Summary
A coumarin derivative apaensin was identified to induce apoptosis of cancer cells through its activation of JNK and p38 MAPK, which act coordinately to activate the Nur77-Bcl-2 apoptotic pathway by inducing Nur77 nuclear export and Nur77 interaction with Bcl-2, respectively.
Abstract
Coumarins are plant-derived natural products with a broad range of known pharmacological activities including anticancer effects. However, the molecular mechanisms by which this class of promising compounds exerts their anticancer effects remain largely unknown. We report here that a furanocoumarin named apaensin could effectively induce apoptosis of cancer cells through its activation of Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK). Apoptosis induction by apaensin in cancer cells was suppressed by chemical inhibitors of JNK and p38 MAPK. Inhibition of the expression of orphan nuclear receptor Nur77 by small interfering RNA (siRNA) approach also abrogated the death effect of apaensin. Molecular analysis demonstrated that JNK activation was required for the nuclear export of Nur77, a known apoptotic event in cancer cells. Although p38 MAPK activation was not involved in Nur77 nuclear export, it was essential for Nur77 mitochondrial targeting through induction of Nur77 interaction with Bcl-2, which is also known to convert Bcl-2 from an antiapoptotic to a proapoptotic molecule. Together, our results identify a new natural product that targets orphan nuclear receptor Nur77 through its unique activation of JNK and p38 MAPK and provide insight into the complex regulation of the Nur77-Bcl-2 apoptotic pathway.
Introduction
Coumarins (2H-1-benzopyran-2-one) consist of a large class of phenolic substances found in a wide variety of natural sources with broad pharmacological activities that are beneficial to human health, such as reducing the risk of cancer, diabetes, cardiovascular and brain diseases (1,2). Many coumarin derivatives are currently being used clinically or under clinical evaluation for their therapeutic applications including photochemotherapy, antitumor anti-HIV therapy, antibacterial, anti-inflammatory and anticoagulant properties (1,2). Psoralen, a furanocoumarin derived from the condensation of a coumarin nucleus with a furan ring, was introduced into clinical practice as early as 1974 for treating psoriasis (3). Since then, several natural and synthetic derivatives of furanocoumarin have been used in the treatment of various skin diseases, psoriasis and cutaneous T-cell lymphoma (1,2). Due to their potent induction of cell differentiation and apoptosis, many new potential therapeutic effects, especially the anticancer activity, are being discovered for this class of compounds. 8-Methoxypsoralen induces apoptosis of HepG2 hepatocellular carcinoma cells through down regulation of the expression of DEC1 gene (4), while imperatorin, a major active furanocoumarin enriched in the root of Angelicae Dahuricae, induces cancer cell apoptosis through death receptor and mitochondria dependent pathways in vitro and in vivo (5). However, the anticancer effects of furanocoumarins and their mechanisms of action remain to be further studied in order to explore their cancer therapeutic potential.
Nur77 (also called NGFI-B or TR3), an orphan member of the nuclear receptor superfamily, is perhaps the most potent apoptotic member of the superfamily (6–9). Accumulating evidence reveals a critical role of Nur77 in the regulation of the growth, survival and apoptosis of cancer cells (6–9). Nur77 is overexpressed in a variety of cancer cells, which in most of case correlates with the growth and survival of cancer cells. Ectopic expression of Nur77 stimulates cell cycle progression and proliferation (10), while silencing Nur77 inhibits the growth, survival and migration of a variety of cancer cells (10–14). Surprisingly, down regulation of Nur77 expression is part of a gene signature predicting tumor metastasis (15), while simultaneous ablation of the genes encoding Nur77 and closely related Nor1 causes acute myeloid leukemia in mice (16). Thus, Nur77 possesses both oncogenic and tumor suppressor activities. The observation that Nur77 is a critical mediator of the apoptotic effect of a variety of death stimuli including chemotherapeutic agents in different types of cancer cells provides a plausible explanation for the somewhat paradox opposing biological activities of this receptor protein (6–9). While the growth promoting effect of Nur77 appears to be dependent on its nuclear action, the death effect of Nur77 involves its translocation from the nucleus to the cytoplasm (6–9). In response to some apoptotic stimuli, Nur77 translocates from the nucleus to the cytoplasm where it targets mitochondria to induce cytochrome c release and apoptosis in various cancer cells (10,17–27). Nur77 targets mitochondria through its interaction with Bcl-2, resulting in conversion of Bcl-2 from an antiapoptotic to a proapoptotic molecule (21–23,25,28,29). Because Bcl-2 is often overexpressed in tumor cells, the ability of Nur77 to convert Bcl-2 from a cancer cell protector to a killer suggests that targeting Nur77-Bcl-2 may lead to selective apoptotic pathway induction in cancer cells, which is therapeutic desirable (6,8).
The diverse and sometimes opposing biological effects of Nur77 is subjected to complex regulations including posttranslational modifications and ligand binding in a cell specific and context dependent manner (6–9). Several natural and synthetic compounds, such as the retinoid-related molecule AHPN (also called CD437 10,18,22), 1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methanes (C-DIMs (30)), the octaketide cytosporone B (31), etoposide (17), 5-fluorouracil (32), certain nonsteroidal antiinflammatory drugs (32), chenodeoxycholic acid derivatives (33), histone deacetylase inhibitors (34), n-butylenephthalide (35), calcium ionophores (17), could target Nur77 to modulate the growth and apoptosis of cancer cells. Cytosporone B (31) and perhaps 1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methanes (C-DIMs (30)) may directly bind to Nur77 to provoke its killing effects. However, most of the apoptotic stimuli that induce Nur77 dependent apoptosis act indirectly through their regulation of the expression of Nur77 and/or posttranslational modification of the Nur77 protein (6–9). Phosphorylation of Nur77 by JNK promotes its cytoplasmic localization and apoptotic effects, while its phosphorylation by AKT has a reverse effect (19). However, how different signaling pathways act coordinately to regulate the Nur77-Bcl-2 pathway remains to be studied.
