Abstract
Background
Mesenchymal stem cells (MSCs) decrease airway eosinophilia, airway hyperresponsiveness (AHR), and remodeling in murine models of acutely induced asthma. We hypothesized that MSCs would diminish these hallmark features in a chronic feline asthma model.
Objective
To document effects of allogeneic, adipose-derived MSCs on airway inflammation, airway hyperresponsiveness (AHR), and remodeling over time and investigate mechanisms by which MSCs alter local and systemic immunologic responses in chronic experimental feline allergic asthma.
Methods
Cats with chronic, experimentally-induced asthma received six intravenous infusions of MSCs (0.36–2.5X10E7 MSCs/infusion) or placebo bimonthly at the time of study enrollment. Cats were evaluated at baseline and longitudinally for one year. Outcome measures included: bronchoalveolar lavage fluid cytology to assess airway eosinophilia; pulmonary mechanics and clinical scoring to assess AHR; and thoracic computed tomographic (CT) scans to assess structural changes (airway remodeling). CT scans were evaluated using a scoring system for lung attenuation (LA) and bronchial wall thickening (BWT). To assess mechanisms of MSC action, immunologic assays including allergen-specific IgE, cellular IL-10 production, and allergen-specific lymphocyte proliferation were performed.
Results
There were no differences between treatment groups or over time with respect to airway eosinophilia or AHR. However, significantly lower LA and BWT scores were noted in CT images of MSC-treated animals compared to placebo-treated cats at month 8 of the study (LA p=0.0311; BWT p=0.0489). No differences were noted between groups in the immunologic assays.
Conclusions and Clinical Relevance
When administered after development of chronic allergic feline asthma, MSCs failed to reduce airway inflammation and AHR. However, repeated administration of MSCs at the start of study did reduce computed tomographic measures of airway remodeling by month 8, though the effect was not sustained at month 12. Further study of MSC therapy including repeated MSC administration is warranted to assess impact on remodeling in chronic asthma.
Keywords: Airway eosinophilia, Airflow limitation, Airway remodeling, Animal model, Allogeneic stem cell therapy
Introduction
Studies in rodent asthma models demonstrated that intravenous mesenchymal stem cells (MSC) attenuate the major pathologic features of asthma including airway inflammation [1-3], airway hyperresponsiveness (AHR) [3-5], and remodeling [5-7]. Most of these studies administered MSC infusions immediately after initial sensitization to allergen followed by allergen challenge for several days to weeks prior to assessing immunologic and pathologic parameters [2, 5, 7, 8]. Human asthmatics who would be candidates for novel therapy such as MSC infusions would likely have chronic established asthma poorly responsive to standard therapies. Thus, the effect of MSC therapy needs to be assessed in chronic, established asthma.
Administration of MSCs in murine models of asthma polarize T lymphocytes from a Th2 to a Th1 phenotype [2, 5], decrease bronchoalveolar lavage fluid (BALF) eosinophilia [1, 7], blunt AHR [2, 4, 5], and improve pulmonary histologic scores [1, 6, 7]. Unfortunately promising therapeutics in murine models of asthma have failed to translate to positive outcomes in human clinical trials, emphasizing a need for better pre-clinical models [9-11]. Cats are the only animal species to spontaneously develop allergic asthma which shares features of human asthma including recurrent symptoms secondary to airflow obstruction, airway hyperresponsiveness (AHR), eosinophilic inflammation and remodeling [12-14]. There are many important parallels in the anatomy, physiology and immunology of cats and humans [15], and both species share close genetic homology [16]. To capitalize on the parallels of spontaneous feline and human asthma, a well-controlled and characterized experimental model of feline asthma has been developed [17] using allergens implicated in pet cats with asthma. A pilot study utilizing this feline model investigated adipose-derived MSCs in acute experimental asthma. This study demonstrated that adipose-derived MSCs significantly diminished indices of airway remodeling and may decrease airway inflammation and hyperresponsiveness [unpublished data].
The purpose of this study was to evaluate the impact of intravenous adipose-derived MSC therapy on airway inflammation, airflow limitation, and remodeling in a chronic feline asthma model followed longitudinally over a 1 year period. Airway inflammation was assessed using bronchoalveolar lavage fluid (BALF) cytology; airflow limitation was determined using clinical scores and ventilator-acquired pulmonary mechanics; and airway remodeling was evaluated using parameters derived from thoracic CT scans. A secondary objective was to investigate mechanisms by which adipose-derived MSCs alter the immunologic response in BALF and blood. We hypothesized that longitudinal evaluation of chronically asthmatic cats administered intravenous allogeneic MSCs would demonstrate decreased airway eosinophilia, airflow limitation, and remodeling compared to cats receiving placebo. We postulated that the mechanism by which this occurs would involve induction of the immunosuppressive cytokine, interleukin (IL)-10, and/or modulation of the T helper (TH2) immune response to allergen.
Materials and Methods
Animals
Nine purpose bred cats [(intact males; 12 months old; median weight 5.8 kg (range 4.6-6.3)] were obtained from a commercial supplier (Liberty Research, Inc., Waverly, NY, USA). These cats were selected from a pool of 24 asthmatic cats based on the strongest clinical response to allergen challenge. Cats were housed individually in runs throughout the study. This study was approved by the University of Missouri Animal Care and Use Committee (Protocol #6912) and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Asthma Induction
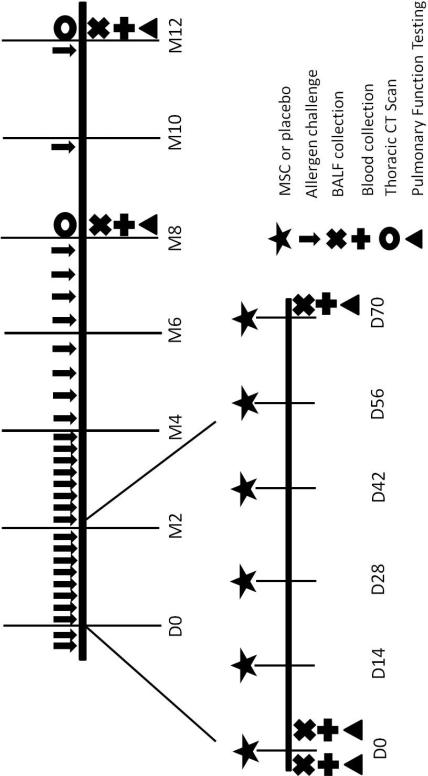
Cats were documented to lack pre-existing sensitization to BGA prior to asthma induction by intradermal testing and BALF analysis. An asthmatic phenotype was induced by sensitizing cats to BGA (Greer laboratories, Lenoir, NC, USA) as previously described [18]. An asthmatic phenotype was confirmed by identifying greater than 17% eosinophils in BALF [17]. After asthma induction, BGA challenges were performed weekly to bimonthly (24 times total) for 9 months prior to study enrollment to mimic the inflammation and remodeling present with chronic asthma. Once administration of MSC or placebo treatments commenced, cats continued to receive BGA aerosol challenges weekly for 4 months after the first MSC infusion. Subsequently, cats were allergen challenged bimonthly between months 4 and 8 and monthly from 8 months until study end to maintain a chronic asthmatic phenotype (Figure 1). Allergen challenges were timed 24 hours prior to any sample collection and 48 hours prior to administration of experimental treatments.
