Abstract
The treatment of acoustic neuromas (AN) usually involves surgical excision or stereotactic radiosurgery. However, for large AN (mean diameter > 3 cm), stereotactic radiosurgery is rarely used, leaving patients with limited noninvasive treatment options. Recently, the use of fractionated stereotactic radiotherapy (FSRT) has been effective in treating small to medium-sized AN. We present a patient with a large AN treated with FSRT. The patient was a 43-year-old man presenting with imbalance, tinnitus, vertigo, and right-sided hearing decline associated with vomiting and hydrocephalus. Magnetic resonance (MR) imaging revealed a large, 3.8-cm, right cerebellopontine-angle tumor compressing the fourth ventricle. Following right frontal ventriculoperitoneal shunt placement, the patient underwent FSRT for treatment of the tumor. Using the Radionics X-Knife 4.0 3D treatment planning system, a total of 54 Gy was delivered in 1.8-Gy daily fractions with the prescription isodose line of 90%. Treatments were delivered using a dedicated Varian 6/100 linear accelerator, and head immobilization was achieved with the Gill-Thomas-Cosman relocatable stereotactic frame. The patient was subsequently evaluated with serial contrast-enhanced MR imaging. Following FSRT, local control (defined as the absence of tumor progression) was achieved, and treatment was well tolerated. There was no hearing-related, trigeminal, or facial-nerve morbidity following FSRT at 63-month follow-up. Treating a patient with a large AN with FSRT resulted in local tumor control, with no trigeminal nerve, facial nerve, or hearing-related morbidity. These results support FSRT as a potential noninvasive treatment modality for AN some would consider too large for single-fraction stereotactic radiosurgery (SRS).
Keywords: Fractionated stereotactic radiotherapy, Acoustic neuroma, Tumor size, Morbidity
Introduction
The emergence of single-fraction stereotactic radiosurgery (SRS) over the past two decades has greatly altered the management of acoustic neuromas (AN)/vestibular schwannomas by neurosurgeons, neuro-oncologists and radiation oncologists. Multiple studies comparing SRS with microsurgical excision have revealed that SRS has less morbidity and is more cost effective, with similar efficacy to surgical resection in controlling tumor progression for AN with an average diameter of less than 3 cm [1–4]. However, for large AN (mean diameter > 3 cm), decreased efficacy along with increasing facial and trigeminal nerve morbidity and hearing loss following SRS make it a less attractive treatment option in comparison with microsurgery [5, 6].
Fractionated stereotactic radiotherapy (FSRT) is a noninvasive alternative treatment modality for tumors of the skull base. Although FSRT requires multiple irradiation sessions, recent reports have indicated that for AN with an average diameter <3 cm, FSRT provides comparable efficacy to SRS with significantly reduced morbidity to adjacent structures, particularly the trigeminal nerve [7–10]. Because of this lower morbidity, FSRT has been utilized for large (>3 cm) AN in a limited number of cases [11]. To further enhance the existing knowledge regarding the safety and efficacy of FSRT for this patient population, we detail our experience in treating a patient with a large acoustic tumor.
Clinical materials and methods
Patient history
Our patient was a 43-year-old man with a past medical history of stroke who presented with a 1–2 year history of worsening imbalance, along with new-onset bilateral tinnitus, vertigo, and right-sided hearing decline associated with vomiting and hydrocephalus. Audiometric testing confirmed right sensorineural hearing loss, and magnetic resonance (MR) imaging revealed a large tumor in the area of the right cerebellopontine angle without definite invasion of the internal auditory canal but with compression of the fourth ventricle. The patient had no facial weakness or changes in facial sensation. Due to his clinical and radiographic findings, he underwent successful placement of a right frontal ventriculoperitoneal shunt. Following shunt placement, he was considered for microsurgery, SRS, or FSRT for treatment of his cerebellopontine-angle lesion. Although microsurgery was presented to the patient, he was reluctant to undergo surgery, and due to the large size of the tumor (mean diameter = 3.8 cm; maximum diameter = 4.1 cm), he chose FSRT over SRS.
Fractionated stereotactic radiotherapy treatment
All FSRT treatments were overseen by the neurosurgeon, radiation oncologist and medical physicist. Following a standardized testing simulation, the patient had FSRT treatment planning for his right AN. After placement of a Gill-Thomas-Cosman relocatable headframe for immobilization (Integra Radionics, Burlington, MA, USA), a computed tomography (CT) scan with contrast was obtained, with the area of tumor scanned by 2-mm slices and the remainder of the head by 4- and 8-mm slices. Treatment planning (Fig. 1) was performed using the Radionics X-Knife 4.0 three-dimensional (3D) planning system (Integra Radionics) as previously described [12]. The size of the tumor was 4.1 cm × 3.5 cm × 3.7 cm (mean diameter = 3.8 cm), which was encompassed with two overlapping isocenters each measuring 4.5 cm in diameter. The patient received daily fractions of 1.8 Gy until a total target dose of 54 Gy was administered. The radiation was prescribed to the 90% isodose line using five rotational arcs and one stationary beam. A mean dose of 0.082 Gy was delivered to the brainstem per daily fraction. Thirty treatments were stereotactically delivered over 6 weeks using a dedicated Varian 6/100 linear accelerator (Varian Medical Systems, Inc., Palo Alto, CA, USA). The patient tolerated the procedure well, with no immediate or delayed posttreatment complications.