We previously reported (36) that several furanocoumarins from the root of A.Dahuricae (5,36) could regulate transactivation of nuclear receptor retinoid X receptor-alpha (36). The aim of the current study was to examine whether and how they induced Nur77-dependent apoptosis in cancer cells known to be sensitive to the Nur77-Bcl-2 apoptotic pathway (10). We report here that one of the furanocoumarins, named as apaensin, could induce apoptosis of NIH-H460 lung cancer and MCF-7 breast cancer cells. Our investigation of its mechanism of action showed that apaensin-induced apoptosis required its activation of JNK and p38 MAPK, which acted coordinately to modulate the Nur77-Bcl-2 apoptotic pathway through induction of Nur77 nuclear export and subsequently its interaction with Bcl-2, respectively.
Materials and methods
Isolation of natural products
Apaensin and other furanocoumarins were isolated from the dry root of A.Dahuricae as described previously (36). The purity of these four compounds is all greater than 95%—determined by nuclear magnetic resonance (NMR) spectrum and High-performance liquid chromatography.
Reagents
Lipofectamine 2000 from Invitrogen, goat anti-rabbit and anti-mouse secondary antibody conjugated to horseradish peroxidase from Thermo Fisher Scientific, anti-mouse/rabbit IgG conjugated with Cy3, anti-mouse/rabbit IgG conjugated with fluorescein isothiocyanate from Chemicon International, anti-Nur77 (3960), anti-P-p38 (9215), anti-p38(9212), anti-P-JNK (9251), anti-JNK (9252), anti-tubulin (2144), anti–poly (ADP ribose) polymerase (PARP) (9542) from Cell Signal Technology, anti-Hsp60 (sc-7150), anti-Bcl-2 (sc-509), anti-Bax (6A7; sc-23959), anti-Myc (9E10; sc-40) from Santa Cruz Biotechnology, anti-β-actin antibody, anti-Flag (M2) antibody, SB203580, SP600125, leptomycin B (LMB) from Sigma, BIRB796 (Doramapimod, 1358) from Axon Medchem, polyvinylidene difluoride membranes from Millipore, enhanced chemiluminescence reagents from GE Healthcare and a cocktail of proteinase inhibitors from Roche were used in this study. Cell Mitochondria Isolation Kit was from Beyotime Institute of Biotechnology, China. All other chemicals used were commercial products of analytic grade obtained from Sigma.
Cell culture
NIH-H460 lung cancer cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, and MCF-7 breast cancer cells were grown in Dulbeccos Modified Eagles (DME) medium supplemented with 10% fetal bovine serum in a humidified atmosphere containing 5% CO2 at 37°C. Cells seeded onto cell culture dishes and kept in medium with 10% fetal bovine serum for 24 h were treated with specified apaensin for the indicated times. When cells were cotreated with chemical inhibitors, they were added 1 h before apaensin. Cell transfections were performed using Lipofectamine 2000 transfection reagent following the manufacturer’s instructions (22). Briefly, plasmid DNAs were mixed with Lipofectamine® Reagent in Opti-MEM® Medium at 1:1 ratio, incubated at room temperature for 15 min before adding to 70–90% confluent cells.
Co-immunoprecipitation assays
For co-immunoprecipitation assay, MCF-7 cells grown in 10cm dishes were transfected with Myc-Nur77 and Flag-Bcl-2. Transfected MCF-7 cells were lysed in 500 μl of NETN (10mM Tris–HCl, pH 8.0, 150mM NaCl, 10mM ethylenediaminetetraacetic acid and 0.2% NP-40) buffer containing protease inhibitors (Sigma). Lysate was incubated with 1 μg anti-Flag monoclonal antibody (Santa Cruz Biotechnology) at 4°C for 2 h. Immuno-complexes were then precipitated with 40 μl of protein A/G-sepharose (Santa Cruz Biotechnology). After extensive washing with NETN buffer, beads were boiled in 40 μl loading buffer and analyzed by western blotting (17,21,22).
Cellular fractionation
Briefly, cells were washed with cold phosphate-buffered saline (PBS), lysed in 0.5ml of buffer A (10mM HEPES-KOH, pH 7.9, 1.5mM MgCl2, 10mM KCl, 0.5mM dithiothreitol, 10mM of NaF and 1mM Na3VO4) with a cocktail of proteinase inhibitors on ice for 5min. The cell lysates were centrifuged at 10 000 r.p.m. for 30 s. The supernatant (cytosolic fraction) was collected. The nuclei-containing pellet was washed with cold PBS and resuspended in buffer B (20mM HEPES-KOH, pH 7.9, 25% glycerol, 420mM NaCl, 1.5mM MgCl2, 0.2mM ethylenediaminetetraacetic acid, 0.5mM dithiothreitol, 10mM NaF and 1mM Na3VO4) to obtain the nuclear fraction. The isolation of mitochondrial fraction was performed using the Cell Mitochondria Isolation Kit (Beyotime Institute of Biotechnology). Briefly, cells were lysed in 100 μl ice-cold mitochondrial lyses buffer for 10min, and cell suspension was then homogenized for 30 strokes using a tight pestle on ice. The homogenate was centrifuged at 600g for 10min at 4°C, and the resulting supernatant was centrifuged again at 11 000g for 10min at 4°C to obtain mitochondria, which was then resuspended in lyses buffer.
Western blotting
Equal amounts of protein lysates (20–40 μg) were electrophoresed on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk in TBST (50mM Tris-HCl, pH 7.4, 150mM NaCl and 0.1% Tween 20) buffer for 1 h, incubated with various primary antibodies for 2 h and detected with either anti-rabbit (1:5000) or anti-mouse (1:5000) secondary antibodies for 1 h, all of which were undertaken under room temperature. The final immunoreactive products were detected by using enhanced chemiluminescence system, and examined by densitometric analysis.
