Abstract
Background
Previous research has shown a reduction in blood pressure (BP) immediately after the practice of alternate nostril yoga breathing (ANYB) in normal healthy male volunteers and in hypertensive patients of both sexes. The BP during ANYB has not been recorded.
Material/Methods
Participants were 26 male volunteers (group mean age ±SD, 23.8±3.5 years). We assessed (1) heart rate variability, (2) non-invasive arterial BP, and (3) respiration rate, during (a) ANYB and (b) breath awareness (BAW) sessions. Each session was 25 minutes. We performed assessments at 3 time points: Pre (5 minutes), during (15 minutes; for ANYB or BAW) and Post (5 minutes). A naïve-to-yoga control group (n=15 males, mean age ±SD 26.1±4.0 years) were assessed while seated quietly for 25 minutes.
Results
During ANYB there was a significant decrease (repeated measures ANOVA) in systolic BP and respiration rate; while RMSSD (the square root of the mean of the sum of squares of differences between adjacent NN intervals) and NN50 (the number of interval differences of successive normal to normal intervals greater than 50 ms) significantly increased. During BAW respiration rate decreased. In contrast, respiration rate increased during the control state. ANYB and BAW were significantly different (2-factor ANOVA) in RMSSD and respiration rate. BAW and control were different with respect to respiration rate.
Conclusions
The results suggest that vagal activity increased during and after ANYB, which could have contributed to the decrease in BP and changes in the HRV.
MeSH Keywords: Arterial Pressure, Heart Rate, Yoga
Background
Heart rate variability is an index of beat-to-beat changes in the heart rate and is a non-invasive assessment of autonomic control of cardiac functions [1]. Reduced heart rate variability has been associated with negative consequences to health, particularly related to negative emotions [2] and mental stress [3]. Voluntarily regulated breathing techniques (pranayamas) have been used either alone or in combination with other interventions to reduce stress-related disorders and conditions such as hypertension [4,5]. These breathing techniques have been practiced most often with the aim of decreasing arousal by correcting the sympatho-vagal imbalance, ie, reducing the sympathetic activity and stimulating vagal efferent activity [4,5]. Alternate nostril yoga breathing (ANYB) is a breathing technique that allows the breath to be modified consciously [6]. It involves breathing alternately through the left and right nostrils. A commentary on a Hatha yoga text (Circa the twelfth century A.D.) claims that alternate nostril yoga breathing has calming effects and helps to attain balance in psychosomatic functioning [7]. Apart from this, scientific studies have determined the physiological effects of alternate nostril yoga breathing.
Persistently elevated blood pressure has been linked to the dysregulation of the autonomic nervous system or increased sympathetic activity [8]. A study on 20 male medical students showed a significant reduction in the systolic blood pressure immediately after 15 minutes of ANYB (Cohen’s d=0.34) [9]. In the study, the magnitude of reduction in systolic blood pressure increased after 8 weeks of training (15 minutes/day) of ANYB (Cohen’s d=0.98). These changes in the blood pressure were supported by a subsequent study [10]. Here, a significant reduction in the heart rate and systolic blood pressure (Cohen’s d=0.83) was observed immediately after 20 minutes of ANYB in 10 participants who were normal in health and naïve-to-breathing practices. There were no changes in 2 sets of matched controls who either relaxed on a couch or practiced quiet sitting and relaxed breathing, with their eyes closed.
Another study compared the immediate effects of right, left, and alternate nostril yoga breathing with breath awareness and normal breathing in 21 healthy male volunteers, with each type of breathing practiced on different days [11]. The participants’ blood pressure, finger plethysmogram amplitude (as an indicator of cutaneous vasomotor tone), skin conductance levels, respiration rate, and heart rate variability were assessed. After alternate nostril yoga breathing for 30 minutes, there was a significant reduction in systolic (Cohen’s d=0.18) and diastolic (Cohen’s d=0.16) blood pressure. The amplitude of finger plethysmogram was lower during the ANYB session, and an increase in the skin conductance levels was observed after the ANYB session. In the study, frequency domain analysis alone was reported for the heart rate variability (HRV). During the ANYB session there was a significant increase in the LF power of the HRV, a decrease in the HF power of the HRV, and an increase in LF/HF ratio. The study suggested that the changes in the finger plethysmogram and HRV were influenced by the decrease in breath rate during the ANYB, as the breath rate had decreased during ANYB. There were no significant changes observed in HRV and blood pressure after breath awareness.
More recently, in a randomized controlled trial, 90 patients of both sexes with essential hypertension who were randomized as ANYB, breath awareness, and control groups, showed a decrease in systolic (Cohen’s d=0.46) and diastolic blood pressure (Cohen’s d=0.17) following 10 minutes of ANYB, while systolic blood pressure alone was reduced after breath awareness and there were no changes in the blood pressure in a control group who read a magazine of neutral content for the same duration [5].
In the studies cited above, the blood pressure was recorded before and after the breathing techniques and it was speculated that the reduction in blood pressure was related to a shift in the autonomic balance towards parasympathetic dominance during the practice. However, as mentioned above, high blood pressure levels are associated with increased sympathetic activity [8]. In the studies cited there was no attempt to record the heart rate variability and blood pressure simultaneously during the actual practice of ANYB.
In the present study, to assess BP changes during ANYB, a CNAP™ Monitor 500 was used to assess the blood pressure. The CNAP™ Monitor 500 is a clinically validated device [12] which allows recording of the arterial blood pressure non-invasively and continuously without any disturbance to the yoga practitioners as caused by repeated cuff inflations.
Apart from this, an earlier cited study [11] which reported changes in the heart rate variability during the practice of ANYB and breath awareness did not report the time domain analysis of the heart rate variability. The components of time domain analysis of the heart rate variability are stronger predictors of parasympathetic activity [13] in comparison to the frequency domain analysis, which is not considered to determine exclusively the sympathetic or parasympathetic tone and is still being investigated [14].
