Abstract
Background
Although (EEN) is a relatively safer route by which to feed patients with severe acute pancreatitis (SAP) or predicted SAP (pSAP) compared to total parental nutrition (TPN), the appropriate starting time for EEN administration after admission is still controversial. This study pooled all relevant studies to assess the complications associated with EEN by stratifying relevant RCTs into subgroups according to the starting time (<24 h or between 24 and 72 h after admission).
Material/Methods
Relevant studies were searched for among 5 databases. The association between intervention and complications, including pancreatic infection, mortality, hyperglycemia, organ failure, and catheter-related septic complications, were assessed by using pooled risk ratio (RR) and the corresponding 95% confidential interval (CI).
Results
Twelve RCTs were identified through our literature search. Pooled analysis showed that EEN, but not TPN or delayed enteral nutrition (DEN), is associated with reduced risk of pancreatic infection, mortality, organ failure, hyperglycemia, and catheter-related septic complications. EEN within 24 h of admission presented significantly better outcome in morality than EEN between 24 and 72 h. However, no significant heterogeneity was observed in the risk of pancreatic infection, organ failure, hyperglycemia, and catheter-related septic complications between the 2 subgroups.
Conclusions
If the patients are reasonably expected to have high compliance to EN therapy, it could be considered as early as possible.
MeSH Keywords: Enteral Nutrition; Meta-Analysis as Topic; Pancreatitis, Acute Necrotizing
Background
The pancreas is an important organ in both the digestive system and the endocrine system. Pancreas injury or disease directly leads to disorders of homeostasis in these 2 systems [1,2]. Acute pancreatitis (AP) is characterized as hypercatabolism with a negative nitrogen balance [3]. Severe acute pancreatitis (SAP) has a high mortality rate due to sepsis, necrosis, and multiple organ failure caused by pancreatic or peripancreatic infection [4]. Approximately 80% of deaths caused by SAP are related to sepsis, necrosis, and multiple organ failure [5].
In the first several hours after onset of AP, the local inflammatory response of injured pancreatic glands consists of increased release of chemokines, cytokines, neutrophils, and other inflammatory mediators [6]. Due to the local inflammatory response, the gut barrier is also damaged, with significantly increased intestinal permeability within 72 h [7]. Through the damaged gut barrier, the bacterial flora in the intestine gain access to systemic circulation, leading to exacerbated systemic inflammatory response and possible infected pancreatic necrosis and sepsis at the early stage of the disease [8]. Thus, maintaining the normal structure and function of the gut barrier is critical to reducing the possibility of organ failure and infected necrosis in AP [9].
Nutrition therapy is an essential component of AP management. During the past decade, early enteral nutrition (EEN) was introduced for SAP patients due to its benefits in modifying the lactulose/mannitol ratio, reducing inflammatory response, maintaining the gut barrier, and lowering bacterial translocation [9]. Previous meta-analyses confirmed that EEN is a relatively safer route to feed patients with SAP or predicted SAP (pSAP) compared to total parental nutrition (TPN) [10,11]. However, the appropriate time at which to start EEN administration it is still controversial. Some scholars thought 72 h was best because the onset of the disease should be the cut-off time [12], while others thought EN provided within 48 h of admission had more definite advantages [13]. One recent meta-analysis even indicated that EEN given within 24 h was associated with more benefits than between 24 h and 48 h [14]. However, that meta-analysis missed several highly randomized controlled studies and only extracted 1 arm’s data of the included studies to make their assessment [14]. For better understanding of the appropriate start time of EEN, the present study tried to pool all relevant studies to assess the complications of EEN by stratifying relevant RCTs into <24 h and 24–72 h groups.
