Abstract
Purpose of review
Blood Oxygen Level Dependent Magnetic Resonance Imaging (BOLD MRI) is a noninvasive tehnique evaluating kidney tissue oxygenation that requires no contrast exposure with the potential to allow functional assessment for patients with Atherosclerotic Renal Artery Stenosis (ARAS). Normal cortical-to-medulla oxygenation gradients are preserved in many patients treated for several years with medical antihypertensive therapy without restoring renal blood flow. The current review is of particular interest as new methods were applied to the analyses of BOLD MRI opening perspective of its wider utilization in the clinical practice.
Recent findings
Recent findings show that more severe vascular compromise ultimately overwhelms these adaptive changes, leading to overt cortical hypoxia and expansion of medullary hypoxic zones. “Fractional kidney hypoxia” method of analysis, developed as an alternative method of BOLD MRI analysis, avoids the assumption of discrete cortical and medullary values and decreases the bias related to operator selection of ROIs.
Summary
We believe that thoughtful application and analysis of BOLD MRI can provide critical insights into changes in renal function prior to the onset of irreversible renal injury and may identify patients most likely to gain from measures to reverse or repair disorders of tissue oxygenation.
Keywords: Blood Oxygen Level Dependent Magnetic Resonance Imaging, Atherosclerotic Renal Artery Stenosis, kidney tissue oxygenation
Introduction
Atherosclerotic renal artery stenosis (ARAS) is a common finding in older patients and remains one of the most common causes of secondary hypertension and reduced kidney function. Severe occlusive ARAS activates pressor systems, and ultimately can lead to renal atrophy (1). The complexity of the renal circulation poses a particular challenge when defining the relationships between arterial blood flow and renal tissue oxygenation (2).
Remarkably, a decrease in renal blood flow (RBF) does not invariably lead to renal hypoxia, likely due to both a surplus of oxygenated blood and a parallel decrease in glomerular filtration rate (GFR) and oxygen consuming tubular reabsorption of sodium. Studies of intra-renal blood flow distribution emphasize that cortex and medulla can be regulated independently under some conditions (3). At some point, renal artery stenosis threatens the viability of the kidney and irreversible kidney injury can occur leading to loss of kidney function (4), tissue fibrosis and end stage kidney disease designated as “ischemic nephropathy”(5). Determining precisely the “no-return” point of occlusive vascular lesion remains an elusive goal.
Direct measurement of renal tissue oxygen tension (pO2) has been achieved experimentally using invasive microelectrodes, but is not readily applicable for human studies.
Blood Oxygen Level-Dependent Magnetic Resonance Imaging (BOLD MRI) is a noninvasive imaging method applied in recent years to examine regional tissue oxygenation within the kidney. While interpretation and application of this technology remains controversial, renal artery stenosis is intuitively an ideal indication for BOLD MRI investigation.
This review summarizes our current understanding of the use of BOLD MRI in renovascular disease.
BOLD MRI principle and validation
BOLD MRI is based on paramagnetic properties of deoxyhemoglobin, whereas oxyhemoglobin is diamagnetic. The presence of deoxyhemoglobin affects the T2* relaxation time of neighboring water molecules and in turn influences the MRI signal of T2*-weighted (gradient echo) images. The rate of spin dephasing R2* (= 1/T2*) thereby is closely related to the tissue content of deoxyhemoglobin. Since the capillary blood oxygen tension (pO2) is normally in equilibrium with the surrounding tissue, changes in R2* levels represent changes in tissue pO2.