Fig. 1.
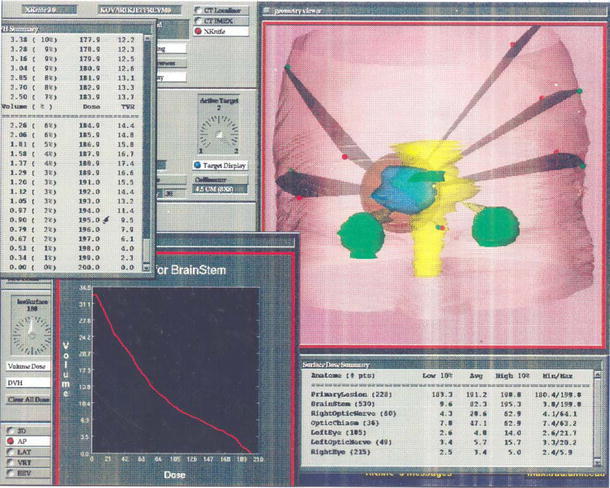
Anterior three-dimensional view on the X-Knife planning software demonstrating the right cerebellopontine-angle acoustic neuroma (blue), the five arcs used for treatment, and the 4.5-cm cone (red) used to encompass the lesion during treatment planning for fractionated stereotactic radiotherapy. The tumor is depicted in relation to the brainstem (yellow), eyes (green), and optic chiasm (green). The measured size of the tumor was 4.1 cm × 3.5 cm × 3.7 cm
Posttreatment course
Following FSRT, the patient was serially evaluated with pre- and post-gadolinium-enhanced MR imaging and the Gardner–Robertson hearing classification system [13]. Local tumor control (defined as the absence of tumor progression) was achieved and sustained. At his last follow-up examination, 63 months after treatment, he continued to demonstrate local tumor control, with no evidence of hearing compromise in comparison with his hearing function prior to FSRT. He experienced no signs or symptoms of trigeminal nerve morbidity, facial nerve morbidity, acute complications, or other signs of radiation injury following FSRT.
Discussion
Presenting typically with unilateral sensorineural hearing loss, hydrocephalus, tinnitus, and/or imbalance, large AN (mean diameter >3 cm) tend to enlarge within 1–2 years from diagnosis, potentially resulting in significant morbidity due to brainstem compression and lower cranial nerve damage by these lesions [14, 15].
The traditional standard of care for the treatment of large AN has been microsurgical excision, which to date has proven to be the treatment modality with the most long-term efficacy compared with SRS or observation alone [6, 14, 16]. However, with the recent advent of FSRT for small and medium-sized AN demonstrating low morbidity and excellent clinical efficacy, it is possible that FSRT may represent an effective noninvasive alternative to microsurgery in patients with large AN [7, 10]. Previously, a small subgroup (five patients with tumors >3 cm) of an extensive acoustic neuroma FSRT series was reported to have experienced adequate tumor control with minimal morbidity [11]. To contribute to the existing knowledge regarding FSRT for large AN, we detailed our experience in a single patient.
Following operative ventriculoperitoneal shunt placement for the treatment of our patient’s symptomatic hydrocephalus, subsequent FSRT resulted in complete local tumor control. This long-term tumor control (>5 years) came without subsequent return of hydrocephalus or compromises in hearing, trigeminal nerve function, or facial nerve function. These findings are comparable to reported results for alternative treatment modalities for AN of diameter >3 cm [6, 14, 17, 18]. This report indicates that there may be a role for FSRT in the treatment of large AN following appropriate management of more immediate associated symptomatology.
Although the findings of this report are encouraging, they must be tempered by the fact that this study involves a single case, thereby limiting the ability to conclude definitively that FSRT is comparable/superior to microsurgery or SRS for asymptomatic large AN. However, the fact that this study supports the positive findings of a previous report involving FSRT for five patients with large AN lends strength to the hypothesis that FSRT may be a useful treatment modality for AN >3 cm [11]. Future prospective randomized controlled studies involving asymptomatic large AN managed by observation, microsurgery, SRS, or FSRT will be required to make a definitive determination of the optimal treatment modality for this patient population.
Conclusion
In our experience of treating a patient with a large AN (maximum diameter = 4.1 cm; mean diameter = 3.8 cm), following FSRT, the patient experienced long-term local tumor control (>5 years) with no trigeminal nerve, facial nerve, or hearing-related morbidity. These findings indicate that FSRT may be a viable noninvasive treatment modality for AN that may be too large to be successfully treated with SRS. Subsequent prospective studies involving larger patient sizes will be needed to adequately address this issue.
Footnotes
No author received financial support in conjunction with the generation of this article.
