Abstract
Objective
Increases in oxidative stress have been consistently reported in younger patients with bipolar disorder (BD) in postmortem brain and blood samples studies. Changes in oxidative stress are also associated with the natural aging process. Thus, the investigation of oxidative stress across the life span of patients with BD is crucial.
Methods
We compared the levels of oxidative damage to proteins and lipids in plasma from 110 euthymic older patients with BD I or II (mean ± SD age: 63.9±9.7) and 75 older healthy individuals (66.0 ± 9.6). To assess protein oxidation, we measured the plasma levels of protein carbonyl (PC) and 3-nitrotyrosine (3-NT) using ELISA technique. To assess lipid peroxidation, we measured plasma levels of lipid hydroperoxide (LPH) and 4-hydroxynonenal (4-HNE) using spectrophotometric assays.
Results
LPH levels were higher in patients than in the comparison healthy individuals, while there were no significant differences for PC, 3-NT and 4-HNE between the two groups.
Conclusions
The increased levels of an early component of the peroxidation chain, LPH, in euthymic older patients with BD support the hypothesis of a persistent effect of reactive species of oxygen in patients with BD into late life.
Keywords: bipolar disorder, aging, oxidative stress, lipid peroxidation, protein oxidation
Introduction
Oxidative stress is the process through which the production of reactive oxygen species (i.e., free radicals) overwhelms the antioxidant system leading to potential damage to proteins, lipids and DNA (1). Oxidative stress damage has been consistently reported in younger patients with bipolar disorder (BD) [for review, see (2, 3)] with evidence both from postmortem brain (4–9) and blood samples (10–17). These studies have demonstrated increased oxidative damage to proteins in postmortem prefrontal cortex from patients with BD (4, 5), while increased nitration–induced damage to protein tyrosine residues has been seen in both BD and schizophrenia (4,5). In addition to protein, lipids are also consistent targets of oxidation (i.e., lipid peroxidation) in patients with BD (18) with increased lipid peroxidation in the anterior cingulate cortex (19) and prefrontal cortex (5). A meta-analysis revealed that increased serum level of lipid peroxidation is the most consistent finding of oxidative stress associated with BD (13). Recent results from our group showed that the increased peripheral levels of lipid hydroperoxide (LPH) --a marker of lipid peroxidation-- is associated with decreased white matter integrity, assessed by diffusion tension imaging (18).
Oxidative stress is also associated with brain aging (20). This is demonstrated by accumulation of oxidative damage to proteins during aging (21) and the negative association between aging and the glutathione enzymes, the major antioxidant system (22). Indeed, studies have identified high levels of oxidative stress markers in patients with mild cognitive impairment (23) and Alzheimer’s disease (24). As discussed above, growing evidence supports that oxidative stress pathways are implicated in the pathophysiology of BD. However, to our knowledge, there has not yet been any published study of oxidative stress markers in older patients with BD. Thus, we evaluated the levels of acute and cumulative damage to protein and lipids in serum samples from older patients with BD. To assess the acute damage due to BD per se, we measured levels of LPH and 3-nitrotyrosine (3NT) as markers of reversible damage to lipid and proteins. To assess the damage due to the aging process and the chronicity of living with BD, we measured 4-hydroxynonenal (4HNE) and protein carbonyl (PC) as markers of cumulative or late damage to lipid and protein (4, 14). We hypothesized that, when compared to healthy individuals, euthymic older patients with BD would have increased levels of 4HNE and PC and no differences in LPH and 3NT.
Material and Methods
Subjects
We compared the levels of oxidative damage to proteins and lipids in 110 euthymic older subjects with BD I or II (78% female) and 75 comparison healthy individuals (53% female). Subjects were 50 years and older and the mean age of the two groups did not differ significantly (mean ± SD age: 63.9 ± 9.7 vs. 66.0 + 9.6; t= −1.46; df=183; p=0.15). Patients and comparison healthy individuals were recruited and assessed in clinics and by ads in Pittsburgh (71 patients; 31 comparison healthy individuals) and Toronto (39 patients; 44 comparison healthy individuals). Similar procedures described elsewhere were followed at both sites (36–38). In brief, psychiatric diagnoses were established or ruled out by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-IV). Exclusion criteria include dementia, presence of neurologic disorder and electro-convulsive therapy or substance abuse or dependence within the past six months. Most patients received treatment in university based clinics where the goals of the pharmacotherapy for BD have been to maximize the use of lithium or divalproex to achieve remission of mood episodes and maintain euthymia, and to limit adjunctive anti-psychotic or antidepressant medications (25, 26). At the time of assessment, patients had to be clinically euthymic for at least four weeks as demonstrated by scores of 10 or less on both the 17-item Hamilton Rating Scale for Depression (HRSD) (27, 28) and on the Young Mania Rating Scale (YMRS) (26). Clinical and demographics characteristics can be found in table 1.
