 A polymer microarray screen identified poly(methylmethacrylate-co-dimethylacrylamide) as a bacteria repelling surface, which substantially reduced binding of bacteria on coated catheters.
A polymer microarray screen identified poly(methylmethacrylate-co-dimethylacrylamide) as a bacteria repelling surface, which substantially reduced binding of bacteria on coated catheters.
Abstract
Nosocomial infections due to bacteria have serious implications on the health and recovery of patients in a variety of medical scenarios. Since bacterial contamination on medical devices contributes to the majority of nosocomical infections, there is a need for redesigning the surfaces of medical devices, such as catheters and tracheal tubes, to resist the binding of bacteria. In this work, polyurethanes and polyacrylates/acrylamides, which resist binding by the major bacterial pathogens underpinning implant-associated infections, were identified using high-throughput polymer microarrays. Subsequently, two ‘hit’ polymers, PA13 (poly(methylmethacrylate-co-dimethylacrylamide)) and PA515 (poly(methoxyethylmethacrylate-co-diethylaminoethylacrylate-co-methylmethacrylate)), were used to coat catheters and substantially shown to decrease binding of a variety of bacteria (including isolates from infected endotracheal tubes and heart valves from intensive care unit patients). Catheters coated with polymer PA13 showed up to 96% reduction in bacteria binding in comparison to uncoated catheters.
Introduction
Nosocomial or hospital related infections, i.e., infection acquired by a patient who was initially admitted for a reason other than infection, are widespread in patients in intensive care units and those requiring long-term catheter use. It has been estimated that in the US 1.7 million patients acquire a nosocomial infection each year, resulting in over 90 000 deaths.1 In the UK, one in ten patients acquire a nosocomial infection costing the economy >£1 billion per year.2 These infections pose major issues for patient care, which are further complicated by the emergence of global antibiotic resistant organisms.
Bacteria account for the majority of nosocomial infections3 and adhesion of bacteria to a surface, such as a catheter, is an essential step in the process.4 Moreover, bacteria on a surface provide a source for reseeding infection, once established in a biofilm.5 Initial bacterial adhesion is affected by the relative charge and hydrophobicity of the surface, and by chemical interactions between the bacteria and the surface and/or secreted components and/or components of biological fluids on the surface.6–8 Attachment is followed by production of extracellular polysaccharides and proteins by the bacteria, as the biofilm establishes.7 The biofilm provides a physical barrier against both the host immune system and antimicrobial therapy, and bacteria within biofilms can have >1000-fold higher resistance to some antibiotics as compared to planktonic bacteria.9 As biofilms are involved in the majority of microbial infections, including chronic infections, prevention of biofilm formation is important in a clinical setting.10
Since medical devices often contribute to nosocomial infections,11 there is an interest to render the surface of these devices resistant to bacterial adhesion and biofilm formation.12 As such, the surface of a medical device can be crafted to contain agents, including antibiotics or bactericides (e.g. silver, iodine or surfactants).13–17 These modified surfaces prevent colonisation by killing bacteria on contact or by the release of antimicrobials in the area surrounding the medical device. When suboptimal, this approach is not ideal since it can contribute to the emergence of resistant strains.14 In addition, some of the agents used on the crafted surfaces are expensive and may not be suitable for use with all patients as they may elicit allergic reactions.7 A more attractive alternative to prevent bacterial adhesion would be to coat the medical device with a polymer, which inherently resists adhesion of bacteria.18
The traditional approaches for the discovery of polymer-based biomaterials with new properties are time-consuming and iterative, involving sequential study of individual polymer for the desired properties, followed by their structural optimisation. As an alternative approach, well-plate based screening methods have been used to identify bacteria-repelling surfaces.19,20 For example, a well-plate based assay allowed the comparison of adhesion of Staphylococcus aureus (S. aureus) on synthetic and biologically derived surgical materials used in plastic surgery.21 However, well-plate based methods are typically limited to relatively small number of surfaces to be assessed in parallel. To enable a high-throughput approach to the discovery of new biomaterials, Bradley22 and Langer23 have developed polymer microarray platforms. These have resulted in the identification of biomaterials by Bradley for various applications such as for the long-term culture of mouse embryonic stem cells,24 culture of human embryonic stem cells and mesenchymal stem cells with thermal harvesting,25,26 and the activation of platelets.27 In addition, polymer microarrays have been used to identify materials able to capture or prevent the binding of Escherichia coli, the major food-borne pathogen Salmonella enterica serovar Typhimurium,28 Pseudomonas aeruginosa, Escherichia coli and S. aureus,29 and the protozoan parasites Giardia lamblia 30 and Cryptosporidium parvum.31
