Abstract
Blocking the MDM2-p53 protein-protein interaction has long been considered to offer a broad cancer therapeutic strategy, despite the potential risks of selecting tumors harboring p53 mutations that escape MDM2 control. In this study, we report a novel small molecule inhibitor of the MDM2-p53 interaction, SAR405838 (MI-77301) that has been advanced into Phase I clinical trials. SAR405838 binds to MDM2 with Ki = 0.88 nM and has high specificity over other proteins. A co-crystal structure of the SAR405838:MDM2 complex shows that in addition to mimicking three key p53 amino acid residues, the inhibitor captures additional interactions not observed in the p53-MDM2 complex and induces refolding of the short, unstructured MDM2 N-terminal region to achieve its high affinity. SAR405838 effectively activates wild-type p53 in vitro and in xenograft tumor tissue of leukemia and solid tumors, leading to p53-dependent cell cycle arrest and/or apoptosis. At well-tolerated dose schedules, SAR405838 achieves either durable tumor regression or complete tumor growth inhibition in mouse xenograft models of SJSA-1 osteosarcoma, RS4;11 acute leukemia, LNCaP prostate cancer and HCT-116 colon cancer. Remarkably, a single oral dose of SAR405838 is sufficient to achieve complete tumor regression in the SJSA-1 model. Mechanistically, robust transcriptional up-regulation of PUMA induced by SAR405838 results in strong apoptosis in tumor tissue, leading to complete tumor regression. Our findings provide a preclinical basis upon which to evaluate SAR405838 as a therapeutic agent in patients whose tumors retain wild-type p53.
Keywords: MDM2, p53, small-molecule inhibitor, protein-protein interaction
INTRODUCTION
Although the tumor suppressor function of p53 can be inactivated through gene mutation or deletion (1), p53 is rarely mutated in some forms of human cancer, and this suggests alternative mechanisms for inhibition of the p53 function. The oncoprotein murine double minute 2 (MDM2) is a primary inhibitor of wild-type p53 (2–5). Upon direct binding, MDM2 induces degradation of p53 by functioning as an E3 ligase and blocks the p53 transactivation domain, rendering p53 ineffective as a transcriptional factor (2–5). MDM2 is overexpressed in many types of human cancers through either gene amplification or other mechanisms (6) and contributes to oncogenesis. Activation of the powerful p53 tumor suppressor function by blocking the MDM2-p53 interaction using small-molecule MDM2 inhibitors is being intensely pursued as a new cancer therapeutic strategy (7–14).
We have previously reported the design of MI-219 as a potent MDM2 inhibitor (8). Although MI-219 demonstrates clear mechanism-based antitumor activity in vitro and in vivo and is well tolerated in extensive toxicity evaluations in animals, it is not suitable for clinical development. In particular, high doses (300 mg/kg) and an intense schedule (twice daily) are needed for MI-219 to achieve strong antitumor activity in vivo. Our optimization efforts have resulted in the discovery of MI-77301 (SAR405838) (Figure 1a), a highly potent and selective MDM2 inhibitor with much improved potency and efficacy over MI-219. We report herein our extensive preclinical characterization of SAR405838.
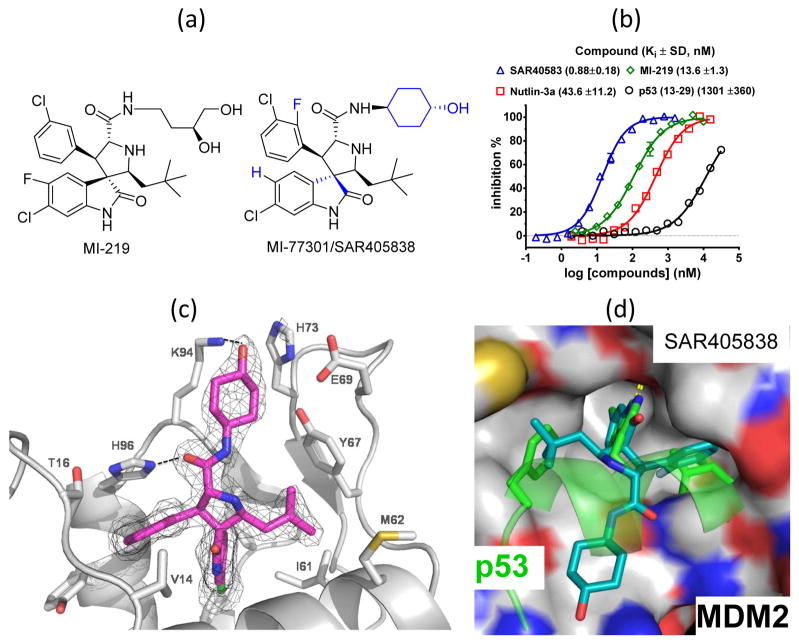
Figure 1.
(a). Chemical structures of SAR405838 and MI-219. (b). Affinities of SAR405838 and control compounds to human MDM2 protein, determined in a fluorescence-polarization binding assay. (c). Co-crystal structure of SAR405838/MDM2 at 2.1 Å resolution. (d). Superposition of SAR405838/MDM2 and p53 peptide/MDM2 co-crystal structures.
MATERIALS AND METHODS
Chemical synthesis of SAR405838
SAR405838 was synthesized using a procedure similar to that used for MI-888 (15) and its purity is >95% by HPLC.
Biochemical binding assays
Binding affinities of MDM2 inhibitors and p53 peptide (residues 13–29) to MDM2 protein were determined using an FP binding assay (15). Binding affinities of SAR405838 to Bcl-2, Bcl-xL, Mcl-1 and β-catenin were determined using our published methods (16) (17), and its binding affinity to MDMx was determined using Biolayer Interferometry technology (18).
Determination of the co-crystal structure of SAR405838: MDM2 complex
Co-crystals of human MDM2 (10–118) protein with SAR405838 were obtained from the Qiagen Ammonium Sulfate Screen condition B8 at 20 °C. Diffraction data were collected at the Advanced Photon Source at Argonne National Laboratory and processed with HKL2000 (19). The co-crystal structure was solved by molecular replacement in Phaser (20) using a structure of MDM2 developed in-house as the search model, fit and refined using Coot (21), and further refined using Buster (22). Details are provided in SI Table S1.
