Abstract
Neuroinflammation contributes to the pathophysiology of diverse diseases including stroke, traumatic brain injury, Alzheimer's Disease, Parkinson's Disease, and multiple sclerosis, resulting in neurodegeneration and loss of neurological function. The response of the microvascular endothelium often contributes to neuroinflammation. One such response is the up-regulation of endothelial adhesion molecules which facilitate neutrophil adhesion to the endothelium and their migration from blood to tissue. Neuregulin-1 (NRG1) is an endogenous growth factor which has been reported to have anti-inflammatory effects in experimental stroke models. We hypothesized that NRG1 would decrease the endothelial response to inflammation, and result in a decrease in neutrophil adhesion to endothelial cells. We tested this hypothesis in an in-vitro model of cytokine-induced endothelial injury, in which human brain microvascular endothelial cells (BMECs) were treated with IL-1β, along with co-incubation with vehicle or NRG1-β. Outcome measures included protein levels of endothelial ICAM-1, VCAM-1, and E-selectin; as well as the number of neutrophils that adhere to the endothelial monolayer. Our data show that NRG1-β decreased the levels of VCAM-1, E-selectin, and neutrophil adhesion to brain microvascular endothelial cells activated by IL1-β. These findings open new possibilities for investigating NRG1 in neuroprotective strategies in brain injury.
Keywords: neuroinflammation, IL-1β, NRG1-β, neutrophil, VCAM-1, E-selectin
Background
Neuroinflammation, the nonspecific immune reaction in the CNS that is characterized by increased microglia activation, presence of pro- and anti-inflammatory cytokines, blood brain barrier (BBB) permeability, and leukocyte invasion, is a key element of many different pathologies. It contributes to the pathophysiology of diverse diseases such as stroke, traumatic brain injury, Alzheimer's Disease, Parkinson's Disease, and multiple sclerosis; and is widely known to amplify the damage of the primary injury, resulting in neurodegeneration and loss of neurological function [1]. Release of both pro- and anti-inflammatory cytokines is a hallmark of the neuroinflammatory cascade and the expression of these cytokines is strongly up-regulated in the acute phase after many forms of brain injury [2, 3]. IL-1β is one of the major cytokines produced in the brain after injury, and IL-1β levels are directly correlated with the severity of inflammation [4-7].
The microvascular endothelium contributes to the process of neuroinflammation through its response to the inciting damage and to circulating cytokines [8]. One such endothelial response is the up-regulation of a class of proteins known as adhesion molecules, which can facilitate the adhesion of leukocytes to the endothelium and their migration from the blood to the tissue [8]. Beneficial effects of leukocyte recruitment include the ability of neutrophils to combat infections, while detrimental effects include the tissue destruction caused by the release of the contents of the neutrophil granules [9]. In many pathological conditions, an excessive inflammatory response to CNS pathologies contributes to secondary brain injury. A means of modulating this inflammatory response may therefore be of therapeutic value.
In this study, we determined the effect of NRG1-β on neutrophil adhesion to the endothelium. Using in an in vitro model of cytokine-induced endothelial injury by incubation of human brain microvascular endothelial cells (BMECs) with IL-1β, we evaluated NRG1-β effect on endothelial levels of VCAM-1, ICAM-1 and e-selectin. Additionally, we assessed the effect of NRG1-β on the extent of neutrophil adhesion to BMECs which have been treated with IL-1β.
Materials and Methods
Animals
Mouse peritoneal neutrophils were obtained from adult CD1 female mice (2-5mo of age; Charles River Laboratories). The mice were housed for 12-h day/night cycles in a pathogen-free facility at Massachusetts General Hospital Institutional Animal Care in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Food and water were given ad libitum All experiments followed the policies and guidelines of the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Human brain microvascular endothelial cells (BMECs) (Cell systems, Kirkland, WA), passage 5 to 12, were grown in EBM-2 Basal Medium supplemented with Endothelial Cell Growth Medium-2 (Lonza, Walkersville, MD) unless otherwise specified. When 80-90% confluent, cells were exposed to 50ng/ml of IL-1β (Sigma, St. Louis, MO); along with different concentrations of NRG1-β (R&D, Minneapolis, MN) or vehicle. The NRG1-β used in all experiments consisted of the active domain of the NRG1-β isoform. The duration of incubation with vehicle or IL-1β was specified for each experiment. Experiments were performed in triplicate wells.
Neutrophil adhesion assay
The assay was performed three times with each condition tested in triplicate wells.
Preparation of endothelial cells
A sterile 24 well plate was coated with 3% Collagen for 2 hours and washed once with phosphate buffer solution. BMEC's were seeded at a density of 1×105/well. When >90% confluent, these cells were treated for 6h with vehicle (PBS) or IL-1β (doses as specified) +/- NRG1-β (doses as specified).
Neutrophil activation and retrieval
CD1 mice (age range 2-5mo) were anesthetized and given an intraperitoneal (IP) injection of 2mL of thioglycolate media (Sigma, St. Louis, MO) using a 27G needle. Eighteen to twenty four hours later, the mice were sacrificed and an IP injection of 8mL of RPMI 1640 + 1% Penicillin-streptomycin (Invitrogen, Grand Island, NY) was injected, then collected with a 16G needle to retrieve the activated neutrophils .
Neutrophil labeling and collection
MitroTracker Red CMXRos (Invitrogen Life Techologies) (1mM) was added to the neutrophil collection at a 1:1000 dilution and incubated for 1h in the cell incubator at 37°C, during which time neutrophils remain floating in the media and macrophages adhere to the bottom of the dish. The media containing the floating neutrophils was collected and cells pelleted by centrifugation at 1000-1500rpm for 5min. Care was taken not to disrupt the surface of the dish in order to exclude the adherent macrophages. The pellet containing neutrophils was resuspended in EBM-2 cell medium.