Table 1.
Clinical and demographics characteristics of patients and comparison healthy individuals.
| Comparison Healthy Individuals | Bipolar Disorder | |
|---|---|---|
| Age (years) | 66.00 ± 9.62 | 63.86 ± 9.70 |
| Gender | 53% female | 78% female |
| BDI/BDII | N/A | 86(46.5%)/23(12.4%) |
| Length of Illness (years) | N/A | 35.55 ± 13.52 |
| YMRS Score | 0.35 ± 0.71 | 2.17 ± 2.31 |
| HRSD Score | 1.43 ± 1.85 | 3.72 ± 2.92 |
| Antidepressants (N, %) | 0, 0% | 62 (56.4%) |
| Antipsychotics | 0, 0% | 48 (43.6%) |
| Lithium | 0, 0% | 30 (27.3%) |
| Other Mood Stabilizers | 0, 0% | 46 (41.8%) |
Experimental Procedures
To assess protein oxidation, we measured the plasma levels of protein carbonyl (PC) and 3-nitrotyrosine (3-NT) using standard ELISA. To assess lipid peroxidation, we measured plasma levels of LPH and 4-hydroxynonenal (4-HNE) using spectrophotometric assays. Experimental procedure details can be found in Andreazza et al, 2009 (13, 14) and Versace et al, 2013 (18).
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was performed using SPSS for Mac Version 19.0. Normal distribution of data was determined using Kolmogorov-Smirnov test. To identify the differences between the BD and control groups we used t-tests or Mann-Whitney tests (see below). Differences were considered significant at the type I error rate of 0.05. The relation of biochemical data with age was measured using Spearman’s correlation and gender by Mann-Whitney test.
Results
Using Shapiro-Wilk (W)test we evaluated the Gaussian distribution of the oxidative stress markers all oxidative stress measures did not follow Gaussian distribution:4-HNE (W=0.810; p<0.001), LPH (W=0.822; p<0.01), 3-NT (W=0.842; p<0.001) and protein carbonyl (W=0.971; p=0.002). Thus, we used non-parametric tests to identify group differences.
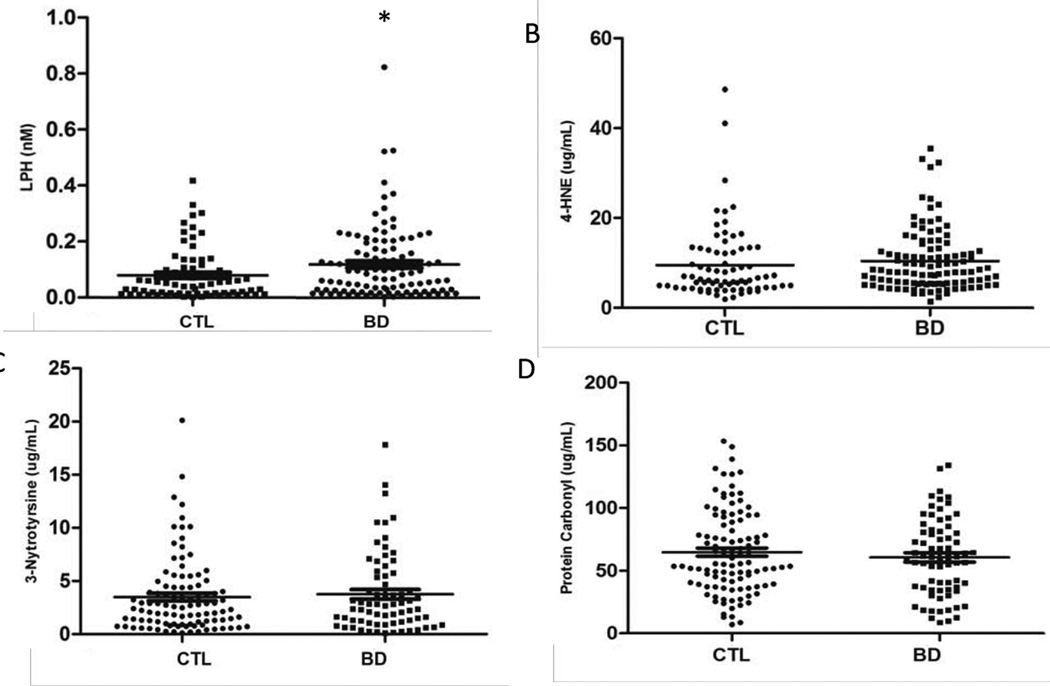
Mean levels of LPH were higher in patients with BD than in the comparison healthy individuals (0.12±0.13 nM vs, 0.08±0.09 nM; U=3085; p=0.017; d= 0.2; Figure. 1A). Levels of 4-HNE demonstrated a trend toward increased levels in patients with BD compared with healthy individuals (10.4±6.9 ug/mL vs. 9.5±8.1 ug/mL; U=3434; p=0.066; d=0.66; Figure. 1B). There was a positive correlation between levels of LPH and 4HNE (r=0.657; N=179, p<0.001). There were no differences in levels of PC and 3NT between patients and comparison healthy individuals (Figures 1C, 1D).
Figure 1.
Serum Oxidative Stress markers in older patients with bipolar disorder (BD) and comparison healthy individuals (CTL). (A) Lipid hydroperoxide (LPH); (B) 4-hydroxynonenal (4-HNE); (C) 3-nitrotyrosine; (D) protein carbonyl.*p=0.017, Mann-Whitney.