Cell lines, cell growth, cell cycle, cell death and apoptosis
Unless otherwise stated, all cell lines were purchased directly from ATCC. HCT-116 p53+/+ and p53−/− cell lines were obtained from Dr. Bert Vogelstein. Cell growth inhibition activity was determined in a water-soluble tetrazolium (WST)-based assay (23). Cell cycle analysis was performed by flow cytometric analysis of DNA content after PI staining, with cell clumps, doublets and subdiploid cells excluded from the analysis. Cell death was measured by trypan blue staining, and apoptosis was determined using an Annexin V-FLUOS staining kit (Roche Applied Science).
Real-Time PCR
cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) with Oligo dt (Eurogentech). Next, TaqMan gene expression assays were performed by gene-specific primer/probe sets (Applied Biosystems) for real-time PCR amplification in an Applied Biosystems 7900 thermocycler. RPL37a was used for normalization using probes and primers from Applied Biosystems. Relative quantification of mRNA was calculated by a comparative cycle threshold (Ct) method.
Western Blotting
For tumor cell lines, the following primary antibodies were used: PUMAα/β (sc-374223) and MDM2 (SMP-14, sc-965) from Santa Cruz; p53 (DO-1, OP43) and MDM2 (OP46) from Millipore; p21 (DCS60) and Caspase-3 (8G10) and PARP (46D11) from the Cell Signaling Technology. For tumor tissues, the following antibodies were used: p53 (OP43, Millipore), MDM2 (sc-965, Santa Cruz), p21 (556431, BD Biosciences), PARP (9542, Cell Signaling Technology), caspase-3 (AAP-113, Stressgen Bioreagents) and HRP-conjugated GAPDH (sc-5778; Santa Cruz).
Immunohistochemistry (IHC) staining
For IHC, the following antibodies were used: p53 (OP43, EMD Millipore) and cleaved caspase-3 (9664, Cell Signaling Technology). p53 was detected by VECTOR Red Alkaline Phosphatase Substrate Kit (AK-5100, Vector) and cleaved caspase-3 was detected by diaminobenzidine (DAB) tetrahydrochloride substrate using a DAB/buffer system (Sigma).
RNA interference and short hairpin RNA (shRNA) knock-down
To down-regulate PUMA, ON-TARGETplus SMARTpools for human BBC3 (encoding PUMA) and non-targeting negative control siRNAs (Dharmacon) were used. Transfection was performed using Lipofectamine RNAiMAX (Invitrogen). SJSA-1 cells were transfected with siRNAs for 2 days, followed by drug treatment.
For stable p53 knock-down, a 19-bp short hairpin RNA interference, corresponding to p53 nucleotides 611–629 (Genbank NM000546) (24) was used, with a scrambled shRNA construct as the control (24). The oligonucleotides were annealed and ligated into a self-inactivating lentiviral vector under the control of the H1 promoter (19) with the GFP reporter gene under control of the human ubiquitin-C promoter. Lentiviral shRNA virus-containing supernatant was used to infect the cells. At 96 hr post-infection, the cells were sorted for GFP fluorescence.
Meso Scale Discovery (MSD) protein immunoassays
Total protein concentrations were determined with the BCA protein assay kit (Thermo scientific) and protein analysis was performed by Meso Scale Discovery (MSD) assays after appropriate dilutions with the following kits: MDM2 (K152FID), p21 (N45ZA-1), p53, cleaved caspase 3 and cleaved PARP (K15102D-1) with a Sector Imager 2400. Results were normalised with total protein concentration.
In vivo microPET and apoptosis imaging
MicroPET scans were done using the microPET FOCUS 120 PET scanner (Siemens Preclinical Solutions). PET-FLT imaging was performed on isoflurane-anesthetized mice starting 60 min after an i.v. injection of 6 to 8 mBq of FLT. Image acquisition time was 12 min. Images were reconstructed using a 2-D ordered subset-expectation maximization reconstruction algorithm (OSEM2D). Standardized uptake value (SUV) was calculated for each tumor with three mice/tumors in each group.
In vivo apoptosis induction was assessed by fluorescence molecular tomography imaging performed with FMT2500 (PerkinElmer). Annexin-Vivo-750 uptake scans were done on Ketamine®/Xylazine® 120/6 mg/kg i.p. anesthetized mice starting at least 4 h after probes i.v. injection. Images were reconstructed and analyzed using TrueQuant software.
In vivo pharmacodynamic and efficacy experiments
To develop xenograft tumors, 5 × 106 tumor cells with 50% Matrigel were injected subcutaneously on the dorsal side of SCID mice (LNCaP in males and other three models in females).
For pharmacodynamic studies, when tumors reached a mean of 200 mm3, 3 mice per group were treated with vehicle control or a single dose of the drug via oral gavage and sacrificed at each time-point, with tumor tissue harvested for analyses.
For in vivo efficacy experiments, when tumors reached 100–200 mm3, mice were randomized into groups of 8 mice, except in the HCT-116 and single dose SJSA-1 efficacy experiments, in which 6 mice per group were used. Vehicle Control (10% PEG400: 3% Cremophor: 87% PBS, or 2%TPGS:98%PEG200), or SAR405838 was given orally once per day for the indicated dose and duration. Tumor sizes and animal weights were measured 2–3 times per week with tumor volume (mm3) = (length×width2)/2. Statistical analyses were done by two-way ANOVA and unpaired two-tailed t test, using Prism (version 4.0, GraphPad). All animal experiments were performed under the guidelines of the University of Michigan Committee for Use and Care of Animals, or of the Sanofi Committee for Use and Care of Animals.
RESULTS
SAR405838 has a much improved binding affinity to MDM2 and chemical stability over MI-219 and good oral pharmacokinetics in animals
SAR405838 has several major structural differences from MI-219: a different stereochemistry in the quaternary carbon atom, different halogen substitution patterns in both phenyl rings and a conformationally constrained cyclohexanol group (Figure 1a).