Neutrophil Adhesion to Endothelial Cells
CSC cells were treated for 6h with PBS or IL-1+/- NRG1-β as described above, after which 25,000 neutrophils (MitoTracker-labeled) were seeded onto the endothelial monolayer and incubated for 90 min at 37°C. Media was then removed, and each well was washed with phosphate buffer solution to remove non-adherent neutrophils. The cells were fixed with 4% paraformaldehyde (PFA) for twenty minutes, and examined by fluorescent microscopy.
Imaging and quantification
3 images (at 200x magnification) were taken at random locations from each well. The number of neutrophils labeled with the fluorescent MitoTracker signal (which binds mitochondria in leukocytes but does not label red blood cells which lack mitochondria) were counted in each field. Only MitoTracker-labeled cells with a corresponding DAPI nuclear signal were counted. For visualization of cell morphology, an aliquot of the neutrophil suspension was concentrated by cytospin, Wright-Giemsa stained, and visualized at 1000x magnification with light microscopy.
Western blot analysis
Cells were lysed with lysis buffer (Cell Signaling Technology, Beverly, MA, USA), and protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Protein lysates (20 μg per lane) were separated in precast 4-12% Tris-glycine SDS-polyacrylamide gels (Invitrogen, Grand Island, NY), and proteins were transferred to PVDF membranes (Invitrogen, Grand Island, NY ). After blocking with 5% non-fat milk, PVDF membranes were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-E-selectin (1:1000 dilution; Santa Cruz, Dallas, TX), rabbit anti-VCAM-1 (1:1000 dilution; Santa Cruz, Dallas, TX), mouse anti-ICAM-1 (1:1000 dilution; Santa Cruz, Dallas, TX). After washing, the membrane was incubated with peroxidase-conjugated secondary antibody at room temperature for 1 hour. Antigen–antibody complexes were visualized with the ECL chemiluminescence system (Amersham, Piscataway, NJ) and exposed to a Kodak X-OMAT film (Kodak, Rochester, NY, USA). The relative densities of bands were analyzed with NIH Image J.
Assessment of Cytotoxicity
IL-1β -induced cytotoxicity was quantified by a standard measurement of lactate dehydrogenase (LDH) release with the use of the LDH assay kit (Boehringer-Mannheim, Ingelheim am Rhein). Percent cytotoxicity was calculated by comparing LDH released into the media to the total LDH content in viable cells and media. In addition, cytotoxicity was also quantified by measurement of the reduction of 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT) to produce a dark blue formazan product. This assay assesses the integrity of mitochondrial function. MTT was added to each culture well at indicated time points after hemoglobin exposure at a final concentration of 0.5% MTT solution (wt/vol). After 3 hours (h) at 37°C, the media was removed and cells were dissolved in dimethyl sulfoxide. The formation of formazan was measured by reading absorbance at a wavelength of 570 nm with a reference setting of 630 nm on a microplate reader (model FL600, Bio-Tek Instruments, Winooski, VT). Both LDH and MTT reduction assays were used to ensure that similar data were obtained after drug-induced cytotoxicity.
Statistical analysis
Statistical analysis was performed by mixed model regression followed by Tukey HSD for multiple comparisons for evaluation of protein levels, and by ANOVA followed by Tukey HSD for evaluation of neutrophil number. Statistical significance was set at p<0.05. Quantitative data for experiments were expressed as mean ± standard deviation (SD).
Results
NRG1-β ameliorates the IL-1β-induced increase in endothelial VCAM-1 and E-selectin
Prior to investigating the effect of IL-1β on endothelial expression of adhesion molecules and on neutrophil expression, we demonstrated that IL-1β did not cause endothelial cell death in this model of cytokine-induced endothelial inflammation (Fig. 1a). Additionally, pilot experiments were performed to determine the dose-dependent effects of IL-1β on adhesion protein levels and on neutrophil adhesion. Based on these pilot data, an IL-1β dose of 50ng/ml and an incubation time of 6h were used for the experiments on adhesion molecule levels.
Fig.1. Incubation of BMEC with IL-1β does not result in an increase in cell death or a decrease in metabolic activity.
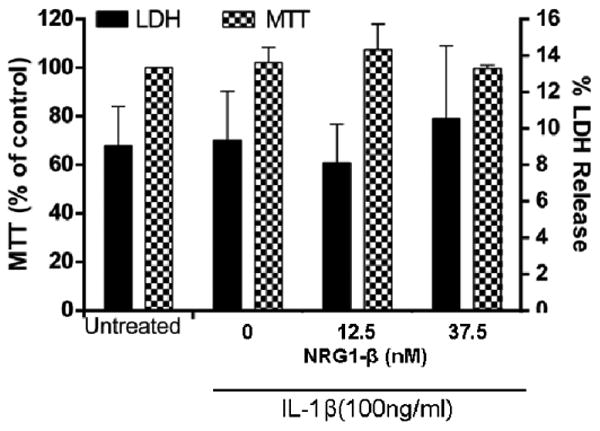
After incubation with IL-1β (100ng/ml) for 18h in the presence of vehicle or NRG1-β (100ng/ml or 300ng/ml), changes in metabolic activity and cell death of BMECs were evaluated by LDH (right axis) or MTT assay (left axis). For MTT results, the data is represented as the percent of metabolic activity compared to the vehicle-treated control (shown as the mean + SEM). For LDH, the results are expressed as the percentage of LDH release compared to vehicle-treated control(shown as the mean + SEM). n=3.
