Abstract
Background
The aim of this study was to investigate differences in glioblastoma RNA gene expression profiles between Uyghur and Han patients in Xinjiang province and to screen and compare differentially expressed genes with respect to their clinical significance in the pathogenesis of high-grade glioma and their relationship to disease prognosis.
Material/Methods
Illumina HT-12mRNA expression profiles microarray was employed to measure the gene expression profiles of 6 patients with advanced glioma and to screen for differentially expressed genes.
Results
GO and KEGG analyses were performed on the differentially expressed genes using Web Gestalt software (P<0.05). Comparison of glioblastoma RNA expression profiles in the Uyghur and Han patients indicated that 1475 genes were significantly differentially expressed, of which 669 showed increased expression, while 807 showed decreased expression. One gene (STRC) corresponded to 2 transcripts, 1 of which showed increased expression and the other showed decreased expression. The differentially expressed genes participate in metabolic processes, biological regulation, stress response, and multi-cellular organic processes, including small GTPase regulatory signaling pathways, Ras signaling pathway, neuronal reactive protein regulation, and myelination of the central nervous system. The genes are also involved in tumor-related signaling pathways, including metabolic pathways, cancer pathways, MAPK signaling pathway, TGF-β signaling pathway, neurotrophic factor signal transduction pathway, and mTOR signaling pathway.
Conclusions
Differentially expressed genes were screened by studying the gene expression profiles in glioblastoma from Uyghur and Han patients. The cellular function and location of these genes were further investigated. Based on related molecular markers of glioblastoma, the differences in the mechanism of initiation and development of glioblastoma between Uyghur and Han patients were investigated for polygenic interactions.
MeSH Keywords: Genetic Association Studies, Glioblastoma, Molecular Biology
Background
As a common malignant tumor of the central nervous system [1,2], glioblastoma patients have poor prognosis and very short life expectancy. With increasing incidences of high-grade glioma (WHO grade 3–4) in recent years the disease has shown the disturbing trend of afflicting younger patients more and more. Furthermore, the mortality rate associated with glioblastoma has increased by nearly 100% [2]. Xinjiang province is home to people of various ethnic backgrounds, among which Uyghur is the largest ethnic group. Preliminary statistics have shown that the incidence of glioblastoma in males is significantly higher than that in females. This is particularly relevant to the Uyghur population, as males make up a larger percentage of the Uyghur population than females. Moreover, the age of onset of glioblastoma is lower among Uyghur patients than among Han patients.
The diversity in the pathogenesis and clinical characteristics of glioblastoma among Uyghur and Han patients indicates the presence of certain differences at the genetic level between these 2 ethnic groups. In this study, based on retrospective analysis and follow-up studies of the clinical characteristics of glioblastoma among Uyghur and Han patients, as well as the molecular-level changes in high-grade glioma [3], we used mRNA expression profiling to screen for differential gene markers of glioblastoma among Uyghur and Han patients. Our goal was to compare the differences in gene expression in glioblastoma and to preliminarily investigate the differences in pathogenesis between these 2 ethnic groups.
Gliomas account for 81% of primary central nervous system malignant tumors, and their 5-year mortality rate is third only to pancreatic cancer and lung cancer. The 2012 Chinese Cancer Registry Annual Report reported that the rate of mortality from brain and central nervous system malignant tumors in China is 3.87/100 000, ranking ninth among the top 10 highest mortality rate tumors [4]. Over half of brain gliomas, which are the most common form of primary brain tumors, take the most malignant form of glioblastoma multiforme (GBM). For GBM patients, the median survival period is unsatisfactory, even when patients are treated with the most active forms of therapy.
Material and Methods
Material
Six fresh specimens of glioblastoma (3 from Uyghur patients and 3 from Han patients) were randomly selected by the Affiliated Tumor Hospital of Xinjiang Medical University between January 2010 and December 2013, of which 2 were taken from males and 4 were from females. The patients were aged 29–56 years, with a median age of 45 years. The histological grades of the specimens were all grade IV. The specimens were rinsed with physiological saline before freezing in liquid nitrogen and transfer into a refrigerator for preservation at −80°C. We verified that there were no duplicate patients, that we had complete clinical data for all patients (age, sex, ethnicity, pathological diagnosis, and imaging features), and that all patients received 2-year post-operation follow-up. None of the patients received radiotherapy or chemotherapy before the operation. For each patient, preliminary imaging (CT or MRI) and post-operative pathological examination were performed by experienced physicians.
Extraction and measurement of total RNA from fresh high-grade gliomas
A suitable quantity of tissue (<30 mg) was wrapped with sterile aluminum foil and frozen in liquid nitrogen. The tissue was crushed with a hammer in liquid nitrogen to preserve it at low temperature. Total RNA was extracted with Trizol (Invitrogen, America) and the RNeasy kit (Qiagen, Germany) according to the manufacturer’s instructions. Qualitative and quantitative measurements of total RNA (concentration and purity) were performed by 1% agarose gel electrophoresis and a NanoDrop 2000 Spectrophotometer (NanoDrop, America). After measuring its concentration, the total RNA was aliquoted and stored in an -low temperature freezer.