SAR405838 has Ki = 0.88 nM to human MDM2 protein in a competitive binding assay (15) and is >10, >50 and >1,000-times more potent than MI-219, nutlin-3a and the p53 peptide, respectively (Figure 1b). To assess its selectivity, we tested SAR405838 for binding to MDMx, a homologue of MDM2. We also tested SAR405838 for its binding to Bcl-2, Bcl-xL, Mcl-1 (25, 26) and β-catenin (17, 27), all of which contain a hydrophobic binding groove on their surface. SAR405838 shows no appreciable binding to these proteins at 10 μM (SI Figure S1). SAR405838 has IC50 values in the μM range at best, for only a few of the >200 receptors/enzymes tested (data not shown). SAR405838 was tested for its stability in cell culture media and two other solutions in which some spiro-oxindoles were shown to isomerize (15) and was found to be stable (SI Figure S2). It has good oral pharmacokinetics (PK) in mice, rats and dogs (SI Table S3).
Hence, SAR405838 is a potent and selective MDM2 inhibitor with good chemical stability and excellent oral bioavailability.
Structural basis of the high affinity binding of SAR405838 to MDM2
We determined the co-crystal structure of the SAR405838:MDM2 complex at 2.1 Å resolution (Figure 1c and SI Table S1). SAR405838 mimics the three key p53 binding residues (Phe19, Trp23 and Leu26) (28) (Figure 1d) in its interaction with MDM2, but captures additional interactions. The Cl atom in the oxindole group of SAR405838 has extensive hydrophobic contacts with MDM2. There is π-π stacking between the His96 of MDM2 and the 2-fluoro-3-chlorophenyl in SAR405838 and a hydrogen bond between the imidazole side chain of His96 and the carboxyl group in SAR405838. Interestingly, the N-terminus of MDM2 (residues 10–18) re-folds and enjoys extensive interactions with SAR405838 through Val14 and Thr16, different from those in the p53:MDM2 (28) and nutlin:MDM2 (7) co-crystal structures.
To investigate the significance of this re-folding, we tested the binding affinities of SAR405838 to several shorter MDM2 proteins (SI Table S2). Removal of the first 9 N-terminal residues in MDM2 has no effect on binding to SAR405838, but further truncation reduces the binding affinity. SAR405838 binds to MDM2 18–118 and 25–118 proteins with Ki = 8.6 nM and 24.0 nM, respectively, being 10- and 25-times weaker than that with the MDM2 1–118 protein. The p53 peptide (13–29) binds to MDM2 25–118 protein with a higher affinity (Ki = 283 nM) than to MDM2 1–118 protein (Ki = 1.3 μM) and nutlin-3a binds to these two MDM2 constructs (1–118 and 25–118) with similar affinities. These data show that residues 10–24 in MDM2 enhance the binding affinity for SAR405838 by a factor of 25, but the binding of p53 peptide or nutlin-3a to MDM2 are unaffected.
SAR405838 potently activates the p53 pathway
Mechanistically, by occupying the p53 pocket in MDM2 (Figure 1c), an MDM2 inhibitor blocks p53-MDM2 interaction and consequently the MDM2-mediated p53 protein degradation, leading to p53 accumulation and transcriptional activation in cells with wild-type p53 but not in cells with mutated or deleted p53 (7, 8). Activation of p53 will transcribe its targeted genes, such as MDM2 and p21, while having no effect on p53 transcription (7, 8). We analyzed SAR405838 for its activity and specificity to activate p53, using MI-219 and/or nutlin-3a as controls.
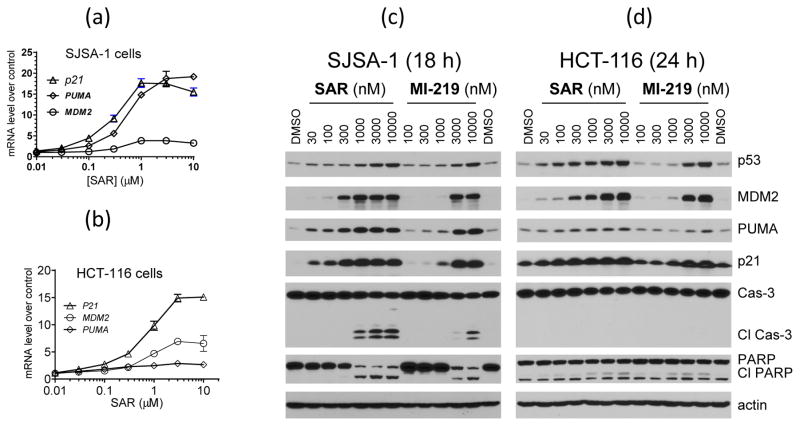
In the SJSA-1 osteosarcoma cell line with wild-type p53 and MDM2 gene amplification, SAR405838 induces dose-dependent up-regulation of MDM2, p21 and PUMA mRNA with EC50 values of 0.3–0.6 μM (Figure 2a), but has no effect on p53 mRNA (SI Figure S3). In the HCT-116 colon cancer cell line with wild-type p53 and no MDM2 gene amplification, SAR405838 also induces a robust, dose-dependent increase of MDM2 and p21 mRNA, with EC50 values of 0.7 μM (Figure 2b), but no increase of p53 mRNA (SI Figure S3). However, SAR405838 only induces a modest increase (~3-fold at 1 and 3 μM) of PUMA mRNA in the HCT-116 cells, in contrast to 19-fold induction at 1 μM in the SJSA-1 cells (Figure 2a). In both cell lines, SAR405838 is 5–10 times more potent than MI-219 and nutlin-3a in induction of p21 and MDM2 transcription (SI Figure S3).
Figure 2.
SAR405838 potently activates p53 in the SJSA-1 and HCT-116 cancer cell lines and strongly induces PUMA up-regulation and cleavage of caspase-3 and PARP in the SJSA-1 cell line but not in the HCT-116 cell line. (a–b). Analysis of dose-dependent transcriptional induction of p21, MDM2 and PUMA in both cell lines. (c–d). Analysis of dose-dependent induction of p53, p21, MDM2 and PUMA proteins and cleavage of caspase-3 and PARP in both cell lines.