References
- 1.Pollock BE, Lunsford LD, Kondziolka D, Flickinger JC, Bissonette DJ, Kelsey SF, Jannetta PJ. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36:215–229. doi: 10.1227/00006123-199501000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Myrseth E, Moller P, Pedersen PH, Vassbotn FS, Wentzel-Larsen T, Lund-Johansen M. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927–935. doi: 10.1055/s-2005-916493. [DOI] [PubMed] [Google Scholar]
- 3.Pollock BE, Driscoll CL, Foote RL, Link MJ, Gorman DA, Bauch CD, Mandrekar JN, Krecke KN, Johnson CH. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77–85. doi: 10.1227/01.NEU.0000219217.14930.14. [DOI] [PubMed] [Google Scholar]
- 4.Wellis G, Nagel R, Vollmar C, Steiger HJ. Direct costs of microsurgical management of radiosurgically amenable intracranial pathology in Germany: an analysis of meningiomas, acoustic neuromas, metastases and arteriovenous malformations of less than 3 cm in diameter. Acta Neurochir (Wien) 2003;145:249–255. doi: 10.1007/s00701-003-0007-4. [DOI] [PubMed] [Google Scholar]
- 5.Flickinger JC, Lunsford LD, Coffey RJ, Linskey ME, Bissonette DJ, Maitz AH, Kondziolka D. Radiosurgery of acoustic neurinomas. Cancer. 1991;67:345–353. doi: 10.1002/1097-0142(19910115)67:2<345::AID-CNCR2820670205>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Kondziolka D, Lunsford LD, Flickinger JC. Acoustic tumors: operation versus radiation–making sense of opposing viewpoints. Part II. Acoustic neuromas: sorting out management options. Clin Neurosurg. 2003;50:313–328. [PubMed] [Google Scholar]
- 7.Combs SE, Volk S, Schulz-Ertner D, Huber PE, Thilmann C, Debus J. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT): long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys. 2005;63:75–81. doi: 10.1016/j.ijrobp.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Fuss M, Debus J, Lohr F, Huber P, Rhein B, Engenhart-Cabillic R, Wannenmacher M. Conventionally fractionated stereotactic radiotherapy (FSRT) for acoustic neuromas. Int J Radiat Oncol Biol Phys. 2000;48:1381–1387. doi: 10.1016/S0360-3016(00)01361-4. [DOI] [PubMed] [Google Scholar]
- 9.Andrews DW, Suarez O, Goldman HW, Downes MB, Bednarz G, Corn BW, Werner-Wasik M, Rosenstock J, Curran WJ., Jr Stereotactic radiosurgery and fractionated radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50:1265–1278. doi: 10.1016/S0360-3016(01)01559-0. [DOI] [PubMed] [Google Scholar]
- 10.Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys. 2003;56:1390–1396. doi: 10.1016/S0360-3016(03)00444-9. [DOI] [PubMed] [Google Scholar]
- 11.Williams JA. Fractionated stereotactic radiotherapy for acoustic neuromas. Acta Neurochir (Wien) 2002;144:1249–1254. doi: 10.1007/s00701-002-0974-x. [DOI] [PubMed] [Google Scholar]
- 12.McClelland S, 3rd, Higgins PD, Gerbi BJ, Orner JB, Hall WA. Fractionated stereotactic radiotherapy for pituitary adenomas following microsurgical resection: safety and efficacy. Technol Cancer Res Treat. 2007;6:177–180. doi: 10.1177/153303460700600304. [DOI] [PubMed] [Google Scholar]
- 13.Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97:55–66. doi: 10.1177/000348948809700110. [DOI] [PubMed] [Google Scholar]
- 14.Bederson JB, Von Ammon K, Wichmann WW, Yasargil MG. Conservative treatment of patients with acoustic tumors. Neurosurgery. 1991;28:646–651. doi: 10.1097/00006123-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Laasonen EM, Troupp H. Volume growth rate of acoustic neurinomas. Neuroradiology. 1986;28:203–207. doi: 10.1007/BF00548193. [DOI] [PubMed] [Google Scholar]
- 16.Karpinos M, Teh BS, Zeck O, Carpenter LS, Phan C, Mai WY, Lu HH, Chiu JK, Butler EB, Gormley WB, Woo SY. Treatment of acoustic neuroma: stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys. 2002;54:1410–1421. doi: 10.1016/S0360-3016(02)03651-9. [DOI] [PubMed] [Google Scholar]
- 17.Cerullo CJ, Grutsch JF, Heiferman K, Osterdock R. The preservation of hearing and facial nerve function in a consecutive series of unilateral vestibular nerve schwannoma surgical patients (Acoustic neuroma) Surg Neurol. 1993;39:485–493. doi: 10.1016/0090-3019(93)90036-Z. [DOI] [PubMed] [Google Scholar]
- 18.Haines SJ, Levine SC. Intracanalicular acoustic neuroma: early surgery for preservation of hearing. J Neurosurg. 1993;79:515–520. doi: 10.3171/jns.1993.79.4.0515. [DOI] [PubMed] [Google Scholar]