Microarray detection of mRNA expression profiles of human HT-12 genome with illumina
RNA specimens were transcribed in vitro to synthesize cDNA. According to the Illumina instructions, after inspecting the cDNA for quality, cDNA was mixed with hybridization reagent to obtain hybrid specimens, which were incubated at room temperature. The samples were then washed 3 times at high temperature with ethanol. Finally, the specimens were desiccated for imaging.
Quality control
Illumina expression profiling is equipped with 6 categories of internal reference genes and 887 probes are available for quality control of each specimen. Quality control items included hybridization controls, negative controls, biotin and high stringency, gene intensity, low stringency, and labeling and background. All specimens passed the quality controls, indicating that the experimental procedure was successful, the probes functioned as expected, and that the chip data were reliable.
Data analysis and normalization
The iSCAN system, which is equipped with 2 laser light sources (532 nm and 635 nm), was used for data analysis. The system enables extremely high resolution (<1 um) by confocal scanning. After changing the setting, the researcher was able to easily read the information [5]. The scanned images of microarray hybridization were imported into GenomeStudio for background correction of the original signal. The corrected data were then normalized by the rsn method found in the lumi package of the R language software. Screening of differentially expressed genes was performed on the normalized data using the limma package of the R language software. Finally, WebGestalt software was used to perform GO and KEGG function annotation for differential genes (through hypergeometric distribution, the P-value was <0.05 after multiple test corrections) [6].
Results
Clinical data and follow-up analysis
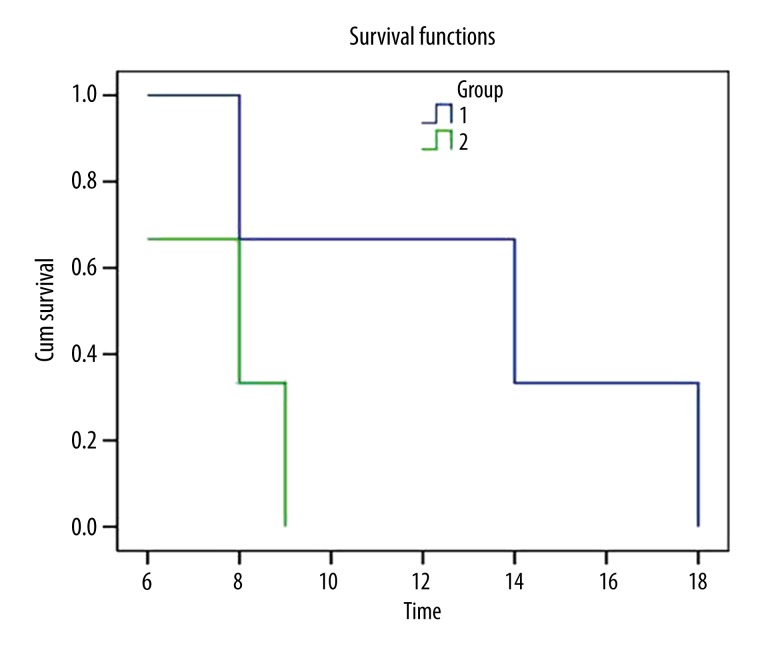
Follow-ups were performed at 3, 6, 12, and 18 months after surgery. The survival period ranged from 3 to 18 months, with an average of 7 months. The survival curves indicate that the survival period of Uyghur patients is significantly longer than that of Han patients, as shown in Table 1 and Figure 1.
Table 1.
Clinical characteristics of the 6 glioblastomas.
| No. | Ethnicity | Sex | Age | Operated position | Maximum diameter (cm) | Grade | Palindromia (month) | Survival period (month) |
|---|---|---|---|---|---|---|---|---|
| 3258 | Uyghur | Female | 29 | Left frontal lobe | 4 | IV | 3 | 18 |
| 6114C | Uyghur | Male | 51 | Left temporal lobe | 2 | IV | – | 14 |
| 6501 | Uyghur | Male | 44 | Right emporal lobe | 4 | IV | 5 | 8 |
| 2968C | Han | Female | 50 | Left frontal lobe | 2 | IV | 3 | 9 |
| 1746C | Han | Female | 56 | Right Parietal lobe | 1 | IV | – | 6 |
| 1414 | Han | Female | 39 | Left frontal lobe | 4 | IV | – | 8 |
Figure 1.
Survival curves of Uyghur and Han patients with glioblastoma (1. Uyghur, 2. Han).
Quantitative and qualitative analysis of total RNA
Extracted RNA from the tissue specimens was measured with the NanoDrop 2000. The A260/A280 ratio was >1.90 (Table 2). The RNA extracted from the fresh high-grade glioma tissues were analyzed by 1% agarose gel electrophoresis, which revealed 2 clear bands – 18s rRNA and 28s rRNA – and the ratio of their intensities was approximately 1:2 (Figure 2). Based on these results, we deemed the total RNA from the extracted tissues had satisfactory integrity, showed no degradation, and were highly pure. Therefore, we used these RNA for subsequent microarray measurements of mRNA expression profiles.
Table 2.