Hence the present study was designed to assess the heart rate variability, breath rate and blood pressure in experienced practitioners of breathing techniques (pranayama) continuously, before, during and after (a) alternate nostril yoga breathing and (b) breath awareness. Breath awareness was chosen as it is an important part of each yoga breathing technique. Also awareness of the breath in a study of the Burmese Buddhist Vipassana meditation technique showed that breath awareness was associated with parasympathetic dominance [15]. Hence it was considered an interesting alternate intervention. The effect of re-testing after the intervention was assessed in a separate group (n=15) of naïve-to-yoga control subjects.
Material and Methods
Participants
Twenty-six male participants, with ages ranging from 18 to 33 years (group average age ±S.D., 23.8±3.5 years) participated in the experimental group. The study was limited to male participants as autonomic and respiratory variables vary with the phases of the menstrual cycle [16]. For the present study the required sample size was calculated from a recent study [5] on alternate nostril yoga breathing. The required sample size (n=18) was obtained using Cohen’s formula for the effect size, 0.46 (medium), with an alpha of 0.05, powered at 0.95 using G power program [17]. The effect size was calculated from the mean and S.D. of the systolic BP values which changed significantly in the study [5]. The participants of the present study were staff of a yoga and Ayurveda center located in north India. They were recruited by announcements on notice boards in the institution. The participants were normal in health, based on a routine clinical examination and were not taking any medication. All of them ate a plant-based diet, were non-smokers and their experience of yoga practice was between 6 and 72 months which was ascertained from their self- reports. Exclusion criteria were any nasal abnormalities, such as nasal polyps or nasal septal deviation. None of the participants were excluded for these reasons. Similar assessments were carried out on an age matched control group (n=15) who were naïve-to-yoga practice. The participants were staff of a security agency within the yoga center and had a comparable lifestyle. Their ages ranged from 19 to 32 years (group mean age ±S.D., 26.1±4.0 years). The group was assessed for quiet sitting to determine any retest effect. None of the participants received any incentive to take part in the study.
Design of the study
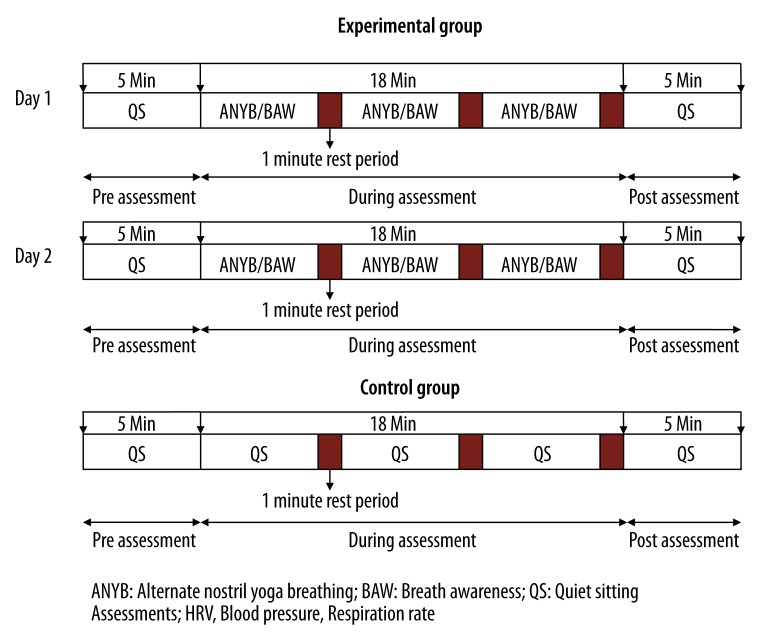
The participants of the experimental group were assessed on 2 separate days for 2 different breathing practices. These were (a) alternate nostril yoga breathing or anulom-vilom pranayama and (b) breath awareness. The order of practice sessions was alternated. The total duration of assessment was 25 minutes. The first 5 minutes of ‘Pre’ ANYB/BAW were followed by 15 minutes of the ‘During’ period when participants practiced ANYB or BAW. This was followed by 5 minutes of ‘Post’ ANYB or BAW. The schematic design of the study is shown in Figure 1. The control group had a single 25 minute session, similar to those of the experimental group, except that for 15 minutes of the period participants were seated and breathed normally. The signed informed consent of each participant was obtained after explaining the study design to the participants. The study was approved by the institution’s ethics committee.
Figure 1.
Schematic presentation of the study design.
Assessments
EKG and respiratory variable
The EKG and respiration rate were assessed using a MP150 data acquisition system (BIOPAC System Inc, U.S.A). The EKG was recorded using Ag/AgCl pre-gelled electrodes (Tyco Healthcare, Germany) and a standard bipolar limb lead III configuration. Limb lead III was chosen to minimize the risk of movement artifacts as the participants used their right hand to manipulate the nostrils. The EKG data were acquired at the sampling rate of 1024 Hz. The respiration rate was recorded using a respiratory stethograph transducer fixed around the trunk about 8 cm below the lower costal margin when the participant sat erect.
Continuous non-invasive blood pressure
The blood pressure was recorded using the CNAP™ MONITOR 500 (CNSystems Medizintechnil AG, Reininghausstrasse 13 8020 Graz, Austria). CNAP finger cuffs of suitable size were placed on the index and middle fingers of the left hand. A NBP cuff was placed on the upper arm of the same hand at the level of the heart and the marker on the NBP cuff was directly above the brachial artery. The hand was placed at the knee and flexed at the elbow.