Western blotting showed that SAR405838 at 30 nM induces a dose-dependent increase in the levels of p53, p21 and MDM2 proteins in both SJSA-1 and HCT-116 cell lines (Figure 2c). While SAR405838 induces clear increase of PUMA protein at 30 nM in the SJSA-1 cell line, it only has a modest effect on PUMA in the HCT-116 cell line even at 10 μM (Figure 2c). SAR405838 also dose-dependently increases the levels of p53, p21, MDM2 and PUMA proteins in the RS4;11 acute leukemia and LNCaP prostate cancer cell lines, with a clear effect at 100 nM (SI Figure S4). SAR405838 is >10-times more potent than MI-219 in activation of p53 in these four cell lines (Figure 2c–2d and SI Figure S4).
We tested the specificity of SAR405838 in cancer cell lines with mutated or deleted p53 (7, 8). SAR405838 has no effect on p21, MDM2, PUMA and p53 proteins in cancer cell lines harboring mutated p53 or with p53 deletion (SI Figure S5), indicating its high cellular specificity.
Taken together, our cellular data provide clear evidence that SAR405838 is a potent and specific MDM2 inhibitor.
SAR405838 potently inhibits cell growth inhibition and induces cell cycle arrest and/or apoptosis in cancer cell lines in a p53-dependent manner
SAR405838 is 5–10 times more potent than MI-219 and nutlin-3a in inhibition of cell growth in wild-type p53 cancer cell lines of diverse tumor types (Table 1). SAR405838 has IC50 ≥10 μM against all cancer cell lines with a mutated or deleted p53 (Table 1), showing high specificity.
Table 1.
SAR405838 potently inhibits cell growth in cancer cell lines of diverse tumor types with wild-type p53 and displays high selectivity over cancer cell lines with mutated or deleted p53.
| Cell Lines | p53 status | Cell growth Inhibition (IC50, μM) | ||
|---|---|---|---|---|
| SAR405838 | MI-219 | Nutlin-3a | ||
| SJSA-1 | Wild Type | 0.092±0.019 | 1.4 ± 0.2 | 1.3± 0.2 |
| RS4;11 | Wild Type | 0.089±0.027 | 1.0 ± 0.2 | 0.57± 0.13 |
| LANCaP | Wild Type | 0.27 ±0.03 | 1.1 ± 0.1 | 1.2 ± 0.4 |
| HCT-116 | Wild Type | 0.20 ± 0.04 | 0.95 ± 0.05 | 0.86 ±0.11 |
| HCT-116 (p53−/−) | Deletion | >20 | >10 | >10 |
| SAOS-2 | Deletion | >10 | ||
| PC-3 | Deletion | >10 | ||
| SW620 | Mutation | >10 | ||
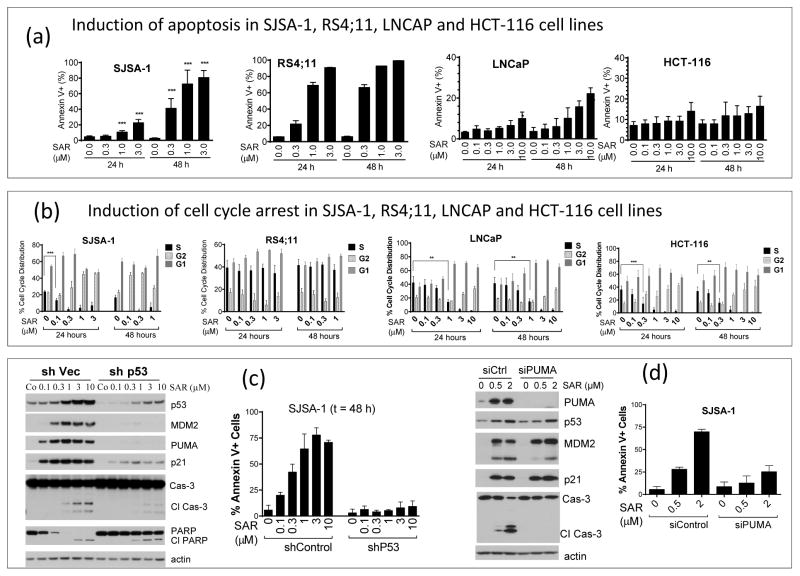
Activation of p53 by nutlin-3a results in cell cycle arrest in all cancer cell lines investigated, but provokes apoptosis only in some cancer cell lines (29). We evaluated by flow cytometry, the effect of SAR405838 on cell cycle progression and apoptosis in four cancer cell lines with wild-type p53 (Figures 3a and 3b). In the SJSA-1 cell line, SAR405838 induces both cell cycle arrest and apoptosis in a dose-dependent manner, but displays kinetics for these two cellular processes, with an early onset for cell cycle arrest and a late onset for apoptosis. In the RS4;11 acute leukemia cell line, SAR405838 induces time- and dose-dependent apoptosis but not cell cycle arrest. In the LNCaP prostate cancer and HCT-116 colon cancer cell lines, SAR405838 effectively inhibits cell cycle progression at 0.3 μM or higher with 24 h treatment, but has a modest or minimal effect on apoptosis induction even at 10 μM.
Figure 3.
SAR405838 induces p53-dependent cell cycle arrest and/or apoptosis in SJSA-1 RS4;11, LNCaP and HCT-116 cancer cell lines. (a). Analysis of apoptosis induction in the four cell lines. (b). Analysis of cell cycle progression in four cell lines. (c). Investigation of the role of p53 in apoptosis induction by SAR405838. (d). Examination of the role of PUMA in apoptosis induction by SAR40583 in the SJSA-1 cell line.
Using the HCT-116 p53+/+ and the isogenic p53−/− cell lines, we showed that the cellular growth inhibitory activity of SAR405838 is p53-dependent (Table 1). To determine if the cellular activity of SAR405838 is also strictly p53-dependent in the SJSA-1, RS4;11 and LNCaP cell lines, we stably knocked down p53 by shRNAi. Efficient knock-down of p53 in SJSA-1 cells effectively attenuates up-regulation of p53, p21, MDM2 and PUMA, as well as cleavage of PARP and caspase-3 (Figure 3c) by SAR405838, reduces its potency in cell growth inhibition by >20-times, and disables its ability to induce apoptosis (Figure 3c) and cell cycle arrest (SI Figure S6). Knock-down of p53 also effectively attenuates cell growth inhibition and apoptosis induction by SAR405838 in the RS4;11 cell line (SI Figure S7), and cell growth inhibition and cell cycle arrest in the LNCaP line by SAR405838 (SI Figure S8). These data firmly establish that the cellular activity of SAR405838 is p53-dependent.