Figure 1. Schematic Overview of Study Design.
After induction of asthma and chronic allergen challenge for 9 months, cats were enrolled in the study and randomized to receive 6 bimonthly infusions of MSC (n=5) or PBS (n=4). During the remaining study period, no further experimental treatments were administered. Allergen challenges were performed weekly for 4 months after the first infusions. Subsequent challenges were performed bimonthly between months 4 and 8 and monthly from 8 months until study end. Blood and BALF were collected and pulmonary function testing was performed at baseline and days 3, 75, 240, and 367. Thoracic CT scans were acquired at month 8 and 12 after the first experimental infusion.
Experimental Treatment
Adipose-derived MSC were chosen for this study as previous studies have demonstrated that adipose-derived MSC are easier to culture in large numbers and share a similar phenotype to bone marrow-derived MSC [19-21]. In addition, several studies have demonstrated that adipose-derived MSCs are capable of reducing airway inflammation, airway hyperresponsiveness, and Th2 cytokine production in a murine [unpublished data] [5, 22, 23]. Prior to performing this study, the effects of MSCs in an acute model of experimental asthma were assessed longitudinally in a pilot study [unpublished data]. After approximately 2 months of administration of adipose-derived MSCs, there was diminished airway inflammation, hyperresponsiveness, and remodeling between 3-9 months after the initial infusion (but not at the early time points). Thus, the current study was modeled after that study.
Allogeneic MSCs were expanded from cryopreserved adipose tissue harvested from healthy donor cats at the Center for Immune and Regenerative Medicine at Colorado State University. The cells were plastic adherent and were assessed as previously described for tri-lineage differentiation and the presence of appropriate cell surface markers to ensure a phenotype consistent with MSCs [20, 24]. Cells were harvested between passages 2 and 5.
Cats were randomized using a random numbers table to receive infusions of MSCs (n=5) or PBS (n=4) [25]. MSCs were shipped live to the University of Missouri overnight the day prior to each infusion. Cells were assessed for viability using trypan blue dye; the average percentage of viable cells was 92% (range 84 – 98%). Washed cells were resuspended in 20 ml of PBS with 200 U of unfractionated heparin and administered through a cephalic catheter over 1 hour. The concentration of live MSCs for each infusion ranged from 3.64×106 – 2.50×107 MSCs (average 1.44×107 live MSCs/infusion). Intravenous placebo (20 ml PBS) was administered to cats over 1 hour. Infusions were performed bimonthly for a total of 6 infusions (Figure 1). To assess safety, cats were monitored for adverse reactions throughout the infusions. Vital parameters (temperature, pulse, respiratory rate) were monitored prior to and after completion of infusions.
Sample Collection
After an overnight fast, blood was collected from the jugular vein into serum and sodium heparin tubes. For serum tubes, blood was allowed to clot for at least 15 minutes at room temperature and centrifuged at 1720 g for 20 minutes. Serum was harvested and aliquots were stored at −20°C for batched analysis at a later date. Sodium heparin tubes were processed within 1 hour of collection to harvest peripheral blood mononuclear cells (PBMCs) by density gradient centrifugation using histopaque 1077 (density 1.077, Sigma-Aldrich, St. Louis, MO, USA) for BGA-specific lymphocyte proliferation assays. Sodium heparinized blood was also processed within 1 hour for a whole blood IL-10 restimulation assay.
BALF was collected in a blind fashion as previously described [17]. An aliquot of BALF was processed for a BALF IL-10 restimulation assay within 1 hour of collection. The remaining BALF sample was centrifuged at 300 g for 10 minutes and the supernatant was harvested in aliquots for storage at −20°C for later batched analysis.
Blood samples and BALF for immunologic assays were collected at baseline and on days 3, 75, 241, and 367 after the first treatment infusion. The time table for sample collection is outlined in Figure 1.
BALF Eosinophil Percentage
Wright's stained cytocentrifuged BALF samples underwent a 200 nucleated cell differential count. Data were reported as percentage of eosinophils.
Clinical Assessment of Airflow Limitation
Airflow limitation was assessed using both a clinical scoring scheme and a visual analog scale (VAS). The clinical scoring scheme is outlined in Table 1. The scores for each individual parameter of the clinical score were combined to create a composite score. The VAS used a 10 cm scale to assess signs observed ranging from no clinical signs (0 cm) to extreme respiratory distress (10 cm). Both the clinical score and VAS were performed by a single investigator [J.E.T]. Assessments were performed during/after aerosol challenge with BGA.
Table 1.
Clinical Respiratory Scoring System
| Respiratory rate (RR) change |
| 0 points - <50% increase in baseline RR |
| 0.5 points - > 50% increase in baseline RR |
| 1 point - >100% increase in baseline RR |
| Auscultation (scored pre- and post-BGA challenge) |
| 0 points – no wheezes |
| 1 point – wheezes |
| Inducible cough (scored pre- and post-BGA challenge) |
| 0 points – no |
| 1 point – yes |
| Time in chamber prior to onset of first clinical respiratory sign |
| 0 points – 10 minutes |
| 0.5 points – 5 to 10 minutes |
| 1 point – 2 to < 5 minutes |
| 1.5 points – 1 to < 2 minutes |
| 2 points - < 1 minutes |
| Clinical signs (post-chamber) |
| 0 points – no signs |
| 0.5 points – mild to moderate increase in respiratory effort but not enough to affect activity or mentation; spontaneous cough |
| 1.0 points – moderate signs of respiratory difficulty (expiratory push or rapid and shallow breathing) |
| 1.5 points – moderate to severe signs of respiratory difficulty (nostrils flaring, impaired activity, obvious distress) |
| 2.0 points – severe signs (open mouth breathing, lateral collapse, obtunded) |
Pulmonary Function Testing
Ventilator-acquired pulmonary mechanics were performed using a commercial ventilator (Engstrom Carestation, GE Healthcare, Fairfield, CT, USA). Cats were sedated with ketamine (30 mg i.v. or i.m; KetaVed, Vedco Inc., St. Joseph, MO, USA) for intravenous catheter placement. Propofol (Propoflo, Abbott Laboratories, Abbott Park, IL, USA) was used for anesthetic induction (6 mg/kg i.v) and maintenance (0.3 mg/kg/min as a CRI). Additional boluses of propofol (0.5-1 mg/kg i.v.) were administered as needed to maintain an appropriate plane of anesthesia. Cats were intubated with identical (internal diameter 4 mm, length 14 cm) cuffed endotracheal tubes to standardize the effect of the endotracheal tube on airway resistance. Mechanical ventilation was performed as previously described [26]. The maximum allowable peak airway pressure was set at 50 cmH20.