Intervention
Alternate nostril yoga breathing
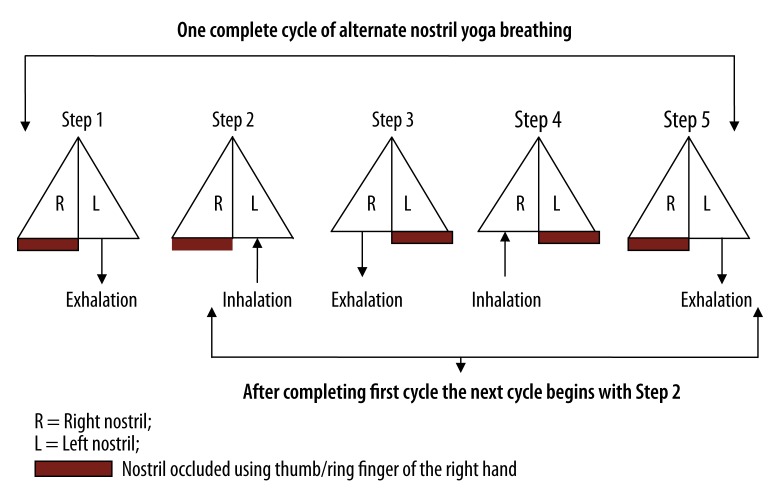
The participants sat cross-legged (sukhasana) with their spine erect and their eyes closed, throughout each session. During ANYB session the participants practiced alternate nostril yoga breathing which involves breathing through both nostrils alternately without retention of the breath. The participants used the thumb and ring finger of the right hand to manipulate the nostrils. The breathing practice begins by exhaling through the left nostril with the right nostril occluded; then inhaling through the left nostril; followed by exhaling through the right nostril with the left nostril occluded; then inhaling through the right nostril and exhaling through the left nostril. This is 1 complete cycle (Figure 2). The approximate duration of 1 cycle is 10–12 seconds [6].
Figure 2.
Schematic diagram of the procedure for alternate nostril yoga breathing.
Breath awareness
During the breath awareness session the participants were asked to be attentive to their breath during inhalation and exhalation without trying to modify their breath. They assumed the same posture as for alternate nostril breathing, which is crossed-legged (sukhasana) with the spine erect and eyes closed.
Quiet sitting (practiced by the control group)
Participants were asked to sit cross-legged (sukhasana) with their spine erect and eyes closed. Though the control group was naïve-to-yoga none of them found this difficult. They were asked to allow their thoughts to wander freely.
Data extraction
The total duration of recording was 25 minutes for each participant. The total duration was divided into 3 recording states as follows: (i) the pre-intervention (5 minutes), (ii) intervention (15 minutes) and (iii) post intervention (5 minutes). The data for each session were analyzed separately. Fast Fourier Transform analysis (FFT) of R-R interval series was done using the software developed by the Biosignal Analysis and Medical Imaging Group, University of Eastern Finland, Kubios HRV. Frequency domain and time domain components of HRV were analyzed separately for each session. The data were visually inspected off-line and only noise free data were included to obtain the heart rate variability spectrum. Two participants of the experimental group were excluded from the final analysis as the data were contaminated at different parts of the record with movement artifact and it was not possible to correct this.
Frequency domain analysis
The energy in the HRV series for 2 specific bands was studied, viz. the low frequency band (0.05–0.15 Hz) and high frequency band (0.15–0.50 Hz). The values of the low frequency (LF) and high frequency (HF) band were expressed as normalized units. The LF/HF ratio was also calculated.
Time domain analysis
The following components of time domain analysis of HRV were obtained: the mean RR interval (the mean of the intervals between adjacent QRS complexes or the instantaneous heart rate); RMSSD (the square root of the mean of the sum of the squares of differences between adjacent NN intervals); NN50 (the number of interval differences of successive normal to normal intervals greater than 50 ms), and pNN50 (the proportion derived by dividing NN50 by the total number of NN intervals).
Respiratory variable
The respiration rate in cycles per minute (CPM) was calculated by averaging the breath rate in 5 minutes states.
Blood pressure
Systolic and diastolic blood pressure values were extracted using the Biopac MP 150 (Biopac, USA) systems and Acqknowledge 4.1 software.
Data Analysis
The experimental group
Repeated measures analyses of variance (ANOVA) followed by post-hoc analyses with Bonferroni adjustment (corrected Bonferroni value of 0.025) were carried out to compare data recorded during and after the 2 practices with pre-intervention data of both practices, using SPSS version 18.0. There were 2 within subject factors (i.e., Factor 1: Sessions; ANYB and BAW, and Factor 2: States; Pre-intervention, During-intervention [D1, D2 and D3] and Post-intervention).
The control group
The data recorded in the control group during and after the session of quiet sitting were compared with the pre data of the respective session using separate paired t tests.
Comparisons between experimental and control groups
A 2-factor ANOVA [where factor 1 was Groups with 3 levels (ANYB, BAW and Control) and factor 2 was States with 5 levels (Pre, During 1, During 2, During 3 and Post)] was performed for each variable to compare the data between experimental and control groups.
Results
The group mean values ± SD for the different variables are given in Tables 1–3.
Table 1.
Frequency domain analysis of the of the heart rate variability and respiration rate (RR). Values are group mean ±S.D.