Measurement of total RNA from 6 fresh glioblastoma tissue samples.
| No. | Specimen No. | ng/ul | Extraction method | 260/280 | 260/230 | Total (ug) | Electrophoresis analysis |
|---|---|---|---|---|---|---|---|
| 1 | 3258 | 1611.0 | Trizol | 2.02 | 2.00 | 39.78 | Qualified |
| 2 | 6114C | 739.8 | Trizol | 1.93 | 2.45 | 19.37 | Qualified |
| 3 | 6501 | 2703.2 | Trizol | 2.01 | 2.12 | 67.08 | Qualified |
| 4 | 2968C | 597.8 | Trizol | 1.92 | 2.12 | 15.54 | Qualified |
| 5 | 1746C | 279.3 | Trizol | 2.03 | 2.25 | 6.94 | Qualified |
| 6 | 1414 | 1588.7 | Trizol | 2.02 | 1.89 | 39.22 | Qualified |
Figure 2.
Agarose gel electrophoresis of total RNA from 6 glioblastoma tissue samples. M: DNA marker; 1–6: specimens.
Comparative analysis on equilibrium of specimen grouping
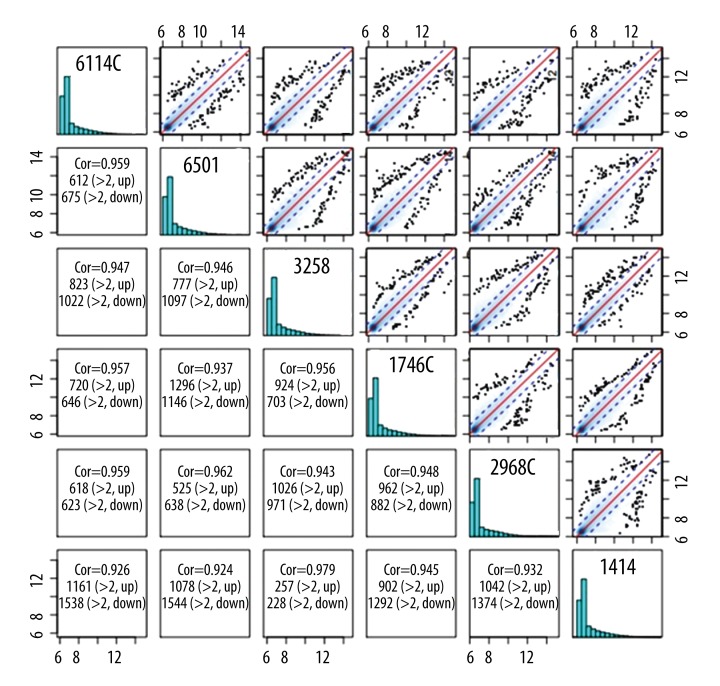
The 6 patients with glioblastoma were divided into 2 groups according to their ethnic background. Analysis of the equilibrium between the 2 groups showed that the data had good symmetry, and the median values were close to each other. Thus, we concluded that the specimens were comparable, the expression modes of the 2 groups were distinct, and the data had high consistency within each group. The original data were normalized, and the results showed high correlation coefficients for 2 arbitrary specimens (>99%), indicating similar overall conditions of the specimens (Figure 3).
Figure 3.
Scatter diagram of the comparative analysis on grouping equilibrium of the 6 specimens.
Microarray hybridization and bioinformatics analysis
The scanned images of microarray hybridization were imported into the Illumina GenomeStudio software for data normalization. The normalized data were then screened for differential genes with the lumi package of the R language software. Comparative analysis of the mRNA expression profiles in glioblastoma from Uyghur and Han patients revealed 1475 genes that are significantly differentially expressed, of which 669 (44.84%) showed increased expression, 807 (55.16%) showed decreased expression, and 1 gene (STRC) corresponding to 2 transcripts showed increased expression of 1 transcript and decreased expression of the other transcript. All differentially expressed genes were all genes that could be found in public-domain databases. Gene Ontology (GO) and Kytoencyclopedia of genes and genomics (KEGG) analyses were used for the screened differentially expressed genes using WebGestalt (P<0.05).
