia whole embryos. (A) Cultivar ‘HAES 835’. Note that the junction is bi-laminate (two cuticles). c, Cuticle; cw, laminate cell wall; ob, oil body; edo, electron-dense object. Scale bar = 200 nm. (B) Cultivar ‘HAES 835’, note electron-dense pectic cuticular peg (p) in middle lamella of epidermal cells, epicuticular wax (ew) between cuticle layers, invaginations (in) of base of cuticular layer. Scale bar = 200 nm. (C) Cultivar ‘HAES 741’. Note convolutions (con) of cuticle (c) adjacent to the middle lamellae junction of epidermis. ob, Oil body. Scale bar = 500 nm.
A clearly defined epidermal cuticle can be seen in TEM images on each cotyledon surface (Figs 3–5).The cotyledons were fused at the junction of two layers of epicuticular wax (Figs 4A and 5B). Each cuticle was bi-laminate with an amorphous cuticle proper (Osborn and Taylor, 1990) overlying a cuticular layer (Figs 4A and 5B). The cuticle proper was more osmophilic than the cuticular layer (Figs 3B and 5B). The cuticular layer was of a reticulate nature (Fig. 5B). A cuticular peg (Osborn and Taylor, 1990) extended between the middle lamellae of adjacent epidermal cells (Figs 3B, 4B and 5A). This peg, due to its electron-dense nature, appeared to be pectic in nature rather than an extension of the cuticle layer (Holloway, 1982). The base of the cuticular layer had a finely invaginated appearance (Figs 4B and C and 5A). A striking feature of the cultivar ‘HAES 741’ was the convoluted cuticle. These convolutions were particularly pronounced near the junction of the periclinal and anticlinal epidermal walls (Fig. 4C).
Fig. 5.

TEM images of cotyledon junction of macadamia kernel, cultivar ‘HAES 741’. Note absence of osmophilic objects in epicuticular wax of this cultivar. (A) Note thickness of epicuticular wax (ew) between cuticles (c). p, Pectic cuticular peg. Scale bar = 500 nm. (B) Note bilaminate cuticle (c) with reticulate cuticular layer (cl) and more osmophilic cuticle proper (cpr). cw, laminate cell wall; ew, epicuticular wax. Scale bar = 200 nm.
Epicuticular wax can be identified as the electron-luscent layers on the adaxial surface of each cuticle. This wax layer varied greatly in thickness, with a thickened region at the epidermal cell junctions (Figs 4B and 5A). The junction of the epicuticular wax layers was punctuated by EDOs of varying size, and epicuticular wax was also thickened at the site of these objects (Figs 4A and B). The larger EDOs had a granular appearance (Figs 3B and 4B). The EDOs in the epicuticular wax were larger and more numerous in the cultivar ‘HAES 835’ than in ‘HAES 741’. A total of 17 EDOs were found in the 29·8 µm length of ‘HAES 835’ cuticle in six samples examined. This contrasted with only nine EDOs in the 44·7 µm length of ‘HAES 741’ cuticle in five samples examined.
DISCUSSION
The tendency of macadamia embryos to remain whole or break into halves (cotyledons) depends strongly on the cultivar, i.e. the genotype of the female parent. For example, ‘HAES 741’ is more likely to break, and ‘HV A16’ is less likely to break, than other cultivars tested. Differences between cultivars in whole kernel percentage were also reported by Stephenson and Gallagher (2000). Clearly, there is a strong genetic influence on macadamia embryo breakage, perhaps due to genetically determined differences in embryo structure.