The aim of this study was to utilise polymer microarrays to identify polymers with anti-adhesive characteristics against a range of clinically important Gram-negative and Gram-positive bacteria. Thus, a library of 381 polyacrylates/acrylamides and polyurethanes was screened for their ability to reduce/prevent attachment of a range of major bacterial pathogens including Campylobacter jejuni (C. jejuni), Clostridium difficile (C. difficile), Clostridium perfringens (C. perfringens), Streptococcus mutans (S. mutans), Klebsiella pneumoniae (K. pneumoniae), Staphylococcus saprophyticus (S. saprophyticus), Enterococcus faecalis (E. faecalis) and S. aureus either individually or in microbial ‘cocktails’. Key bacteria repellent ‘hit’ polymers, identified using the microarray screens, were scaled-up and their ability to resist bacterial attachment was confirmed on larger surfaces. Two polymers were chosen for detailed study and used to coat two types of commercially available intravenous catheters. Confocal microscopy and scanning electron microscopy (SEM) analysis were used to study and quantify bacteria binding on the coated and uncoated catheters.
Materials and methods
All chemicals were of analytical grade and used without further purification. Sodium cacodylate trihydrate, formaldehyde, monomers for polymer synthesis, and silane-prep glass slides were purchased from Sigma-Aldrich. Glutaraldehyde (2.5% w/v) and osmium tetroxide (1% w/v) were from Electron Microscopy Sciences. Well plates were purchased from Nunc, glass coverslips from VWR, and GeneFrames from Thermo Scientific. Polyurethane large bore multi-lumen central intravenous catheterisation sets (Cath-1) were purchased from Arrow International (CS12123E), and silicone double lumen catheter sets with stylet (Cath-2) from Baxter Healthcare Corporation (ECS1320).
Polymer synthesis and characterisation
For coating of the catheters, PA13 (M w 411 kDa, PDI 3.4), PA515 (M w 90 kDa, PDI 2.7) and PA155 (M w 9.5 kDa, PDI 1.2) were synthesised by free-radical polymerisation and characterised by gel permeation chromatography (GPC) and infrared spectroscopy (IR) (see ESI†). GPC was conducted on an Agilent GPC instrument, fitted with a PLgel 5 μm MIXED-C column (300 × 7.5 mm), with NMP as eluent (flow rate 1 mL min–1), pre-calibrated using polystyrene standards. IR analysis was conducted using a Brucker Tensor 27 spectrometer.
Coating of the catheters
Depending on the concentration/viscosity of the polymer solutions, two methods of coating were employed, with polymer loading confirmed by increase in weight of the catheter pieces after coating and drying (ESI†). For the binding studies by confocal microscopy, 2.5% polymer solutions in acetone (w/v) were used. The catheter pieces were placed into a small glass vial (5 mL) and 1 mL of the polymer solution was added for 2 min. The excess polymer solution was removed and the catheter pieces were dried overnight in vacuo at 40 °C. For the SEM studies, catheter coating was achieved by immersing the pieces into 10% PA13 solution in acetone (w/v) for ∼30 seconds. The coated pieces were air dried for 30 min and then re-coated by immersing into the polymer solution. The pieces were dried overnight at room temperature.
Preparation of bacteria and seeding on catheters
K. pneumoniae, S. saprophyticus, S. aureus, E. faecalis, and S. mutans were cultured on agar-coated Petri dishes with brain–heart–infusion (BHI) medium in a microaerobic environment (85% N2, 10% CO2, 5% O2) at 37 °C. For S. mutans strain NCTC 10923 was used. All other strains were clinical isolates obtained from infected medical devices and identified by PCR genotyping. Frozen stock of the bacteria was prepared and used for all experiments, to enable seeding known count of bacteria and ensuring consistency between experiments. For preparing the frozen stocks, overnight cultures of each strain were set up: bacteria were inoculated into 50 mL of BHI and incubated in a Falcon tube in a microaerobic environment at 37 °C for 12 h, except for S. mutans, which was incubated for 36 h. Cultures were frozen after addition of 10% glycerol, and the number of colony forming units (CFU) per mL in the frozen stock determined through serial dilution.