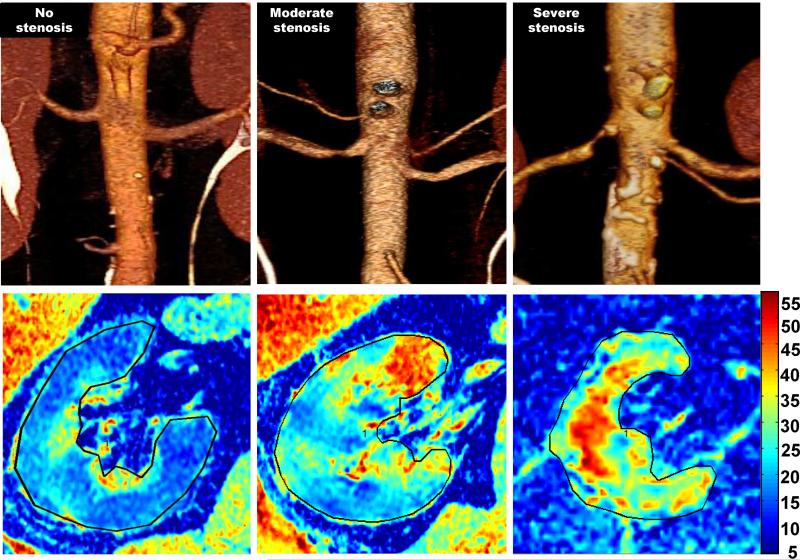
Parametric maps of R2* illustrate the R2* translation of renal structures and has been applied to focus selection of local ROIs and exclude artifacts induced by adjacent tissue outside the kidneys. [FIGURE 1] Typically, cortex can be identified by lower R2* values, while a gradient of higher R2* levels develops in the medullary sections (6-9) (TABLE 1).
Figure-1.
CT angiographic images (upper row) of the right kidneys illustrating three patients with 1) no renal artery stenosis 2) moderate renal artery stenosis and 3) severe renal artery stenosis. Below each in the bottom row are corresponding R2* parametric maps illustrating higher fraction of axial images with elevated deoxyhemoglobin evident with progressively more severe disease.
Table 1.
Example of recent reports of BOLD MR R2* values described for cortical and medullary regions obtained at (3 Tesla) magnet strength in human subjects
| Study | Year | Cortex | Medulla | Comments |
|---|---|---|---|---|
| Li et at. (29) | 2004 | 21.8 | 37.4 | Healthy volunteers |
| Tumkur et al. (30) | 2006 | 14.5 | 30.3 | Baseline normal |
| Pruijm et al. (31) | 2010 | 18.2 | 28.1 | Normal, Low NA |
| Pruijm et al. (31) | 2010 | 17.8 | 31.3 | Normal, High NA |
| Gloviczki et al. (9) | 2011 | 17.8 | 36.8 | Essential Hypertension, 150 mEq NA |
| Gloviczki et al. (9) | 2011 | 15.7 | 37.8 | Moderate ARAS |
| Gloviczki et al. (9) | 2011 | 21.6 | 39.1 | Severe ARAS |
| Xin-Long et al. (32) | 2012 | 18.79 | 25.07 | CKD |
| Pruijm et al. (22) | 2012 | 17.9 | 28.7 | Type 2 diabetic patients |
| Vivier et al. (11) | 2013 | 16.8-17.5 | 27.9-28.3 | Healthy volunteers |
Experimental swine data using oxygen sensing electrodes within renal cortical and medullary locations demonstrate tissue oxygen levels consistent with deoxyhemoglobin levels identified using BOLD MRI (10). Average levels of tissue oxygen tension in the animal models ranged from 50 – 55 mmHg in cortex to as low as 15 to 20 mmHg in the deep sections of the medulla with 45% to 50% fall in oxygen level moving from cortex to deep medullary regions.
Factors influencing tissue oxygenation signal intensity in BOLD MRI studies
Deoxyhemoglobin level and tissue oxygenation can be modified by renal blood flow changes, but also by tubular solute transport, hydration state, arteriovenous shunts, antihypertensive drugs, temperature, blood pH and hematocrit. Recently, it has been shown that the contribution of a water load to R2* can be important, especially in the renal cortex (11).