This study has produced the first description of the ultrastructure of mature macadamia embryos. Light microscopy and electron microscopy revealed a well-developed cuticle covering the epidermis of the embryonic cotyledons. The cuticle covering the cotyledons of macadamia embryos results in a double cuticle in the cotyledon junction area (Figs 3B, 4A and 5C). The double cuticle is of particular interest to this study as breakage appears to occur in the cuticle region between the cotyledons. Breakage can be seen between the cuticle and the cell wall (Fig. 5A), but other images (Figs 3–5) indicate this may be an artefact of sectioning rather than a regular occurrence. Cuticle has been identified on the epidermis of other embryos (Wood and Vaughan, 1976; Lyshede, 1992; Yeung et al., 1996) but as far as is known, the presence and structure of a double cuticular layer between adjoining cotyledons has not previously been described. Macadamia has a thick layer of epicuticular wax between the cotyledons, composed of the adjoined wax layer from each of the two cotyledons (Figs 4A and 5B). The waxy surfaces of the two cuticles may be a weak zone that allows breakage between the cotyledons.
Three structural differences were found in the cuticular break zone of a high whole cultivar and a low whole cultivar (‘HAES 835’ and ‘HAES 741’, respectively) that may be implicated in breakage of the macadamia embryo. The first of these differences was the thickness of the cuticle. The high whole cultivar (‘HAES 835’) had a narrower double cuticle at 342 nm compared with 443 nm for ‘HAES 741’. A thicker cuticle may be a zone of weakness that is prone to breakage. The second difference between the cultivars was the EDOs in the epicuticular wax between the cotyledons. These EDOs were larger and more numerous in the high whole kernel cultivar (‘HAES 835’) than in the low whole kernel cultivar. The third difference was the convolutions and air spaces in the cuticle. Convolutions in the cuticle were observed in the low whole kernel cultivar (‘HAES 741’) but not the high whole kernel cultivar. The convolutions may result in air spaces in the wax layer between the cotyledons, facilitating breakage.
The embryonic axis of seeds is composed of the embryonic root or radicle at one end and the embryonic shoot, the epicotyl, at the other. In macadamia the embryonic axis is situated at the apex of the kernel, and the cotyledons of many kernels appear to be strongly joined in this area. In Fig. 2D, provascular tissue can be seen to fork and extend into the cotyledons, as in a typical embryonic axis (West and Harada, 1993). At 20 weeks after anthesis the radicle of macadamia is enveloped by the expanding cotyledons (Stroschen, 1986). The present results showed that at maturity (33 weeks) the cotyledons have completely enclosed the radicle and extend into the micropyle (Fig. 2D). Many kernels joined only by the embryonic axis region with a gap extending along the rest of the axis have been observed. In such cases separation of the two cotyledons tears a piece away from one cotyledon adjacent to the embryonic axis. The provascular tissue and associated tissue extending from the embryonic axis into the cotyledons may provide an important zone of strength which helps maintain the integrity of the embryo.
The ultrastructure studies revealed many oil bodies and protein bodies in the parenchymatous tissue of the mature macadamia embryo. Pate et al. (1986) state that protein bodies are evenly distributed throughout the macadamia embryo and contain inclusions that stain with toluidine blue. In the present study, protein bodies were numerous in the parenchyma but absent from the central inner layers of epidermis. The protein bodies usually contained one large globoid inclusion of type E as described by Lott (1981).
The key results of this study were that breakage of macadamia embryos depends on the cultivar, i.e. genotype of the female, and ultrastructural differences exist in the break zone between cultivars. To ensure commercial benefits, cultivar breeding programmes could focus on selection of germplasm with structural characteristics that ensure high percentages of whole kernel.
Acknowledgments
We thank Horticultural Research and Development Corporation and the Australian Macadamia Society for funding, Rick Webb of the University of Queensland for advice and help with microscopy, and Stephen Trueman and two anonymous referees for helpful comments on the manuscript.