PUMA mediates apoptosis induction by SAR405838 in tumor cells
Although SAR405838 can robustly activate p53 in both the SJSA-1 and HCT-116 cell lines, it selectively induces apoptosis in the SJSA-1 cells. Since SAR405838 effectively increases the mRNA and protein levels of PUMA in SJSA-1 cells, but not in HCT-116 cells, we examined the role of PUMA, a strong pro-apoptotic protein (30). While efficient knock-down of PUMA by shRNAi in SJSA-1 cells has no effect on induction of p53, p21 and MDM2 proteins by SAR405838, it effectively reduces the cleavage of caspase-3 and apoptosis by SAR405838 (Figure 3d).
In addition to PUMA, p53 can also directly regulate the expression of pro-apoptotic Bax and Noxa (31, 32), both of which play a role in p53-mediated apoptosis (33). qRT-PCR analysis, however, showed that SAR405838, MI-219 and nutlin-3a have no significant effect on the mRNA levels of Bax and Noxa in the SJSA-1 cell line (SI Figure S3). Hence, our data show that PUMA plays a key role in apoptosis induction by SAR405838 in SJSA-1 cells.
SAR405838 strongly activates p53 in tumor tissues
To gain insights into the mechanism of action of SAR405838 in vivo, we performed pharmacodynamics (PD) analyses in the SJSA-1, HCT-116, RS4;11 and LNCaP xenograft tumors in mice treated with a single, oral dose of SAR405838.
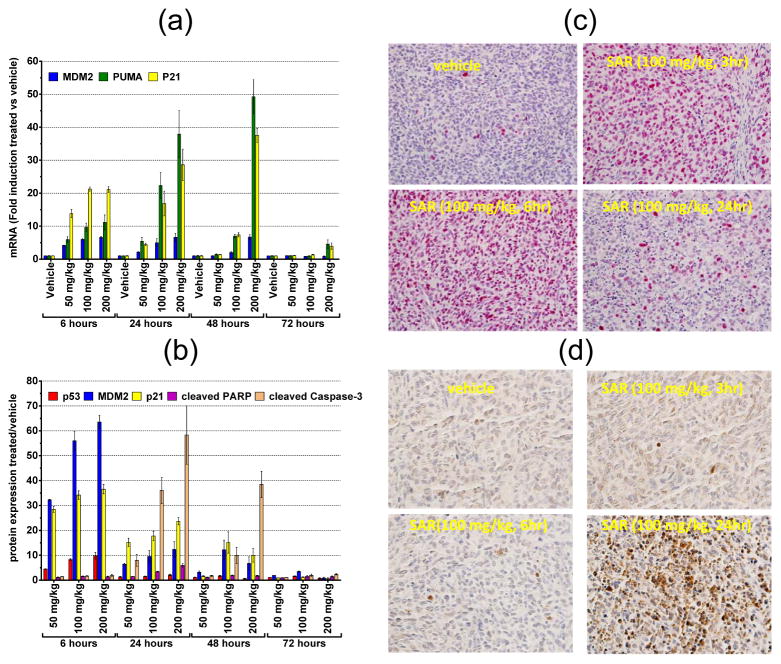
In the SJSA-1 tumor tissue, qRT-PCR analysis showed that SAR405838 induces dose-dependent and robust up-regulation for p21 and PUMA mRNA, but a relatively moderate increase for MDM2 mRNA (Figure 4a). Induction for p21, PUMA and MDM2 mRNA is already robust at 6 h at all doses tested and the strong effect persists longer with higher doses, increasing from 24 h with 50 mg/kg to 72 h with 200 mg/kg (Figure 4a). On the protein level, SAR405838 induces dose-dependent increases of MDM2, p21 and p53 proteins at both 6 h and 24 h time-points (Figure 4b). However, unlike the kinetics observed for transcriptional induction, the protein level for each of MDM2, p21 and p53 peaks at 6 h at all three doses and is minimally elevated at 72 h (Figure 4b). IHC staining confirmed that the p53 protein is strongly upregulated at 3 h and 6 h but greatly diminished at 24 h in tumor tissue (Figure 4c). Compared to up-regulation of p53, MDM2 and p21 proteins, cleavage of caspase-3 is a delayed event, minimal at 6 h with all the three doses but very robust at 24 hr (Figure 4b). Cleavage of PARP and caspase-3 was very clear at 24 h in the tumor tissue treated with a single dose of SAR405838 at 100 mg/kg (SI Figure S9). These in vivo PD data indicate that SAR405838 induces dose- and time-dependent p53 activation and strong apoptosis in the SJSA1-1 tumor tissue.
Figure 4.
SAR405838 strongly activates p53 and induces apoptosis in SJSA-1 xenograft tumors in mice. (a). qRT-PCT analysis of mRNA induction of MDM2, p21 and PUMA by SAR405838 at different doses and time-points in SJSA-1 tumor tissue. (b). Mesoscale analysis of induction of p53, p21, MDM2, cleaved caspase-3 and cleaved PARP with a single dose of SAR405838 at different doses and time-points in SJSA-1 tumor tissue. (c). Immunohistochemical (IHC) staining of p53 in SJSA-1 tumor tissue. (d). IHC staining of cleaved caspase-3 in SJSA-1 tumor tissue.
In HCT-116 xenograft tumor tissue, SAR405838 induces dose- and time-dependent transcription of p21, PUMA and MDM2 genes (SI Figure S11a). However, the magnitude of induction of p21 and MDM2 mRNA by SAR405838 is 3–4 times lower than that observed in the SJSA-1 tumor tissue. More strikingly, the maximum induction of PUMA mRNA by SAR405838 is only 3-fold over the control even at 200 mg/kg, as compared to the >40-fold increase in the SJSA-1 tumor tissue. On the protein level, SAR405838 dose-dependently increases MDM2, p21 and p53 proteins in the HCT-116 tumor tissue with kinetics similar to those in the SJSA-1 tumor (SI Figure S10b). However, SAR405838 induces minimal caspase-3 cleavage in the HCT-116 tumor in all doses and at all time-points.