IL-1β challenge resulted in an increase in VCAM-1, ICAM-1, and E-selectin. VCAM-1 protein level in IL-1β treated cells was 5 (5.1 +/- 1.0, p<0.05) times that of untreated controls. Addition of 100ng/ml (12.5nM) NRG1-β did not lead to a statistically significant difference from IL-1β treated cells. Addition of a higher dose (300ng/ml or 37.5nM) of NRG1-β resulted in a decrease in VCAM-1 to 3.5 (3.5 +/-1.2) times that of IL-1β treated cells in the absence of NRG1-β, a statistically significant reduction of 32% (p<0.05), (Fig. 2a). Based on this result, a NRG1-β dose of 300ng/ml (37.5 nM) was used in subsequent experiments for evaluating the NRG1 effect on IL-1β induced increases in ICAM-1 and E-selectin protein levels. The protein level of ICAM-1 in IL-1β treated cells was 5 (4.9 +/-1.5, p<0.05) times that of untreated controls. In the presence of NRG1, ICAM-1 protein level was 4.2x that of IL-1β treated cells, which did not represent a statistically significant difference (Fig. 2b). The protein level of E-selectin in IL-1β treated cells was 3.3 (3.3 +/- 0.6; p<0.05) times that of untreated controls. Addition of NRG1-β resulted in a decrease in E-selectin levels to 2.4 (2.4 +/- 0.8) times that of IL-1β - treated cells, a statistically significant decrease of 27% (p<0.05) (Fig. 2c).
Fig. 2. NRG1-β decreases IL-1β induced increases in protein levels of endothelial intercellular adhesion molecules.
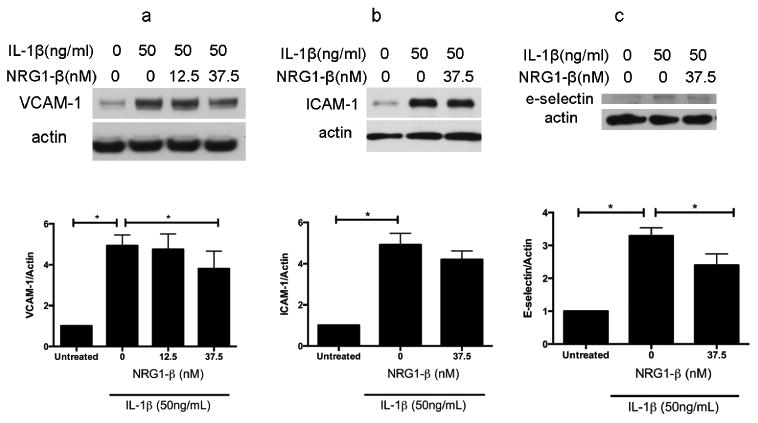
BMECs were treated with IL-1β (50ng/ml) and co-incubated addition vehicle (PBS) or NRG1-β (12.5nM and/or 37.5nM). Western blots of cell lysates were analyzed for protein levels of adhesion molecules. n=3.
2a. 6h incubation with IL-1β resulted in an increase in a 5-fold (5.1 +/- 1.0, p<0.05) increase in VCAM-1. Co-incubation with 100ng/ml (12.5nM) NRG1-β did not result in a statistically significant difference from IL-1β - treated cells; however, addition of 300ng/ml (37.5nM) NRG1-β resulted in a decrease in VCAM-1 levels to 3.5 (3.5 +/- 1.2) times that of IL-1β - treated cells that were co-incubated with vehicle, which is a statistically significant reduction of 32% (p<0.05).n=3.
2b. 6h incubation with IL-1β resulted in a 5-fold (4.9 +/-1.5, p<0.05) increase in endothelial ICAM-1 levels compared to vehicle-treated controls. BMECs that were co-incubated with NRG1 (300ng/ml or 37.5nM) had ICAM-1 protein levels which were 4.2 x that of IL-1β –treated cells, which does not represent a statistically significant difference.
2c. 6h incubation with IL-1β resulted in an increase in endothelial E-selectin expression, averaging 3.3-fold (3.3 +/- 0.6; p<0.05) increase compared to baseline (*p < .0.01). Co-incubation with 300ng/ml (37.5nM) NRG1-β resulted in a decrease in E-selectin levels to 2.4 (2.4 +/- 0.8) times that of IL-1β treated cells that were co-incubated with vehicle, a statistically significant decrease of 27% (p<0.05) (Fig. 2c). n=3.
NRG1-β reduces the IL-1β-induced increase in neutrophil adhesion in human brain endothelial cells
We further explored whether the effect of NRG1-β on endothelial adhesion molecules extended to a change in the level of neutrophil adhesion. In a neutrophil adhesion assay, activated mouse peritoneal neutrophils were used. Light microscopy of Wright/Giemsa stained cells in the suspension revealed that >90% of the leukocytes had morphology consistent with neutrophils (Fig.3a, d). Variable numbers of erythrocytes were present in different preparations, depending on the amount of capillary blood entrained into the fluid collected during the neutrophil retrieval process. However, erythrocytes do not contain mitochondria and hence do not incorporate the fluorescent MitoTtracker signal, while the labeled neutrophils appear brightly fluorescent (Fig.3b). DAPI staining showed that the MitoTracker labeling corresponded to cells rather than to non-specific debris.
Fig. 3. Representative images of neutrophils and endothelial cells in the neutrophil adhesion assay.