Material and Methods
Search strategy
Relevant studies were searched for in PubMed, EMBASE, MEDLINE, the Cochrane Library, and ClinicalTrials.com from Jan 1990 to May 2014 by 2 authors independently (XL and FM). The following terms and strategy were applied for searching in the databases: (“enteral nutrition” OR “nasojejunal” OR “nasogastric”) AND (“acute pancreatitis) AND (“randomized controlled trial” OR “RCT” OR “clinical trial” OR “trial”) in titles and abstracts. No language restriction was set during searching. To avoid missing qualified trials, manual searching with backward snowballing method was used to check the reference lists of included studies, relevant meta-analyses, and reviews.
Selecting criteria
Studies included in this meta-analysis had to fulfill the following criteria: (1) randomized comparative trials (RCTs); (2) consecutive patients with acute pancreatitis; (3) patients were randomized assigned to experimental EEN group initiated within 72 h of admission or control group with TPN or DEN (beyond 72 h). Studies without detailed information for required clinical outcomes were excluded.
Data Extraction and quality assessment
The following information was extracted from the included RCTs: first author, year of publication, start time and route of EN administration, intervention of the control group, and number of participants in experimental and control groups. To compare the clinical outcomes in the 2 groups, cases of pancreatic infection, mortality, hyperglycemia, organ failure, and catheter-related septic complications were extracted. Stratified analysis was performed by the starting time of EN after admission (within 24 h or 24–72 h). Quality of the included RCTs was assessed according to methodological criteria of the Cochrane Handbook for Systematic Reviews of Interventions. Publication bias was assessed by visually inspecting of funnel plots of RR of organ failure, which was reported in most of the studies included.
Data synthesis and analysis
RevMan 5.2 (Cochrane Collaboration) was used for data synthesis and analysis. Data of discontinuous outcomes extracted from original RCTs were pooled to estimate risk ratios (RR) and corresponding 95% confidence intervals (CIs). Subgroup analysis was performed by stratifying the studies into <24 h and 24–72 h subgroups. Between-studies heterogeneity was measured with chi-square-based Q test and I2. P<0.1 or I2>50% indicate significant heterogeneity. To identify the level of heterogeneity, a primary analysis was performed with a fixed-effects model. If no significant heterogeneity observed, a fixed-effects model was used to make estimates, otherwise a random-effects model was applied. For the pooled estimates, p value <0.05 of the Z test was considered as a significant difference.
Results
Characteristics of trials included
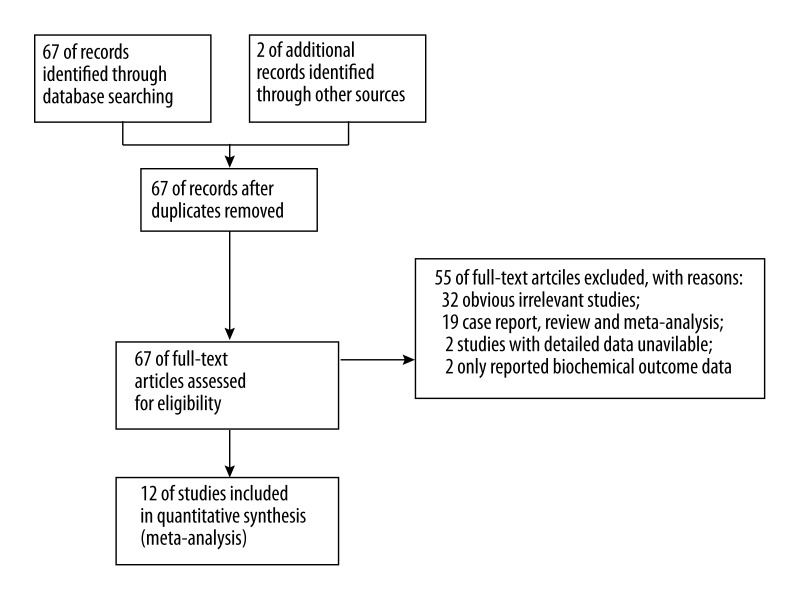
Through searching databases, 12 studies were included in the final meta-analysis [12,15–25]. The searching and screening of eligible studies is summarized in a PRISMA flow diagram in Figure 1. The key characteristics of included studies are summarized in Table 1. Among the 12 studies included, 4 provided EEN to patients within 24 h after admission [15–18], while 8 studies provided EEN to patients at 24–72 h after admission [12,19–25]. Except for Eckerwall’s study [16], which used EN delivered through the nasogastric-feeding route, all other studies used the nasojejunal feeding route. Only 2 studies had DEN in the control group [12,24], while the remaining studies all used TPN in the control group. A total of 625 participants were included in the 12 studies, with 301 patients in the EEN group and 324 in the control group.