Immunofluorescence microscopy
Cells mounted on glass slides were permeabilized with PBS containing 0.1% Triton X-100 and 0.1M glycine for 15min on ice, and blocked with 1% BSA in PBS for 30min at room temperature. The cells were incubated with primary antibody (anti-Nur77) (1:200) or anti-Bcl-2 (1:200) respectively at 37°C for 1 h and detected by anti-rabbit IgG conjugated with Cy3 (1:200) or anti-mouse IgG conjugated with fluorescein isothiocyanate (1:200) at room temperature for 30 min. Cells were co-stained with 4',6'-diamidino-2-phenylindole (DAPI) to visualize nuclei. To detect mitochondria, cells were treated with MitoTracker® Red (Invitrogen) before adding the cell lysates. The images were taken using the LSM-510 confocal laser scanning microscope system (Carl Zeiss, Oberkochen, Germany (17,21,22)).
Statistical analysis
Data were expressed as mean ± SD. Each assay was repeated in triplicate in three independent experiments. Statistical significance of differences between groups was analyzed by using Student’s t-test or analysis of variance. P < 0.05 was considered significant.
Results
Apaensin induces cancer cell PAPR cleavage
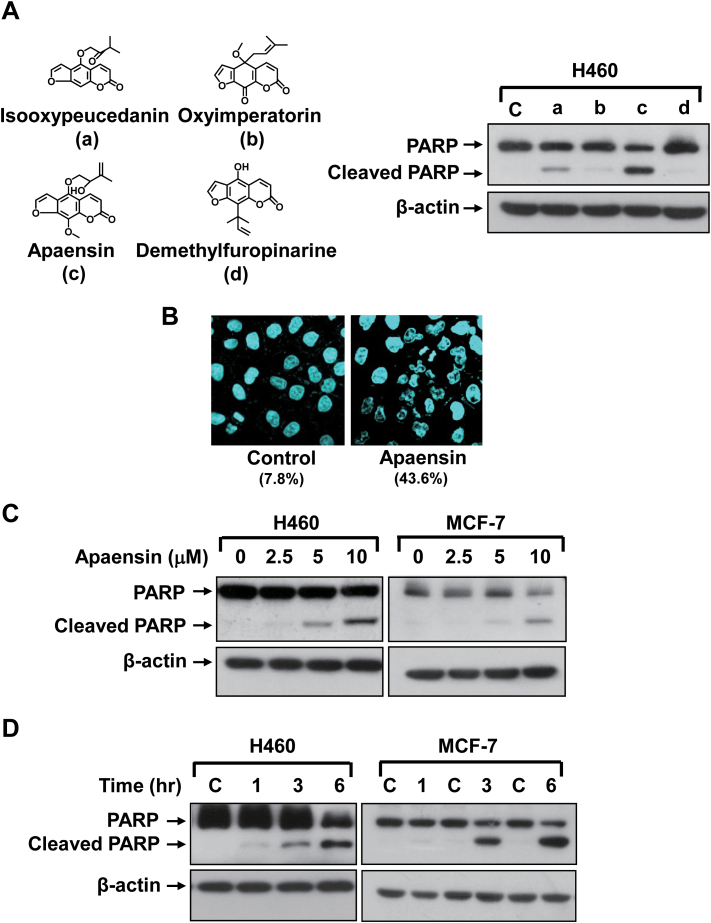
Four furanocoumarin, including isooxypeucedanin, oxyalloimperatorin, apaensin and demethylfuropinarine, were studied for their apoptotic effect in NIH-H460 lung cancer cells known to be sensitive to the Nur77 dependent apoptotic effect (10). Apoptosis was examined for their ability to induce the cleavage of PARP protein, a sensitive apoptotic marker occurring early in the apoptotic response (Figure 1A). Although various degrees of PARP cleavage was observed when cells were treated with 10 μM compound for 6 h, apaensin was the most potent inducer of apoptosis under the conditions used (Figure 1A). The apoptotic effect of apaensin was also confirmed by using DAPI staining that visualizes nuclear morphology of cells before and after apaensin treatment (Figure 1B). Although apoptotic nuclear morphology such as nuclear condensation and fragmentation was seldom seen in the untreated cells (7.8%), 43.6% of NIH-H460 cells displayed the apoptotic nuclear morphology upon treatment with apaensin for 6 h (Figure 1B). Dose response study showed that the induction of PARP cleavage by apaensin was apparent in NIH-H460 lung cancer cells treated with 5 μM apaensin for 3 h, which was further increased when treated with higher concentration of apaensin (Figure 1C). Similar results were obtained in MCF-7 breast cancer cells, in which Nur77 translocation and mitochondrial targeting were observed in response to apoptin (24). Time-course analysis demonstrated that PARP cleavage was observed in both NIH-H460 and MCF-7 cells when they were treated with 10 μM apaensin for as early as 3 h (Figure 1D). Thus, apaensin induces cell apoptosis in a dose- and time-dependent manner.
Fig. 1.
Induction of apoptosis by apaensin. (A) Structure of four coumarin derivatives and their apoptotic effect. NIH-H460 cells treated with 10 μM of isooxypeucedanin (a), oxyalloimperatorin (b), apaensin (c) and demethylfuropinarine (d) for 6h were analyzed for the cleavage of PARP by western blotting. (B) Apoptotic effect of apaensin revealed by DAPI staining. NIH-H460 cells treated with apaensin (10 μM) for 6h were subjected to DAPI staining. Upon apaensin treatment, about 43.6% of cells displaying apoptotic nuclear morphology cells, while 7.8% of cells showed apoptotic nuclear morphology in the absence of treatment. (C) Dose dependent effect of apaensin. NIH-H460 cells or MCF-7 cells treated with apaensin (2.5, 5 and 10 μM) for 3h were analyzed by western blotting. (D) Time-course analysis. NIH-H460 cells or MCF-7 cells treated with apaensin (10 μM) for 1, 3 and 6h were analyzed by western blotting. One of three to five similar experiments is shown.