After a 5 minute equilibration period, saline was nebulized for 30 seconds through an in-line nebulizer (Aeroneb, Pro, Aerogen, Mountain View, CA, USA) and measurements were obtained to establish baseline values. This was followed by bronchoprovocation using increasing concentrations of BGA (doubling doses from 1.25 to 40 μg/ml) nebulized for 30 seconds. Data collection was performed for 2 minutes after each dose of BGA. Data collection ended after the dose of BGA that resulted in an increase in airway resistance by 150% above saline aerosol (baseline). Data were expressed as the effective concentration of BGA increasing baseline airway resistance by 150% (EC150Raw), as determined by linear interpolation of the log-log plot of the dose response curve.
Thoracic CT Imaging
Thoracic CT scans were obtained with cats under general anesthesia in sternal recumbency using a 64 row detector CT scanner (Aquilion 64, Toshiba America Medical Systems, Inc., Tustin, CA, USA). Contiguous transverse images were acquired with 0.5 mm collimation, matrix of 512 × 512, pitch of 1.0, small focal spot size, 10-12 cm field of view, 120 kVp and 250 mAs during both inspiration and exhalation. Images were reconstructed with a high-resolution algorithm and 1-mm-thick sections, no interval. Images were transferred to an Empiric PACS system (Encompass, Fujifilm Medical Systems USA Inc., Tokyo, Japan), viewed using OsiriX v.3.9.4 32 bit (Pixmeo, Geneva, Switzerland) and displayed with a constant window width of 1600 Hounsfield units (HU) and level of −550 HU.
Inspiratory CT images were blindly evaluated by a board-certified radiologist [I.M.]. A semi-quantitative scoring system for lung parenchymal abnormalities adapted from Warrick et al (Table 2) was used [27]. A global lung attenuation (LA) score was calculated by combining the score for each individual parameter that was assessed. Bronchial wall thickening (BWT) was determined by comparing wall thickness in treated cats to the mid-range value derived from a series of six clinically healthy non-asthmatic cats as has been done previously [28]. The number of thickened bronchi and severity [mild (1), moderate (2), severe (3)] of the thickening were determined for each lung lobe and summed to obtain a global score.
Table 2.
Semi-quantitative Scoring Method for Lung Attenuation Adapted from Warrick et al [27]
| Abnormality | Grading for Each Abnormality | Anatomical Regions Scored (Lobes scored independently) | |
|---|---|---|---|
| % Disease Extent | Score | ||
| Parenchymal band or linear opacity | 0 | 0 | Left cranial; cranial segment, caudal segment |
| Hazy or ground-glass opacity | 1-25% | 1 | Right cranial |
| 26-50% | 2 | Right middle | |
| Complete attenuation or consolidation | 51-75% | 3 | Left caudal |
| >75% | 4 | Right caudal | |
| Right accessory | |||
Allergen-specific IgE Concentration
Analysis of BGA-specific IgE was determined by ELISA using a validated polyclonal chicken anti-feline IgE antisera [29]. Serum and BALF BGA-specific IgE, assayed in triplicate, were determined from batched serum and BALF samples as previously described [29, 30]. The positive control consisted of pooled sera or BALF from cats sensitized to BGA in a separate study. Negative controls consisted of PBS and pooled sera or BALF supernatant from cats prior to induction of asthma. The polyclonal anti-feline antibody was diluted to 1:15,000, the secondary antibody (biotinylated donkey anti-chicken IgG, (cat no. 703-065-155, Jackson Immunoresearch, West Grove, PA, USA) was diluted 1:20,000, and avidin-horse-radish peroxidase (cat no. 016-030-084, Jackson Immunoresearch, West Grove, PA, USA) was diluted 1:1000 with 0.5% BSA in wash buffer (PBS-Tween 0.5%). Samples were incubated for 1 hour at 37°C after the addition of each antibody and washed prior to the addition of the next antibody. After the addition of substrate (o-phenylenediamine; cat no. P-1526, Sigma Aldrich, St. Louis, MO, USA), plates were incubated at room temperature for 10 minutes for BALF samples and 20 minutes for serum samples. The plates were read at dual wavelengths of 450–650 nm on a spectrophotometer (SpectraMax® Plus 384 Microplate Spectrophotometer, Molecular Devices, Sunnyvale, CA, USA). Samples were diluted as necessary to ensure that results fell within the working range of the assay. Coefficients of variation were less than 15%. Results were reported as an optical density and converted to ELISA units (EU). To convert to EU, a standard curve using the pooled positive control was generated using doubling dilutions. The undiluted pooled positive control sample was arbitrarily set as 1000 EU and the maximally diluted sample (1:64) was set as 15.6 EU. For patient samples, the EU was then determined by plotting the optical density versus EU, accepting values that fell within the linear portion of the standard curve.
IL-10 Restimulation Assay
For determination of IL-10 production, whole blood was diluted 1:2 in warmed cRPMI culture media (990μl) with lipopolysaccharide (LPS; 10 μg). BALF samples were standardized to ensure that the number of mononuclear cells per well was consistent between samples. Cells were plated at a final volume of 50 μl in 500 μl of cRPMI with 1μg/ml of LPS with a target of 5×105 cells/well.
Samples were incubated at 37°C, 5% CO2 for 24 hours. Plates were centrifuged at 400 g for 7 minutes. Supernatant was harvested and banked at −20°C until batched analysis of IL-10 concentration could be performed. Determination of IL-10 concentrations was performed on samples in triplicate using a commercial ELISA kit (cat no. DY736, R&D systems, Minneapolis, MN, USA) according to the manufacturer's instructions; range of assay detection was 125-8000 pg/ml. Samples below the limit of detection were reported as 125 pg/ml for statistical analysis.