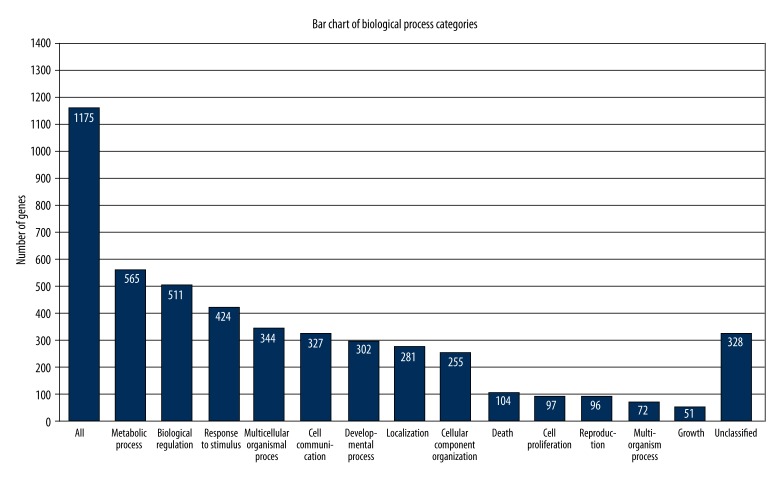
Among the 1475 differentially expressed genes, 1175 are included in the GO database and fall within 3 branches: biological process, cellular component, and molecular function. By collecting the numbers of differentially expressed genes within each GO item, we were able to perform enrichment analysis based on statistical tests to calculate the significance of the enrichment of differentially expressed genes for each GO item. Within the branch of biological processes, differentially expressed genes were mainly enriched in metabolic processes (48.09%, 565/1175), biological regulation (43.49%, 511/1175), stress response (36.01%, 424/1175), multi-cellular organic processes (29.28%, 344/1175), cell communication (27.83%, 327/1175), and cell proliferation (8.26%, 97/1175). These results are shown below in Figure 4 and Table 3. Within the cellular component branch, differentially expressed genes were mainly enriched in constitution of cell membrane, cell nucleus, and extracellular matrix. Within the molecular function branch, the genes were enriched in functions involving a nucleotide-binding domain, ATP or protein binding site, and catalytic activity.
Figure 4.
Distribution of differentially expressed genes in biological processes.
Table 3.
Biological processes involving differentially expressed genes.
| GO-ID | Biological process | Number | Enrichment ratio | rawP | adjP |
|---|---|---|---|---|---|
| GO: 0007264 | Small GTPase signal transduction | 61 | 1.68 | 4.75e-05 | 0.0266 |
| GO: 0007265 | Ras signal protein transduction | 42 | 1.90 | 4.71e-05 | 0.0266 |
| GO: 0001508 | Action potential regulation | 22 | 2.70 | 1.86e-05 | 0.0134 |
| GO: 0019228 | Neural action potential regulation | 21 | 3.57 | 2.63e-07 | 0.0013 |
| GO: 0007272 | Neuron inlayer | 18 | 3.62 | 1.55e-06 | 0.0026 |
| GO: 0008366 | Axonal inlayer | 18 | 3.62 | 1.55e-06 | 0.0026 |
| GO: 0042552 | Myelination | 17 | 3.54 | 4.05e-06 | 0.0051 |
| GO: 0032291 | Central nervous system axon formation | 7 | 8.34 | 6.43e-06 | 0.0054 |
| GO: 0022010 | Central nervous system myelination | 7 | 8.34 | 6.43e-06 | 0.0054 |
The KEGG pathway analysis revealed that the differentially expressed genes also participate in several signal pathways relating to tumor development. Among these, the differentially expressed genes were particularly enriched in 28 pathways, including metabolic pathways, cancer pathways, MAPK signaling pathway, TGF-β signaling pathway, neurotrophic factor signal transduction pathway, and mTOR signaling pathway (Table 4).
Table 4.
Distribution of the differentially expressed genes as analyzed by KEGG.
| Pathway | Number of genes | Enrichment ratio | rawP | adjP |
|---|---|---|---|---|
| Metabolic pathway | 69 | 2.24 | 5.84e-10 | 1.14e-07 |
| Cancer pathway | 25 | 2.81 | 4.16e-06 | 0.0002 |
| MAPK signaling pathway | 20 | 2.74 | 5.39e-05 | 0.0018 |
| TGF-β signaling pathway | 10 | 4.37 | 9.65e-05 | 0.0024 |
| Neurotrophic factor signal transduction pathway | 11 | 3.18 | 0.0007 | 0.0125 |
| Wnt signaling pathway | 11 | 2.69 | 0.0028 | 0.0284 |
| mTOR signaling pathway | 6 | 4.24 | 0.0028 | 0.0284 |
Cluster analysis
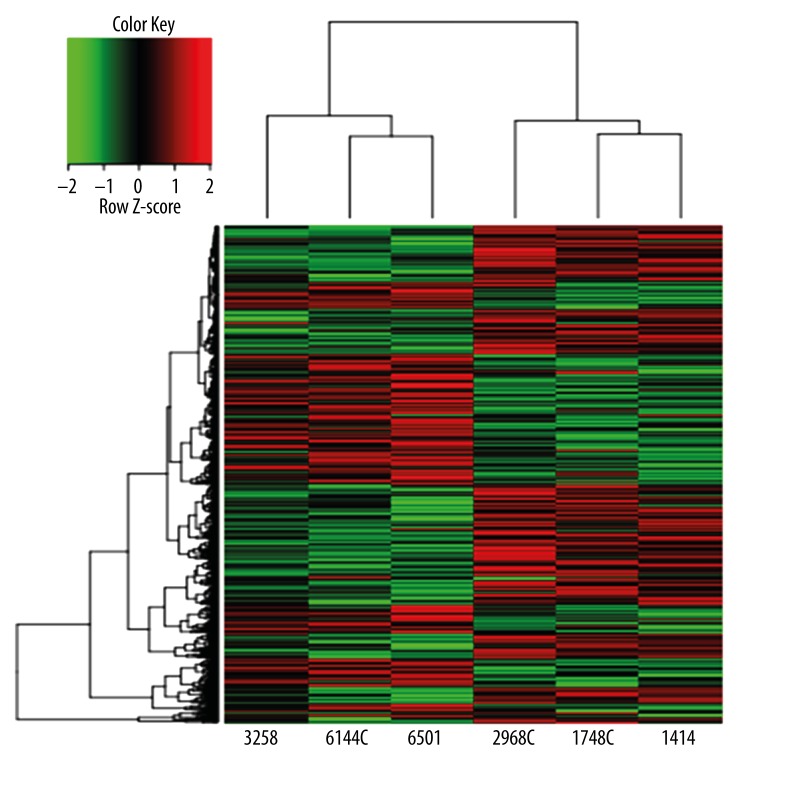
We applied 2-way hierarchical cluster analysis to the 1475 differentially expressed genes from Uyghur and Han patients, with red representing increased expression and green indicating reduced expression. The color scale shows the variation of the level of gene expression from relative low (green) to high (red). The 6 glioblastomas were divided into 2 groups according to the ethnic background of the patient – Uyghur 3258, 6144C, 6401, and Han 2968C, 1746C, 1414. Figure 5 shows that the specimens from each group cluster with each other. This indicates high consistency within each group, and statistically significant differences between the 2 groups. Both the red and green areas appear in distinct clusters, suggesting that the expression of the differentially expressed genes are distinct in the 2 groups, as shown in Figure 5. Data cluster analysis of the 1475 differentially expressed genes demonstrate that individuals with glioblastoma have similar characteristics, while those in different categories show large variability.