| Sessions | Variables | Pre | During | Post | ||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | ||||
| ANYB | LF (n.u) | 53.55±16.28 | 53.18±23.55 | 56.45±19.80 | 53.88±20.92 | 48.05±15.97 |
| HF (n.u) | 46.45±16.28 | 46.83±23.55 | 43.55±19.80 | 46.13±20.92 | 51.95±15.97 | |
| LF/HF | 1.45±0.96 | 2.03±2.23 | 2.17±2.46 | 1.97±2.51 | 1.29±1.47 | |
| RR (CPM) | 14.85±0.95 | 11.09±2.29*** | 11.96±2.12*** | 11.63±1.87*** | 13.45±1.43*** | |
| BAW | LF (n.u) | 53.49±21.79 | 51.70±20.10 | 51.83±18.79 | 58.61±20.81 | 56.37±19.50 |
| HF (n.u) | 46.51±21.77 | 48.30±20.10 | 48.18±18.79 | 41.39±20.81 | 43.63±19.50 | |
| LF/HF | 2.10±2.56 | 2.12±3.19 | 1.79±2.34 | 2.28±2.22 | 2.07±2.18 | |
| RR (CPM) | 15.51±1.81 | 13.88±1.97** | 14.81±1.76 | 14.58±1.65 | 14.77±1.25 | |
| CTRL | LF (n.u) | 45.7±18.0 | 40.9±15.0 | 46.1±18.0 | 45.5±17.8 | 46.2±16.8 |
| HF (n.u) | 54.3±18.0 | 59.1±15.0 | 53.9±18.0 | 54.5±17.8 | 53.8±16.8 | |
| LF/HF | 1.1±0.9 | 0.9±0.8 | 1.1±1.0 | 1.2±1.2 | 1.1±1.1 | |
| RR (CPM) | 15.38±2.64 | 16.35±1.98# | 16.39±2.60 | 16.55±2.66 | 15.97±3.07 | |
p<0.01;
p<0.01, post-hoc analyses with Bonferroni adjustment compared with pre;
p<0.05, paired t-test with respective pre values.
ANYB – alternate nostril yoga breathing; BAW – breath awareness; CTRL – quiet sitting with eyes closed.
Table 3.
Systolic (SBP) and diastolic (DBP) blood pressure. Values are group mean ±S.D.
| Sessions | Variables | Pre | During | Post | ||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | ||||
| ANYB | SBP (mm Hg) | 119.8±9.2 | 117.4±10.4* | 114.2±12.8 | 114.2±11.3* | 116.8±14.5 |
| DBP (mm Hg) | 73.9±7.3 | 73.4±7.8 | 72.9±8.9 | 71.2±11.9 | 73.1±8.8 | |
| BAW | SBP (mm Hg) | 118.3±10.4 | 117.5±11.8 | 116.8±12.6 | 115.3±13.0 | 117.3±13.0 |
| DBP (mm Hg) | 75.3±6.2 | 74.5±6.3 | 74.6±6.8 | 74.3±6.4 | 74.6±7.0 | |
| CTRL | SBP (mm Hg) | 118.7±11.9 | 119.5±12.1 | 118.9±13.0 | 117.4±14.4 | 118.8±15.1 |
| DBP (mm Hg) | 73.8±11.9 | 74.6±11.3 | 73.3±12.0 | 72.1±12.3 | 72.7±11.6 | |
p<0.05, post-hoc analyses with Bonferroni adjustment compared with pre.
ANYB – alternate nostril yoga breathing; BAW – breath awareness; CTRL – quiet sitting.
Repeated measures analyses of variance (ANOVA)
Time domain analysis of HRV
The mean RR interval showed a significant difference between States (F=6.171, df=3.237, 74.442, Huynh-Feldt epsilon=0.809, p<0.01) and an interaction between Sessions x States (F=9.165, df=2.319, 53.329, p<0.001, Huynh-Feldt epsilon=0.580). For the RMSSD there was a significant difference between Sessions (F=6.503, df=1, 23, p<0.05) and States (F=3.681, df=3.299, 75.866, p<0.05, Huynh-Feldt epsilon=0.825). For the NN50 there was a significant difference between States (F=6.096, df=2.432, 55.937, p<0.01, Huynh-Feldt epsilon=0.563) and an interaction between Sessions x States (F=3.087, df=2.252, 51.798, p<0.05, Huynh-Feldt epsilon=0.563). The pNN50 showed a significant difference between States (F=4.344, df=2.624, 60.360, p<0.05, Huynh-Feldt epsilon=0.656).
Frequency domain analysis of HRV
No significant changes were observed between Session, States and the interaction between Session and States for the LF, HF and LF/HF.
Blood pressure
For the systolic blood pressure there was a significant difference between States (F=3.184, df=2.234, 55.849, p<0.05, Huynh-Feldt epsilon=0.558).
No significant changes were observed in Sessions, States and the interaction between Sessions and States for the diastolic blood pressure.
Respiratory variable
The mean respiration rate (CPM) showed a significant difference between (i) Sessions [F=50.844, df=1, 25, Huynh-Feldt epsilon=1.000, p<0.001, (ii) States [F=31.946, df=2.980, 74.503, Huynh-Feldt epsilon=0.745, p<0.001] and (iii) Sessions × States [F=8.293, df=3.025, 75.622, Huynh-Feldt epsilon=0.756, p<0.001].
Comparisons between experimental and control groups (2 factor ANOVA)
Time domain analysis of HRV
For the Mean RR, ANOVA value for between group comparison in the following States (i) Pre (F=0.014, df=2, 60, p=0.987), (ii) During 1 (F=0.667, df=2, 60, p=0.517), (iii) During 2 (F=1.460, df=2, 60, p=0.240), (iv) During 3 (F=0.752, df=2, 60, p=0.476) and (iv) Post (F=0.747, df=2, 60, p=0.478) did not show any significant differences.
For the RMSSD, ANOVA value for between-group comparison in the following States: (i) During 1 (F=0.26, df=2, 60, p=0.045), (ii) During 2 (F=5.795, df=2, 60, p=0.005), and (iii) During 3 (F=3.652, df=2, 60, p=0.032) revealed significant differences.
For the NN50, ANOVA value for between group comparison in the following States (i) Pre (F=1.318, df=2, 60, p=0.275), (ii) During 1 (F=1.151, df=2, 60, p=0.323), (iii) During 2 (F=2.044, df=2, 60, p=0.138), (iv) During 3 (F=2.326, df=2, 60, p=0.106) and (v) Post (F=1.349, df=2, 60, p=0.267) did not show any significant differences.