Figure 1.
The searching and screening process.
Table 1.
The key characteristics of RCTs included.
| Study | Severity of AP | EN route | EN start time after admission | Control group | No. patients | |
|---|---|---|---|---|---|---|
| EEN | Control | |||||
| Petrov 2006 | pSAP | NJ | <24 h | TPN | 35 | 34 |
| Eckerwall 2006 | pSAP | NG | <24 h | TPN | 24 | 26 |
| Gupta 2003 | pSAP | NJ | <24 h | TPN | 8 | 9 |
| Louie 2005 | SAP | NJ | <24 h | TPN | 10 | 18 |
| Olah 2002 | AP | NJ | <48 h | TPN | 41 | 48 |
| McClave 1997 | MAP | NJ | <48 h | TPN | 16 | 16 |
| Kalfarentzos 1997 | SAP | NJ | <48 h | TPN | 18 | 20 |
| Casas 2007 | SAP | NJ | <48 h | TPN | 11 | 11 |
| Qin 2008 | SAP | NJ | <48 h | TPN | 36 | 38 |
| Sun 2013 | SAP | NJ | <48 h | DEN | 30 | 30 |
| Abou-Assi 2002 | AP | NJ | <72 h | TPN | 26 | 27 |
| Zou 2014 | AP | NJ | <72 h | DEN | 46 | 47 |
AP – acute pancreatitis; EEN – early enteral nutrition; DEN – delayed enteral nutrition; TPN – total parenteral nutrition; NJ – nasojejunal feeding; NG – nasogastric feeding; AP – acute pancreatitis; MAP – mild AP; SAP – severe AP; pSAP – predicted SAP.
The methodological quality of the RCTs was generally high, but only 2 studies had blind design for intervention. The studies by Louie et al. [18] and Qin et al. [25] were both single-blind; this design deficiency is largely due to the nature of the intervention. Allocation concealment was adequate in 6 studies (50%). Only 1 study [15] had 1 participant withdraw after randomized allocation. Therefore, nearly all patients randomized in the RCTs completed the study and thus provided complete data of composite endpoints. Detailed quality information of the RCTs is given in Table 2.
Table 2.
Quality assessment of trails included.
| Study | Adequate sequence generation | Adequate allocation concealment | Blinding | Incomplete outcome data adequately addressed | Free of selective reporting | Free of other bias |
|---|---|---|---|---|---|---|
| Petrov 2006 | + | ? | − | + | + | ? |
| Eckerwall 2006 | + | + | − | + | + | + |
| Gupta 2003 | + | + | − | + | + | + |
| Louie 2005 | + | + | + | + | + | − |
| Olah 2002 | ? | ? | − | + | + | + |
| McClave 1997 | ? | ? | − | + | + | + |
| Kalfarentzos 1997 | + | + | − | + | + | + |
| Casas 2007 | + | + | − | + | + | ? |
| Abou-Assi 2002 | ? | ? | − | + | + | + |
| Qin 2008 | + | + | + | + | + | − |
| Sun 2013 | ? | ? | − | + | + | + |
| Zou 2014 | ? | ? | − | + | + | ? |
+ is “yes”, − is “no”, ? is “unclear”.