For the catheter experiments, BacMix-1 and BacMix-2 were prepared from frozen stocks. BacMix-1 was prepared with K. pneumoniae, S. saprophyticus, and S. aureus, approximately 5 × 105 CFU of each bacteria in 50 mL of BHI medium. BacMix-2 was prepared with K. pneumoniae, S. mutans, S. aureus, and E. faecalis, with all the strains other than S. mutans maintained at 5 × 105 CFU per 50 mL of BHI medium. Due to its lower proliferation rate, S. mutans was seeded in approximately 2-fold higher density.
For studies via confocal microscopy, pieces (n = 3) of uncoated catheters Cath-1 and Cath-2, those coated with polymers PA13, PA515 or control PA155 were placed in agarose coated 24-well plates, sterilised by UV (20 min), and inoculated with 1 mL of either BacMix-1 or BacMix-2. After seeding, the plates were incubated for 72 h in a microaerobic environment at 37 °C, with agitation at 50 rpm. The media was supplemented with 0.5 mL of BHI at 24 h and 48 h. For quantification of bacterial binding using SEM, catheters were seeded with 2 mL of bacterial stocks and incubated in medium for 12 days with agitation at 50 rpm.
Quantification of bacteria binding
Confocal microscopy analysis
Catheter samples (n = 3) were washed with PBS (2 × 2 mL) after incubation with bacteria. The cells were fixed with 10% paraformaldehyde in PBS for 30 min, and washed with PBS (1 mL). The bacteria on catheter pieces were stained with DAPI (1 μg mL–1) for 20 min and washed again with PBS (1 mL). Confocal imaging was performed on a Leica SP5 confocal microscope using λ ex 405 nm and 414–502 nm emission filter, and images analysed using Image-pro plus 7.0, to obtain percentage of area of bacterial coverage (ESI†).
SEM analysis
SEM imaging was conducted using a Philips XL30 CP electron microscope. After incubation with bacteria, the pieces (n = 3) were washed with PBS (2 × 1 mL) before being transferred into 48-well plates containing 10% formaldehyde in PBS for 30 min. After fixing, the pieces were washed with PBS (1 mL). The catheters were then loaded on stubs, dried overnight at room temperature, and gold coated using a Bal-Tec SCD050 sputter coater prior to imaging.
Results and discussion
High-throughput identification of bacteria repelling polymers
Polymer microarrays with 381 polymer members (each printed in quadruplicate) were fabricated by contact printing of preformed polymers as described previously.22 Individual cultures (C. jejuni, C. difficile, C. perfringens, and S. mutans) and two clinically relevant bacteria mixtures, BacMix-1 (consisting of K. pneumoniae, S. saprophyticus and S. aureus) and BacMix-2 (consisting of K. pneumoniae, S. mutans, S. aureus, and E. faecalis), were incubated overnight on the arrays. The strains in BacMix-1 and BacMix-2 were obtained from endotracheal tubes from ICU patients or from patients exhibiting infectious endocarditis, often associated with cardiovascular implants. After washing and staining, bacterial adhesion on each of the polymer features was evaluated via nucleoid staining, allowing polymer ranking (ESI, Table S1†). Detailed analysis of the arrays showed differences in the bacterial repellence of specific polymers for different strains, with 11 polyacrylates/acrylamides and 11 polyurethanes showing strong repellence for C. jejuni, C. difficile, C. perfringens, and S. mutans, as well as the mixtures BacMix-1 and BacMix-2 (ESI, Fig. S1–S8†).