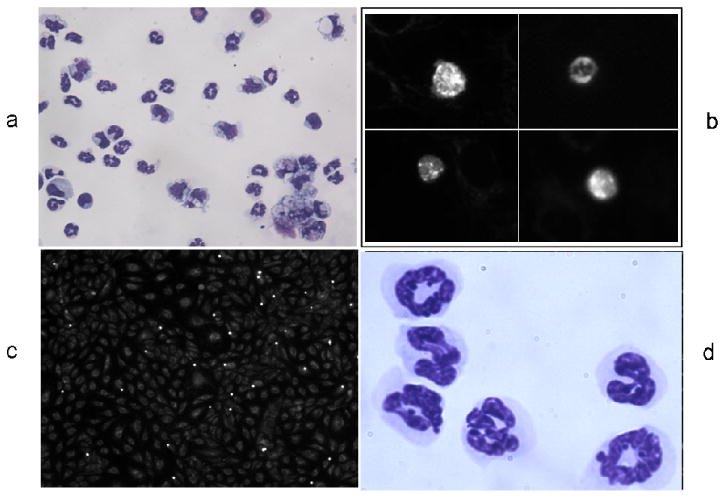
3a. Cytopsin of PFA-fixed aliquot of mouse peritoneal neutrophils, Wright-Giemsa stained, photographed at 400x.
3b. High magnification view of neutrophils with fluorescent Mitotracker signal, photographed at 400x.
3c. Neutrophils and endothelial cells in neutrophil adhesion assay, photographed at 200x.
3d. mouse peritoneal neutrophils, Wright-Giemsa stained, photographed at 1000x.
Within the pilot series of experiments to determine the appropriate IL-1β dosage for the neutrophil adhesion assay, IL-1β doses of 10ng/ml, 50ng/ml, and 100ng/ml were tested. There was a dose-dependent increase in the number of adherent neutrophils, and addition of NRG1-β reduced neutrophil adhesion at each IL-1β concentration (Fig. 4a-g and Fig.4h). Because this was a pilot series, three separate experiments were not performed at all doses; however the consistency of NRG-1β effect across the different IL1-β concentrations supports the hypothesis that NRG1 has an effect on neutrophil adhesion.
Fig. 4. IL-1β dose-finding pilot experiment for neutrophil adhesion assay.
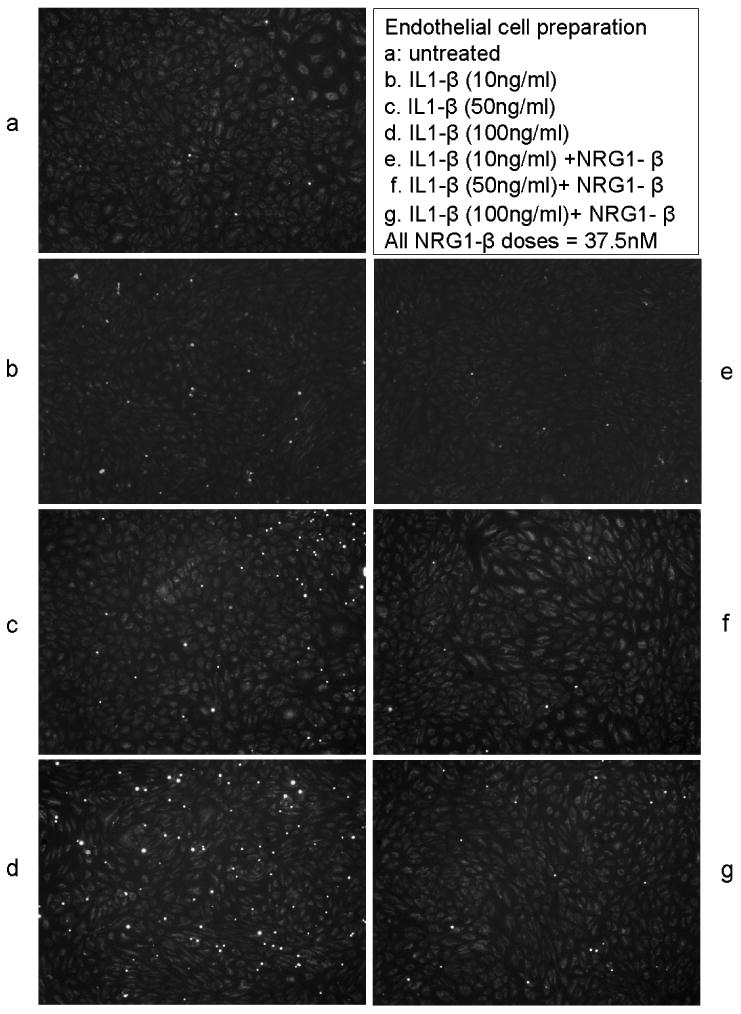
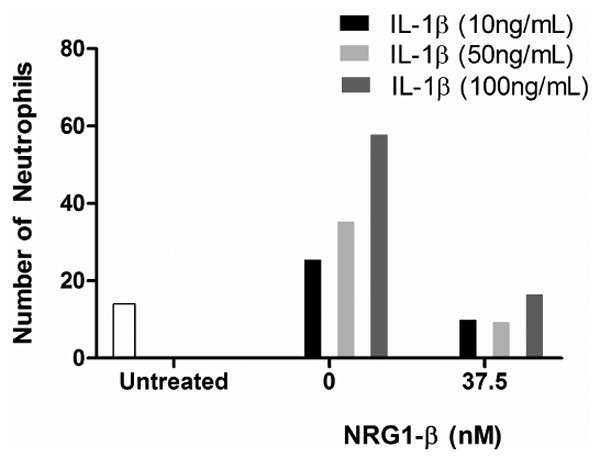
At 10ng/ml (n=2), IL-1β increased the neutrophil adhesion 1.8 fold that of the baseline level; at 50ng/ml (n=3): 2.5 fold; at 100ng/ml (n=1): 4 fold. Co-incubation of NRG1- β (300ng/ml or 37.5nM) reduced neutrophil adhesion at each dose of IL-1β.
a-g. Representative images of the neutrophil adhesion assay with the conditons listed.