Effect of EEN on pancreatic infection
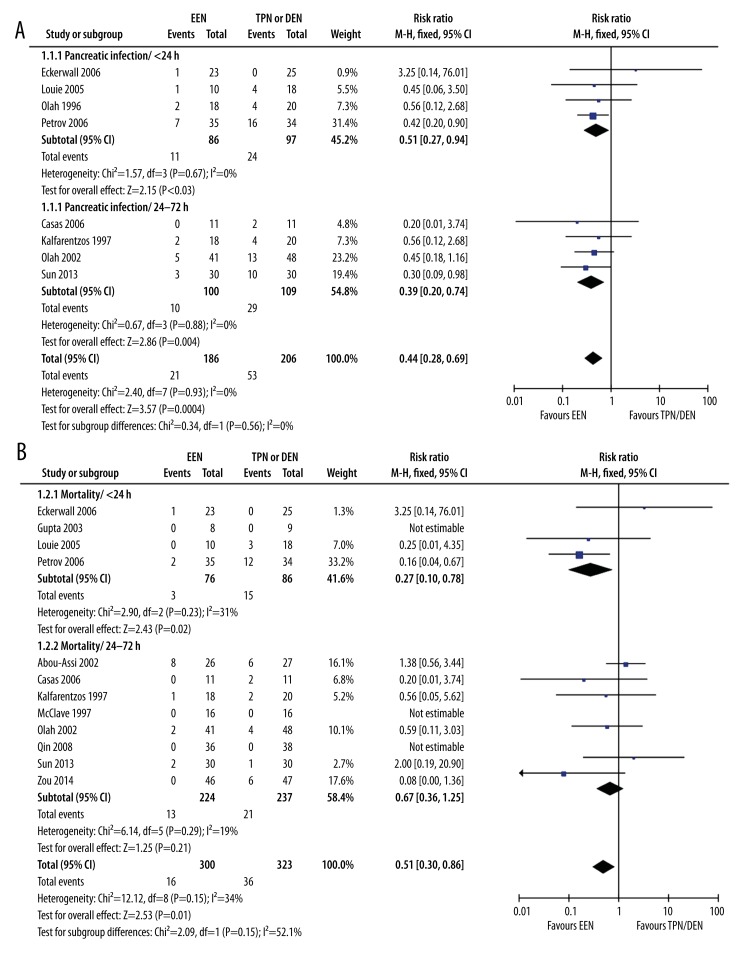
Eight studies reported the outcome of pancreatic infection – 4 in the <24 h subgroup and 4 in the 24–72 h subgroup. The pooled analysis showed that EEN was generally associated with lower risk of pancreatic infection than TPN or DEN (21/186 vs. 53/206) (RR: 0.44, 95% CI: 0.28–0.69, p=0.0004, I2=0%). Subgroup analysis confirmed a similar trend in both <24 h (11/86 vs. 24/97) (RR: 0.51, 95% CI 0.27–0.94, p=0.03, I2=0%) and 24–72 h subgroup (10/100 vs. 29/109) (RR: 0.39, 95% CI: 0.20–0.74, p=0.004, I2=0%). Although the EEN in the 24–72 h subgroup was associated with lower RR than in the <24 h group, the difference was not significant (p=0.56, I2=0%) (Figure 2A).
Figure 2.
EEN vs. TPN or DEN in pancreatic infection and mortality (A). EEN vs. TPN or DEN in pancreatic infection (B). EEN vs. TPN or DEN in mortality.
Effect of EEN on mortality
All of the studies included reported the outcome of mortality. The pooled analysis showed that EEN was generally associated with lower mortality rate than TPN or DEN (16/300 vs. 36/323) (RR: 0.51, 95% CI: 0.30–0.86, p=0.01, I2=34%) (Figure 2B). Subgroup analysis observed significantly decreased risk of mortality in the <24 h subgroup (3/76 vs. 15/86) (RR: 0.27, 95% CI: 0.10–0.78, p=0.02, I2=31%), but not in the 24–72 h subgroup (13/224 vs. 21/237) (RR: 0.67, 95% CI: 0.36–1.25, p=0.29, I2=19%). Significant subgroup heterogeneity was observed (p=0.15, I2=52.1%) (Figure 2B).