Activation of JNK and p38 MAPK is required for apaensin-induced apoptosis
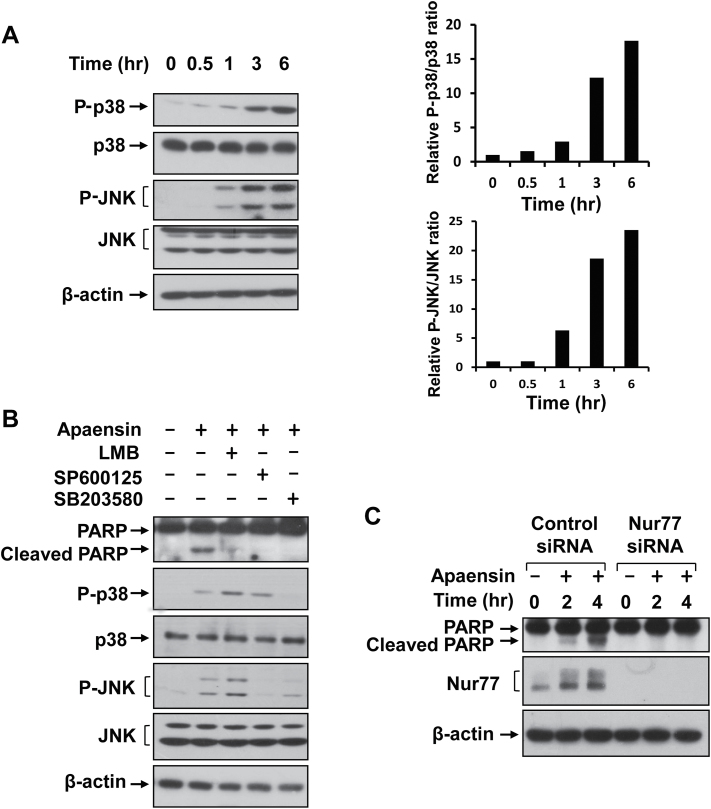
Apoptosis induction in cancer cells by some coumarins was associated with MAPK signaling pathway (37). We therefore investigated whether apaensin could activate JNK and p38 MAPK in NIH-H460 cells by western blotting. Figure 2A showed that both p38 MAPK and JNK were activated upon treatment with 10 μM apaensin in a time-dependent manner. JNK activation was clearly seen after 1 h apaensin treatment, which was further activated when treated for 3 or 6 h. Activation of p38 MAPK by apaensin appeared slightly delayed, with an apparent activation after cells were treated for 3 or 6 h. We next examined whether apaensin activation of JNK or p38 MAPK was required for its apoptotic effect by using chemical inhibitors of JNK and p38 MAPK. NIH-H460 cells treated with apaensin in the absence or presence of the JNK inhibitor SP600125 or the p38 MAPK inhibitor SB203580 were analyzed for PARP cleavage (Figure 2B). Treatment of cells with SP600125 completely blocked apaensin activation of JNK, while it had little effect on the activation of p38 MAPK, whereas treatment of cells with SB203580 inhibited the activation of p38 MAPK but not JNK by apaensin. When the PARP cleavage was assessed, the induction of PARP cleavage by apaensin was completely suppressed when cells were cotreated with either SP600125 or SB203580. These data demonstrated that the activation of JNK and p38 MAPK by apaensin was essential for its apoptotic effect.
Fig. 2.
Role of apaensin activation of p38 MAPK and JNK. (A) Activation of p38 MAPK and JNK by apaensin. NIH-H460 cells treated with 5 μM of apaensin for 0, 0.5, 1, 3, 6h were analyzed for the activation of JNK and p38 MAPK by western blotting using antibodies recognizing phosphorylated (P-) JNK and p38 MAPK. The levels of β-actin were used for loading control. Right panels represent densitometric analyses of relative pJNK/JNK and P-p38/p38 MAPK ratio. (B) Role of p38 MAPK, JNK and LMB. NIH-H460 cells were incubated for 3h with apaensin (10 μM), in the presence or absence of SP600125 (10 μM), SB203580 (10 μM) or LMB (10ng/ml), and analyzed by western blotting for PARP cleavage. (C) Nur77 expression is required for the death effect of apaensin. NIH-H460 cells were transfected with control or Nur77 siRNA for 48h, then treated with or without 5 μM apaensin for the indicated time. Lysates prepared were analyzed for PARP cleavage and Nur77 expression by western blotting. One of three to five similar experiments is shown.
Nur77 expression and its cytoplasmic localization are essential for the death effect of apaensin
JNK plays a critical role in both death receptor-initiated extrinsic and mitochondrial intrinsic apoptotic pathways by upregulating the expression of proapoptotic genes or modulating the activities of mitochondrial pro- and antiapoptotic proteins through phosphorylation (38,39). One of the downstream targets of JNK is Nur77 that translocates from the nucleus to the cytoplasm upon JNK phosphorylation (10,19). To determine whether Nur77 mediated the death effect of apaensin, we first asked whether the nuclear export of proteins was required for the effect of apaensin on inducing apoptosis. Treatment of cells with LMB, an inhibitor of CRM1-dependent nuclear export (17,22), indeed abrogated the death effect of apaensin (Figure 2B), suggesting that the nuclear export of apaensin-targeting protein(s) was essential for its death effect. We next determined whether the expression of Nur77 was required for the death effect of apaensin by siRNA approach. NIH-H460 cells expressed a significant amount of Nur77, which was further increased upon treatment with apaensin (Figure 2C). Transfection with Nur77 siRNA completely suppressed the expression of Nur77 either in the absence or presence of apaensin. When the effect of apaensin on PARP cleavage was examined, we found that apaensin could induce PARP cleavage in cells transfected with the control siRNA but not in cells transfected with the Nur77 siRNA. Thus, Nur77 represents a critical intracellular target mediating the death effect of apaensin. It is worth noting that we could detect PARP cleavage even in cells transfected with Nur77 siRNA when treated with apaensin for longer time, suggesting that targets other than Nur77 might also play a role.