4h. Graphical representation of the results of the dose-finding pilot experiment.
In the neutrophil adhesion assay using an IL-1β dose of 50ng/ml, an average of 14 (14 +/- 6.5) adherent neutrophils were seen per field of BMECs treated with vehicle (PBS). In IL-1β treated endothelial cells, the number of adherent neutrophils increased to 35 (35+/- 12.9) per high-powered field, a 2.5 fold increase (p<0.05) over baseline. In endothelial cells that were treated with IL-1β and100ng/ml (12.5nM) NRG1-β, the number of adherent neutrophils decreased to 14 (14.6 +/- 4.7), a significant decrease back to baseline levels (p<0.05). In endothelial cells treated with IL-1β and 300ng/ml (37.5nM) NRG1-β, the number of adherent neutrophils was 9.9 (9.9 +/- 4.0), again a significant decrease from that in cells treated with IL-1β without NRG1-β (p<0.05), (Fig. 5).
Fig. 5. NRG1-β reduces the IL-1β induced increase in neutrophil adhesion to human brain endothelial cells.
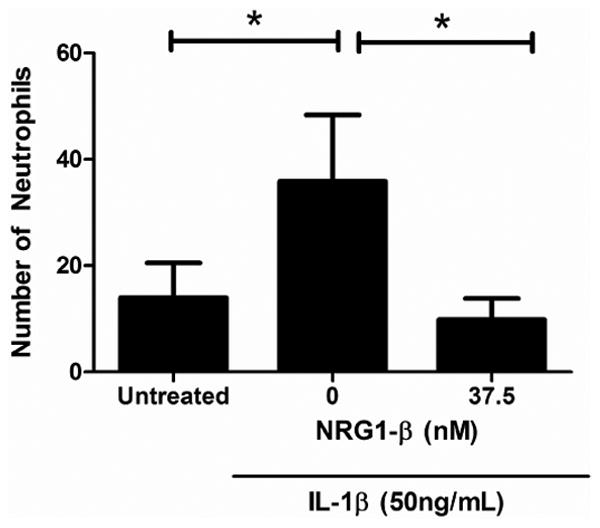
In PBS-treated BMECs, an average of 14 (14 +/- 6.5) neutrophils were adherent to the endothelial monolayer in each high-powered (10x) field. In IL-1β treated endothelial cells, the number of adherent neutrophils increased to 35 (35+/- 12.9), a 2.5 fold increase (p<0.05). In BMECs treated with IL-1β and co-incubated with NRG1-β (100ng/ml or 12.5nM) NRG1-β, the number of adherent neutrophils decreased to the baseline level of 14 (14.6 +/- 4.7; p<0.05compared to BMECs treated with IL-1β and co-incubated with PBS). In endothelial cells treated with IL-1β and 300ng/ml (37.5nM) NRG1-β, the number of adherent neutrophils was 9.9 (9.9 +/-4.0), again a significant decrease from that in cells treated with IL-1β without NRG1-β (p<0.05).
Discussion
Our previous data show that NRG1-β prevents endothelial hyper-permeability during IL-1β induced injury, and ameliorates acute increases in BBB permeability following brain trauma [10]. Here we show that NRG1-β reduces the IL-1β induced increase in protein levels of VCAM-1 and E-selectin, and in neutrophil adhesion to endothelial cells.
IL-1β is upregulated rapidly in the brain after diverse forms of brain injury, especially in the hippocampus and cortical areas. After subarachnoid hemorrhage and severe head trauma in humans, IL-1β levels peak on day 2 at 26.9 ± 4.5pg/ml, followed by a gradual decline [11]. Analysis of IL-1β levels from brain microdialysates from TBI patients yielded a mean IL-1β concentration of 10.4+/-14.7pg/mL [12]. The IL-1β dose used in our study is of a higher level than that reported from these clinical series; however, it is consistent with the range of concentrations used in other in-vitro experiments that study the interaction of endothelial cells with IL-1β. It is possible that when a single type of cell is being studied in in-vitro experiments without the normal components of the neurovascular unit, synergistic cellular interactions that amplify cellular responses do not occur, and therefore a higher concentration of IL-1β is required to obtain a clear signal above background. In a study investigating the effect of IL-1β on endothelial permeability, the half-maximal effective concentration of IL-1β used to induce endothelial permeability during a 6h incubation was 53ng/ml. [13]. In studies involving the IL-1β effect on endothelial activation and expression of adhesion molecules, IL-1β doses used ranged from pg/ml [14] to ng/ml [15]. In our dose-finding experiment, activating endothelial cells with IL-1β doses of 10ng/ml, 50ng/ml, and 100ng/ml resulted in a dose-dependent increase in neutrophil adhesion. In order to obtain a reasonable signal to background ratio, an IL -1β dose of 50ng/ml was used.