Effect of EEN on organ failure
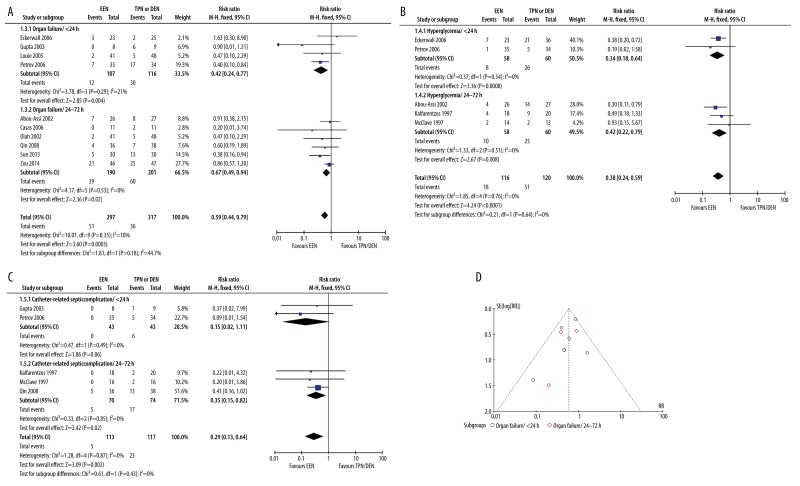
Ten studies reported the outcome of organ failure, 4 in the <24 h subgroup and 6 in the 24–72 h subgroup. The pooled analysis showed that EEN was generally associated with lower risk of organ failure than TPN or DEN (51/297 vs. 90/317) (RR: 0.59, 95% CI: 0.44–0.79, p=0.0003, I2=10%) (Figure 3A). Subgroup analysis observed significantly decreased risk of organ failure in both the <24 h subgroup (12/107 vs. 30/116) (RR: 0.42, 95% CI: 0.24–0.77, p=0.004, I2=21%) and the 24–72 h subgroup (39/190 vs. 60/201) (RR: 0.67, 95% CI: 0.49–0.94, p=0.02, I2=0%). No significant heterogeneity was observed between the results of these 2 subgroups (p=0.18, I2=44.7%) (Figure 3A).
Figure 3.
EEN vs. TPN or DEN in other complications and publication bias (A). EEN vs. TPN or DEN in organ failure (B). EEN vs. TPN or DEN in hyperglycemia (C). EEN vs. TPN or DEN in catheter-related septiccomplications (D). Assessment of publication bias by using organ failure data.
Effect of EEN on hyperglycemia
Five studies reported the outcome of hyperglycemia, 2 in the <24 h subgroup and 3 in the 24–72 h subgroup. The pooled analysis showed that EEN was generally associated with lower risk of hyperglycemia than TPN or DEN (18/116 vs. 51/120) (RR: 0.38, 95% CI: 0.24–0.59, p<0.0001, I2=0%) (Figure 3B). Subgroup analysis observed significantly decreased risk of hyperglycemia in both the <24 h subgroup (8/58 vs. 26/60) (RR: 0.34, 95% CI: 0.18–0.64, p=0.0008, I2=0%) and the 24–72 h subgroup (10/58 vs. 25/60) (RR: 0.42, 95% CI: 0.22–0.79, p=0.008, I2=0%). No significant heterogeneity was observed between the results of these 2 subgroups (p=0.64, I2=0%) (Figure 3B).