Lithium has been reported to have antioxidant properties, therefore we evaluated whether patients taken lithium had a different profile of oxidative stress markers from those not taken lithium. Patients taking lithium had significantly higher levels of LPH than the comparison healthy individuals (0.14±0.17 vs. 0.08±0.09; U=738; p=0.018), but their levels did not differ from patients not taking lithium (0.10±0.11; U=1010; p=0.40). We also investigate whether patients taken antidepressants or antipsychotics or other mood stabilizers could affect the levels of oxidative stress markers. We did not find any changes in levels of oxidative stress markers in patients taken or not taken antidepressants or antipsychotics or other mood stabilizers. Since samples were collected at two different sites, we explored the levels of LPH at each site. Results were not significant once samples were analyzed per site due to smaller samples, site interactions, or site differences (Toronto: U=581; p=0.079; Pittsburgh: U=1051; p=0.721). However, the same relationships trends were observed at both sites (i.e., patients had higher levels of LPH than comparison healthy individuals in both centers). Finally, we found no significant relationship between oxidative stress markers and age, gender, clinical scores on the YMRS and HAMD or duration of illness, or diagnosis of BD I vs. BD II.”
Discussion
To our knowledge, this is the first study to evaluate oxidative stress in older patients with BD. We found that patients had higher measures of lipid peroxidation (i.e., LPH) than comparison healthy individuals but the two groups did not differ in terms of protein nitration (i.e., 3NT), protein carbonylation and sub-products of lipid peroxidation (i.e., 4HNE). Our results are consistent with the literature in younger patients with BD that has consistently reported increased levels of lipid peroxidation (12, 16, 29) and heterogeneous findings about protein oxidation and nitration (12, 16, 29). Also, while two studies have shown increased levels of protein oxidation in postmortem prefrontal cortex from patients with BD (4, 5), protein oxidation results in peripheral samples are less consistent. For instance, Kapczisnki et al (17) found increased serum levels of protein oxidation in adult patients with BD during mania or depressive episodes, while Andreazza et al (14) found no difference between adult patients in the early stage of BD (less than 3 years since onset of illness) vs. the late stage (over 10 years since onset of illness).