Tubular sodium transport and medications
Experimental studies using either microelectrodes or BOLD MRI, establish that furosemide acutely lowers medullary R2* (therefore lowering deoxyhemoglobin) as a function of inhibiting solute transport in the ascending limb of Henle's loop. Acetazolamide, a diuretic acting on the proximal tubule, induces little change in the medullary oxygenation (6, 10).
Renal Blood Flow
Renal blood flow (RBF) is higher than any other organ with respect to organ weight, consistent with its primary function for blood filtration. The kidney has minimal overall oxygen consumption reflected in the smallest net arteriovenous difference in oxygen tension (12). The largest portion of RBF is directed towards the cortical glomeruli. The anatomical structure of medulla with its functional requirement of large amount of oxygen generates a progressively hypoxic milieu. Nearly constant oxygen depletion in this region makes it particularly susceptible to ischemic injury. Cortical tissue oxygenation can be maintained relatively constant when RBF is decreasing and is independent of the changes in the renal oxygen consumption (13).
In ARAS, when a “critical” 70% to 80% degree of stenosis is attained, renal hypoperfusion leads to a cascade of events from activation of the renin-angiotensin system to the rarefaction of small renal vessels, kidney fibrosis, loss of function, and atrophy (1). Studies of oxygen delivery and consumption in animal model indicate that tissue oxygen levels are stable even if cortical blood flow is reduced by up to 40%, thanks to reduced filtration and oxygen consumption (14). Medullary oxygenation may be compromised during moderate to severe acute cortical ischemia even when medullary blood flow is maintained (15). Low medullary pO2 reflects combined effects of reduced blood flow and increased oxygen consumption, as demonstrated using furosemide administration (10, 11). Local gradients of cortical and medullary oxygenation are closely regulated, but sometimes independently from each other. Numerous vasoactive systems intervene to compensate renal blood flow changes. In a rat model, for example, angiotensin II infusion produces a 40% decrease of cortical perfusion, but medullary perfusion can remain unchanged, apparently protected by prostaglandin E2 synthesis (16). Modification of arteriovenous shunting in response to changes in renal blood flow appear to maintain the oxygen tension and adjust local areas to blood supply, but also may render some focal regions more susceptible to hypoxia (14).
Comparison of 1.5 and 3T BOLD MRI to study kidney oxygenation
Studies comparing 1.5 and 3.0 Tesla magnetic fields indicate that BOLD MRI measurements at high field strength amplify differences between cortical and inner medullary regions of the kidney. The stronger magnetic field increases the sensitivity to deoxyhemoglobin acting as a natural contrast agent (2, 21).
Maneuvers that reduce oxygen consumption related to tubular solute transport (e.g. furosemide administration or water load) allow functional evaluation of transport-related activity as a determinant of tissue oxygenation (6, 11) .
Methods for analyzing BOLD MRI
Interpretation of BOLD MRI data related to renovascular disease processes has been challenging and we believe that failure to define meaningful interpretation has limited its application. Few studies describe precisely the BOLD MRI acquisition protocol, tissue volumes definition and quantification of the oxygenation level. Most often, images are sampled within a 5-8 mm thick axial, sagittal or coronal slice. Volume and slice orientation definition is important as what is represented as a bi-dimensional image corresponds in reality to tridimensional slice of renal parenchyma.
ROI selection
By convention, individual T2*-weighted images are selected to define regions of interest (ROIs) within the cortex and medulla. Most commonly, a single average R2* value for each region is determined, either from a single slice, or as the average of several samples and/or slices (8, 10, 17).
The ROIs can be limited to small regions (cortical or medullary) (6, 8, 10) or can encircle the entire kidney (17). Selection of local ROI's in diseased kidneys is subject to wide variation and sensitive to operator bias. Most analytical methods assign a single “best” or “mean” value for R2* associated with either cortex or medulla. In reality, levels of R2* vary gradually from the cortex to the medulla, reaching a “most hypoxic” zone in the deepest sections of medullary pyramids. Hence, the precision, and reproducibility of R2* values are affected by the size and location of ROI. Larger ROIs that include the entire medullary compartments may provide more representative and less variable mean values, but often include multiple medullary and cortico-medullary overlap zones with different hemodynamics. Small, selective ROIs are less vulnerable to volume averaging, but may be skewed by fluctuations caused by spatial and temporal heterogeneity in oxygen distribution within the kidney, particularly in the medulla.