For the pNN50, ANOVA value for between group comparison in the following States (i) Pre (F=1.001, df=2, 60, p=0.374), (ii) During 1 (F=0.571, df=2, 60, p=0.568), (iii) During 2 (F=1.294, df=2, 60, p=0.282), (iv) During 3 (F=1.903, df=2, 60, p=0.158) and (v) Post (F=1.356, df=2, 60, p=0.266) did not show any significant differences.
Frequency domain analysis of HRV
For the low frequency (LF), ANOVA value for between group comparison in the following States (i) Pre (F=0.973, df=2, 60, p=0.384), (ii) During 1 (F=1.834, df=2, 60, p=0.169), (iii) During 2 (F=1.386, df=2, 60, p=0.258), (iv) During 3 (F=1.944, df=2, 60, p=0.152) and (v) Post (F=2.016, df=2, 60, p=0.142) did not show any significant differences.
For the high frequency (HF), ANOVA value for between group comparison in the following States (i) Pre (F=0.977, df=2, 60, p=0.382), (ii) During 1 (F=1.834, df=2, 60, p=0.169), (iii) During 2 (F=1.386, df=2, 60, p=0.258), (iv) During 3 (F=1.944, df=2, 60, p=0.152) and (v) Post (F=2.016, df=2, 60, p=0.142) did not show any significant differences.
For the LF/HF, ANOVA value for between group comparison in the following States (i) Pre (F=1.682, df=2, 60, p=0.195), (ii) During 1 (F=1.419, df=2, 60, p=0.250), (iii) During 2 (F=1.034, df=2, 60, p=0.362), (iv) During 3 (F=1.242, df=2, 60, p=0.296) and (v) Post (F=1.806, df=2, 60, p=0.173) did not show any significant differences.
Blood pressure
For the Systolic blood pressure, ANOVA value for between group comparison in the following States (i) Pre (F=0.135, df=2, 60, p=0.874), (ii) During 1 (F=0.208, df=2, 60, p=0.813), (iii) During 2 (F=0.655, df=2, 60, p=0.523), (iv) During 3 (F=0.304, df=2, 60, p=0.739) and (v) Post (F=0.109, df=2, 60, p=0.897) did not show any significant differences.
For the Diastolic blood pressure, ANOVA value for between group comparison in the following States (i) Pre (F=0.264, df=2, 60, p=0.769), (ii) During 1 (F=0.145, df=2, 60, p=0.866), (iii) During 2 (F=0.253, df=2, 60, p=0.777), (iv) During 3 (F=0.639, df=2, 60, p=0.531) and (v) Post (F=0.283, df=2, 60, p=0.755) did not show any significant differences.
Respiratory variable
For the Respiration rate, ANOVA value for between group comparison in the following States (i) During 1 (F=31.158, df=2, 64, p<0.001), (ii) During 2 (F=23.598, df=2, 64, p<0.001), (iii) During 3 (F=31.496, df=2, 64, p<0.001) and (iv) Post (F=9.035, df=2, 64, p<0.001) revealed significant differences.
Post-hoc analyses (Comparisons were between during/after values with respective pre values)
HRV time domain analysis
A significant increase (p<0.001) was observed in the mean RR interval after the ANYB session with 95% CI of [−0.023, −0.094] compared with the respective pre values. During the ANYB session a significant decrease was observed in (i) NN50 (p<0.05) with 95% CI of [−0.583, −74.001] and (ii) RMSSD (p<0.05) with 95% CI of [−10.573, −264.361] compared with the pre-intervention values.
Blood pressure
A significant decrease (p<0.05) in systolic blood pressure was recorded during the ANYB session with 95% CI of [10.381, 0.712] compared with the respective pre-intervention values.
Respiratory variable
There was a significant decrease in the respiration rate during ANYB (p<0.001) with 95% CI of [5.205, 2.309] and after ANYB (p<0.001) with 95% CI of [2.265, 0.531] compared with pre-intervention values. A significant decrease (p<0.01) in the respiration rate was observed during the BAW session with 95% CI of [2.676, 0.575] compared with pre-intervention values.
Control group
There was a significant increase in respiration rate during (p<0.05) the quiet sitting session when compared with pre using paired t-test.
Post-hoc analyses (2-factor ANOVA)
For RMSSD, a significant difference was observed in During 2 for (i) ANYB vs. BAW (p<0.05) with 95% CI of [218.023, 14.427; ANYB>BAW], and (ii) ANYB vs. Control (p<0.05) with 95% CI of [255.456, 23.321; ANYB>Control].
For Respiration rate, a significant difference (in all cases, ANYB<BAW, ANYB < Control and BAW<Control) was observed in (A) During 1 for (i) ANYB vs. BAW (p<0.001) with 95% CI of [1.358, 4.225], (ii) ANYB vs. Control (p<0.001) with 95% CI of [3.586, 6.937] and (iii) BAW vs. Control (p<0.01) with 95% CI of [−0.795, 4.145], (B) During 2 for (i) ANYB vs. BAW (p<0.001) with 95% CI of [−1.404, −4.281], (ii) ANYB vs. Control (p<0.001) with 95% CI of [−2.742, −6.105], (C) During 3 for (i) ANYB vs. BAW (p<0.001) with 95% CI of [−1.591, 4.315], (ii) ANYB vs. Control (p<0.001) with 95% CI of [−3.327, −6.511] and (iii) BAW vs. Control (p<0.05) with 95% CI of [−0.373, −3.558], and (D) Post for (i) ANYB vs. BAW (p<0.05) with 95% CI of [−0.49, −2.592], (ii) ANYB vs. Control (p<0.001) with 95% CI of [−1.031, −4.005].
Discussion
During alternate nostril yoga breathing (ANYB), RMSSD (the square root of the mean of the sum of the squares of differences between adjacent normal to normal intervals) and NN50 (the number of interval differences of successive normal to normal intervals greater than 50 ms) were increased and systolic blood pressure decreased by an average of 4.5 mmHg. Following ANYB there was a significant increase in the mean RR interval. The rate of respiration decreased during and after ANYB and during breath awareness (BAW).