Lymphocyte Proliferation Assay
BGA-specific CD4+ T cell proliferation using PBMCs in a flow cytometric assay was determined as previously described [31]. PBMCs were harvested from sodium heparinized blood and resuspended in cRPMI at a concentration of 2×105 cells/well in a final volume of 200 μl/well. Cells were incubated for 7 days either with or without BGA (BGA 25 μg/ml). On the 6th day of incubation, 5-bromo-2’-deoxyuridine (BrdU B5002, Sigma-Aldrich, St. Louis, MO, USA) was added at a final concentration of 10 μM/well. For flow cytometric analysis, PBMCs were labeled with fixed viability dye efluor 780 (eBioscience, San Diego, CA, USA), anti-feline CD4 PE (clone 3-4F4, Southern Biotech, Birmingham, AL, USA), anti-feline CD5 biotin (clone f43, Southern Biotech) and streptavidin APC (cat no. 7100, Southern Biotech). Cells were permeabilized using FoxP3/transcription factor staining buffer set (cat no 00-5523-00, eBioscience) followed by labeling with anti-BrdU FITC (cat no. 11-6071-42, eBioscience). Flow cytometry was performed on a CyAn ADP Flow Cytometer (Becton Dickenson, Franklin Lakes, NJ, USA) with a minimum of 20,000 gated events collected. Cells which fell in the lymphocyte gate on the forward vs. side scatter plot and which were live (i.e., negative for fixable viability dye) were subsequently analyzed. The percentage of CD5+ cells (pan T cell marker in cats) that were also positive for CD4 and BrdU were determined on samples cultured with and without BGA. The data are expressed as the percentage of proliferating CD4+ CD5+ T lymphocytes in response to BGA above baseline (% proliferating cells incubated in BGA minus % proliferating cells incubated in media alone).
Statistics
Data were assessed for normality using a Shapiro-Wilk test. A two way repeated measures ANOVA was used to assess changes over time in each treatment group for EC150Raw, BALF eosinophil percentage, VAS and composite clinical score, serum and BALF IgE, serum and BALF IL-10 restimulation, and lymphocyte proliferation. Post-hoc multiple comparisons were performed as appropriate. A Student's t test for parametric data and a Mann-Whitney Rank Sum test for non-parametric data was used to compare LA and BWT scores between MSC-treated and placebo-treated cats at 8 and 12 months. Statistical analysis was performed using commercially available software (SigmaPlot 12, Systat Software Inc, San Jose, CA, USA).
Results
MSC therapy fails to decrease airway inflammation
Airway inflammation in allergic asthma is characterized by infiltration of eosinophils [32]. To assess alterations in airway inflammation in response to MSC therapy in this study, the eosinophil percentage in BALF was determined at baseline, immediately after the first MSC administration (Day 3), after all MSC infusions were given (Day 75) and months 8 and 12. The mean BALF eosinophil percentage for each treatment group at all time points is presented in Table 3. The BALF eosinophil percentage was not significantly different between MSC-treated and placebo-treated cats (p=0.300). In addition, there was not a statistically significant difference over time (p=0.438). Thus, in this model, MSC therapy did not appear to dampen eosinophilic airway inflammation.
Table 3.
Selected Blood and Bronchoalveolar Lavage Assays for MSC-treated and Placebo-treated Asthmatic Cats
| Assay | Txa | Baseline | Day 3 | Day 75 | Month 8 | Month 12 |
|---|---|---|---|---|---|---|
| BALFb eosinophil % | PBSc | 33 ± 14 | 45 ± 27 | 55 ± 8 | 40 ± 6 | 44 ± 8 |
| MSCd | 37 ± 11 | 64 ± 14 | 50 ± 22 | 41 ± 19 | 57 ± 12 | |
| Composite Clinical score | PBS | 3.3 ± 0.5 | 4.1 ± 0.9 | 4.3 ± 0.9 | 4.3 ± 0.9 | 4.3 ± 1.3 |
| MSC | 4.0 ± 0.5 | 3.4 ± 0.8 | 3.7 ± 1.3 | 3.2 ± 1.7 | 3.2 ± 1.2 | |
| VASe | PBS | 4.5 ± 2.2 | 7.4 ± 0.6 | 6.3 ± 1.4 | 5.2 ± 1.1 | 4.3 ± 2.2 |
| MSC | 5.9 ± 1.3 | 5.2 ± 2.0 | 5.2 ± 3.1 | 3.8 ± 3.3 | 2.8 ± 1.8 | |
| EC150RAWf | PBS | 1.3 (1.2-30.2) | 1.3 (1.2-1.9) | 1.7 (1.2-3.1) | 1.2 (0.3-1.9) | 3.2 (2.2-4.4) |
| MSC | 2.3 (1.2-12.9) | 1.3 (1.3-17.7) | 1.3 (1.2-13.7) | 1.3(1.1-49.5) | 4.0 (1.8-6.2) | |
| BALF IgE (EUg) | PBS | 258 (144-609) | 725 (242-1645) | 331 (121-706) | 997 (304-1776) | 220 (125-535) |
| MSC | 315 (123-1181) | 745 (103-1023) | 468 (105-1424) | 288 (146-1006) | 261 (79-2139) | |
| Serum IgE (EU) | PBS | 93 (17-603) | 448 (196-12271) | 587 (125-803) | 120 (19-2301) | 173 (24-11422) |
| MSC | 15 (14-256) | 16 (14-743) | 242 (19-3583) | 17 (15-2716) | 143 (17-19694) | |
| WBh IL-10 restimulation (pg/ml) | PBS | 406 (345-770) | 377 (177-680) | 125 (125-125) | 125 (125-125) | 134 (125-197) |
| MSC | 125 (125-136)* | 357 (158-865) | 125 (125-141) | 125 (125-125) | 221 (125-258) | |
| BALFi IL-10 restimulation (pg/ml) | PBS | 492 (158-828) | 635 (252-3197) | 405 (125-2423) | 646 (125-1917) | 546 (179-887) |
| MSC | 1306 (535-2204) | 864 (487-1569) | 569 (482-763) | 391 (149-936) | 824 (462-1368) | |
| BGAj-specific lymphocyte proliferation % | PBS | 3.9 ± 3.8 | 1.8 ± 2.6 | 1.2 ± 0.3 | 1.4 ± 1.2 | 0.7 ± 0.9 |
| MSC | 3.4 ± 3.1 | 1.5 ± 1.2 | 1.8 ± 1.6 | 1.3 ± 1.0 | 0.4 ± 0.5 |
Parametric data are expressed as mean ± standard deviation and non-parametric data are expressed at median (interquartile range).
p<0.05 in MSC-treated cats compared with placebo-treated cats.
Tx – treatment group
BALF – bronchoalveolar lavage fluid
PBS – placebo-treated asthmatic cats
MSC – MSC-treated asthmatic cats
VAS – visual analog scale of respiratory symptoms
EC150RAW – dose of BGA increasing baseline airway resistance by 150%
EU – ELISA units
WB – whole blood
BALF – bronchoalveolar lavage fluid
BGA – Bermuda grass allergen
MSC therapy does not attenuate airway hyperresponsiveness in response to allergen challenge
One of the defining characteristics of both human and feline asthma is bronchoconstriction in response to allergen exposure resulting in signs of respiratory distress [14]. Clinical signs of asthma and ventilator-acquired pulmonary mechanics were used to assess airway hyperresponsiveness at baseline and longitudinally after MSC administration. Improvement in clinical symptoms of asthma were evaluated in MSC- and placebo-treated cats by using both a composite clinical scoring scheme and a VAS score (Table 3). For both scores, there were no significant differences observed between treatment groups (clinical score p=0.327; VAS p=0.384) or over time (clinical score p=0.934; VAS p=0.216). Thus, the symptoms observed in response to allergen challenge were not decreased after MSC therapy.