Compartments model
To partially overcome these limitations, Ebrahimi et al.(18) developed a model assuming the R2* populations in cortex and medulla could be defined by two distinct mathematical distributions. Using this assumption, the R2* values were separated into two renal compartments by fitting the histogram of the R2* data acquired from a large ROI encompassing both cortex and medulla to the corresponding distribution functions. A single numerical R2* value was assigned for cortex and another for medulla. The limitations of this method are related to medullary heterogeneity and to the reliability of the histogram curve depending on the amount of data available, which compromises the precision or accuracy when these are limited. When relatively highly oxygenated medullary regions do not differ from cortex, or when kidneys are severely diseased, these boundaries may be blurred.
“Fractional kidney hypoxia” method
Saad et al. (19) recently developed an alternative method of BOLD MRI analysis with the aim of avoiding the assumption of discrete cortical and medullary values and decreasing the bias related to operator selection of ROIs. The renal tissue oxygenation was evaluated in both essential hypertensive and ARAS patients using a method to depict “fractional kidney hypoxia” determined by measuring the percentage of voxels from the whole kidney ROI with R2* values above 30 sec−1 taking the average of all available slices. Levels of cortical oxygenation were similar to those from methods with small ROIs. Estimates of medullary deoxygenation using fractional kidney hypoxia were substantially higher in in post-stenotic kidneys and were demonstrably related to hemodynamic severity of vascular lesions. The response to furosemide in the stenotic kidney was markedly blunted as compared with the contralateral kidney, essential hypertension kidneys and previous studies. The variability of “fractional hypoxia” in 2-4 axial slices was substantial at baseline and after furosemide administration, while cortical R2* values were quite reproducible. We believe that this variability reflects true biological differences between functional zones with different deep medullary representation, particularly in diseased kidneys.
Application of Renal BOLD MRI
Human studies suggest that BOLD MRI could identify kidney alterations in different conditions: after administration of nephrotoxic contrast, allograft injury, water loading (11), and occlusive renal arterial disease (8). Some authors had postulated that local hypoxia is a “final common pathway” related to kidney injury. Surprisingly, normal or low deoxyhemoglobin in both cortical and medullary regions can be observed in conditions that severely limit tubular metabolic activity, such as acute interstitial inflammation associated with transplant rejection, acute tubular necrosis, or even renal atrophy beyond an occluded vessel (8, 20). BOLD MRI has been recently used to show that Angiotensin II receptor blocker could partially ameliorate intrarenal hypoxia in chronic kidney disease patients (21), whereas, blockade of renin-angiotensin system in patients with type 2 diabetes does not seem to increase renal tissue oxygenation (22).
Studies in hypertensive African Americans demonstrate higher R2* levels associated with increased medullary volume and sodium reabsorption as compared with Caucasian patients. These data support a role increased oxidative stress in African Americans that may accelerate hypertension and target organ injury (34).
BOLD MRI found a particular application in renal artery stenosis (RAS) having a direct consequences for intrarenal oxygenation. Warner et al. showed that graded reduction in blood flow acutely decrease tissue oxygenation measured by oxygen electrodes that also appear as changes in R2* signal, more pronounced in the medulla (23).
In our previous study, we found that in ARAS patients’ compared with age-matched group of essential hypertensive patients, the cortical and medullary oxygenation was preserved despite significant reductions in blood flow in the stenotic kidneys sufficient to elevate plasma renin activity (7).
Another BOLD MRI study included three groups of patients: essential hypertension, “moderate” ARAS, and “severe” ARAS with Doppler velocities >384 cm/s and loss of functional renal tissue (8). Cortical R2* levels were significantly higher in severe ARAS group, showing the limits of kidney adaptation. BOLD analysis utilizing the “fractional hypoxia” method (19) confirmed that severe renovascular occlusion does indeed produce cortical hypoxia (FIGURE 1).