In the RS4;11 xenograft tissue, SAR405838 induces dose-dependent transcriptional up-regulation of MDM2, p21 and PUMA mRNA (SI Figure S11a), similar to that observed in the SJSA-1 xenograft tissue. Strong apoptosis induction was evident at 100 and 200 mg/kg and 6 h and 24 h based upon cleavage of caspase-3 and PARP (SI Figure S11b).
In the LNCaP xenograft tissue, SAR405838 induces dose- and time-dependent transcriptional up-regulation of MDM2, p21 and PUMA (SI Figure S12a). The magnitude of the transcriptional up-regulation with p21 and MDM2 was similar to that observed in the other three models. The induction of PUMA mRNA by SAR405838 in the LNCaP tumor tissue is weaker than in the SJSA-1 and RS4;11 tumor tissues, but stronger than in the HCT-116 tumor tissue. On the protein level, SAR405838 induces robust accumulation of MDM2, p21 and p53 in a time- and dose-dependent manner (SI Figure S12b). While the level of p53 protein peaks at the 6 h time-point, the strong up-regulation of p21 and MDM2 proteins persists much longer. There is, however, a minimal amount of cleaved caspase-3 in the LNCaP tumor tissue (SI Figure S11b), indicating a low level of apoptosis induction.
Hence, in all the four models examined, a single, oral dose of SAR405838 activates p53 in tumor tissues in a dose- and time-dependent manner and is capable of inducing strong apoptosis in the SJSA-1 and RS4;11 xenograft tumors, but only minimal or modest apoptosis in the HCT-116 and LNCaP xenograft tumors.
SAR405838 achieves complete and durable SJSA-1 tumor regression
We tested the efficacy of SAR405838 in the SJSA-1 xenograft model, which has MDM2 gene amplification and has been used to evaluate other MDM2 inhibitors in vivo (7, 8, 34, 35).
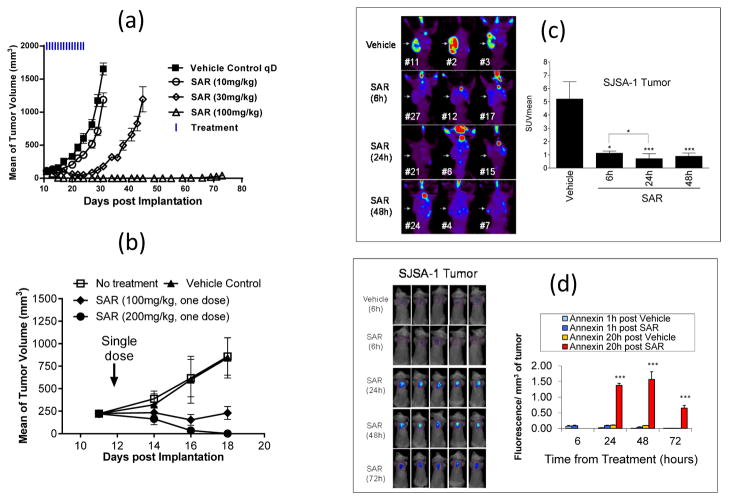
With daily, oral administration for 2 weeks, SAR405838 at 10 mg/kg has no significant activity but at 30 mg/kg, it induces partial tumor regression (Figure 5a). At 100 mg/kg, it achieves rapid, complete and persistent tumor regression (Figure 5a). After only four doses, the average tumor volume shrinks by >90% and after nine doses, all tumors completely regress, becoming undetectable. The complete tumor regression is durable; 31 days after the last dose, at day 55, all 8 mice remain tumor free, and at day 73, 2 months after the last dose, 5 out of 8 (63%) of the animals are still tumor free.
Figure 5.
SAR405838 induces strong apoptosis and achieves complete tumor regression in the SJSA-1 osteosarcoma xenograft model. (a). Antitumor activity of SAR405838 with daily dosing for 2 weeks. (b). Antitumor activity of a single-dose of SAR405838. (c). FLT-PET imaging analysis of cell proliferation in the SJSA-1 tumor tissue. (d). Enhanced fluorescent Annexin-V imaging uptake analysis of apoptosis in the SJSA-1 tumor tissue.
Since a single dose of SAR405838 induces strong apoptosis in the SJSA-1 tumor tissue, we tested whether a single, oral dose of SAR405838 can achieve strong antitumor activity (Figure 5b). Remarkably, a single 100 mg/kg oral treatment with SAR405838 effectively inhibits tumor growth and a single oral dose of 200 mg/kg induces complete tumor regression in 100% of the mice.
To gain further insights into the complete tumor regression achieved with a single dose of SAR405838 in the SJSA-1 tumor model, we employed the 18F-L-thymidine micro PET (FLT-PET) technique to image tumor proliferation (Figure 5c) and an Annexin-V 750 kit to image tumor apoptosis in mice. The imaging data showed that a single dose of SAR405838 effectively inhibits tumor cell proliferation and induces strong apoptosis, with the effect persisting for 72 h (Figure 5d).
SAR405838 is highly efficacious in xenograft models of multiple tumor types lacking MDM2 gene amplification
Since MDM2 gene amplification is only found in, on average, 5–7% of human tumors (6), we tested SAR405838 in RS4;11, LNCaP and HCT-116 tumor models, which have a wild-type p53 status but which all lack MDM2 gene amplification.
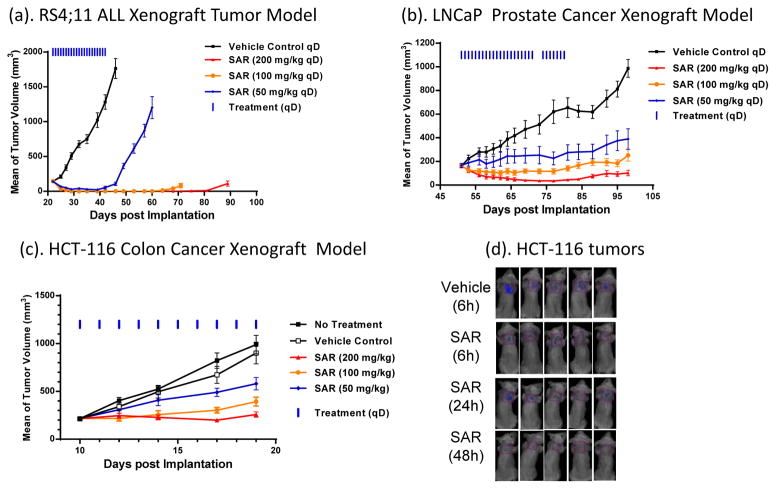
SAR405838 achieves partial tumor regression at 50 mg/kg and complete tumor regression at 100 mg/kg or 200 mg/kg daily dosing in the acute lymphoblastic leukemia RS4;11 model (Figure 6a). The tumor regression achieved by SAR405838 at 200 mg/kg in the RS4;11 model is durable; at day 71, 25 days after the last dose, all 8 mice remained tumor free.