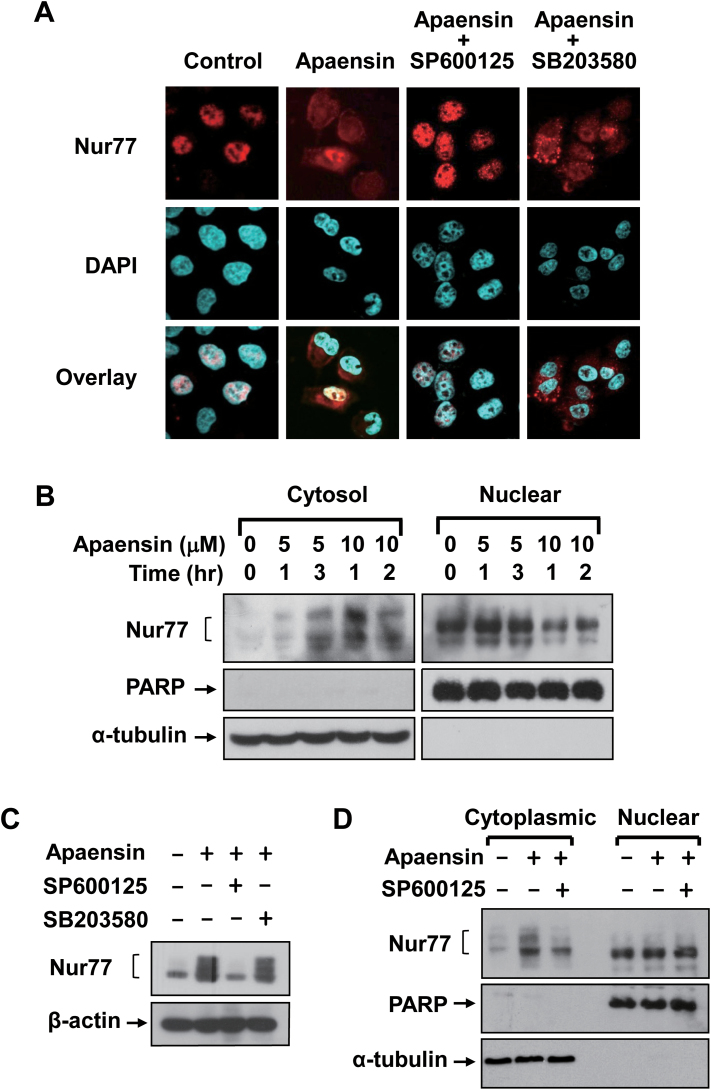
The apoptotic effect of Nur77 in NIH-H460 cells was shown to involve its cytoplasmic localization and subsequent mitochondrial targeting (10,22). We therefore examined whether apaensin regulated the subcellular localization of Nur77 in NIH-H460 cells by immunostaining using anti-Nur77 antibody. In the absence of the apaensin treatment, Nur77 was exclusively localized in the nucleus of the cells (Figure 3A). However, Nur77 was diffusely distributed throughout the cells upon treatment of apaensin, suggesting that apaensin induced the cytoplasmic localization of Nur77. To determine the role of JNK, cells were cotreated with SP600125. Cotreatment with SP600125 could almost completely suppress the effect of apaensin on inducing Nur77 nuclear export. By contrast, the p38 MAPK inhibitor SB203580 had no such an effect (Figure 3A). To confirm the ability of apaensin on inducing Nur77 nuclear export, NIH-H460 cells were treated with 5 or 10 μM apaensin for different periods of time. Cytoplasmic and nuclear fractions were then prepared and analyzed for the presence of Nur77 by immunoblotting (Figure 3B). The purity of the fractions was verified by their selective expression of nuclear protein PARP or cytoplasmic protein α-tubulin. Nur77 was mainly detected in the nuclear but not in the cytoplasmic fraction prepared from control cells (Figure 3B). Upon apaensin treatment, however, the level of Nur77 protein in the nuclear fractions decreased, which was accompanied with an increased level of Nur77 protein in the cytoplasmic fraction.
Fig. 3.
Induction and regulation of Nur77 nuclear export by apaensin. (A) Effect of apaensin on the subcellular localization of Nur77. NIH-H460 cells were treated with apaensin (5 μM) in the presence or absence of SP600125 (10 μM) or SB203580 (10 μM) for 3h. The subcellular localization of Nur77 was examined by immunostaining using anti-Nur77 antibody and revealed by immunofluorescence microscopy. About 95% of control cells showed exclusive nuclear staining of Nur77, while approximately 80–85% of apaensin-treated cells displayed diffused distribution of Nur77 shown. (B) Effect of apaensin on the subcellular localization of Nur77 revealed by cellular fractionation. Nuclear and cytoplasmic fractions were prepared from NIH-H460 cells treated with apaensin (5 μM for 1 and 3h or 10 μM for 1 and 2h) and subjected to western blotting analysis using anti-Nur77 antibody. The purity of cellular fraction was confirmed by using anti-PARP and anti-α-tubulin antibodies. One of three similar experiments is shown. (C) JNK phosphorylation of Nur77. NIH-H460 cells treated with 5 μM apaensin in the presence or absence of 10 μM SP600125 or 10 μM SB203580 were analyzed for Nur77 expression by western blotting using anti-Nur77 antibody. (D) JNK activation is required for induction of Nur77 nuclear export. Nuclear and cytoplasmic fractions were prepared from NIH-H460 cells treated with apaensin (5 μM for 3h) in the presence or absence of SP600125 (10 μM) and subjected to western blotting analysis using anti-Nur77 antibody. The purity of cellular fraction was confirmed by using anti-PARP and anti-α-tubulin antibodies. One of three similar experiments is shown.
Nur77 displayed multiple bands on sodium dodecyl sulfate–polyacrylamide gel electrophoresis upon apaensin treatment (Figure 2C), suggesting its post-translational modifications. Interestingly, the slow-migrating Nur77 band was only seen in the cytoplasmic but not nuclear fraction of cells treated with apaensin (Figure 3B). These observations were consistent with the ability of apaensin to activate JNK known to phosphorylate Nur77 and induce Nur77 nuclear export (19). We therefore examined whether the expression of the slow-migrating Nur77 band was due to apaensin activation of JNK. Indeed, immunoblotting showed that apaensin treatment induced the expression of the slow-migrating Nur77 band, which was inhibited when cells were cotreated with SP600125 but not SB203580 (Figure 3C). Consistently, the presence of the slow-migrating Nur77 protein in the cytoplasmic fraction by apaensin treatment was inhibited when cells were cotreated with SP600125 (Figure 3D). Together, these results demonstrate that apaensin could induce nuclear export of Nur77 through its activation of JNK.