A separate publication from the United Kingdom in patients with ARAS used an analysis attributing a single average R2* value over entire coronal slice and showed that a higher level of R2* with preserved kidney volumes was associated with favorable response to renal revascularization (17).
Future Directions and Limitations
Despite its simplicity and appeal, clinical application of BOLD MRI is not yet standardized or routine. Some authors suggest that intrinsic limitations related to spatial distribution of blood and/or magnetic field non-homogeneities may limit the utility of BOLD MRI (24). In a recent publication Michaely et al. (25) failed to identify a correlation between R2* values and estimated GFR in patients with different stages of CKD in a large cohort with a variety of kidney diseases. However, these BOLD MRI studies were undertaken without standardized conditions such as control of sodium intake and defined medications (especially ACEI/ARBs or diuretics). Also, renal tissue oxygenation likely depends not only on the severity of CKD but also on the etiology of the underlying kidney disease (26).
Further studies of renal oxygenation over time and changes observed with medical therapy or revascularization in ARAS will be essential to define more precisely how variation in tissue oxygenation is related to tissue injury in this disorder. More precise definition of the level of oxygenation associated with renovascular occlusive disease may identify those patients most likely to benefit from renal revascularization, thereby clarifying results from negative treatment trials such as the ASTRAL and STAR trials (4, 27, 28).
Conclusion
We believe that BOLD MRI could be a valuable tool to evaluate changes in intra-renal oxygenation and potentially to identify kidneys at risk from vascular injury that may benefit from renal revascularization and/or adjunctive measures to repair the kidney before irreversible parenchymal damage.
Key Points.
Blood Oxygen Level Dependent Magnetic Resonance Imaging (BOLD MRI) is the only noninvasive technique evaluating kidney tissue oxygenation.
In Atherosclerotic Renal Artery Stenosis (ARAS) initial human studies showed a preserved cortical-to-medulla oxygenation gradients in many patients treated with medical antihypertensive therapy without restoring renal blood flow.
More severe vascular compromise ultimately overwhelms kidney adaptive potential in some individuals, leading to overt cortical hypoxia and expansion of medullary hypoxic zones.
New methods of BOLD MRI analysis will improve this evaluation sensitivity to detect changes in renal function prior to the onset of irreversible renal injury.
Acknowledgements
These studies were supported by PO1HL85307 from the National Heart Lung and Blood Institute and National Institutes of Health (NIH)/National Center for Research Resources CTSA grant UL1 RR024150. The content is solely the responsibility of the author and does not necessarily represent the official views of the NHLBI or the NIH.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to declare.
References
- 1.Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010;23(11):1159–69. doi: 10.1038/ajh.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Connor PM, Evans RG. Structural antioxidant defense mechanisms in the mammalian and nonmammalian kidney: different solutions to the same problem? Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R723–7. doi: 10.1152/ajpregu.00364.2010. [DOI] [PubMed] [Google Scholar]
- 3**.Evans RG, Eppel GA, Anderson WP, Denton KM. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J Hypertens. 2004;22(8):1439–51. doi: 10.1097/01.hjh.0000133744.85490.9d. [Key reference for physiopathology of renal cortical and medullary blood flow.] [DOI] [PubMed] [Google Scholar]