Figure 5.
Results of cluster analysis for the 1475 differentially expressed genes of 6 glioblastoma specimens.
Discussion
Pathogenic characteristics of glioblastoma in Uyghur and Han patients
Preoperative diagnosis of GBM is mainly based on clinical manifestations and imaging examination [7–9]. Imaging diagnosis of glioma primarily includes an enhanced MRI scan, with CT scan as an auxiliary technique. Enhanced MRI scans are able to distinguish gliomas from other benign pathological changes and can also be used to grade the glioma and observe the tumor in real-time during surgery to determine the invasive range of the glioma [10]. Therefore, MRIs are useful in selecting the area of tumor stereotaxic biopsies, and in removing the glioma and evaluating the patient’s prognosis. MRI scans of high-grade gliomas usually show a focus with mixed intensities, showing isointense or low signal intensity on T1WI, and nonuniform high signal intensity on T2W1. The tumor is always spread along white matter fiber bundles.
Enhanced MRI shows nodes or irregular ring-shaped enhancements. Tumor vessels are obvious. Gliomas mostly show no enhancement or slight plaque enhancement. Post-operative grading of glioblastoma by the WHO depends on pathological histology. As the most invasive glioma, glioblastoma (WHO grade IV) has the following characteristics by microscopy: abundant cells in tumor tissues, large tumor cells, obviously abnormal cell morphology and karyotype, more nuclear fissure, vascular endothelial cell hyperplasia, substantial immature vessels, and large areas of hemorrhage and necrosis [11]. Previous studies have shown that glioma of various grades have distinct molecular changes, drug uptake ability, and associated prognosis [12,13].
Primary glioblastoma usually affects adults and occurs preferentially in the hemicerebrum. Most glioblastomas appear and develop rapidly, without low-potential malignancy precancerous changes [14]. Recent studies of brain gliomas from Uyghur and Han patients in Xinjiang province focused on the expression of proteins such as TP53, MGMT, and VEGF [15,16] and few studies have looked at changes at the genetic level. The diversity in pathogenesis and clinical characteristics of glioblastoma in Uyghur and Han patients suggested the existence of certain differences at the genetic level. In this study, based on retrospective analysis and follow-up study of the clinical characteristics of glioblastoma from Uyghur and Han patients, it was revealed that male patients account for the majority of Uyghur patients, and their average age of onset was younger than that of the Han patients. We considered 85 cases of glioblastoma: Uyghur patients made up 29.4% (25/85) of the cases, while Han patients accounted for 70.6% (60/85) of the cases. Since the establishment of our hospital, patient intake for glioma consisted of 56% Han and 33% Uyghur, without obvious differences between the 2 groups. However, in terms of glioblastoma, we saw twice as many Han patients as Uyghur patients. Nevertheless, a follow-up study of the survival rate of the patients showed that Uyghur patients had significantly longer survival rates, which suggested to us that there are certain “protection factors” at the molecular level in Uyghur patients present during the incidence and development of glioblastoma.
Diversity and significance of gene expression profiles of glioblastoma in Uyghur and Han patients
Investigating pathogenesis at the genetic level is a natural trend in the field of glioma research. As with all malignant tumors, the initiation and development of glioblastoma are the consequences of a series of molecular changes. Specifically, these are changes induced by the abnormal expression of many tumor-related genes or by the deactivation of many tumor-suppressing genes. As a high-throughput gene screening technique and microarrays now allow researchers to screen the differential expressions of a variety of genes under the same experimental conditions, the techniques have enabled rapid and accurate investigations of genomic information. This has effectively provided massive amounts of information that can help determine predisposing factors behind the mechanisms of tumor formation and development and may allow for early-stage diagnosis and treatment, and evaluation of patient prognosis [17]. Since Schena et al. published the first paper on the gene expression profiling microarray in 1995, the gene-microarray method has been extensively applied to study various human malignant tumors, such as oophoroma, mammary cancer, prostatic carcinoma, cervical carcinoma [18], and glioblastoma. However, to date, no studies have been performed to screen the differential expression profiles of glioblastoma among Uyghur and Han patients.