Apaensin induces Nur77 mitochondrial targeting, Nur77 colocalization with Bcl-2 and BAX activation
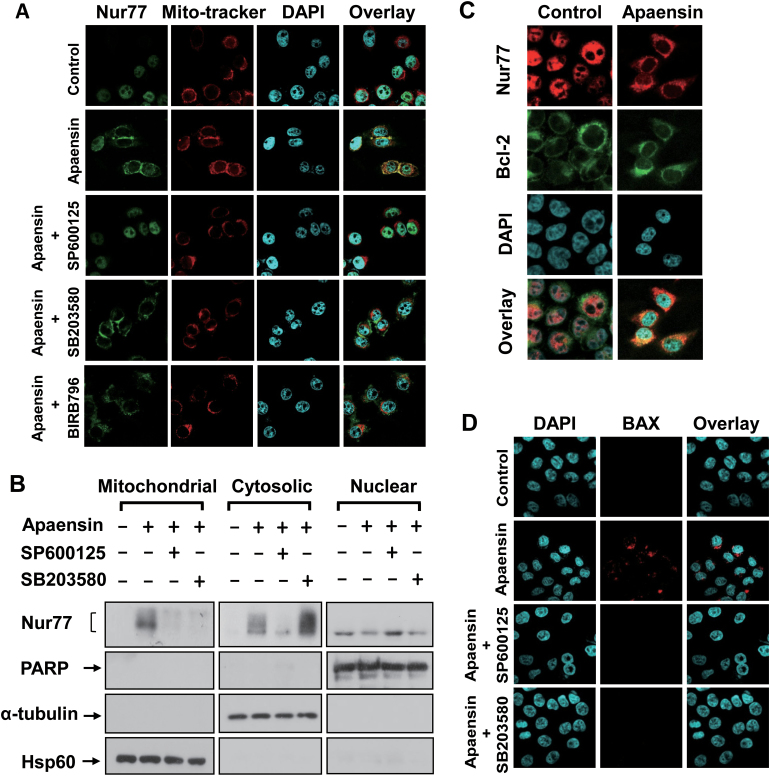
The above observation that apaensin could induce cytoplasmic localization of Nur77, the hallmark of the Nur77-mediated apoptotic pathway (17), prompted us to examine whether the cytoplasmic Nur77 could target mitochondria. Thus, NIH-H460 cells treated with or without apaensin were immunostained with anti-Nur77 antibody and Mito-Tracker® Red that specifically recognizes mitochondria. Confocal microscopy analysis revealed an extensive overlapping of the distribution patterns of Nur77 and Mito-Tracker® Red when cells were treated with apaensin (Figure 4A), demonstrating that the cytoplasmic Nur77 resided mainly at mitochondria. SP600125 cotreatment resulted in exclusive nuclear localization of Nur77, consistent with its inhibitory effect on Nur77 nuclear export (Figure 3A). Interestingly, cotreatment with SB203580 or another p38 MAPK inhibitor BIRB796 (Doramapimod) only impaired the colocalization of Nur77 with Mito-tracker while it had no effect on the cytoplasmic localization of Nur77. These results suggested that apaensin activation of p38 MAPK, unlike its activation of JNK, might act to promote Nur77 mitochondrial targeting but not its nuclear export. To confirm the effect of p38 MAPK and JNK, mitochondrial, cytosolic and nuclear fractions of cells treated with apaensin in the presence or absence of SP600125 or SB203580 were prepared and analyzed for the presence of Nur77 (Figure 4B). In the absence of apaensin treatment, endogenous Nur77 was only found in the nuclear fraction. Upon apaensin treatment, a significant amount of Nur77 was detected in both the mitochondrial fraction and cytosolic factions. Cotreatment of cells with either SP600125 or SB203580 similarly inhibited the presence of Nur77 in the mitochondrial fraction. However, cotreatment with SP600125 but not SB203580 could also reduce the level of Nur77 in the cytosolic fraction. Together, these results demonstrated that apaensin activation of JNK induced Nur77 nuclear export, whereas its activation of p38 MAPK promoted Nur77 mitochondrial targeting.
Fig. 4.
Effect of apaensin on Nur77 mitochondrial targeting and BAX activation. (A) Induction and regulation of Nur77 mitochondrial targeting by apaensin. NIH-H460 cells treated with apaensin (5 μM) for 3h in the presence or absence of SP600125 (10 μM), SB203580 (10 μM) or BIRB796 (1 μM). Endogenous Nur77 was immunostained with anti-Nur77 antibody. Cells were co-stained with Mito-tracker that recognizes the mitochondria and with DAPI to visualize the nuclei. Fluorescent microscopy was used to determine the overlap of Nur77 and Mito-tracker. About 95% of control cells showed exclusive nuclear staining of Nur77, and approximately 65% of apaensin-treated cells displayed colocalization with mitochondria shown. Most of cells displayed exclusive nuclear staining of Nur77 upon cotreatment of apaensin with SP600125, whereas 50–55% of cells exhibited cytoplasmic Nur77 incapable of colocalizing with Mito-tracker when cotreated with apaensin and p38 MAPK inhibitor (SB203580 or BIRB796). (B) Apaensin activation of p38 MAPK is required for Nur77 mitochondrial targeting. Nuclear, cytosolic and mitochondrial fractions prepared from NIH-H460 cells treated with apaensin (5 μM for 3h) in the presence or absence of SP600125 (10 μM) or SB203580 (10 μM) were subjected to western blotting analysis using anti-Nur77 antibody. The purity of cellular fractions was confirmed by using anti-PARP antibody, anti-α-tubulin and Hsp60 antibody. (C) Induction of Nur77 colocalization with Bcl-2 by apaensin. NIH-H460 cells were treated with apaensin (5 μM) for 3h, and the localization of Nur77 and Bcl-2 was examined by immunostaining and revealed by immunofluorescence microscopy. Approximately 75% apaensin-treated cells displayed colocalization with Bcl-2 shown. (D) Induction of BAX activation by apaensin. NIH-H460 cells treated with apaensin (5 μM) for 6h were examined for BAX activation by antibody against BAX (6A7). Cells were also stained with DAPI to visualize the nucleus. About 85% of apaensin-treated cells displayed various degrees of BAX staining shown.