The time domain measures of the heart rate variability (HRV), i.e., RMSSD, NN50 and the mean R-R interval have been recognized as stronger predictors of vagal modulation than frequency domain measures [13]. Hence the increase in RMSSD and NN50 during ANYB and in the mean R-R interval following ANYB are suggestive of vagal dominance during and after ANYB. The shift towards vagal dominance during and after ANYB could be 1 of the factors responsible for the reduction in blood pressure in essential hypertension after practicing ANYB [5].
There were no significant changes observed in the measures of frequency domain analysis of HRV. Generally it is emphasized that the LF power of the HRV reflects the cardiac sympathetic activity and HF power determines the cardiac parasympathetic activity [18,19]. However, this is still under investigation. It has been subsequently reported that neither the LF power band nor the HF power band are exclusive indices of cardiac sympathetic and parasympathetic tone, respectively [14]. There have been studies to explore this. The LF power reduced after selective cardiac parasympathectomy and did not totally disappear after β-adrenoceptor blockade resulting from denervation [20]. Apart from this the sympathetic activity can also influence the HF power but to a lesser extent compared to the effect of parasympathetic activity on the LF power [21]. Therefore, the changes in LF and LF/HF must be interpreted cautiously. In the present study there were no changes in the LF, HF, and LF/HF values. This may be related to the fact that there was vagal stimulation during and after ANYB but the effects of ANYB on sympathetic activity are less clear, as are the interpretation of the LF power.
It has been recommended to monitor HRV and respiration simultaneously given the fact that quiet respiration is associated with parallel phasic changes in activity of medullary vagal cardiac and spinal muscle sympathetic motonuclei in humans [22]. There was a decrease in breath rate during and after ANYB and during the initial stage of BAW. The changes during BAW were not sustained throughout the session. The breath rate is associated with several physical as well as psychological factors [23]. Generally a decrease in breath rate has been associated with relaxation and could be related with the increased vagal tone or overall reduction in arousal during and after ANYB in the present study. In contrast, the respiration rate increased during the quiet sitting control session. These changes could be related to the fact that the participants might have felt a sense of monotony and boredom during the session, which is often associated with a higher breath rate [24].
Two earlier studies on healthy male volunteers with approximately a similar duration of ANYB practice (15 min) as in the present study reported a significant reduction in the systolic blood pressure alone following ANYB [9,10]. Another study on healthy male volunteers cited earlier, reported a reduction in both systolic (by 1.14 mmHg) and diastolic blood pressure (by 0.67 mmHg) following 30 minutes of ANYB [11]. In the present study systolic blood pressure alone decreased during ANYB. Hence, the present study resembles the results of 2 earlier studies where the duration of ANYB as well as the characteristics (healthy young adults) of the participants was comparable to the present study. Since the results of present study resembles the findings of 2 earlier studies [9,10], it would be ideal to compare the ages of the participants of the 2 earlier studies with the participants of the present study, as aging is associated with decline in cardiovascular and autonomic function and levels of brain-derived neurotropic factor [25]. In the 2 earlier studies [9,10] the age range of the participants was between 17–22 years and 17–20 years respectively; while in the present study the age range of the participants is 18–33 years with group average age ±S.D., 23.8±3.5 years. In a recent study done by Pal et al., 2014, 3 groups were categorized based on their age range (20–29 years, 30–39 years, 40–49 years) and the groups differed in cardiovascular and autonomic functions, and brain-derived neurotropic factor levels [25]. This difference in cardiovascular and autonomic function, and brain-derived neurotropic factor levels was reduced after 3 months of yoga and the senior/older age group showed less age-related differences in cardiovascular and autonomic functions, and brain-derived neurotropic factor levels. An age-related difference is not anticipated in the present study. The average ages of the 3 groups (2 previous studies [9,10] and the present study) are comparable based on the categorization given by Pal et al., 2014 [25]. Also in the present study participants were experienced in yoga (6–72 months), which, if one extrapolates the findings of Pal et al., 2014 [25], suggests that differences caused by age would be markedly reduced.
The absence of changes in the diastolic blood pressure could be related to the fact that diastolic blood pressure is considered a more stable component of the blood pressure [26] and it may be speculated that the duration of practice could have an influence on it. More recently, a study was conducted on 20 trained participants who practiced left (chandra nadi, chandra bhedana); right (surya nadi, surya bhedana); alternate nostril breathing (nadi shuddhi); and normal breathing [27]. Hence, each participant was assessed on 6 separate days. Following 9 rounds of alternate nostril breathing (nadi shuddhi) there was a significant decrease in heart rate, rate-pressure product, and the product of heart rate x Mean pressure/100 (called double product). The changes were suggestive of reduced sympathetic activity and improved vagal tone. While this recent report was in participants with normal health, another recent study assessed the effect of yoga as a therapy for persons with heart failure (i.e., with systolic and/or diastolic dysfunction, having ejection fraction of 30 to 50% and fulfilling international criteria for heart failure [28]. The study assessed the longitudinal effect (during 12 weeks) of a yoga program which included yoga postures (asanas) and regulated breathing (pranayamas). At the end of 12 weeks there was a significant decrease in the heart rate, blood pressure, and the rate-pressure product in 44 patients who practiced yoga compared to 48 who were in the control group and did not practice yoga. Also LF (nu) and LF-HF ratio decreased significantly while HF (nu) increased significantly in the yoga group compared to the control group. This study on patients with heart failure differed from the present study on healthy participants, in several aspects. Perhaps the most obvious is that the study on patients used multiple yoga techniques and assessed their effects over 12 weeks of practice, compared to the present study, which involved a single yoga breathing technique (i.e., ANYB) practiced for 15 minutes. In another study, Krishna et al., 2014 demonstrated, that 12 weeks of yoga practice, in a between groups (yoga vs. control) comparison improved left ventricular ejection fraction, Tei index, and NT pro BNP [29].