Lipids are susceptible to oxidative stress damage, which leads to disrupted cell membranes and generation of toxic sub-products (30). Reactive oxygen species can react with lipids generating the initial product of lipid peroxidation – the LPH, which can be detoxified from the cells by antioxidant enzymes (1, 31–33). The progression of this cascade will promote LPH reaction with another fatty acid resulting in the formation of sub-products of lipid peroxidation including 4-HNE, 8-isoprostane and acrolein (1, 24). In turn, these lipid peroxidation sub-products can form covalent adducts with cysteine, lysine, or histidine residues of proteins and can induce changes in their function (1, 25–27). To prevent the formation of these sub-products a natural response of the cellular system is to increase antioxidant system or accelerate the elimination rate, in case the first antioxidant response fails (34–36). Thus, our finding of increased LPH levels with a trend toward increased 4-HNE levels (and a positive correlation between them) suggest that in older patients with BD, the antioxidant system might adapt to prevent formation of toxic sub-products of lipid peroxidation. In support of this hypothesis, we did not find increased levels of oxidative damage to proteins (i.e., PC and 3-NT), which contrast with some results in adult patients with BD (2, 13). Of significance of the role of lipid peroxidation in BD, a recent study from our laboratory demonstrated that peripheral levels of LPH were associated with white matter abnormalities in euthymic adult patients (18). Of significance, the levels of LPH found in adults patients with BD during euthymia (1–10nM) by Versace et al (2013) are higher then those levels described in this study in older patients with BD (0.1–1nM). Other studies also reported increased levels of lipid peroxidation in adult patients with BD (12,13,17) however they used other markers of lipid peroxidation which make difficult to compare the levels with those found in this study. In the aggregate, our findings and other results in the literature are congruent and support a crucial role for lipid peroxidation in the pathophysiology of BD.
Though an increase in markers of oxidative stress, especially lipid peroxidation, is a replicated finding in BD (2, 13, 16), we do not know whether oxidative damage is a risk factor for BD, the result of comorbid medical illness, or a consequence of cumulative biological changes due to illness or life style (e.g., diet). Our finding of increased levels of lipid peroxidation, LPH, in euthymic older patients support the hypothesis of a continuous effect of reactive species of oxygen in patients with BD. Our results need to be replicated in an independent sample and further investigation of the effect of lipid peroxidation across the life span inpatients with BD is needed. Indeed, aging is often accompanied by an increase in glucose consumption and sedentary life style, factors associated with a redox-swift that contributes to mitochondrial dysfunction and increase in reactive oxygen species production. Brewer (37) has described this process as the “epigenetic oxidative redox shift theory of aging”. BD is associated with mitochondrial dysfunction and increased oxidative stress (3, 5), as discussed above and demonstrated in this manuscript. This makes patients with BD more vulnerable to consequences of cumulative oxidative stress damage than subjects without BD. Longitudinal studies investigating the consequences of cumulative damage of oxidative stress are warranted. These follow up studies with larger samples size may provide valuable information on how oxidative stress can be used to track illness progression, guide treatment and, ultimately, improve the illness outcome.
Acknowledgment
This work is supported by grants from the Canadian Institutes of Health Research (A.C.A. and L.T.Y.; B.H.M. and T.K.R..), NARSAD Young Investigator awards (A.C.A.), and US National Institute on Mental Health (A..G.[P30 MH90333] and B.H.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 2.Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PV, Kauer-Sant'Anna M, Klamt F, et al. Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res. 2011;45:156–161. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Andreazza AC, Young LT. The neurobiology of bipolar disorder: identifying targets for specific agents and synergies for combination treatment. Int J Neuropsychopharmacol. 2013:1–14. doi: 10.1017/S1461145713000096. [DOI] [PubMed] [Google Scholar]
- 4.Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- 5.Andreazza AC, Wang JF, Salmasi F, Shao L, Young LT. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J Neurochem. 2013 doi: 10.1111/jnc.12316. [DOI] [PubMed] [Google Scholar]
- 6.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 7.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Yatham LN, Young LT. Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. Int J Neuropsychopharmacol. 2011;14:1069–1074. doi: 10.1017/S1461145711000617. [DOI] [PubMed] [Google Scholar]