In this study, a microarray was performed to measure mRNA expression profiles using 47323 probes corresponding to 31 330 genes was employed to detect the differences in gene expression between 6 glioblastomas (3 Uyghur and 3 Han). The results suggested that the glioblastomas from Uyghur patients and those from Han patients shared 29 855 (95.3%) genes with similar expression levels and included genes belonging to proto-oncogenes, anti-oncogenes, mismatch repair genes, and protein synthesis genes. We also found 1475 (4.7%) differentially expressed genes. Software analysis indicated that among the 1475 differentially expressed genes (P<0.05), 669 (44.84%) showed up-regulated expression, while 807 (55.16%) showed down-regulated expression. The gene STRC had 2 transcripts, 1 of which showed increased expression and the other showed decreased expression. The differentially expressed genes participate in several biological processes, including metabolic processes, biological regulation, stress response, and multi-cellular organismal processes. The latter includes several functional nodes, including the small GTPase signaling pathway, Ras signaling pathway, neuronal reactive protein regulation, and myelination of the central nervous system. The differentially expressed genes are involved in many signal pathways related to tumorigenesis as well, including metabolic pathways, cancer pathways, MAPK signaling pathway, TGF-β signaling pathway, neurotrophic factor signal transduction pathway, and mTOR signaling pathway. Studies on a single candidate gene only allow the evaluation of the expression a single gene, while deconvolution of an entire pathway could allow researchers to assess the interactions between groups of genes. The KEGG pathway analysis that we performed indicated that 28 pathways were significantly enriched in our differentially expressed genes, among which changes in gene expression within metabolic and cancer pathways were likely key factors in the initiation and development of glioblastoma.
In terms of differences in gene expression and pathway functions, the glioblastomas from Uyghur and Han patients not only shared a large percentage of common genetic profiles, they each also had a particular set of differentially expressed genes, indicating that both genetic changes and intra-group diversity exist during the initiation and development of glioblastoma. Investigating the genes that share similar expression in both Uyghur and Han people may help us better understand the molecular mechanisms and signal transduction pathways behind the initiation and development of glioblastoma, while studying the differentially expressed genes in each ethnic group will allow us to elucidate ethnic differences in the pathogenesis of glioblastoma.
Clinical significance of differentially expressed genes in glioblastomas from Uyghur and Han patients
In our study of the gene expression profiles of high-grade glioma from Uyghur and Han patients, we selected the differentially expressed genes and further analyzed their function and cellular location. The differences in the mechanisms of glioblastoma initiation and development mechanism were investigated from the perspective of polygenic interactions. As of this study, the screened differentially expressed genes included common molecular markers (e.g., MGMT, TP53, and IDH) found in The Glioma Molecular Diagnostic Guidelines. Previous research [19–21] has suggested that mutations in IDH and MGMT gene promoter methylation are the most representative biomarkers of glioblastoma.
The differentially expressed genes also consist of many proteins of great interest, such as BMP2, ADH5, ERCC1, DAPK2, GAB2, HLA-DP/DR, IL family proteins, MMP16, MAPKAPK5, NOTCH2, PIK family proteins, PCNA, RASSF1, RB1, S100A1, TF, VEGFA, and TP73L.
Human leukocyte antigen (HLA) family proteins form the primary histocompatibility complex in humans and include genes coding for HLA types I, II, and III. The HLA type II genetic region is located near the chromosomal kinetochore and mainly consists of 3 sub-regions: HLA-DR, DQ, and DP. HLA is a key molecule in tumor antigen recognition and presentation, which plays an essential role in organismal immune response and immunoregulation. The expressions of these genes are regulated at the transcriptional level, and genetic polymorphism may be a factor in the formation of malignant tumors [22–28]. Immunotherapy has great potential to intervene in glioblastoma disease progression. Complementation analysis of HLA-A 02 negative tumors not only aids in the discovery of novel shared glioblastoma antigens, but most importantly, it also provides opportunities for individualized immunotherapy by tumor-associated peptide cocktails.
Because of their function in repairing DNA damage, DNA repair genes play an important role in chemotherapy-resistant glioblastoma. As an important gene in the DNA repair system, ERCC1 is responsible for damage recognition and excision. A number of studies [30–32] have indicated that in multiple malignant tumors such as non-small cell lung cancer, oophoroma, and esophageal cancer, the expression level of ERCC1 is related to chemotherapy resistance to platinum drugs. Studies in glioblastoma cell cultures have also demonstrated that over-expression of ERCC1 is associated with chemotherapy resistance to alkyl drugs. Nitrosourea and platinum drugs have been extensively used in brain glioma patients, but the efficacy of clinical chemotherapy remains low due to the resistance of glioblastoma to anti-cancer drugs. ERCC1 is of great significance in understanding the molecular pathways related to glioma drug resistance and in improving the effectiveness of chemotherapy.