Analysis of the chemical composition of the ‘hit’ polyacrylates/acrylamides revealed some common structural characteristics, with 10 out of the 11 hits containing tertiary amino groups, with aliphatic amines (7 out of the 10) rendering the surface positively charged in biological media. Interestingly, one of the most repellent co-polymers PA13 (poly(methylmethacrylate-co-dimethylacrylamide)) did not contain amines but contained amide functionalities, neutral at physiological pH. The majority of the best non-binding polyurethanes lacked charged functionalities and were found to contain either poly(butyleneglycol)2000 or poly(ethyleneglycol)900/2000 (ESI, Table S2 and S3†), consistent with the fact that polyglycol groups are known to contribute to resistance to bacterial attachment.32,33 Whether the ability of the polymer surface to resist adhesion of bacteria can be predicted from chemical composition of the polymer is an interesting question. Adhesion of bacteria to polymeric surfaces is a result of complex interplay between characteristics of the bacteria, physicochemical properties of the polymer surface and nature of the medium in which the bacteria and the surface interact. In addition, roughness and morphology of the surface affect the adhesion.34 Although there is some vague similarity between the resistant polymers, correlating bacteria resistance to chemical composition of the polymers is problematic,35 further highlighting the advantages of the high-throughput discovery process, which is designed to give leads for further optimisation.
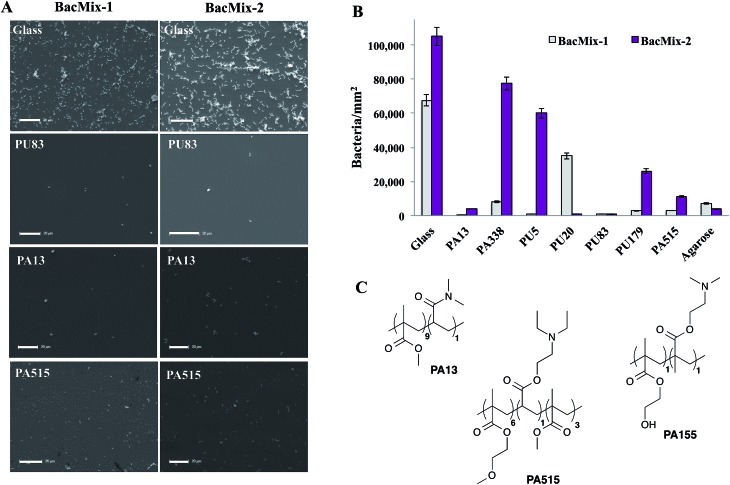
To validate the ‘hits’ and to further explore the bacteria-repellent activity of the polymers on a larger scale, the most promising polyacrylates/acrylamides PA13, PA515, and PA338 and polyurethanes PU5, PU20, PU83 and PU179 (see Table 1) were spin-coated onto glass coverslips and assessed for binding by BacMix-1 and BacMix-2. Scanning Electron Microscopy (SEM) was used to quantify the number of bacteria attached to each polymer surface (ESI, Fig. S9 and S10†). Polyacrylates PA13 and PA515 and polyurethane PU83 were found to exhibit the lowest bacteria binding (Fig. 1A and B).
Table 1. ‘Hit’ polymers used in scaled-up studies.
| Polymer | Description |
| PA13 | Co-polymer of methylmethacrylate and dimethylacrylamide (9 : 1 monomer ratio) a |
| PA515 | Co-polymer of methoxyethylmethacrylate, diethylaminoethylacrylate and methylmethacrylate (6 : 1 : 3 monomer ratio) |
| PA338 | N-Methylaniline functionalised co-polymer of methylmethacrylate and glycidylmethacrylate (1 : 1 monomer ratio) |
| PU5 | Polyurethane synthesised from poly(butyleneglycol)2000 and 1,6-diisocyanatohexane (1 : 1 monomer ratio) |
| PU20 | Polyurethane synthesised from poly(butyleneglycol)2000 and 4,4′-methylenebis(phenylisocyanate) (1 : 1 monomer ratio) |
| PU83 | Polyurethane synthesised from poly(ethyleneglycol)900 and 4,4′-methylenebis(cyclohexylisocyanate), with 1,4-butanediol as a chain extender (1 : 2 : 1 monomer ratio) |
| PU179 | Polyurethane synthesised from poly(butyleneglycol)2000 and 1,6-diisocyanatohexane, with 2-nitro-2-methyl-1,3-propanediol as a chain extender (1 : 2 : 1 monomer ratio) |
aRefers to the monomer ratio used in the synthesis of the polymer.