- 8.Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 9.Konradi C, Sillivan SE, Clay HB. Mitochondria, oligodendrocytes and inflammation in bipolar disorder: evidence from transcriptome studies points to intriguing parallels with multiple sclerosis. Neurobiol Dis. 2012;45:37–47. doi: 10.1016/j.nbd.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, et al. DNA damage in bipolar disorder. Psychiatry Res. 2007;153:27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LM, Nardin P, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res. 2007;41:523–529. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Andreazza AC, Kauer-Sant'anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Andreazza AC, Kapczinski F, Kauer-Sant'Anna M, Walz JC, Bond DJ, Goncalves CA, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34:263–271. [PMC free article] [PubMed] [Google Scholar]
- 15.Frey BN, Martins MR, Petronilho FC, Dal-Pizzol F, Quevedo J, Kapczinski F. Increased oxidative stress after repeated amphetamine exposure: possible relevance as a model of mania. Bipolar Disord. 2006;8:275–280. doi: 10.1111/j.1399-5618.2006.00318.x. [DOI] [PubMed] [Google Scholar]
- 16.Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein BI, Frye MA, et al. Biomarkers in bipolar disorder: A positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust N Z J Psychiatry. 2013 doi: 10.1177/0004867413478217. [DOI] [PubMed] [Google Scholar]
- 17.Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PV, Kauer-Sant'Anna M, Klamt F, et al. A systemic toxicity index developed to assess peripheral changes in mood episodes. Mol Psychiatry. 2010;15:784–786. doi: 10.1038/mp.2009.112. [DOI] [PubMed] [Google Scholar]
- 18.Versace A, Andreazza AC, Young LT, Fournier JC, Almeida JR, Stiffler RS, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry. 2013 doi: 10.1038/mp.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JF, Shao L, Sun X, Young LT. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009;11:523–529. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 20.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Onorato JM, Thorpe SR, Baynes JW. Immunohistochemical and ELISA assays for biomarkers of oxidative stress in aging and disease. Ann N Y Acad Sci. 1998;854:277–290. doi: 10.1111/j.1749-6632.1998.tb09909.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones DP, Mody VC, Carlson JL, Lynn MJ, Sternberg P. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 23.Diniz BS, Reynolds CF, Begley A, Dew MA, Anderson SJ, Lotrich F, et al. Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: a longitudinal study. J Psychiatr Res. 2014;49:96–101. doi: 10.1016/j.jpsychires.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelfriet PM, Jansen EH, Picavet HS, Dollé ME. Biochemical Markers of Aging for Longitudinal Studies in Humans. Epidemiol Rev. 2013 doi: 10.1093/epirev/mxs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young RC, Gyulai L, Mulsant BH, Flint A, Beyer JL, Shulman KI, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12:342–357. doi: 10.1176/appi.ajgp.12.4.342. [DOI] [PubMed] [Google Scholar]
- 26.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Bezchlibnyk YB, Wang JF, McQueen GM, Young LT. Gene expression differences in bipolar disorder revealed by cDNA array analysis of post-mortem frontal cortex. J Neurochem. 2001;79:826–834. doi: 10.1046/j.1471-4159.2001.00628.x. [DOI] [PubMed] [Google Scholar]
- 29.Andreazza AC, Kauer-Sant'Anna M, Frey BN, Stertz L, Zanotto C, Ribeiro L, et al. Effects of mood stabilizers on DNA damage in an animal model of mania. J Psychiatry Neurosci. 2008;33:516–524. [PMC free article] [PubMed] [Google Scholar]
- 30.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. discussion 24S–25S. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1:1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4 ed. Oxford: Clarendon Press; 2007. [Google Scholar]
- 34.Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15:2011–2035. doi: 10.1089/ars.2010.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui J, Shao L, Young LT, Wang JF. Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience. 2007;144:1447–1453. doi: 10.1016/j.neuroscience.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 37.Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol. 2010;45:173–179. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gildengers AG, Butters MA, Chisholm D, Anderson SJ, Begley A, Holm M, Rogers JC, Reynolds CF, 3rd, Mulsant BH. Cognition in older adults with bipolar disorder versus major depressive disorder. Bipolar Disord. 2012 Mar;14(2):198–205. doi: 10.1111/j.1399-5618.2012.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lotrich FE, Butters MA, Aizenstein H, Marron MM, Reynolds CF, III, Gildengers AG. The relationship between interleukin-1 receptor antagonist and cognitive function in older adults with bipolar disorder. Int J Geriatr Psychiatry. doi: 10.1002/gps.4048. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajji TK, Voineskos AN, Butters MA, Miranda D, Arenovich T, Menon M, Ismail Z, Kern RS, Mulsant BH. Am J Geriatr Psychiatry. 2013 Feb;21(2):108–118. doi: 10.1016/j.jagp.2012.10.011. Epub 2013 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]