Ventilator-acquired pulmonary mechanics allows for objective assessment of AHR. Changes in airway resistance in response to nebulization with BGA were used to determine the EC150Raw (i.e., the dose of BGA inducing a 150% increase in airway resistance over baseline). Cats with less responsive airways will have a higher EC150Raw. This was evaluated at baseline, days 3 and 75, and months 8 and 12 (Table 3). There was no significant difference between treatment groups (p=0.480) or over time (p=0.234). Thus, MSC therapy did not attenuate airflow limitation in response to allergen bronchoprovocation.
MSC therapy reduces CT-derived indices of airway remodeling
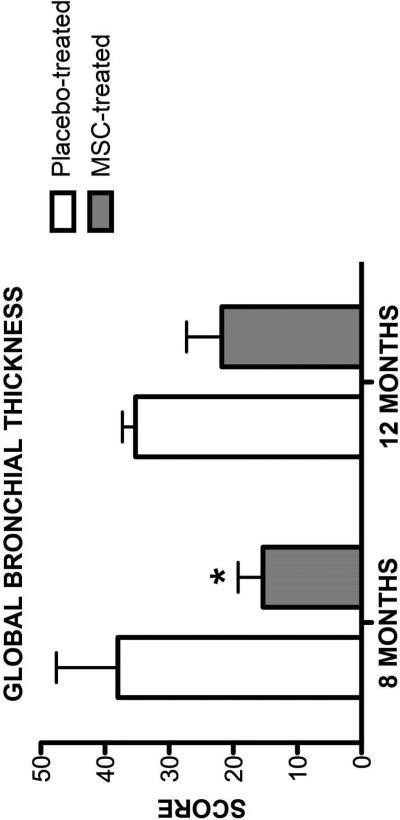
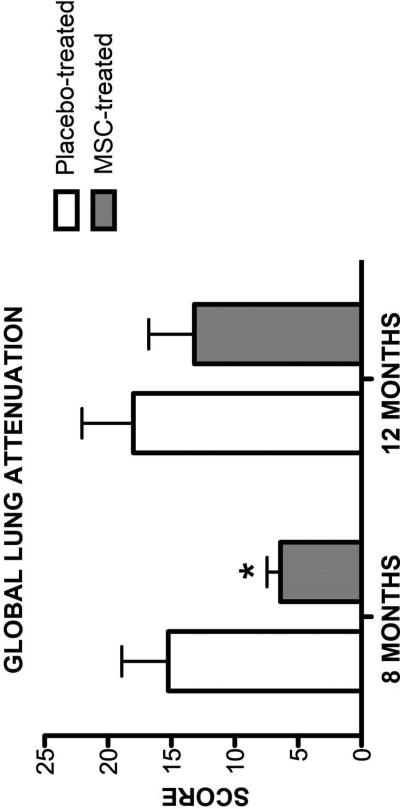
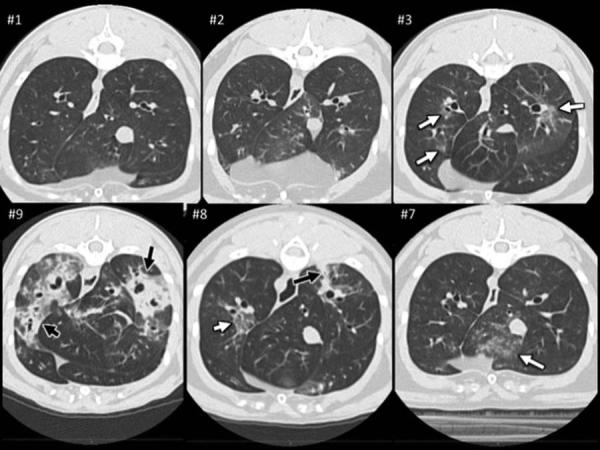
Airway remodeling causes long term declines in lung function in asthmatic patients [33]. It has been proposed the MSC therapy may attenuate or reverse airway remodeling [6]. However, changes in remodeling after administration of MSC therapy in rodent models have not been repeatedly assessed over long time periods. Additionally, sacrifice has been required in rodent models. To overcome these limitations, we assessed changes in airway remodeling using indices derived from thoracic CTs at months 8 and 12 (6 and 10 months after the last MSC infusion; Figures 2 and 3). Representative CT images from both MSC- and placebo-treated cats are presented in Figure 4. There was a significant difference in both global scores between MSC- and placebo-treated cats 8 months after the first infusion (LA score p=0.031; BWT score p=0.049); however, this difference was no longer present at month 12 of the study (LA score p=0.406; BWT score p=0.077). MSC therapy appears to reduce CT derived indices of remodeling over time in chronic established feline asthma. However, the effect is not sustained indefinitely.
Figure 2. Global Bronchial Wall Thickness Score on Thoracic CT.
Mean ± standard deviation of bronchial wall thickness scores were determined during inspiration in MSC- (n=5) and placebo-treated (n=4) cats at month 8 and 12 after experimental treatment. Cats given experimental treatments and were compared to a healthy cat with a mid-range score as has been done previously [27]. *p=0.049
Figure 3. Global Lung Attenuation Score on Thoracic CT.
Mean ± standard deviation of global lung attenuation scores determined during inspiration in MSC-(n=5) and placebo-treated (n=5) cats at month 8 and 12 after experimental treatment. *p=0.031
Figure 4. Representative Thoracic CT Images from MSC- and Placebo-treated Cats.
Cross-sectional CT images of six asthmatic cats at the level of the caudal and accessory lung lobes which represented the three least and three most affected cats based on objective CT grading. Top panel (from left to right): Images of lungs from cats (#1-3) with the lowest final scores; all three of these cats were MSC-treated. Note a few lesions (white arrows) in cat #3 qualified as “hazy” in both caudal lung lobes. Cat #1 and 2 had lesions in the cranial lung lobes but not at this level. Bottom panel (from left to right): Images of lung from cats (#9 −7) with the highest final scores; two of these (#8 and #9) were placebo treated. Illustrated in images from cat #8 and #9 are lesions with severe increase in lung attenuation (black arrows) in the caudal and accessory (cat #9 only) lung lobes. These three cats also had also hazy pulmonary lesions distributed in various lung lobes of which a few are seen at this level of the thorax (white arrows).
Effect of treatment on immunologic parameters
To investigate the influence of MSC therapy on the abnormal immune response observed in asthma, several immunologic assays were performed. Results of all immunologic assays are presented in Table 3.