Figure 6.
SAR405838 demonstrates strong antitumor activity in RS4;11, LNCaP and HCT-116 models. (a). Antitumor activity of SAR405838 in the RS4;11 xenograft model. (b). Antitumor activity of SAR405838 in the LNCaP xenograft model. (c). Antitumor activity of SAR405838 in the HCT-116 xenograft model. (d). Enhanced fluorescent Annexin-V imaging uptake analysis of apoptosis in the HCT-116 tumor tissue.
SAR405838 shows strong antitumor activity in the LNCaP prostate cancer model (Figure 6b). Administered daily at 50 mg/kg for 4 weeks, it inhibits tumor growth by 70% over the vehicle control, at 100 mg/kg it completely inhibits tumor growth and at 200 mg/kg, it induces 80% tumor regression.
Although SAR405838 fails to achieve tumor regression in the HCT-116 colon cancer model, it effectively inhibits tumor growth (Figure 6c). SAR405838 at 100 mg/kg daily dosing inhibits tumor growth by >80% over the vehicle control at the end of the treatment, and at 200 mg/kg, completely inhibits tumor growth. Annexin-V in vivo imaging consistently showed that SAR405838 induces minimal apoptosis in the HCT-116 tumor tissue in mice (Figure 6d).
SAR405838 is well tolerated in these efficacy experiments, producing less than 10% weight loss in all dose-scheduled tests (SI Figure S13) and causing no signs of toxicity in mice.
DISCUSSION
Our extensive optimization of MI-219 has yielded SAR405838, which is >10-times more potent than MI-219 in binding to MDM2 and in activation of p53 in tumor cells with wild-type p53. SAR405838 has good chemical stability in solution, thus overcoming a significant issue associated with our earlier MDM2 inhibitors (36). SAR405838 has good oral pharmacokinetics in rodents and non-rodents and achieves much stronger and more sustained p53 activation than MI-219 in tumor tissues with oral administration. This translates into an impressive antitumor activity for SAR405838, including complete and persistent tumor regression with oral, daily administration in the SJSA-1 tumor model. Remarkably, a single, oral dose of SAR405838 at 200 mg/kg is capable of achieving complete SJSA-1 tumor regression in 100% of mice. Although genetic studies have shown that tissue-specific p53 activation by the deletion of the MDM2 gene results in complete regression of established tumors (37–39), previously reported MDM2 inhibitors, including MI-219, nutlin-3a and recently reported more potent MDM2 inhibitors, such as AM-8533 (34) and RG-7112 (35), all fail to achieve complete tumor regression in the SJSA-1 tumor model. Consistently, in vivo imaging showed that a single dose of SAR405838 induces sustained apoptosis persisting for 3 days in SJSA-1 tumors. Although only 5–6% of human cancers have an amplified MDM2 gene (6), a higher frequency of MDM2 gene amplification occurs in certain tumor types, including soft tissue tumors (20%), osteosarcomas (16%), and esophageal carcinomas (13%), and well-differentiated liposarcomas (>80%) (41, 42). The complete tumor regression achieved by SAR405838 in the SJSA-1 xenograft model suggests its strong therapeutic potential as a single agent for the treatment of human tumors with MDM2 gene amplification.
SAR405838 also shows strong antitumor activity in xenograft models of different tumor types which possess wild-type p53 but lack MDM2 gene amplification. SAR405838 achieves complete tumor regression in the RS4;11 acute leukemia model, partial (80%) tumor regression in the LNCaP prostate cancer model, and complete tumor growth inhibition in the HCT-116 colon cancer model. The tumor regression correlates with the ability of SAR405838 to induce strong apoptosis in tumors. Our data suggest that SAR405838 may also have a therapeutic potential for the treatment of human cancers containing a wild-type p53 but lacking MDM2 gene amplification.
While SAR405838 induces strong up-regulation of p21 and MDM2 in all tested cancer cell lines with wild-type p53, it has a different effect on PUMA in vitro and in vivo. SAR405838 induces robust up-regulation of PUMA in SJSA-1 and RS4;11 tumor cells, but has a modest effect in LNCaP and HCT-116 tumor cells. Furthermore, strong up-regulation of PUMA by SAR405838 correlates with apoptosis induction in vitro and in vivo and complete tumor regression in vivo, suggesting the critical role of PUMA in mediating apoptosis and tumor regression. Indeed, knock-down of PUMA by sRNAi in SJSA-1 cells effectively reduces activation of caspase-3 and apoptosis induction by SAR405838, without affecting induction of p53, MDM2 and p21 proteins. Although Noxa and Bax are also p53-targeted genes, they are not induced by SAR405838 in SJSA-1 cells. These data suggest that PUMA is a key mediator for apoptosis induction by MDM2 inhibitors in tumor cells, and that strong up-regulation of PUMA induced by SAR405838 results in complete tumor regression in vivo.
The SAR405838:MDM2 co-crystal structure provides insights into the high affinity binding. In addition to capturing all the key hydrogen bonding and hydrophobic contacts between the three p53 key binding residues with MDM2 (28), SAR405838 enjoys several additional interactions with MDM2 that are not observed in the p53:MDM2 (28) or nutlin:MDM2 co-crystal structures (9). In particular, SAR405838 induces re-folding of the unstructured extreme N-terminus of MDM2 (residues 10–25), which further enhances their interactions and enhances the binding affinity by 25-fold but makes no significant contribution to the binding of nutlin-3a and the p53 peptide to MDM2.
In summary, our present study demonstrates that SAR405838 is a potent and highly efficacious MDM2 inhibitor. Based upon these promising preclinical data, SAR405838 was selected for clinical development and is currently being evaluated in clinical trials for the treatment of human cancer.