Effect of EEN on catheter-related septic complications
Five studies reported the outcome of catheter-related septic complications, 2 in the <24 h subgroup and 3 in the 24–72 h subgroup. The pooled analysis showed that EEN was generally associated with lower risk of catheter-related septic complications than TPN or DEN (5/113 vs. 23/117) (RR: 0.29, 95% CI: 0.13–0.64, p=0.002, I2=0%) (Figure 3C). Subgroup analysis revealed significantly decreased risk of hyperglycemia in both the <24 h subgroup (0/43 vs. 6/43) (RR: 0.15, 95% CI: 0.02–1.11, p=0.06, I2=0%) and the 24–72 h subgroup (5/70 vs. 17/74) (RR: 0.35, 95% CI: 0.15–0.82, p=0.02, I2=0%). No significant heterogeneity was observed between the results of these 2 subgroups (p=0.43, I2=0%) (Figure 3C).
Publication bias
Because most of the included studies reported outcome of organ failure, funnel plots of OR of organ failure were used to assess publication bias. The plots demonstrate asymmetric distribution at the bottom of the funnel, suggesting potential of publication bias (Figure 3D). However, since the number of studies involved in this meta-analysis was relatively small, it is difficult to estimate the publication bias accurately.
Discussion
This meta-analysis based on 12 RCTs showed that EEN is generally associated with reduced risk of pancreatic infection, mortality, organ failure, hyperglycemia, and catheter-related septic complications compared to TPN or DEN. EEN within 24 h of admission presented significantly better outcome in reducing morality than EEN administered at 24–72 h. However, no significant subgroup difference was observed in the risk of pancreatic infection, organ failure, hyperglycemia, or catheter-related septic complications between the 2 subgroups. Patients included in the RCTs had high levels of compliance to the EEN since only 1 patient withdrew from the study. Therefore, the risk of withdraw bias is low in this study. Findings of this meta-analysis are consistent with previous retrospective studies. One large retrospective study based on 197 patients with pSAP showed that EEN started within 48 h after admission contributed to reduced infected pancreatic necrosis, respiratory failure, intensive care unit admission, and mortality [26].
Critically ill patients have high risk of malnutrition [27]. Poor nutrition management at the early stage increases the risk of infections. Therefore, for patients with SAP or pSAP, nutrition support should be initiated as soon as possible. Generally, the cut-off time points for EN after onset of symptoms is 24 h according to the ESPEN (European Society for Parenteral and Enteral Nutrition) [28] and 48 h according to the ASPEN (American Society for Parenteral and Enteral Nutrition) guidelines [29].
Infection complications are the leading causes of poor outcomes of AP. Previous studies proposed that EEN could help reduce infection complications through 2 aspects [30]: firstly, to maintain the integrity of the intestinal mucosa barrier and thus to lower the possibility of bacteria translocation from small intestine to systemic circulation at the early phase of the disease [31]; and secondly, to reduce TPN-associated high rates of catheter-related infection [23]. In this meta-analysis, pooled evidence showed that EN could simultaneously reduce the risk of pancreatic infection and catheter-related infection better than TPN or DEN.
Higher intestinal permeability is not only associated with increased bacteria translocation, but is also associated with increased serum endotoxin level and cytokine level [32]. Therefore, systemic inflammatory response syndrome (SIRS) might involve remote organs or even cause remote organ failure. In fact, the integral gut mucosa acts as a barrier against bacteria translocation and spreading of toxins and inflammation factors. Since increased permeability of the gut barrier was observed during the first 24 h of SAP, the importance of maintaining the gut barrier integrity was already emphasized in previous studies [33]. EEN initiated within 24 h may reasonably be supposed to more strongly protect the gut barrier. However, this study also failed to demonstrate significantly greater benefits of EEN within 24 h in reducing the risk of pancreatic infection, hyperglycemia, and catheter-related septic complications than at 24–72 h. Although the <24 h subgroup had slightly lower RR of organ failure than the 24–72 h subgroup, the difference was not significant. Interestingly, the mortality rate of the <24 h subgroup was significantly lower than that in the 24–72 h subgroup, suggesting that EEN within 24 h might have some unidentified benefits.