Nur77 interaction with Bcl-2 is known to mediate Nur77 mitochondrial targeting and apoptosis (21,22). To address whether apaensin induction of Nur77 mitochondrial targeting and apoptosis involved Nur77 interaction with Bcl-2, we first examined whether Nur77 and Bcl-2 co-localized by immunostaining NIH-H460 cells with anti-Nur77 and anti-Bcl-2 antibodies. As shown in Figure 4C, Bcl-2 resided predominantly in the cytoplasm, while Nur77 was mainly nuclear in control cells. However, when cells were treated with apaensin, the distribution patterns of Nur77 and Bcl-2 overlapped extensively in the cytoplasm. Thus, Nur77 might target mitochondria through its interaction with Bcl-2. The interaction of Bcl-2 with Nur77 converts Bcl-2 from an antiapoptotic to a proapoptotic molecule, which acts as a BH3-only molecule to activate BAX (21,22). Thus, we analyzed whether BAX activation was involved in apaensin-induced apoptosis by immunostaining NIH-H460 cells with anti-BAX (6A7) antibody that only recognizes the active form of BAX protein (21). In the absence of apaensin treatment, BAX was not active as the 6A7 anti-BAX antibody failed to show any staining (Figure 4D). However, a significant BAX staining was observed in cells treated with apaensin, demonstrating its activation by apaensin. Consistent with their inhibitory effect on apaensin-induced PARP cleavage, cotreatment of cells with either SP600125 or SB203580 completely inhibited BAX staining in apaensin-treated cells. Thus, BAX activation is important for the apoptotic effect of apaensin.
p38 MAPK activation promotes Bcl-2-Nur77 interaction
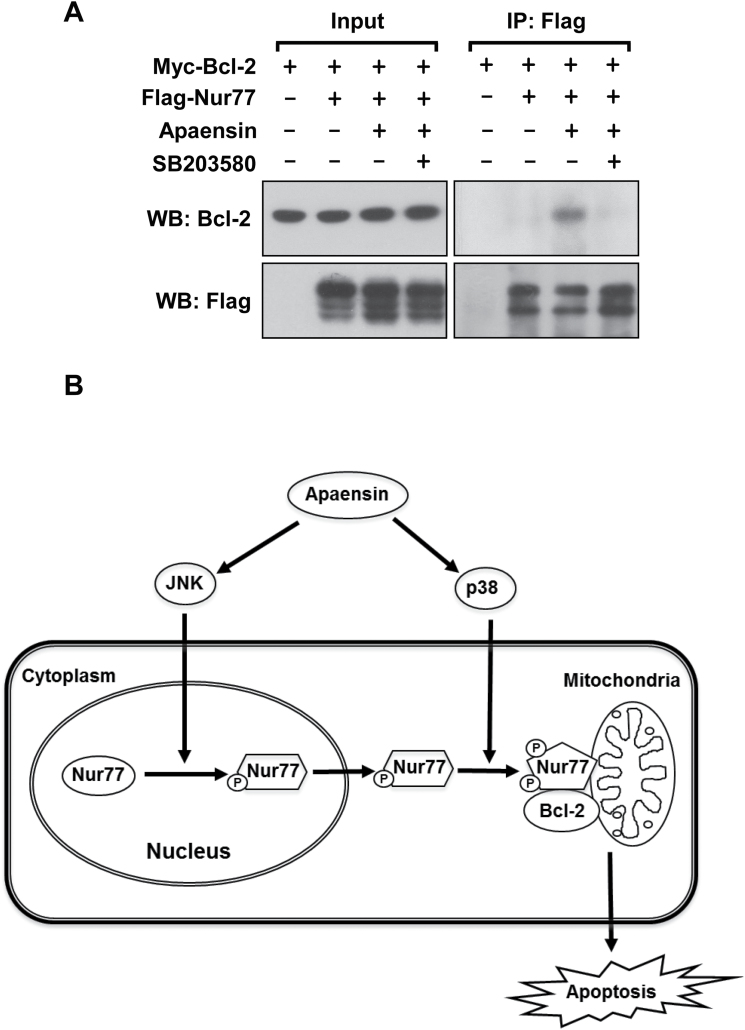
The p38 MAPK inhibitor SB203580 could not affect the ability of apaensin to induce the cytoplasmic localization of Nur77 (Figure 3A), and yet it potently inhibited the effect of apaensin on inducing Nur77 mitochondrial targeting (Figure 4A and B), PARP cleavage (Figure 2B) and BAX activation (Figure 4D). To determine the possibility that apaensin activation of p38 MAPK induced BAX activation and apoptosis through its modulation of Nur77 interaction with Bcl-2, we examined the effect of SB203580 on the interaction of Nur77 with Bcl-2 by co-immunoprecipitation assay. Thus, Myc-tagged Bcl-2 (Myc-Bcl-2) and Flag-tagged Nur77 (Flag-Nur77) were co-transfected into MCF-7 cells, which were then treated with apaensin and/or SB203580 and assayed for the interaction between Myc-Bcl-2 and Flag-Nur77 by co-immunoprecipitation assay (Figure 5A). Myc-Bcl-2 was co-immunoprecipitated by anti-Flag antibody only in cells treated with apaensin, demonstrating that apaensin could promote the interaction of Myc-Bcl-2 with Flag-Nur77. When cells were cotreated with SB203580, apaensin-induced Bcl-2-Nur77 interaction was largely abolished. Thus, p38 MAPK activation by apaensin is crucial for its promotion of the Bcl-2-Nur77 interaction.
Fig. 5.