Fig. 1. (A) SEM images of glass and selected polymer surfaces after incubation with bacteria (scale bar = 20 μm). (B) The average number of bacteria per mm2 on the polymers, agarose and glass (n = 4) after incubation with bacteria. (C) Structures of the random co-polymers PA13, PA515 and PA155. The ratio of monomers used in the synthesis is given in the brackets.
In general, polyacrylates/acrylamides offer a flexible coating material since they can be used either as a solution of preformed polymers or synthesised in situ on surfaces using a range of polymerisation techniques. Therefore, PA13 and PA515 (methoxyethylmethacrylate-co-diethylaminoethylacrylate-co-methylmethacrylate) were chosen to coat two commercially available types of catheter to study whether the polymers render the surface resistant to bacterial adhesion. Another polyacrylate PA155 (poly(hydroxyethylmethacrylate-co-dimethylaminoethylmethacrylate)), identified in a previous study to offer good bacterial binding,28 was chosen as a control (Fig. 1C). Due to their clinical relevance, BacMix-1 and BacMix-2 were used for binding studies on the coated catheters.
Coating of catheters
A polyurethane-based multi-lumen central intravenous catheter (Cath-1) and a silicone-based double lumen catheter (Cath-2) were coated and evaluated. The catheters were cut into cylindrical pieces (and the dimensions of the catheter analysed, ESI†) and coated with PA13, PA515, and the control PA155. A range of solvents was evaluated both for their ability to dissolve the polymers and for compatibility with the catheters. Thus, pieces of catheters were immersed in solvents for 12 h and evaluated for any visual changes (e.g. cloudiness or swelling, ESI, Fig. S12†). Both catheter materials tolerated acetone, which due to its low boiling point and fast evaporation, is an ideal solvent to achieve uniform coating. Polymers PA13, PA515 and PA155 were fully soluble in acetone. Polymer loading on the surfaces was established by treating both Cath-1 and Cath-2 with 10% (w/v) PA13. Cath-1–PA13 showed an average weight increase of 36% and Cath-2–PA13 an average increase of 11%, suggesting that better coating was achieved on Cath-1.
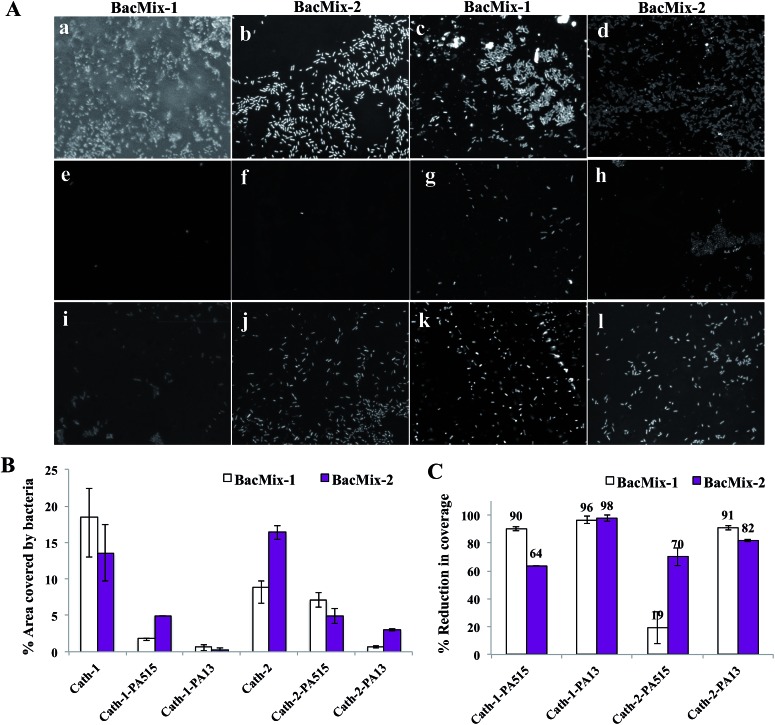
Uncoated pieces of Cath-1 and Cath-2, and those coated with polymers PA13, PA515 and PA155 in acetone, were inoculated with either BacMix-1 or BacMix-2. After incubation, the catheters were washed, and the bacteria fixed and stained with DAPI. Confocal images of the catheter pieces showed significantly improved resistance to bacterial binding on the PA13 and PA515 coated catheters, compared to the uncoated catheters (Fig. 2A), whereas coating with control PA155 resulted in high bacterial binding (data not shown).