This study has several limitations. Firstly, there is still no consensus about the definition of “early” EN. Therefore, in this study, RCTs were arbitrarily divided into <24 h and 24–72 h subgroup according to previous clinical guidelines. Secondly, some of the RCTs included did not clearly report the interval between onset of disease symptoms and patient admission, which might be a factor confounding accurate classification of EEN. However, overall and subgroup analysis all had small heterogeneity, suggesting the influence of this limitation was not large. Thirdly, the intervention of the control group was not consistent in all RCTs. Two RCTs had DEN in the control group, while TPN was applied in controls in other studies. Fourthly, only 5 studies reported the outcome of hyperglycemia and catheter-related septic complications. Due to the small sample size of these 2 outcomes, the statistical power of pooled analysis is relatively weak. In addition, since various studies might be based on a small sample of patients and were not reported, the possible reporting bias should not be ignored.
Conclusions
This study showed that EEN is associated with reduced risk of pancreatic infection, mortality, organ failure, hyperglycemia, and catheter-related septic complications compared to TPN or DEN. EEN within 24 h of admission presented significantly better effect in reducing morality than EEN at 24–72 h. However, no significant difference was observed in the risk of pancreatic infection, organ failure, hyperglycemia, and catheter-related septic complications between the 2 subgroups. If a patient may reasonably expected to have good compliance with EN therapy, it should be considered as early as possible.
Footnotes
Source of support: Departmental sources
References
- 1.Rui X, Xu B, Su J, et al. Differential pattern for regulating insulin secretion, insulin resistance, and lipid metabolism by osteocalcin in male and female T2DM patients. Med Sci Monit. 2014;20:711–19. doi: 10.12659/MSM.890130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lees C, Howie S, Sartor RB, Satsangi J. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology. 2005;129(5):1696–710. doi: 10.1053/j.gastro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Halonen KI, Pettila V, Leppaniemi AK, et al. Multiple organ dysfunction associated with severe acute pancreatitis. Crit Care Med. 2002;30(6):1274–79. doi: 10.1097/00003246-200206000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Jha RK, Ma Q, Sha H, Palikhe M. Acute pancreatitis: a literature review. Med Sci Monit. 2009;15(7):RA147–56. [PubMed] [Google Scholar]
- 5.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354(20):2142–50. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 6.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127–51. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83(3):461–66. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 8.Rahman SH, Ammori BJ, Holmfield J, et al. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg. 2003;7(1):26–35. doi: 10.1016/S1091-255X(02)00090-2. discussion 35–26. [DOI] [PubMed] [Google Scholar]
- 9.Rebours V. [Acute pancreatitis: An overview of the management]. Rev Med Interne. 2014;35(10):649–55. doi: 10.1016/j.revmed.2014.04.009. [in French] [DOI] [PubMed] [Google Scholar]
- 10.Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;(1):CD002837. doi: 10.1002/14651858.CD002837.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Xu Y, Lu T, et al. Meta-analysis of enteral nutrition versus total parenteral nutrition in patients with severe acute pancreatitis. Ann Nutr Metab. 2008;53(3–4):268–75. doi: 10.1159/000189382. [DOI] [PubMed] [Google Scholar]
- 12.Zou L, Ke L, Li W, et al. Enteral nutrition within 72 h after onset of acute pancreatitis vs. delayed initiation. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.164. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Petrov MS, Pylypchuk RD, Uchugina AF. A systematic review on the timing of artificial nutrition in acute pancreatitis. Br J Nutr. 2009;101(6):787–93. doi: 10.1017/S0007114508123443. [DOI] [PubMed] [Google Scholar]