DNA methylation may result in the inactivation of tumor suppressors and the activation of oncogenes [33]. RASSF1 is classified as a tumor suppressor, and abnormal methylation at its promoter region was frequently observed in colon cancer, gastric cancer, and esophageal cancer. However, studies on the RASSF1 gene in adult glioblastoma showed that RASSF1 methylation appeared to be an independent specific event, with negligible influence on glioblastoma [42].
After the idea that tumor development depends on blood vessel growth and proliferation was proposed, anti-angiogenesis therapies were believed to be a potential alternative anti-glioblastoma therapy. VEGF is a key factor in tumor angiogenesis. However, clinical trials of anti-angiogenesis therapy on glioblastoma showed that Avastin, an anti-angiogenic therapy, does produce the anticipated result. A gene is rarely ever involved in just 1 pathway with a single function. The 1475 differentially expressed genes that we found in glioblastoma participate in various biologic processes, molecular functions, and metabolic pathways. Moreover, many crucial molecules were found as part of this screen. Researchers must further investigate and validate these differentially expressed genes to discover the specific functions they play in pathways and the molecular changes that are associated with glioblastoma pathogenesis, as well as the differences in gene expression between gliomas from Uyghur and Han patients.
Conclusions
The incidence of glioblastoma has been increasing in recent years and a greater percentage of younger people are affected by this disease. Glioblastoma is a serious disease and presents with surgical challenges, postoperative recurrence, poor effectiveness of radiotherapy and chemotherapy, and extremely low cure rate. Through screening of differentially expressed genes in glioblastoma tissues from Uyghur and Han patients based on gene expression profiles by microarray, the mechanism of disease development and the differences in the molecular pathogenic mechanism among Uyghur and Han patients can be investigated from the perspective of multi-gene interactions. This approach may ultimately provide researchers with a new molecular target for glioblastoma therapy. Therefore, our research presents a meaningful way to improve the survival rate and life quality of glioblastoma patients.
The differentially expressed genes screened in this study are involved in several biological processes. Our data lay a solid foundation for the investigation of specific molecular markers. With intensive follow-up studies of these genes, it is expected that more target genes will be found to be associated with the onset of glioblastoma, thereby providing markers for early detection, diagnosis, therapy, and prognosis of glioblastoma.
Footnotes
Conflict of interest
None declared.
Source of support: This work was supported by grant No. XJC201271 from the Innovation Foundation of Xinjiang Medical University
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Nagarajan RP, Costello JF. Molecular epigenetics and genetics in neuro-oncology. Neurotherapeutics. 2009;6(3):436–46. doi: 10.1016/j.nurt.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macy ME, Birks DK, Barton VN, et al. Clinical and molecular characteristics of congenital glioblastoma. Neuro Oncol. 2012;14(7):931–41. doi: 10.1093/neuonc/nos125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24(13):1547–48. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 6.Smyth GK, Yang YH, Speed T. Statistical issues in cDNA microarray data analysis. Methods Mol Bio. 2003;224:111–36. doi: 10.1385/1-59259-364-X:111. [DOI] [PubMed] [Google Scholar]
- 7.Onishi M, Kurozumi K, Ichikawa T, et al. Gene expression profiling of the anti-glioma effect of Cilengitide. Springerplus. 2013;2(1):160. doi: 10.1186/2193-1801-2-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liau LM, Lallone RL, Seitz RS, et al. Identification of a human glioma-associated growth factor gene, granulin, using differential immuno-absorption. Cancer Res. 2000;60(5):1353–60. [PubMed] [Google Scholar]
- 9.van den Boom J, Wolter M, Kuick R, et al. Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. Am J Pathol. 2003;163(3):1033–43. doi: 10.1016/S0002-9440(10)63463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toussaint LR, Nilson AE, Goble JM, et al. Galectin-1, a gene preferentially expressed at the tumor margin, promotes glioblastoma cell invasion. Mol Cancer. 2012;11:32. doi: 10.1186/1476-4598-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haque T, Faury D, Albrecht S, et al. Gene expression profiling from formalin-fixed paraffin-embedded tumors of pediatric glioblastoma. Clin Cancer Res. 2007;13(21):6284–92. doi: 10.1158/1078-0432.CCR-07-0525. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Oberg AL, Asmann YW, et al. Genome-wide transcriptional profiling reveals microRNA-correlated genes and biological processes in human lymphoblastoid cell lines. PLoS One. 2009;4:e5878. doi: 10.1371/journal.pone.0005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunez-Iglesias J, Liu CC, Morgan TE, et al. Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer’s disease cortex reveals altered miRNA regulation. PLoS One. 2010;5:e8898. doi: 10.1371/journal.pone.0008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Dougherty ER, Shmulevich I, et al. Identification of combination gene sets for glioma classification[J] Mol Cancer Ther. 2002;1(13):1229–36. [PubMed] [Google Scholar]