Neuregulin1-β (NRG1-β) is an endogenous growth factor which plays multiple functions in the embryonic and postnatal central nervous system [16-25]. NRG1/erbB signaling is known to be involved in many functions in neurons and glia [17, 18, 26], and its involvement in the peripheral microvascular endothelium has been increasingly clarified [27-29]. It was recently demonstrated that NRG1/erbB signaling is active in microvascular endothelial cells of the brain [30] and that NRG1-β decreases pathological increases in endothelial permeability induced by IL-1β [10]. Our current data show that NRG1-β decreases the expression of VCAM-1 and E-selectin in BMECs that have been activated by IL-1β, and modulates the IL-1β induced increase in neutrophil adhesion to endothelial cells.
These findings are consistent with previous reports of the anti-inflammatory actions of NRG1. In in-vitro studies, the NRG1 isoform GGF2 was reported to reduce free radical release from activated microglial cells [31]. A separate group also found that NRG-1 induces a decrease in the pro-inflammatory cytokines TNF-α and IL-6 in microglial cells challenged with lipopolysaccharide [32]. In animal studies, reduced levels of type III NRG1 transcripts were observed in immunodeficient mice [33], and NRG1 was found to attenuate inflammatory responses induced by ischemic stroke [34]. Findings from human studies yielded consistent data in this regard. Studies using cell lines from patients with schizophrenia and from controls suggest that NRG1-α affects the migration of immune cells [35]. In another human study, a mutation in the transmembrane domain of the NRG-1 protein was linked to dysregulation of the immune system resulting in an increase of pro-inflammatory cytokines IL-6, TNF-α, and IL-1β as well as a reduced expression of the anti-inflammatory cytokine IL-4 [36]. On the other hand, NRG1 actions may differ depending on the cell type and context. There have been reports that NRG1/erbB signaling may increase inflammation in some conditions – for instance, NRG1/erbB signaling has been reported to promote microglial proliferation and chemotaxis in an animal model of peripheral nerve injury [37]. In patients with acute lung injury, elevated bronchial levels of NRG1 was reported to be associated with level of inflammation, although it is not clear whether there is a causal relationship [38].
Our current in-vitro model is consistent with NRG1 effects in the setting of acute inflammation. It is important to keep in mind that neuronflammation has both deleterious and protective properties in the different phases after injury. In the acute phase, inflammation may exacerbate tissue damage; while in the recovery phase inflammation may promote endogenous repair [39]. However, prolonged inflammation may outweigh endogenous regenerative reactions [40]. Given these considerations, further translational studies will be needed to determine the optimal time for exogenous administration of NRG1 as its anti-inflammatory activity effects during the acute phase may impact on the balance between beneficial and deleterious effects of the inflammatory response in the acute and recovery phase.
Although this study focuses on the effect of NRG1 on the expression of endothelial adhesion molecules and the subsequent adhesion of neutrophils, one might speculate on possible NRG1 effects on other physiological processes associated with these adhesion molecules. A relevant example is that of the migration of bone marrow derived endothelial progenitor cells (EPC) to the site of brain injury, since adhesion molecules such as VCAM-1 are involved in this process [41]. The question may arise as to whether the modification of adhesion molecule expression by NRG1 could render the environment less conducive to EPC migration towards damaged tissue. Although NRG1's effect on EPC migration towards a site of CNS injury has not been studied, there is evidence that NRG1 is involved in EPC function. Transcripts of NRG1 receptors erbB2 and erbB3 have been found in EPCs isolated from rat bone marrow, human whole blood, and an embryonic EPC (eEPCs) cell line [42], and NRG1 has been reported to affect survival of eEPCs by inhibiting serum deprivation-induced apoptosis [42]. These findings suggest that NRG1 signaling has the potential to support EPC function during the recovery phase of injury, although it is not known whether this benefit is mediated through an effect on EPC survival, migration, or other angiogenic processes. Separate from its potential interactions with EPC migration, NRG1 effect on blood flow recovery in a rodent model of limb ischemic injury suggests that it has favorable actions on the vasculature after ischemia. In this model, Hedhli et al. [43] reported that deletion of endothelial NRG decreased blood flow recovery and arteriogenesis, while administration of exogenous NRG1-β enhanced blood flow recovery.
There are limitations to our study. Our in-vitro experiments, which utilize a cell line, do not capture the complex events that occur in-vivo during an inflammatory process. Interactions with other cellular elements, such as macrophages, lymphocytes, and platelets are not included, or with proteins in the serum. We have focused on one time-point (6 h) in the IL-1β interaction with BMECs and one time point (90 min) in the process of neutrophil/endothelial adhesion, when both of these events likely involve many dynamic changes. The study of a single cell type does not recapitulate the complex interactions within the neurovascular unit. The use of one dose of IL-1β used (50ng/ml) does not accurately capture the different types of inflammatory microvenironments in the setting of various CNS pathologies.
Conclusion
Neuroinflammation can lead to many immediate and long-term harmful sequelae. Neutrophil migration into the tissue is a central event in neuroinflammation. Our data suggest that NRG1-β reduces cytokine-induced neutrophil adhesion, one of the initial steps in the process of neutrophil recruitment and migration. These findings may open new possibilities for investigating NRG1's potential in neuroprotection in inflammatory conditions in the CNS.
Acknowledgments
This work is supported by the following grants:
R37NS037074-13 (EHL)
R01NS076694-02(EHL)
P01NS055104-05 (EHL)
K08N5057339-04 (JL)
China Scholarship Council (#201206170107; LW)
The authors thank Dr. Mark Vangel and Dr. Hang Lee (Harvard Catalyst) for statistical support.
Abbreviations
- ICAM-1
intercellular cell adhesion molecule
- IL-1β
interleukin-1 beta
- NRG1-β
neuregulin 1-beta
- VCAM-1
vascular cell adhesion molecule-1 or vascular cell adhesion protein-1
Footnotes
Limin Wu, Samantha Walas, Wendy Leung, David Sykes, Jiang Wu, Eng H. Lo, and Josephine Lok declare that they have no conflict of interest.