There have been other studies which have used a combination of yoga practices to reduce the blood pressure. Individual studies have demonstrated that yoga practice (several techniques in combination as well as single techniques) can reduce blood pressure. However, when critically reviewed in a meta-analysis on yoga for hypertension, it appeared that the current evidence is low in quality and requires larger studies to confirm the use of yoga in the management of hypertension [30].
The mechanism by which breathing through a particular nostril has different effects on the autonomic nervous system is not clear. The nasal cycle was recognized as early as 1895 when Kayser described that change in the volume of blood flowing through the vascular nasal mucosa varies for the 2 nostrils alternately [31]. The nasal mucosa when engorged with blood would reduce the patency of the nostril. The cyclical manner in which the patency and efficiency of the 2 nostrils varied resulted in the nasal cycle being considered an ultradian rhythm [32]. There have been several attempts to understand the periodicity of this rhythm. One such study assessed the time periods of multiple systems during sleep and waking rest were studied in 3 healthy adults with 10 variables, including the nasal cycle [33]. Time series across all variables detected periods at 115–145, 70–100, and 45–65 minutes across all variables. Hence, spontaneous changes in nasal patency are considered a possibility, though the mechanisms are not clear [34]. The nasal blood vessels influence nasal airflow and nasal airflow is regulated by autonomic and central control. This is related to the fact that sympathetic innervation to the nasal mucosal vasculature is regulated by the hypothalamus and vasomotor areas of the brainstem. Hence, ANYB may be influencing the autonomic nervous system by inducing changes in what is a spontaneous rhythm. The way in which ANYB influences the regulatory centers remains unclear despite several theories of neural connections between the nasal mucosa and the hypothalamus [32].
Conclusions
The study is limited by the following factors: (i) It would have been ideal to have the same participants practice the 3 interventions in a 3-armed study, in addition to having a non-yoga group. One of the reasons this was difficult to implement was that participants were reluctant to come to the laboratory for 3 days in a row. (ii) The study was limited to males to avoid changes in autonomic balance due to hormonal fluctuations in females. Extending the study to include females with necessary adjustments to control for hormonal changes would help to generalize the findings.
Table 2.
Time domain analysis of the heart rate variability. Values are group mean ±S.D.
| Sessions | Variables | Pre | During | Post | ||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | ||||
| ANYB | Mean RR (s) | 0.84±0.12 | 0.81±0.15 | 0.80±0.11 | 0.82±0.13 | 0.89±0.16*** |
| RMSSD (ms) | 55.31±54.04 | 218.18±285.19* | 192.78±217.17* | 215.93±298.75 | 147.81±261.29 | |
| NN50 (count) | 71.42±54.81 | 108.71 ±57.23* | 108.29±58.22* | 109.54±71.70 | 84.33±52.49 | |
| PNN50 (%) | 21.20±17.20 | 30.77±19.31 | 30.29±17.29 | 31.65±21.33 | 26.91±18.52 | |
| BAW | Mean RR (s) | 0.84±0.11 | 0.85±0.11 | 0.85±0.10 | 0.85±0.11 | 0.85±0.13 |
| RMSSD (ms) | 58.52±63.08 | 102.20±172.37 | 76.55±77.33 | 81.29±131.16 | 100.28±198.08 | |
| NN50 (count) | 70.46±62.65 | 83.46±60.65 | 76.42±57.08 | 74.29±54.30 | 75.33±50.98 | |
| PNN50 (%) | 21.02±20.14 | 24.92±19.91 | 22.78±18.50 | 22.17±17.68 | 22.32±16.42 | |
| CTRL | Mean RR (s) | 0.83±0.11 | 0.84±0.12 | 0.85±0.12 | 0.86±0.12 | 0.85±0.12 |
| RMSSD (ms) | 55.62±23.31 | 58.67±26.87 | 53.39±23.45 | 64.04±26.36 | 62.50±29.75 | |
| NN50 (count) | 98.80±55.30 | 97.40±53.48 | 101.60±53.72 | 108.13±56.73 | 103.87±56.83 | |
| PNN50 (%) | 28.78±17.01 | 28.57±17.23 | 30.00±17.15 | 32.31±18.14 | 32.01±19.52 | |
p<0.05;
p<0.001, post-hoc analyses with Bonferroni adjustment compared with pre.
ANYB – alternate nostril yoga breathing; BAW – breath awareness; CTRL – quiet sitting.