Allergen-specific IgE is the prototypical antibody associated with allergic asthma [32], and has previously been shown to decrease with MSC-therapy in murine models [1, 7, 34]. Thus, we investigated changes in both systemic and local production of allergen-specific IgE in this study. BGA-specific IgE was evaluated in serum and BALF at baseline, days 3 and 75, and months 8 and 12. There was no significance difference for serum or BALF IgE between MSC-treated and placebo-treated cats (serum p=0.927; BALF p=0.856). In addition, there was no significant difference noted over time for serum or BALF IgE levels (serum p=0.762; BALF p=0.067).
We next sought to evaluate the effect of MSC therapy on IL-10 production after stimulation with LPS. Previous murine studies have demonstrated that BALF IL-10 concentrations are increased after MSC administration [3, 7, 35] presumably secondary to upregulation of T regulatory cell proliferation [3]. IL-10 decreases activation of dendritic cells, induces T cell anergy, and inhibits IgE class switching [36], which may be beneficial in the restoring immune tolerance in asthma. IL-10 concentrations were determined in whole blood and BALF after ex vivo stimulation with LPS. For whole blood, there was a statistically significant difference between treatment groups (p=0.037). This difference was only observed at baseline (p=0.003) and at no other time point throughout the study; MSC-treated cats had significantly lower IL-10 concentrations than placebo-treated cats. For BALF IL-10 concentrations after ex vivo stimulation, there was no significant difference noted between treatment groups (p=0.943) or over time (p=0.393).
This feline model has previously been shown to demonstrate allergen-specific increases in CD4+ T lymphocyte proliferation [17]. While MSCs are thought to have direct effects on T cell function resulting in down regulation of T lymphocyte proliferation or skewing to a TH1 phenotype, the current evidence is limited [37]. Thus, we investigated the effect of MSC therapy on T lymphocyte proliferation. The percentage of CD4+ T lymphocytes in whole blood that proliferated in response to BGA was determined ex vivo. There was no significant difference noted between MSC-treated or placebo-treated cats (p=0.942) or over time (p=0.979). Thus, MSC therapy did not attenuate overall CD4+ T lymphocyte proliferation.
Safety
No adverse reactions were noted as assessed by changes in vital parameters noted before and after each infusion. Approximately 1 month after study conclusion, one cat that received MSC treatment developed a scrotal mass. A biopsy was consistent with an aggressive sarcoma. Blood testing for feline immunodeficiency virus and feline leukemia virus was negative. The cat was subsequently humanely euthanized. Post-mortem examination confirmed a spindle cell sarcoma with no evidence of any other malignancies or metastatic disease. Immunohistochemistry for FeLV (gp70 and gp 27) were both negative. There were no subjective histopathologic characteristics consistent with an injection site sarcoma.
Discussion
In chronic, established feline asthma, intravenous adipose-derived feline MSC infusions resulted in significant reduction in CT-derived indices of airway remodeling when assessed serially. In particular, there was a significant decrease in BWT and LA obtained on CT examination at month 8, roughly 6 months after the final MSC infusion. This effect was not sustained at month 12 suggesting that additional MSC treatments might be necessary to slow progression of remodeling. In contrast to studies in rodent asthma models investigating the use of MSCs, in our feline model there was no reduction of eosinophilic airway inflammation or AHR. Although disappointing, a lack of effect of adipose-derived MSCs on airway inflammation and AHR does not dampen enthusiasm for a treatment which may reduce airway remodeling, a key feature of asthma that current therapies do not consistently address.
In previous rodent asthma models, MSC therapy has diminished airway eosinophilia and AHR [1-3, 6]. Decreased airway eosinophilia has been one of the most consistent effects of MSC therapy in rodent models noted regardless of the model used, source of MSCs, and method of administration. While inconsistent with prior rodent studies, the lack of an effect of MSCs on airway eosinophilia in this study may be related to the more complex genetics and immune system in cats compared to rodents. Some other interventions that have initially appeared promising in rodent models ultimately failed to provide a benefit in human clinical trials underscoring the need for more relevant pre-clinical models [9-11]. The feline model may be more representative of asthma in human disease [14].
Airway hyperresponsiveness, defined as an excessive narrowing of airways in response to an inhaled substance that fails to elicit such a response in a normal person, is a hallmark feature of asthma. While AHR has not been extensively evaluated after MSC-treatment in murine asthma models, a few studies have demonstrated a reduction in this parameter [3-5]. In the present study, no change in AHR was noted between MSC-treated and placebo-treated groups at any time period assessed. Aside from differences in protocols for MSC administration, reasons for discrepancies between results from murine studies and this feline study could include differences in methods to assess AHR, bronchoprovocants and/or key features of the models (i.e., complexity and chronicity of the asthmatic phenotype, unidentified species differences in MSC function or secretome under specific model conditions). In the majority of rodent models, barometric whole body plethysmography has been employed, which uses the unitless parameter of enhanced pause (Penh) to assess AHR and has been criticized for not directly measuring airway resistance [38, 39]. In the present study, direct measurement of airway resistance was performed using ventilator-acquired pulmonary mechanics. To the authors’ knowledge, all previous murine models assessing AHR have used the bronchoprovocant methacholine [2-5], which directly acts on smooth muscle causing contraction [39]. In this study, BGA was used for bronchoprovocation, which is more likely to mimic airflow limitation that occurs in naturally-occurring asthma [39].
Airway remodeling has long been a significant concern in asthmatic patients as this is one reason for worsening clinical signs that ultimately are refractory to therapy [40]. Many rodent models have evaluated MSC therapy in acutely induced asthma and have found a decrease in airway remodeling [3, 4, 7, 34]. However, MSC intervention in humans would not occur prior to induction of asthma and would be less likely to be administered early in the course of disease when conventional therapies would be prescribed. While treatment in chronic asthma is unlikely to be able to reverse long-standing pathologic changes such as fibrosis and smooth muscle hypertrophy, slowing progression of remodeling is an important therapeutic goal.
There have been few studies in rodent models to investigate the effect of MSC therapy given after chronic asthma has been established [1, 6]. Firinci et al [6] performed aerosolized ovalbumin challenges on 3 days per week for a total of 8 weeks. Bonfield et al [1], performed intranasal challenges every other day for 4 weeks. In both studies, mice were given a single intravenous infusion of MSCs at the end of the challenge period and then sacrificed 1 and 2 weeks later for histopathologic analysis of airway remodeling. While both studies demonstrated significant improvement in remodeling including a decrease in goblet cell hyperplasia and epithelial and basement membrane thickening compared to placebo-treated mice, the length of time that challenges were administered may not be representative of what occurs in naturally-developing chronic asthma. In addition, the short time period between MSC administration and histopathologic evaluation does not allow assessment of the duration of MSC effect. In this study, we showed that in chronically asthmatic cats, CT-derived indices of airway remodeling, specifically lung attenuation and bronchial wall thickness were diminished with MSC therapy compared with placebo up to 8 months after initial infusions. Because baseline CT scans were not performed we cannot say if the pathologic changes were reversed or if the progression was simply delayed, although the latter seems more feasible given MSCs appear not to engraft into the pulmonary tissue leading to regeneration [22, 41, 42].