Fig. 2. (A) Confocal fluorescence images (× 40 magnification, λ ex 405 nm, λ em 414–502 nm) of catheter surfaces showing bacteria binding/non-binding. The images were obtained after 72 h incubation with bacteria, followed by washing, fixing and staining with DAPI (1 μg mL–1). (a and b) uncoated Cath-1, (c and d) uncoated Cath-2, (e and f) Cath-1–PA13, (g and h) Cath-2–PA13, (i and j) Cath-1–PA515, (k and l) Cath-2–PA515. (B) The percentage of surface area covered by bacteria after 72 h incubation with BacMix-1 and BacMix-2, and (C) percentage of reduction in bacteria binding, obtained by comparing the area of bacteria coverage to the area of the image (n = 3, see ESI†).
Analysis of the confocal images showed that PA13 had better repellent characteristics than PA515 (Fig. 2B and C). Reduction in bacterial binding with the polymer coatings varied with the catheter type and bacterial mixture used. For Cath-1, coating with PA515 resulted in 64% reduction in bacterial coverage with BacMix-2, while coating with PA13 offered ≥96% reduction in coverage for both bacterial mixtures. Similarly for Cath-2, coating with PA515 resulted in a moderate 19% reduction in bacterial coverage with BacMix-1, while PA13 coating yielded ≥82% reduction with both BacMix-1 and BacMix-2, therefore making PA13 the coating polymer of choice.
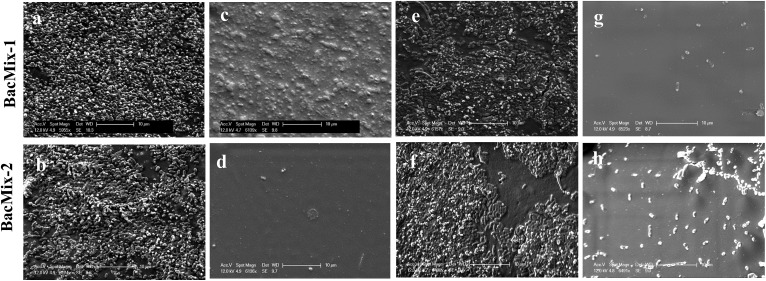
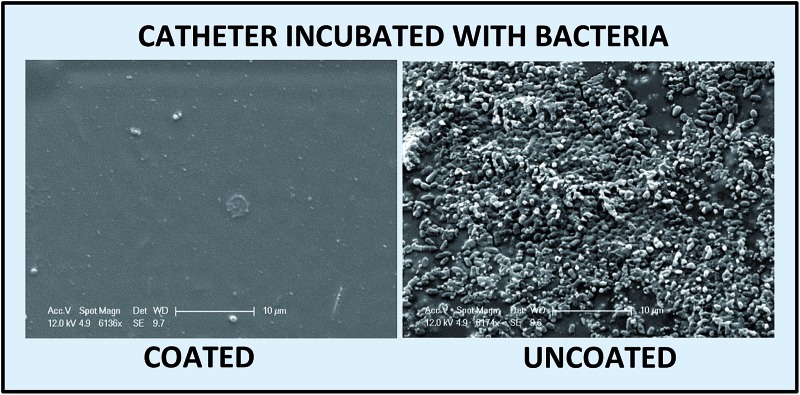
SEM analysis was performed to study the reduction of bacterial adhesion on Cath-1–PA13 and Cath-2–PA13. Coated (10% w/v PA13 in acetone) and uncoated catheter pieces were incubated with BacMix-1 and BacMix-2 for 12 days, to mimic the duration of use in a clinical environment. The catheter pieces were then washed, gold coated and imaged. SEM analysis showed that coating with PA13 significantly reduced bacteria binding on the surface of both catheter types (Fig. 3). Also, the surfaces of the coated catheters looked smooth and considerably different from the uncoated catheter surfaces, confirming uniform coating with PA13. The thickness of the polymer coating was measured by cutting the coated catheters and imaging by SEM (ESI, Fig. S13†). Coating with PA13 resulted in a 92 μm and 54 μm layer on Cath-1 and Cath-2, respectively.