Compliance with Ethics Requirements: This article does not contain any studies with human subjects.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Brothers HM, Bardou I, Hopp SC, Marchalant Y, Kaercher RM, Turner SM, et al. Time-Dependent Compensatory Responses to Chronic Neuroinflammation in Hippocampus and Brainstem: The Potential Role of Glutamate Neurotransmission. J Alzheimers Dis Parkinsonism. 2013;3:110. doi: 10.4172/2161-0460.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter CD, Iannotti F, Pringle AK, Trikkas C, Clough GF, Church MK. A microdialysis method for the recovery of IL-1beta, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Methods. 2002;119(1):45–50. doi: 10.1016/s0165-0270(02)00153-x. S016502700200153X[pii] [DOI] [PubMed] [Google Scholar]
- 3.Woodroofe MN, Sarna GS, Wadhwa M, Hayes GM, Loughlin AJ, Tinker A, et al. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol. 1991;33(3):227–36. doi: 10.1016/0165-5728(91)90110-s. [DOI] [PubMed] [Google Scholar]
- 4.Boato F, Rosenberger K, Nelissen S, Geboes L, Peters EM, Nitsch R, et al. Absence of IL-1beta positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J Neuroinflammation. 2013;10:6. doi: 10.1186/1742-2094-10-6. 1742-2094-10-6[pii]10.1186/1742-2094-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA, et al. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J Neurosci. 2013;33(11):5053–64. doi: 10.1523/JNEUROSCI.4361-12.2013. 33/11/5053[pii]10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: The role and consequences. Neurosci Res. 2013 doi: 10.1016/j.neures.2013.10.004. S0168-0102(13)00225-3[pii]10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289(2):H738–43. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevilacqua MP, Gimbrone MA., Jr Inducible endothelial functions in inflammation and coagulation. Semin Thromb Hemost. 1987;13(4):425–33. doi: 10.1055/s-2007-1003519. [DOI] [PubMed] [Google Scholar]
- 9.Finnie JW. Neuroinflammation: beneficial and detrimental effects after traumatic brain injury. Inflammopharmacology. 2013;21(4):309–20. doi: 10.1007/s10787-012-0164-2. [DOI] [PubMed] [Google Scholar]
- 10.Lok J, Zhao S, Leung W, Seo JH, Navaratna D, Wang X, et al. Neuregulin-1 effects on endothelial and blood-brain-barrier permeability after experimental injury. Translational stroke research. 2012;3(Suppl 1):S119–24. doi: 10.1007/s12975-012-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellergard P, Aneman O, Sjogren F, Saberg C, Hillman J. Differences in cerebral extracellular response of interleukin-1beta, interleukin-6, and interleukin-10 after subarachnoid hemorrhage or severe head trauma in humans. Neurosurgery. 2011;68(1):12–9. doi: 10.1227/NEU.0b013e3181ef2a40. discussion 9. 10.1227/NEU.0b013e3181ef2a4000006123-201101000-00003[pii] [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson PJ, O'Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24(10):1545–57. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 13.Rigor RR, Beard RS, Jr, Litovka OP, Yuan SY. Interleukin-1beta-induced barrier dysfunction is signaled through PKC-theta in human brain microvascular endothelium. Am J Physiol Cell Physiol. 2012;302(10):C1513–22. doi: 10.1152/ajpcell.00371.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridder DA, Lang MF, Salinin S, Roderer JP, Struss M, Maser-Gluth C, et al. TAK1 in brain endothelial cells mediates fever and lethargy. J Exp Med. 2011;208(13):2615–23. doi: 10.1084/jem.20110398. jem.20110398[pii]10.1084/jem.20110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mako V, Czucz J, Weiszhar Z, Herczenik E, Matko J, Prohaszka Z, et al. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1beta, TNF-alpha, and LPS. Cytometry A. 2010;77(10):962–70. doi: 10.1002/cyto.a.20952. [DOI] [PubMed] [Google Scholar]
- 16.Schaff U, Mattila PE, Simon SI, Walcheck B. Neutrophil adhesion to E-selectin under shear promotes the redistribution and co-clustering of ADAM17 and its proteolytic substrate L-selectin. J Leukoc Biol. 2008;83(1):99–105. doi: 10.1189/jlb.0507304. jlb.0507304[pii]10.1189/jlb.0507304. [DOI] [PubMed] [Google Scholar]
- 17.Falls DL. Neuregulins: functions, forms, and signaling strategies. Experimental cell research. 2003;284(1):14–30. doi: 10.1016/s0014-4827(02)00102-7. S0014482702001027[pii] [DOI] [PubMed] [Google Scholar]
- 18.Rieff HI, Raetzman LT, Sapp DW, Yeh HH, Siegel RE, Corfas G. Neuregulin induces GABA(A) receptor subunit expression and neurite outgrowth in cerebellar granule cells. J Neurosci. 1999;19(24):10757–66. doi: 10.1523/JNEUROSCI.19-24-10757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19(1):39–50. doi: 10.1016/s0896-6273(00)80346-3. S0896-6273(00)80346-3[pii] [DOI] [PubMed] [Google Scholar]