LITERATURE CITED
- Ako H, Okuda K, Gray BA. 1995. Healthful new oils from macadamia nuts. Nutrition 11: 286–288. [PubMed] [Google Scholar]
- Braga GC, Couto SM, Hara T, Almeida Neto JTP. 1999. Mechanical behaviour of macadamia nut under compression loading. Journal of Agricultural Engineering Research 72: 239–245. [Google Scholar]
- Douglas AW. 1995. Affinities. In: McCarthy P, ed. Flora of Australia. Vol. 16. Elaeagnaceae, Proteaceae 1. Melbourne: CSIRO, 6–14. [Google Scholar]
- Francis WD. 1928. The anatomy of the Australian bush nut (Macadamia ternifolia). Proceedings of the Royal Society of Queensland 39: 48–52. [Google Scholar]
- Gross CL. 1995. Macadamia. In: McCarthy P, ed. Flora of Australia. Vol. 16. Elaeagnaceae, Proteaceae 1. Melbourne: CSIRO, 419–425. [Google Scholar]
- Holloway PJ. 1982. Structure of cuticular membranes. In: Cutler DF, Alvin KL, Price CE, eds. The plant cuticle. London: Academic Press, 1–32. [Google Scholar]
- Johansen DA. 1940.Plant microtechnique. New York: McGraw Hill. [Google Scholar]
- Jones WW, Shaw L. 1943. The process of oil formation and accumulation in the macadamia. Plant Physiology 18: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott JNA. 1981. Protein bodies in seeds. Nordic Journal of Botany 1: 421–432. [Google Scholar]
- Lyshede OB. 1992. Studies on mature seeds of Cuscuta pedicellata and C. campestris by electron microscopy. Annals of Botany 69: 365–371. [Google Scholar]
- McDonald JA, Ismail R. 1995.Macadamia erecta (Proteaceae), a new species from Sulawesi. Harvard Papers in Botany 7: 7–10. [Google Scholar]
- Mason RL. 2000. Macadamia nut quality research: the processing challenge. Food Australia 52: 416–419. [Google Scholar]
- Meyers NM, Morris SC, McFadyen LM, Huett DO, McConchie CA. 1999. Investigation of sampling procedures to determine macadamia fruit quality in orchards. Australian Journal of Experimental Agriculture 39: 1007–1012. [Google Scholar]
- O'Brien TP, McCully ME. 1981.The study of plant structure: principles and selected methods. Melbourne: Temacarphi. [Google Scholar]
- Osborn JM, Taylor TN. 1990. Morphological and ultrastructural studies of plant cuticular membranes. 1. Sun and shade leaves of Quercus velutina (Fagaceae). Botanical Gazette 151: 465–476. [Google Scholar]
- Pate JS, Rasins E, Rullo J, Kuo J. 1986. Seed nutrient reserves of Proteaceae with special reference to protein bodies and their inclusions. Annals of Botany 57: 747–770. [Google Scholar]
- Sedgley M. 1981. Early development of the Macadamia ovary. Australian Journal of Botany 29: 185–193. [Google Scholar]
- Stephenson RA, Gallagher EC. 2000.Selecting better Macadamia varieties. Brisbane: DPI Publications. [Google Scholar]
- Stroschen B. 1986. Contributions to the biology of useful plants. 4. Anatomical studies of fruit development and fruit classification of the macadamia nut (Macadamia integrifolia Maiden and Betche). Angewandte Botanik 60: 239–247. [Google Scholar]
- Trueman SJ, Turnbull CGN. 1994. Fruit set, abscission and dry matter accumulation on girdled branches of Macadamia Annals of Botany 74: 667–774. [Google Scholar]
- Trueman SJ, Richards S, McConchie CA, Turnbull CGN. 2000. Relationship between kernel oil content, fruit removal force and abscission in macadamia. Australian Journal of Experimental Agriculture 40: 859–866. [Google Scholar]
- Wallace HM, Vithanage V, Exley EM. 1996. The effect of supplementary pollination on nut set of Macadamia (Proteaceae). Annals of Botany 78: 865–883. [Google Scholar]
- West AL, Harada J. 1993. Embryogenesis in higher plants: an overview. The Plant Cell 5: 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B, Vaughan JG. 1976. Studies on the ultrastructure at the interface of the cotyledon and the testa of cashew nut (Anacardium occidentale L.), before and after industrial processing. Annals of Botany 40: 213–222. [Google Scholar]
- Yeung EC, Zee SY, Ye XL. 1996. Embryology of Cymbidium sinense: embryo development. Annals of Botany 78: 105–110. [Google Scholar]