- 4.Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int. 2013;83(1):28–40. doi: 10.1038/ki.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Gloviczki ML, Keddis MT, Garovic VD, Friedman H, Herrmann S, McKusick MA, et al. TGF Expression and Macrophage Accumulation in Atherosclerotic Renal Artery Stenosis. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.06460612. [Important study correlating hemodynamic changes in renovascular disease with intrarenal TGF beta expression, fibrosis and macrophages accumulation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94(12):3271–5. doi: 10.1161/01.cir.94.12.3271. [BOLD MRI state of the art publication describing the prinicples of this method.] [DOI] [PubMed] [Google Scholar]
- 7.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55(4):961–6. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, et al. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension. 2011;58(6):1066–72. doi: 10.1161/HYPERTENSIONAHA.111.171405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gloviczki ML, Lerman LO, Textor SC. Blood oxygen level-dependent (BOLD) MRI in renovascular hypertension. Curr Hypertens Rep. 2011;13(5):370–7. doi: 10.1007/s11906-011-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol. 2011;46(1):41–7. doi: 10.1097/RLI.0b013e3181f0213f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Vivier PH, Storey P, Chandarana H, Yamamoto A, Tantillo K, Khan U, et al. Renal Blood Oxygenation Level-Dependent Imaging: Contribution of R2 to R2* Values. Invest Radiol. 2013 doi: 10.1097/RLI.0b013e3182823591. [Study showing the importance of water intake in BOLD MRI evaluation, especially for cortical R2* estimates.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Evans RG, Gardiner BS, Smith DW, O'Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008;295(5):F1259–70. doi: 10.1152/ajprenal.90230.2008. [Comprehensive review of kidney tissue oxygeantion regulation factors such as renal blood flow, arteriovenous shunts and oxygen consumption.] [DOI] [PubMed] [Google Scholar]
- 13.Evans RG, Goddard D, Eppel GA, O'Connor PM. Stability of tissue PO2 in the face of altered perfusion: a phenomenon specific to the renal cortex and independent of resting renal oxygen consumption. Clin Exp Pharmacol Physiol. 2011;38(4):247–54. doi: 10.1111/j.1440-1681.2011.05494.x. [DOI] [PubMed] [Google Scholar]
- 14.Evans RG, Eppel GA, Michaels S, Burke SL, Nematbakhsh M, Head GA, et al. Multiple mechanisms act to maintain kidney oxygenation during renal ischemia in anesthetized rabbits. Am J Physiol Renal Physiol. 2010;298(5):F1235–43. doi: 10.1152/ajprenal.00647.2009. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor PM, Kett MM, Anderson WP, Evans RG. Renal medullary tissue oxygenation is dependent on both cortical and medullary blood flow. Am J Physiol Renal Physiol. 2006;290(3):F688–94. doi: 10.1152/ajprenal.00275.2005. [DOI] [PubMed] [Google Scholar]
- 16*.Sadowski J, Badzynska B. Specific features and roles of renal circulation: angiotensin II revisited. J Physiol Pharmacol. 2006;57(Suppl 11):169–78. [Important review on renin-angiotensin system and renal circulation.] [PubMed] [Google Scholar]
- 17*.Chrysochou C, Mendichovszky IA, Buckley DL, Cheung CM, Jackson A, Kalra PA. BOLD imaging: a potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27(3):1013–9. doi: 10.1093/ndt/gfr392. [Interesting approach looking for the biomarker predicting renal artery revascularization results. BOLD MRI analysis was done in this study attributing a unique R2* value to the whole kidney coronal image.] [DOI] [PubMed] [Google Scholar]