- 14.Bakker OJ, van Brunschot S, Farre A, et al. Timing of enteral nutrition in acute pancreatitis: Meta-analysis of individuals using a single-arm of randomised trials. Pancreatology. 2014;14(5):340–46. doi: 10.1016/j.pan.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23(5–6):336–44. doi: 10.1159/000097949. discussion 344–45. [DOI] [PubMed] [Google Scholar]
- 16.Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: A clinical, randomized study. Ann Surg. 2006;244(6):959–65. doi: 10.1097/01.sla.0000246866.01930.58. discussion 965–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R, Patel K, Calder PC, et al. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II > or =6) Pancreatology. 2003;3(5):406–13. doi: 10.1159/000073657. [DOI] [PubMed] [Google Scholar]
- 18.Louie BE, Noseworthy T, Hailey D, et al. 2004 MacLean-Mueller prize enteral or parenteral nutrition for severe pancreatitis: a randomized controlled trial and health technology assessment. Can J Surg. 2005;48(4):298–306. [PMC free article] [PubMed] [Google Scholar]
- 19.Olah A, Pardavi G, Belagyi T, et al. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition. 2002;18(3):259–62. doi: 10.1016/s0899-9007(01)00755-9. [DOI] [PubMed] [Google Scholar]
- 20.McClave SA, Greene LM, Snider HL, et al. Comparison of the safety of early enteral vs. parenteral nutrition in mild acute pancreatitis. JPEN J Parenter Enteral Nutr. 1997;21(1):14–20. doi: 10.1177/014860719702100114. [DOI] [PubMed] [Google Scholar]
- 21.Kalfarentzos F, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84(12):1665–69. [PubMed] [Google Scholar]
- 22.Casas M, Mora J, Fort E, et al. [Total enteral nutrition vs. total parenteral nutrition in patients with severe acute pancreatitis]. Rev Esp Enferm Dig. 2007;99(5):264–69. doi: 10.4321/s1130-01082007000500004. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 23.Abou-Assi S, Craig K, O’Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: results of a randomized comparative study. Am J Gastroenterol. 2002;97(9):2255–62. doi: 10.1111/j.1572-0241.2002.05979.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun JK, Mu XW, Li WQ, et al. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol. 2013;19(6):917–22. doi: 10.3748/wjg.v19.i6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin HL, Zheng JJ, Tong DN, et al. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr. 2008;62(7):923–30. doi: 10.1038/sj.ejcn.1602792. [DOI] [PubMed] [Google Scholar]
- 26.Wereszczynska-Siemiatkowska U, Swidnicka-Siergiejko A, Siemiatkowski A, Dabrowski A. Early enteral nutrition is superior to delayed enteral nutrition for the prevention of infected necrosis and mortality in acute pancreatitis. Pancreas. 2013;42(4):640–46. doi: 10.1097/MPA.0b013e318271bb61. [DOI] [PubMed] [Google Scholar]
- 27.Kiss CM, Byham-Gray L, Denmark R, et al. The impact of implementation of a nutrition support algorithm on nutrition care outcomes in an intensive care unit. Nutr Clin Pract. 2012;27(6):793–801. doi: 10.1177/0884533612457178. [DOI] [PubMed] [Google Scholar]
- 28.Kreymann KG, Berger MM, Deutz NE, et al. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr. 2006;25(2):210–23. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Druyan ME, Compher C, Boullata JI, et al. Clinical Guidelines For the Use of Parenteral and Enteral Nutrition in Adult and Pediatric Patients: applying the GRADE system to development of A.S.P.E.N. clinical guidelines. J Parenter Enteral Nutr. 2012;36(1):77–80. doi: 10.1177/0148607111420157. [DOI] [PubMed] [Google Scholar]
- 30.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10(6):350–56. doi: 10.1016/j.surge.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pupelis G, Selga G, Austrums E, Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. 2001;17(2):91–94. doi: 10.1016/s0899-9007(00)00508-6. [DOI] [PubMed] [Google Scholar]
- 32.Koh YY, Jeon WK, Cho YK, et al. The effect of intestinal permeability and endotoxemia on the prognosis of acute pancreatitis. Gut Liver. 2012;6(4):505–11. doi: 10.5009/gnl.2012.6.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capurso G, Zerboni G, Signoretti M, et al. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46(Suppl):S46–51. doi: 10.1097/MCG.0b013e3182652096. [DOI] [PubMed] [Google Scholar]