- 20.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25(41):9378–83. doi: 10.1523/JNEUROSCI.2100-05.2005. 25/41/9378[pii]10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19(3):612–8. doi: 10.1093/cercor/bhn107. bhn107[pii]10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-beta induces expression of an NMDA-receptor subunit. Nature. 1997;390(6661):691–4. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- 23.Tan GH, Liu YY, Hu XL, Yin DM, Mei L, Xiong ZQ. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci. 2012;15(2):258–66. doi: 10.1038/nn.3005. nn.3005[pii]10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- 24.Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31(1):15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. 31/1/15[pii]10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen Y, Planel E, Herman M, Figueroa HY, Wang L, Liu L, et al. Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J Neurosci. 2008;28(10):2624–32. doi: 10.1523/JNEUROSCI.5245-07.2008. 28/10/2624[pii]10.1523/JNEUROSCI.5245-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(1):96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 27.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337(6100):1357–60. doi: 10.1126/science.1220845. 337/6100/1357[pii]10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iivanainen E, Paatero I, Heikkinen SM, Junttila TT, Cao R, Klint P, et al. Intra-and extracellular signaling by endothelial neuregulin-1. Experimental cell research. 2007;313(13):2896–909. doi: 10.1016/j.yexcr.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Kalinowski A, Plowes NJ, Huang Q, Berdejo-Izquierdo C, Russell RR, Russell KS. Metalloproteinase-dependent cleavage of neuregulin and autocrine stimulation of vascular endothelial cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(7):2567–75. doi: 10.1096/fj.08-129072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. The American journal of physiology. 1999;277(6 Pt 2):H2205–11. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 31.Lok J, Wang H, Murata Y, Zhu HH, Qin T, Whalen MJ, et al. Effect of neuregulin-1 on histopathological and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2007;24(12):1817–22. doi: 10.1089/neu.2007.0372. [DOI] [PubMed] [Google Scholar]
- 32.Dimayuga FO, Ding Q, Keller JN, Marchionni MA, Seroogy KB, Bruce-Keller AJ. The neuregulin GGF2 attenuates free radical release from activated microglial cells. J Neuroimmunol. 2003;136(1-2):67–74. doi: 10.1016/s0165-5728(03)00003-1. S0165572803000031[pii] [DOI] [PubMed] [Google Scholar]
- 33.Mencel M, Nash M, Jacobson C. Neuregulin upregulates microglial alpha7 nicotinic acetylcholine receptor expression in immortalized cell lines: implications for regulating neuroinflammation. PLoS One. 2013;8(7):e70338. doi: 10.1371/journal.pone.0070338. 10.1371/journal.pone.0070338PONE-D-13-12930[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asp L, Beraki S, Kristensson K, Ogren SO, Karlsson H. Neonatal infection with neurotropic influenza A virus affects working memory and expression of type III Nrg1 in adult mice. Brain Behav Immun. 2009;23(6):733–41. doi: 10.1016/j.bbi.2009.04.004. S0889-1591(09)00106-8[pii]10.1016/j.bbi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322(2):440–6. doi: 10.1016/j.bbrc.2004.07.149. 10.1016/j.bbrc.2004.07.149S0006-291X(04)01627-4[pii] [DOI] [PubMed] [Google Scholar]
- 36.Kanakry CG, Li Z, Nakai Y, Sei Y, Weinberger DR. Neuregulin-1 regulates cell adhesion via an ErbB2/phosphoinositide-3 kinase/Akt-dependent pathway: potential implications for schizophrenia and cancer. PLoS One. 2007;2(12):e1369. doi: 10.1371/journal.pone.0001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marballi K, Quinones MP, Jimenez F, Escamilla MA, Raventos H, Soto-Bernardini MC, et al. In vivo and in vitro genetic evidence of involvement of neuregulin 1 in immune system dysregulation. J Mol Med (Berl) 2010;88(11):1133–41. doi: 10.1007/s00109-010-0653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, et al. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci. 2010;30(15):5437–50. doi: 10.1523/JNEUROSCI.5169-09.2010. 30/15/5437[pii]10.1523/JNEUROSCI.5169-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finigan JH, Mishra R, Vasu VT, Silveira LJ, Nethery DE, Standiford TJ, et al. Bronchoalveolar lavage neuregulin-1 is elevated in acute lung injury and correlates with inflammation. Eur Respir J. 2013;41(2):396–401. doi: 10.1183/09031936.00004912. 09031936.00004912[pii]10.1183/09031936.00004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maki T, Hayakawa K, Pham LD, Xing C, Lo EH, Arai K. Biphasic mechanisms of neurovascular unit injury and protection in CNS diseases. CNS Neurol Disord Drug Targets. 2013;12(3):302–15. doi: 10.2174/1871527311312030004. CDTCNSND-EPUB-20130227-12[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond DM, et al. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One. 2013;8(1):e53376. doi: 10.1371/journal.pone.0053376. 10.1371/journal.pone.0053376PONE-D-12-28513[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–28. doi: 10.1016/j.pneurobio.2011.08.005. S0301-0082(11)00150-X[pii]10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safa RN, Peng XY, Pentassuglia L, Lim CC, Lamparter M, Silverstein C, et al. Neuregulin-1beta regulation of embryonic endothelial progenitor cell survival. Am J Physiol Heart Circ Physiol. 2011;300(4):H1311–9. doi: 10.1152/ajpheart.01104.2009. ajpheart.01104.2009[pii]10.1152/ajpheart.01104.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hedhli N, Dobrucki LW, Kalinowski A, Zhuang ZW, Wu X, Russell RR, 3rd, et al. Endothelial-derived neuregulin is an important mediator of ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res. 2012;93(3):516–24. doi: 10.1093/cvr/cvr352. cvr352[pii]10.1093/cvr/cvr352. [DOI] [PMC free article] [PubMed] [Google Scholar]