MSCs are believed to modulate the local environment through cell to cell interactions and release of soluble mediators [43]. Several factors are thought to contribute to airway remodeling in human asthmatics including, but not limited to chronic airway inflammation, soluble mediators and mechanical stresses [44, 45]. In this study, there were no significant differences between treatment groups in airway eosinophilia, allergen-specific IgE, allergen-specific T lymphocyte proliferation or stimulated IL-10 production, making it less likely that the decrease in airway remodeling was related to attenuation of the TH2-predominating environment. Additionally, there were no significant differences between treatment groups in clinical response to allergen challenge (clinical scoring system or VAS) or in direct measurement of pulmonary mechanics in response to BGA bronchoprovocation. Thus, allergen-driven mechanical stresses also fail to provide an explanation for remodeling. Further study needs to be performed to specifically identify the mechanism for delayed progression of remodeling.
The nature of the changes in airway remodeling cannot be defined in this study. CT scans were used as a non-invasive serial surrogate marker of airway remodeling. Animal sacrifice was prohibited by the granting institution for this particular study, thus histopathologic changes could not be assessed. Histopathologic examination in combination with thoracic CT scans should be performed to define what features of remodeling are altered by MSC therapy.
No adverse reactions were noted during any MSC infusion suggesting that repeated infusions were safe. It was interesting to note that one cat developed a scrotal sarcoma subsequent to study end. Known factors predisposing cats to development of sarcomas include injection-associated sarcomas [46] and feline retroviruses [47]. The cat had no history of injections in the region, and the tumor did not have histopathologic characteristics suggestive of an injection-associated sarcoma [48]. Furthermore, this cat tested negative both in blood and tumor tissue for feline leukemia virus and feline immunodeficiency virus, the two retroviruses commonly associated with neoplastic transformation in the cat [47]. Thus, there is concern that the development of this tumor could potentially be related to administration of MSCs. A previous study has evaluated the effect of high dose MSC infusion in immunodeficient mice; no tumor development was noted for up to 26 weeks [49]. In addition, MSCs are being used in a number of human clinical trials with no reported tumor developments noted to date suggesting low tumorigenicity of MSCs; however, these studies have primarily utilized autologous bone marrow-derived MSCs [50]. Unfortunately, based on the information available at this time, it is impossible to determine if this neoplasm developed spontaneously or was related to MSC infusion.
In conclusion, the most striking results of this trial in a feline chronic allergic asthma model were attenuated CT-derived indices of airway remodeling noted six months past the last infusion of allogeneic MSCs. Longitudinal evaluation of hallmark asthmatic features was possible in this model and demonstrated that reduction of CT parameters was not permanent; this holds relevance for future studies to determine how often dosing of MSCs might be necessary. In contrast to rodent models of asthma, we were unable to document blunted airway inflammation or AHR once inflammation and AHR were established. Despite this, the reduction of CT evidence of airway remodeling by adipose-derived MSCs in this study may hold promise for human asthmatics in whom remodeling contributes to airflow limitation and where no current treatment consistently reduces this key feature of asthma.
Acknowledgements
Funding for this study was provided by grants from the Winn Feline Foundation and the George and Phyllis Miller Trust at The San Francisco Foundation (Grant # MT11-002, MT12-003, MT13-002). Partial funding was provided by Frankie's Fund for Feline Stem Cell Research at the Center for Immune and Regenerative Medicine, CSU. This work was also supported, in part, by grant K08AI071724 from the National Institute of Allergy and Infectious Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors declare they have no competing interests
References
- 1.Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L760–70. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–48. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–31. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Jang AS, Kwon JH, Park SK, Won JH, Park CS. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res. 2011;3:205–11. doi: 10.4168/aair.2011.3.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HK, Cho KS, Park HY, Shin DH, Kim YK, Jung JS, Park SK, Roh HJ. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010;19:1811–8. doi: 10.1089/scd.2009.0513. [DOI] [PubMed] [Google Scholar]
- 6.Firinci F, Karaman M, Baran Y, Bagriyanik A, Ayyildiz ZA, Kiray M, Kozanoglu I, Yilmaz O, Uzuner N, Karaman O. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol. 2011;11:1120–6. doi: 10.1016/j.intimp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652–7. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ou-Yang HF, Han XP, Zhao F, Ti XY, Wu CG. The role of bone marrow-derived adult stem cells in a transgenic mouse model of allergic asthma. Respiration. 2012;83:74–80. doi: 10.1159/000330013. [DOI] [PubMed] [Google Scholar]
- 9.Harding J, Roberts RM, Mirochnitchenko O. Large animal models for stem cell therapy. Stem Cell Res Ther. 2013;4:23. doi: 10.1186/scrt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hein WR, Griebel PJ. A road less travelled: large animal models in immunological research. Nat Rev Immunol. 2003;3:79–84. doi: 10.1038/nri977. [DOI] [PubMed] [Google Scholar]
- 11.Krug N, Rabe KF. Animal models for human asthma: the perspective of a clinician. Curr Drug Targets. 2008;9:438–42. doi: 10.2174/138945008784533598. [DOI] [PubMed] [Google Scholar]
- 12.Dye JA, McKiernan BC, Rozanski EA, Hoffmann WE, Losonsky JM, Homco LD, Weisiger RM, Kakoma I. Bronchopulmonary disease in the cat: historical, physical, radiographic, clinicopathologic, and pulmonary functional evaluation of 24 affected and 15 healthy cats. J Vet Intern Med. 1996;10:385–400. doi: 10.1111/j.1939-1676.1996.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 13.Moise NS, Wiedenkeller D, Yeager AE, Blue JT, Scarlett J. Clinical, radiographic, and bronchial cytologic features of cats with bronchial disease: 65 cases (1980-1986). J Am Vet Med Assoc. 1989;194:1467–73. [PubMed] [Google Scholar]
- 14.Reinero CR, DeClue AE, Rabinowitz P. Asthma in humans and cats: is there a common sensitivity to aeroallegens in shared environments? Environ Res. 2009;109:634–40. doi: 10.1016/j.envres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Reinero CR. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet J. 2011;190:28–33. doi: 10.1016/j.tvjl.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien SJ, Wienberg J, Lyons LA. Comparative genomics: lessons from cats. Trends Genet. 1997;13:393–9. doi: 10.1016/s0168-9525(97)01297-3. [DOI] [PubMed] [Google Scholar]