Footnotes
Source of support: Departmental sources
References
- 1.Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–22. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- 2.Alvares GA, Quintana DS, Kemp AH, et al. Reduced heart rate variability in social anxiety disorder: associations with gender and symptom severity. PLoS One. 2013;8:7. doi: 10.1371/journal.pone.0070468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder EB, Liao D, Chambless LE, et al. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42:1106–11. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 4.van Dixhoorn J. Cardiorespiratory effects of breathing and relaxation instruction in myocardial infarction patients. Biol Psychol. 1998;49:123–35. doi: 10.1016/s0301-0511(98)00031-3. [DOI] [PubMed] [Google Scholar]
- 5.Telles S, Yadav A, Kumar N, et al. Blood pressure and Purdue pegboard scores in individuals with hypertension after alternate nostril breathing, breath awareness, and no intervention. Med Sci Monit. 2013;19:61–66. doi: 10.12659/MSM.883743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramdev S. Pranayama Rahasya. Haridwar: Divya Prakshan; 2009. [Google Scholar]
- 7.Saraswati SN. Prana pranayama pranavidya. Munger: Bihar School of Yoga; 1994. [Google Scholar]
- 8.Guzzetti S, Piccaluga E, Casati R, et al. Sympathetic predominance in essential hypertension: a study employing spectral analysis of heart rate variability. J Hypertens. 1988;6:711–17. doi: 10.1097/00004872-198809000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava RD, Jain N, Singhal A. Influence of alternate nostril breathing on cardiorespiratory and autonomic functions in healthy young adults. Indian J Physiol Pharmacol. 2005;49:475–83. [PubMed] [Google Scholar]
- 10.Subbalakshmi NK, Saxena SK, Urmimala, et al. Immediate effect of ‘nadishodhana pranayama’ on some selected parameters of cardiovascular, pulmonary, and higher functions of brain. Thai J Physiol Sci. 2005;18:10–16. [Google Scholar]
- 11.Raghuraj P, Telles S. Immediate effect of specific nostril manipulating yoga breathing practices on autonomic and respiratory variables. Appl Psychophysiol Biofeedback. 2008;33:65–75. doi: 10.1007/s10484-008-9055-0. [DOI] [PubMed] [Google Scholar]
- 12.Hahn R, Rinösl H, Neuner M, Kettner SC. Clinical validation of a continuous non-invasive haemodynamic monitor (CNAP™ 500) during general anaesthesia. Br J Anaesth. 2012;108:581–85. doi: 10.1093/bja/aer499. [DOI] [PubMed] [Google Scholar]
- 13.Wennerblom B, Lurje L, Tygesen H, et al. Patients with uncomplicated coronary artery disease have reduced heart rate variability mainly affecting vagal tone. Heart. 2000;83:290–94. doi: 10.1136/heart.83.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malliani A, Julien C, Billman GE, et al. Cardiovascular variability is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:684–88. doi: 10.1152/japplphysiol.00562.2006. [DOI] [PubMed] [Google Scholar]
- 15.Telles S, Mahapatra RS, Naveen KV. Heart rate variability spectrum during Vipassana mindfulness meditation. J Indian Psychol. 2005;23:1–5. [Google Scholar]
- 16.Yildirir A, Kabakci G, Akgul E, et al. Effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variability. Ann Noninvasive Electrocardiol. 2002;7:60–63. doi: 10.1111/j.1542-474X.2001.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]
- 18.Doğru MT, Başar MM, Yuvanç E, et al. The relationship between serum sex steroid levels and heart rate variability parameters in males and the effect of age. Turk Kardiyol Derns Ars. 2010;38:459–65. [PubMed] [Google Scholar]
- 19.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explore in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 20.Randall DC, Brown DR, Raisch RM, et al. SA nodal parasympathectomy delineates autonomic control of heart rate power spectrum. Am J Physiol. 1991;260:H985–88. doi: 10.1152/ajpheart.1991.260.3.H985. [DOI] [PubMed] [Google Scholar]
- 21.Telles S, Raghavendra BR, Naveen KV, et al. Changes in autonomic variables following 2 meditative states described in yoga texts. J Altern Complement Med. 2013;19:35–42. doi: 10.1089/acm.2011.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol. 1985;365:181–96. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson I, Ripley HS. Variations in respiration and in respiratory symptoms during changes in emotion. Psychosom Med. 1952;14:476–90. doi: 10.1097/00006842-195211000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Chetta A, Foresi A, Marangio E, Olivieri D. Psychological implications of respiratory health and disease. Respiration. 2005;72:210–15. doi: 10.1159/000084056. [DOI] [PubMed] [Google Scholar]
- 25.Pal R, Singh SN, Chatterjee A, Saha M. Age-related changes in cardiovascular system, autonomic functions, and levels of BDNF of healthy active males: role of yogic practice. Age (Dordr) 2014;36:9683. doi: 10.1007/s11357-014-9683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pede S, Lombardo M. [Cardiovascular risk stratification. Systolic, diastolic or pulse pressure?]. Ital Heart J Suppl. 2001;2:356–58. [in Italian] [PubMed] [Google Scholar]
- 27.Bhavanani AB, Ramanathan M, Balaji R, Pushpa D. Differential effects of uninostril and alternate nostril pranayamas on cardiovascular parameters and reaction time. Int J Yoga. 2014;7:60–65. doi: 10.4103/0973-6131.123489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishna BH, Pal P, GKP, et al. Effect of yoga therapy on heart rate, blood pressure and cardiac autonomic function in heart failure. J Clin Diagn Res. 2014;8:14–16. doi: 10.7860/JCDR/2014/7844.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishna BH, Pal P, Pal G, et al. A Randomized Controlled Trial to Study the Effect of Yoga Therapy on Cardiac Function and N Terminal Pro BNP in Heart Failure. Integr Med Insights. 2014;9:1–6. doi: 10.4137/IMI.S13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer H, Haller H, Lauche R, et al. A systematic review and meta-analysis of yoga for hypertension. Am J Hypertens. 2014;27:1146–51. doi: 10.1093/ajh/hpu078. [DOI] [PubMed] [Google Scholar]
- 31.Kayser R. Die exacte messung der luftdurchgangigkeit der nase. Arch Laryng Rhinol. 1895;3:101–20. [Google Scholar]
- 32.Stoksted P. Rhinometric measurements for determination of the nasal cycle. Acta Otolaryngol Suppl. 1953;109:159–75. doi: 10.3109/00016485309132516. [DOI] [PubMed] [Google Scholar]
- 33.Shannahoff-Khalsa DS, Yates FE. Ultradian sleep rhythms of lateral EEG, autonomic, and cardiovascular activity are coupled in humans. Int J Neurosci. 2000;101:21–43. doi: 10.3109/00207450008986490. [DOI] [PubMed] [Google Scholar]
- 34.Ohki M, Ogoshi T, Yuasa T, et al. Extended observation of the nasal cycle using a portable rhinoflowmeter. J Otolaryngol. 2005;34:346–49. doi: 10.2310/7070.2005.34509. [DOI] [PubMed] [Google Scholar]