Fig. 3. SEM images of uncoated and coated catheters after incubation with BacMix-1 and BacMix-2 (scale bar = 10 μm). (a and b) Cath-1, (c and d) Cath-1–PA13, (e and f) Cath-2, (g and h) Cath-2–PA13. See ESI† for additional images of Cath-1–PA13 with BacMix-1 (Fig. S14†).
Conclusions
A 381-member polymer library was screened on polymer microarrays with bacterial strains (C. jejuni, C. difficile, C. perfringens, and S. mutans) and two clinically relevant bacteria mixtures, BacMix-1 (consisting of K. pneumoniae, S. saprophyticus and S. aureus) and BacMix-2 (consisting of K. pneumoniae, S. mutans, S. aureus, and E. faecalis). This high-throughput screen identified polyacrylate/acrylamide and polyurethane-based substrates that prevented the adhesion of both Gram-negative and Gram-positive bacteria. Scale-up studies with the two clinically relevant bacterial mixtures revealed that polyurethane PU83 and polyacrylates/acrylamides PA13 and PA515 exhibited broad-spectrum bacteria repellence, suggesting potential for use as coatings for medical devices. PA13 and PA515 were used to uniformly coat both a polyurethane-based and a silicone-based intravenous catheteter. Coating with PA13 was found to significantly reduce bacterial binding when compared to uncoated catheters as analysed by confocal microscopy and SEM. This study shows that polymers such as PA13, identified through a high-throughput screening approach, have potential as antibiotic-free bacteria-repellent coatings for medical devices.
Acknowledgments
The authors thank EASTBIO (the East of Scotland BioScience Doctoral Training Partnership funded by the BBSRC) for funding (S. V.). Dr David Kelly of the Wellcome Trust Centre for Cell biology (Optical Instrumentation Laboratory), the SEM facility management at school of geosciences (University of Edinburgh), and Mark Leonard and Dr Robert Thomson (Heriot-Watt University) are thanked for their help with the imaging experiments.
Footnotes
References
- Klevens R. M., Edwards J. R., Richards C. L., Horan T. C., Gaynes R. P., Pollock D. A., Cardo D. M. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inweregbu K., Dave J., Pittard A. Continuing Education in Anaesthesia, Critical Care & Pain. 2005;5:14–17. [Google Scholar]
- Ducel G., Fabry J. and Nicolle L., Prevention of hospital-acquired infections – A practical guide, WHO, 2002. [Google Scholar]
- Costerton J. W., Stewart P. S., Greenberg E. P. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Wang R., Khan B. A., Cheung G. Y. C., Bach T. L., Jameson-Lee M., Kong K., Queck S. Y., Otto M. J. Clin. Invest. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole G. O., Kaplan H. B., Kolter R. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- An Y. H. and Friedman R. J., Handbook of bacterial adhesion: Principles, methods and applications, Humana Press, 2000, pp. 73–90 and 581–589. [Google Scholar]
- An Y. H., Friedman R. J. J. Biomed. Mater. Res., Part B. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Danese P. N. Chem. Biol. 2002;9:873–880. doi: 10.1016/s1074-5521(02)00192-8. [DOI] [PubMed] [Google Scholar]
- Mah T. F., O'Toole G. A. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Guggenbichler J. P., Assadian O., Boeswald M., Kramer A. GMS Krankenhaushyg. Interdiszip. 2011;6:1–19. doi: 10.3205/dgkh000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoccia D., Montanaro L., Arciola C. R. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- Noimark S., Dunnill C. W., Wilson M., Parkin I. P. Chem. Soc. Rev. 2009;38:3435–3448. doi: 10.1039/b908260c. [DOI] [PubMed] [Google Scholar]