- 15.Zhengquan ZHU, Zhe SUN, Liang LIU, et al. The clinical research of the expression of VEGF in glioma tissue of Uygur people in Xinjiang. Chinese Journal of Experimental and Clinical Virology. 2012;26(3):208–10. [Google Scholar]
- 16.Haicheng XIA, Zhengquan ZHU, Liang LIU. The clinical research of the MGMT expression levels in glioma pations. Chinese Journal of Experimental and Clinical Virology. 2011;25(4):265–67. [PubMed] [Google Scholar]
- 17.Wang H, Zhao Y, Zhang C, et al. Rab27a was identified as a prognostic biomaker by mRNA profiling, correlated with malignant progression and subtype preference in gliomas. PLoS One. 2014;9:e89782. doi: 10.1371/journal.pone.0089782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian M, Fang J, Han D, et al. Microarray gene expression analysis of tumorigenesis and regional lymph node metastasis in laryngeal squamous cell carcinoma. PLoS One. 2013;8:e84854. doi: 10.1371/journal.pone.0084854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell JE, Henders AK, McRae AF, et al. The Brisbane Systems Genetics Study: genetical genomics meets complex trait genetics. PLoS One. 2012;7:e35430. doi: 10.1371/journal.pone.0035430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song W, Ruder AM, Hu L, et al. Genetic epidemiology of glioblastoma multiforme: confirmatory and new findings from analyses of human leukocyte antigen alleles and motifs. PLoS One. 2009;4:e7157. doi: 10.1371/journal.pone.0007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piperi C, Themistocleous MS, Papavassiliou GA, et al. High incidence of MGMT and RARbeta promoter methylation in primary glioblastomas: association with histopathological characteristics, inflammatory mediators and clinical outcome. Mol Med. 2010;16(1–2):1–9. doi: 10.2119/molmed.2009.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Arbab AS. Neovascularization in Glioblastoma: Current Pitfall in Anti-angiogenic therapy. Zhong Liu Za Zhi. 2013;1(3):16–19. [PMC free article] [PubMed] [Google Scholar]
- 23.McKean-Cowdin R, Barnholtz-Sloan J, Inskip PD, et al. Associations between polymorphisms in DNA repair genes and glioblastoma. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1118–26. doi: 10.1158/1055-9965.EPI-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:e3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 25.Kowalczyk A, Pomorska-Mol M, Kwit K, et al. Cytokine and chemokine mRNA expression profiles in BALF cells isolated from pigs single infected or co-infected with swine influenza virus and Bordetella bronchiseptica. Vet Microbiol. 2014;170(3–4):206–12. doi: 10.1016/j.vetmic.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Kamei M, Miyajima A, Fujisawa M, et al. Effects of postnatal dexamethasone treatment on mRNA expression profiles of genes related to alveolar development in an emphysema model in mice. J Toxicol Sci. 2014;39(4):665–70. doi: 10.2131/jts.39.665. [DOI] [PubMed] [Google Scholar]
- 27.Burns TA, Dours-Zimmermann MT, Zimmermann DR, et al. Imbalanced expression of Vcan mRNA splice form proteins alters heart morphology and cellular protein profiles. PLoS One. 2014;9(2):e89133. doi: 10.1371/journal.pone.0089133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bielecki P, Muthukumarasamy U, Eckweiler D, et al. In vivo mRNA profiling of uropathogenic Escherichia coli from diverse phylogroups reveals common and group-specific gene expression profiles[J] MBio. 2014;5(4):e1014–e1075. doi: 10.1128/mBio.01075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neidert MC, Schoor O, Trautwein C, et al. Natural HLA class I ligands from glioblastoma: extending the options for immunotherapy. J Neurooncol. 2013;111(3):285–94. doi: 10.1007/s11060-012-1028-8. [DOI] [PubMed] [Google Scholar]
- 30.Umasuthan N, Wan Q, Revathy KS, et al. Molecular aspects, genomic arrangement and immune responsive mRNA expression profiles of two CXC chemokine receptor homologs (CXCR1 and CXCR2) from rock bream, Oplegnathus fasciatus. Fish Shellfish Immunol. 2014;40(1):304–18. doi: 10.1016/j.fsi.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Jain P, Bhalla US. Transcription control pathways decode patterned synaptic inputs into diverse mRNA expression profiles. PLoS One. 2014;9:e95154. doi: 10.1371/journal.pone.0095154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabouret E, Chinot O, Sanson M, et al. Predictive biomarkers investigated in glioblastoma. Expert Rev Mol Diagn. 2014;14(7):883–93. doi: 10.1586/14737159.2014.945436. [DOI] [PubMed] [Google Scholar]
- 33.Helmig S, Dopp E, Wenzel S, et al. Induction of altered mRNA expression profiles caused by fibrous and granular dust. Mol Med Rep. 2014;9(1):217–28. doi: 10.3892/mmr.2013.1765. [DOI] [PubMed] [Google Scholar]
- 34.Dong YS, Hou WG, Li XL, et al. Genetic association of CHEK2, GSTP1, and ERCC1 with glioblastoma in the Han Chinese population. Tumour Biol. 2014;35(5):4937–41. doi: 10.1007/s13277-014-1648-z. [DOI] [PubMed] [Google Scholar]