- 18**.Ebrahimi B, Gloviczki M, Woollard JR, Crane JA, Textor SC, Lerman LO. Compartmental analysis of renal BOLD MRI data: introduction and validation. Invest Radiol. 2012;47(3):175–82. doi: 10.1097/RLI.0b013e318234e75b. [New method of BOLD MRI data analysis based on the mathematical compartment model. This could be important step when developing routine clinical applications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Saad A, Crane J, Glockner JF, Herrmann SM, Friedman H, Ebrahimi B, et al. Estimating fractional tissue hypoxia to analyze Blood Oxygen Level Dependent (BOLD) MR in human renovascular disease. Radiology. 2013 doi: 10.1148/radiol.13122234. In press. [Validation study of a new method of BOLD MRI analysis. “Fractional tissue hypoxia” analysis could render BOLD MRI more sensitive to show evolving renal hypoxia in atherosclerotic renal artery stenosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SY, Kim CK, Park BK, Huh W, Kim SJ, Kim B. Evaluation of transplanted kidneys using blood oxygenation level-dependent MRI at 3 T: a preliminary study. AJR Am J Roentgenol. 2012;198(5):1108–14. doi: 10.2214/AJR.11.7253. [DOI] [PubMed] [Google Scholar]
- 21.Manotham K, Ongvilawan B, Urusopone P, Chetsurakarn S, Tanamai J, Limkuansuwan P, et al. Angiotensin II receptor blocker partially ameliorated intrarenal hypoxia in chronic kidney disease patients: a pre-/post-study. Intern Med J. 2012;42(4):e33–7. doi: 10.1111/j.1445-5994.2011.02610.x. [DOI] [PubMed] [Google Scholar]
- 22.Pruijm M, Hofmann L, Zanchi A, Maillard M, Forni V, Muller ME, et al. Blockade of the renin-angiotensin system and renal tissue oxygenation as measured with BOLD-MRI in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012 doi: 10.1016/j.diabres.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 23*.Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, et al. Regional decreases in renal oxygenation during graded acute renal arterial stenosis: a case for renal ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R67–71. doi: 10.1152/ajpregu.90677.2008. [Important study showing the renal oxygenation decrease concomittant to the acute renal artery stenosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Evans RG, Leong CL, Anderson WP, O'Connor PM. Don't be so BOLD: potential limitations in the use of BOLD MRI for studies of renal oxygenation. Kidney Int. 2007;71(12):1327–8. doi: 10.1038/sj.ki.5002321. author reply 8. [Important review of BOLD MRI analysis limitations.] [DOI] [PubMed] [Google Scholar]
- 25.Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012;81(7):684–9. doi: 10.1038/ki.2011.455. [DOI] [PubMed] [Google Scholar]
- 26.Neugarten J. Renal BOLD-MRI and assessment for renal hypoxia. Kidney Int. 2012;81(7):613–4. doi: 10.1038/ki.2011.462. [DOI] [PubMed] [Google Scholar]
- 27**.The ASTRAL Investigators Revascularization versus Medical Therapy for Renal-Artery Stenosis. N Engl J Med. 2009;361:1953–62. doi: 10.1056/NEJMoa0905368. [Randomized controlled clinical trial included 806 patients with ARAS and failed to demonstrate benefits of revascularization procedure as compared to medical treatment alone. This trial results have to be understood within the frame of the specific protocol including patients uncertain indication for revascularization and often with renal artery stenosis between 50% and 70%.] [DOI] [PubMed] [Google Scholar]
- 28.Textor SC. Issues in renovascular disease and ischemic nephropathy: beyond ASTRAL. Curr Opin Nephrol Hypertens. 2011;20(2):139–45. doi: 10.1097/MNH.0b013e328342bb35. [DOI] [PubMed] [Google Scholar]
- 29.Li LP, Vu AT, Li BS, Dunkle E, Prasad PV. Evaluation of intrarenal oxygenation by BOLD MRI at 3.0 T. J Magn Reson Imaging. 2004;20(5):901–4. doi: 10.1002/jmri.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumkur SM, Vu AT, Li LP, Pierchala L, Prasad PV. Evaluation of intra-renal oxygenation during water diuresis: a time-resolved study using BOLD MRI. Kidney Int. 2006;70(1):139–43. doi: 10.1038/sj.ki.5000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, et al. Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension. 2010;55(5):1116–22. doi: 10.1161/HYPERTENSIONAHA.109.149682. [DOI] [PubMed] [Google Scholar]
- 32.Xin-Long P, Jing-Xia X, Jian-Yu L, Song W, Xin-Kui T. A preliminary study of blood-oxygen-level-dependent MRI in patients with chronic kidney disease. Magn Reson Imaging. 2012;30(3):330–5. doi: 10.1016/j.mri.2011.10.003. [DOI] [PubMed] [Google Scholar]