- Hetrick E. M., Schoenfisch M. H. Chem. Soc. Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- Rupp M. E., Lisco S. J., Lipsett P. A., Perl T. M., Keating K., Civetta J. M., Mermel L. A., Lee D., Dellinger E. P., Donahoe M., Giles D., Pfaller M. A., Maki D. G., Sherertz R. Ann. Intern. Med. 2005;143:570–580. doi: 10.7326/0003-4819-143-8-200510180-00007. [DOI] [PubMed] [Google Scholar]
- Greenfeld J., Sampath L., Popilskis S., Brunnert S., Stylianos S., Modak S. Crit. Care Med. 1995;23:894–900. doi: 10.1097/00003246-199505000-00018. [DOI] [PubMed] [Google Scholar]
- Tcholakian R., Raad I. I. Antimicrob. Agents Chemother. 2001;45:1990–1993. doi: 10.1128/AAC.45.7.1990-1993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Yang C., Lim T. P., Hsu L. Y., Engler A. C., Hedrick J. L., Yang Y.-Y. Biomaterials. 2012;33:6593–6603. doi: 10.1016/j.biomaterials.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Peng F., Hoek E. M. V., Damoiseaux R. J. Biomol. Screening. 2010;15:748–754. doi: 10.1177/1087057110374992. [DOI] [PubMed] [Google Scholar]
- Stafslien S. J., Bahr J. A., Feser J. M., Weisz J. C., Chisholm B. J., Ready T. E., Boudjouk P. J. Comb. Chem. 2006;8:156–162. doi: 10.1021/cc050047m. [DOI] [PubMed] [Google Scholar]
- Nyame T. T., Lemon K. P., Kolter R., Liao E. C. Plast. Reconstr. Surg. 2011;128:1061–1068. doi: 10.1097/PRS.0b013e31822b65af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourniaire G., Collins J., Campbell S., Mizomoto H., Ogawa S., Thaburet J.-F., Bradley M. Chem. Commun. 2006:2118–2120. doi: 10.1039/b602009g. [DOI] [PubMed] [Google Scholar]
- Anderson D. G., Levenberg S., Langer R. Nat. Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- Zhang R., Liberski A., Sanchez-Martin R., Bradley M. Biomaterials. 2009;30:6193–6201. doi: 10.1016/j.biomaterials.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Zhang R., Mjoseng H. K., Hoeve M. A., Bauer N. G., Pells S., Besseling R., Velugotla S., Tourniaire G., Kishen R. E. B., Tsenkina Y., Armit C., Duffy C. R. E., Helfen M., Edenhofer F., de Sousa P. A., Bradley M. Nat. Commun. 2013;4(1335):1–10. doi: 10.1038/ncomms2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy C. R. E., Zhang R., How S.-E., Lilienkampf A., de Sousa P. A., Bradley M. Biomaterials. 2014;35:5998–6005. doi: 10.1016/j.biomaterials.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Hansen A., McMillan L., Morrison A., Petrik J., Bradley M. Biomaterials. 2011;32:7034–7041. doi: 10.1016/j.biomaterials.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Pernagallo S., Wu M., Gallagher M. P., Bradley M. J. Mater. Chem. 2011;21:96–101. [Google Scholar]
- Hook A. L., Chang C.-Y., Yang J., Luckett J., Cockayne A., Atkinson S., Mei Y., Bayston R., Irvine D. J., Langer R., Anderson D. G., Williams P., Davies M. C., Alexander M. R. Nat. Biotechnol. 2012;30:868–875. doi: 10.1038/nbt.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering H., Wu M., Bradley M., Bridle H. Environ. Sci. Technol. 2012;46:2179–2186. doi: 10.1021/es203637e. [DOI] [PubMed] [Google Scholar]
- Wu M., Bridle H., Bradley M. Water Res. 2012;46:1715–1722. doi: 10.1016/j.watres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Kingshott P., Wei J., Bagge-Ravn D., Gadegaard N., Gram L., Lyngby D. Langmuir. 2003;209:6912–6921. [Google Scholar]
- Park K. D., Kim Y. S., Han D. K., Kim Y. H., Lee E. H., Suh H., Choi K. S. Biomaterials. 1998;19:851–859. doi: 10.1016/s0142-9612(97)00245-7. [DOI] [PubMed] [Google Scholar]
- Decuzzi P., Ferrari M. Biomaterials. 2010;31:173–179. doi: 10.1016/j.biomaterials.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Tuson H. H., Weibel D. B. Soft Matter. 2013;9:4368–4380. doi: 10.1039/C3SM27705D. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.