Supplementary Material
Acknowledgments
Financial support: This work was supported with funding from the National Institutes of Health (R01CA121279, P50CA069568, P30CA046592), Ascenta Therapeutics Inc. and Sanofi SA. Use of the Advanced Photon Source was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
Footnotes
Conflict of interest disclosure statement: MI-77301 has been licensed by Ascenta and Sanofi from the University of Michigan for clinical development and SW, WS, YZ, SS, SY are inventors on the patents and receive royalties. SW owns stock in Ascenta.
References
- 1.Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Advances in cancer research. 2000;77:81–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–32. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cellular and molecular life sciences: CMLS. 1999;55:96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Momand J, Wu HH, Dasgupta G. MDM2--master regulator of the p53 tumor suppressor protein. Gene. 2000;242:15–29. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 5.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Current cancer drug targets. 2005;5:3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 6.Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic acids research. 1998;26:3453–9. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 8.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3933–8. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassilev LT. p53 Activation by small molecules: application in oncology. J Med Chem. 2005;48:4491–9. doi: 10.1021/jm058174k. [DOI] [PubMed] [Google Scholar]
- 10.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends in molecular medicine. 2007;13:23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14:5318–24. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Zhao Y, Bernard D, Aguilar A, Kumar S. Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapeutics. Top Med Chem. 2012;8:57–80. [Google Scholar]
- 13.Carry JC, Garcia-Echeverria C. Inhibitors of the p53/hdm2 protein-protein interaction- Path to the clinic. Bioorg Med Chem Letters. 2013;23:2480–5. doi: 10.1016/j.bmcl.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Bernard D, Wang S. Small Molecule inhibitors of MDM2-p53 and MDMX-p53 interaction as new cancer therapeutics. Bio Discovery. 2013:8. [Google Scholar]
- 15.Zhao Y, Yu S, Sun W, Liu L, Lu J, McEachern D, et al. A Potent Small-Molecule Inhibitor of the MDM2-p53 Interaction (MI-888) Achieved Complete and Durable Tumor Regression in Mice. J Med Chem. 2013;56 doi: 10.1021/jm4005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou H, Chen J, Meagher JL, Yang CY, Aguilar A, Liu L, et al. Design of Bcl-2 and Bcl-xL Inhibitors with Subnanomolar Binding Affinities Based upon a New Scaffold. J Med Chem. 2012;55:4664–82. doi: 10.1021/jm300178u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto SA, Thompson AD, Coleska A, Nikolovska-Coleska Z, Yi H, Wang S. Analysis of the interaction of BCL9 with beta-catenin and development of fluorescence polarization and surface plasmon resonance binding assays for this interaction. Biochemistry. 2009;48:9534–41. doi: 10.1021/bi900770z. [DOI] [PubMed] [Google Scholar]
- 18.Concepcion J, Witte K, Wartchow C, Choo S, Yao D, Persson H, et al. Label-free detection of biomolecular interactions using BioLayer interferometry for kinetic characterization. Comb Chem High Throughput Screen. 2009;12:791–800. doi: 10.2174/138620709789104915. [DOI] [PubMed] [Google Scholar]
- 19.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–72. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 20.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Applied Crystallography. 2007;40:658–67. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallographica Section D Biological Crystallography. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 22.Bricogne G, Blanc E, Brandl M, Flensburg C, Keller P, Paciorek W, et al. BUSTER. Cambridge; United Kingdom: 2011. [Google Scholar]
- 23.Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. Journal of molecular medicine. 2007;85:1175–86. doi: 10.1007/s00109-007-0221-2. [DOI] [PubMed] [Google Scholar]
- 24.Verhaegen M, Bauer JA, Martín de la Vega C, Wang G, Wolter KG, Brenner JC, et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Research. 2006;66:11348–59. doi: 10.1158/0008-5472.CAN-06-1748. [DOI] [PubMed] [Google Scholar]
- 25.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 26.Friberg A, Vigil D, Zhao B, Daniels RN, Burke JP, Garcia-Barrantes PM, et al. Discovery of potent myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods and structure-based design. Journal of Medicinal Chemistry. 2013;56:15–30. doi: 10.1021/jm301448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu WQ. Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Molecular Cell. 2006;24:293–300. doi: 10.1016/j.molcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948–53. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 29.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1888–93. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Delft MF, Huang DCS. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Research. 2006;16:203–13. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 31.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–9. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 32.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 33.Shibue T, Suzuki S, Okamoto H, Yoshida H, Ohba Y, Takaoka A, et al. Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO Journal. 2006;25:4952–62. doi: 10.1038/sj.emboj.7601359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rew Y, Sun DQ, De Turiso FGL, Bartberger MD, Beck HP, Canon J, et al. Structure-based design of novel inhibitors of the MDM2-p53 interaction. J Med Chem. 2012;55:4936–54. doi: 10.1021/jm300354j. [DOI] [PubMed] [Google Scholar]
- 35.Tovar C, Graves B, Packman K, Filipovic Z, Higgins B, Xia M, et al. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73:2587–97. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Liu L, Sun W, Lu J, McEachern D, Li X, et al. Diastereomeric spirooxindoles as highly potent and efficacious MDM2 inhibitors. J Am Chem Soc. 2013;135:7223–734. doi: 10.1021/ja3125417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–34. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 40.Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, et al. Discovery of RG7388, a Potent and Selective p53-MDM2 Inhibitor in Clinical Development. Journal of Medicinal Chemistry. 2013;56:5979–83. doi: 10.1021/jm400487c. [DOI] [PubMed] [Google Scholar]
- 41.Weaver J, Downs-Kelly E, Goldblum JR, Turner S, Kulkarni S, Tubbs RR, et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Modern Pathology. 2008;21:943–9. doi: 10.1038/modpathol.2008.84. [DOI] [PubMed] [Google Scholar]
- 42.Weaver J, Goldblum JR, Turner S, Tubbs RR, Wang WL, Lazar AJ, et al. Detection of MDM2 gene amplification or protein expression distinguishes sclerosing mesenteritis and retroperitoneal fibrosis from inflammatory well-differentiated liposarcoma. Mod Pathol. 2009;22:66–70. doi: 10.1038/modpathol.2008.153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.