- 17.Norris Reinero CR, Decile KC, Berghaus RD, Williams KJ, Leutenegger CM, Walby WF, Schelegle ES, Hyde DM, Gershwin LJ. An experimental model of allergic asthma in cats sensitized to house dust mite or bermuda grass allergen. Int Arch Allergy Immunol. 2004;135:117–31. doi: 10.1159/000080654. [DOI] [PubMed] [Google Scholar]
- 18.Reinero C, Lee-Fowler T, Chang CH, Cohn L, Declue A. Beneficial cross-protection of allergen-specific immunotherapy on airway eosinophilia using unrelated or a partial repertoire of allergen(s) implicated in experimental feline asthma. Vet J. 2012;192:412–6. doi: 10.1016/j.tvjl.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Jang HJ, Cho KS, Park HY, Roh HJ. Adipose tissue-derived stem cells for cell therapy of airway allergic diseases in mouse. Acta Histochem. 2011;113:501–7. doi: 10.1016/j.acthis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Quimby JM, Webb TL, Habenicht LM, Dow SW. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther. 2013;4:48. doi: 10.1186/scrt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg. 2012;14:165–8. doi: 10.1177/1098612X11429224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinas-Pardo L, Mirones I, Amor-Carro O, Fraga-Iriso R, Lema-Costa B, Cubillo I, Rodriguez Milla MA, Garcia-Castro J, Ramos-Barbon D. Mesenchymal stem cells regulate airway contractile tissue remodeling in murine experimental asthma. Allergy. 2014;69:730–40. doi: 10.1111/all.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Gonzalez I, Cruz MJ, Moreno R, Morell F, Munoz X, Aran JM. Human Mesenchymal Stem Cells Resolve Airway Inflammation, Hyperreactivity, and Histopathology in a Mouse Model of Occupational Asthma. Stem Cells Dev. 2014 doi: 10.1089/scd.2013.0616. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quimby JM, Webb TL, Gibbons DS, Dow SW. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: a pilot study. J Feline Med Surg. 2011;13:418–26. doi: 10.1016/j.jfms.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel WW. Biostastistics: A foundation for analysis in the health sciences. 7th Edn. John Wiley and Sons, Inc; New York, NY: 1999. [Google Scholar]
- 26.Nafe LA, Guntur VP, Dodam JR, Lee-Fowler TM, Cohn LA, Reinero CR. Nebulized lidocaine blunts airway hyper-responsiveness in experimental feline asthma. J Feline Med Surg. 2013;15:712–6. doi: 10.1177/1098612X13476705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warrick JH, Bhalla M, Schabel SI, Silver RM. High resolution computed tomography in early scleroderma lung disease. J Rheumatol. 1991;18:1520–8. [PubMed] [Google Scholar]
- 28.Ketai L, Coutsias C, Williamson S, Coutsias V. Thin-section CT evidence of bronchial thickening in children with stable asthma: bronchoconstriction or airway remodeling? Acad Radiol. 2001;8:257–64. doi: 10.1016/S1076-6332(03)80535-4. [DOI] [PubMed] [Google Scholar]
- 29.Chang CH, Lee-Fowler TM, Declue AE, Cohn LA, Robinson KL, Reinero CR. The impact of oral versus inhaled glucocorticoids on allergen specific IgE testing in experimentally asthmatic cats. Vet Immunol Immunopathol. 2011;144:437–41. doi: 10.1016/j.vetimm.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Lee-Fowler TM, Guntur V, Dodam J, Cohn LA, DeClue AE, Reinero CR. The tyrosine kinase inhibitor masitinib blunts airway inflammation and improves associated lung mechanics in a feline model of chronic allergic asthma. Int Arch Allergy Immunol. 2012;158:369–74. doi: 10.1159/000335122. [DOI] [PubMed] [Google Scholar]
- 31.Reinero CR, Liu H, Chang CH. Flow cytometric determination of allergen-specific T lymphocyte proliferation from whole blood in experimentally asthmatic cats. Vet Immunol Immunopathol. 2012;149:1–5. doi: 10.1016/j.vetimm.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Buc M, Dzurilla M, Vrlik M, Bucova M. Immunopathogenesis of bronchial asthma. Arch Immunol Ther Exp (Warsz) 2009;57:331–44. doi: 10.1007/s00005-009-0039-4. [DOI] [PubMed] [Google Scholar]
- 33.Halwani R, Al-Muhsen S, Hamid Q. Airway remodeling in asthma. Current opinion in pharmacology. 2010;10:236–45. doi: 10.1016/j.coph.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Effect of mesenchymal stem cells on inhibiting airway remodeling and airway inflammation in chronic asthma. J Cell Biochem. 2013;114:1595–605. doi: 10.1002/jcb.24501. [DOI] [PubMed] [Google Scholar]
- 35.Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Intratracheal transplantation of bone marrow-derived mesenchymal stem cells reduced airway inflammation and up-regulated CD4(+)CD25(+) regulatory T cells in asthmatic mouse. Cell Biol Int. 2013;37:675–86. doi: 10.1002/cbin.10084. [DOI] [PubMed] [Google Scholar]
- 36.Orihara K, Dil N, Anaparti V, Moqbel R. What's new in asthma pathophysiology and immunopathology? Expert Rev Respir Med. 2010;4:605–29. doi: 10.1586/ers.10.57. [DOI] [PubMed] [Google Scholar]
- 37.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–78. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 38.Bates J, Irvin C, Brusasco V, Drazen J, Fredberg J, Loring S, Eidelman D, Ludwig M, Macklem P, Martin J, Milic-Emili J, Hantos Z, Hyatt R, Lai-Fook S, Leff A, Solway J, Lutchen K, Suki B, Mitzner W, Pare P, Pride N, Sly P. The use and misuse of Penh in animal models of lung disease. Am J Respir Cell Mol Biol. 2004;31:373–4. doi: 10.1165/ajrcmb.31.3.1. [DOI] [PubMed] [Google Scholar]
- 39.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138:4S–10S. doi: 10.1378/chest.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durrani SR, Viswanathan RK, Busse WW. What effect does asthma treatment have on airway remodeling? Current perspectives. J Allergy Clin Immunol Pract. 2011;128:439–48. doi: 10.1016/j.jaci.2011.06.002. quiz 49-50. [DOI] [PubMed] [Google Scholar]
- 41.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–42. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasef A, Ashammakhi N, Fouillard L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 2008;3:531–46. doi: 10.2217/17460751.3.4.531. [DOI] [PubMed] [Google Scholar]
- 44.Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–15. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 45.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–72. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 46.McEntee MC, Page RL. Feline vaccine-associated sarcomas. J Vet Intern Med. 2001;15:176–82. doi: 10.1892/0891-6640(2001)015<0176:fvs>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann K. Clinical aspects of feline retroviruses: a review. Viruses. 2012;4:2684–710. doi: 10.3390/v4112684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aberdein D, Munday JS, Dyer CB, Knight CG, French AF, Gibson IR. Comparison of the histology and immunohistochemistry of vaccination-site and non-vaccination-site sarcomas from cats in New Zealand. N Z Vet J. 2007;55:203–7. doi: 10.1080/00480169.2007.36769. [DOI] [PubMed] [Google Scholar]
- 49.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 50.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]