Role of p38 MAPK in Nur77-Bcl-2 interaction. (A) Induction of Nur77 interaction with Bcl-2 by apaensin and its inhibition by SB203580. Myc-Bcl-2 (2 μg) and Flag-Nur77 (2 μg) alone or together were transfected into MCF-7 cells. After 16h, cells were treated with apaensin (5 μM) in the presence or absence of SB203580 (10 μM) for 3h. Cell lysates were immunoprecipitated by using monoclonal anti-Flag antibody. Lysates and immunoprecipitates were examined by immunoblotting using anti-Flag and anti-Myc antibody. The same membranes were blotted with anti-Flag antibody to determine immunoprecipitation specificity and efficiency. Input represents 5% of cell lysates used in the co-immunoprecipitation assays. (B) Role of apaensin activation of JNK and p38 MAPK in the regulation of the Nur77-Bcl-2 apoptotic pathway. Our results demonstrate that apaensin activation of the Nur77-Bcl-2 apoptotic pathway involves its activation of JNK and p38 MAPK. While apaensin activation of JNK promotes Nur77 nuclear export through phosphorylation of Nur77, apaensin activation of p38 MAPK induces interaction of cytoplasmic Nur77 with Bcl-2. The coordinated action of JNK and p38 MAPK and perhaps other undefined apaensin-regulated cellular factors results in Nur77 mitochondrial targeting, leading to apoptosis of cancer cells.
Discussion
Coumarin and derivatives represent a class of potential anticancer drugs deserving further investigation of their anticancer activities and mechanisms (1,2). Recent studies have demonstrated that certain coumarin derivatives could regulate important biological functions by direct or indirectly acting on several nuclear receptors including estrogen receptor (40,41), peroxisome proliferator-activated receptor-γ (42), androgen receptor (43), progesterone receptor (44,45), pregnane X receptor (46), retinoic acid-related orphan receptor γt (47) and retinoid X receptor-alpha (36). Coumarin derivatives, SP500263 (40) and SS5020 (41), binds to estrogen receptor with high affinity and acts as a coumarin-based selective estrogen receptor modulators to inhibit the growth and breast cancer cells in vitro and in animals. We report here that apaensin, one of the coumarins that we isolated previously (36), induces apoptosis of cancer cells by targeting the Nur77-Bcl-2 apoptosis pathway through its activation of JNK and p38 MAPKs (Figure 5B). Thus, coumarins with their unique structures represent a rich source for the discovery of new nuclear receptor modulators for studying nuclear receptor function and the underlying mechanisms.
Our results demonstrated that apaensin could induce apoptosis in NIH-H460 lung cancer and MCF-7 breast cancer cells in a time- and dose-dependent manner (Figure 1). Previous studies also reported that several coumarins, especially furanocoumarins such as imperatorin (5) and 8-methoxypsoralen (4), could potently induce apoptosis of various cancer cells. Apoptosis induction is therefore likely an important mechanism by which this class of compounds exerts their anticancer effects. How coumarins induce apoptosis of cancer cells remains largely unknown. Results presented in this study demonstrated that apaensin activation of JNK and p38 MAPK was required for its apoptotic effect in cancer cells. JNK and p38 MAPK function in a cell context-specific and cell type-specific manner to integrate signals that affect proliferation, differentiation, apoptosis and survival (38,48). Whether the activation of JNK and p38 MAPK leads to cell proliferation or apoptosis is dependent not only on the stimuli, but also on the cellular context (38,48). Our finding that cotreatment of cancer cells with either the JNK inhibitor SP600125 or the p38 MAPK inhibitor SB203580 inhibited the apoptotic effect of apaensin (Figure 2B) demonstrated that the activation of JNK and p38 MAPK by apaensin was apoptotic at least in cancer cells studied.
Our results also demonstrated that orphan nuclear receptor Nur77 was required for the apoptotic effect of apaensin in cancer cells. One of the apoptotic downstream events of JNK involves its induction of the migration of orphan nuclear receptor Nur77 from the nucleus to mitochondria (10,19). Transfection of Nur77 siRNA, which inhibited the expression of Nur77 in cancer cells, abrogated the death effect of apaensin (Figure 2C). Consistent with the death effect of Nur77 nuclear export, the nuclear export inhibitor LMB abrogated the apoptotic effect of apaensin (Figure 2B). In addition, Nur77, which was predominantly nuclear in control cells, was detected in the cytoplasm upon apaensin treatment (Figure 3A). In response to certain apoptotic stimuli, cytoplasmic Nur77 could target mitochondria through its interaction with Bcl-2, resulting in the conversion of Bcl-2 from an antiapoptotic to a proapoptotic molecule (21,22). Proapoptotic Bcl-2 acts as a BH3-only protein to induce activation of BAX (21). We found that apaensin induced cytoplasmic Nur77 targeted mitochondria (Figure 4A and B) and colocalized with Bcl-2 (Figure 4C). Co-immunoprecipitation assays showed revealed a strong interaction between Nur77 and Bcl-2 in cells treated with apaensin (Figure 5A). Apaensin treatment also resulted in activation of BAX revealed by immunostaining (Figure 4D). Together, these results demonstrate that apaensin is a new activator of the Nur77-Bcl-2 apoptotic pathway.
In studying how apaensin activation of JNK and p38 MAPK was involved in the regulation of the Nur77-Bcl-2 apoptotic pathway, we showed that activation of JNK but not p38 MAPK promoted the nuclear export of Nur77. SP600125 but not SB203580 inhibited the effect of apaensin on inducing cytoplasmic localization of Nur77 (Figure 3A). The role of JNK in inducing Nur77 nuclear export was also demonstrated by our cellular fractionation assays, which showed the presence of a hyperphosphorylated form of Nur77 in the cytoplasmic fraction but not in the nuclear fraction (Figure 3B). Such hyperphosphorylated form of Nur77 was likely caused by JNK phosphorylation as it disappeared in cells treated with SP600125 but not with SB203580 (Figure 3C). These results, which were consistent with our previous observations (10,19), demonstrated that apaensin activation of JNK regulated the Nur77-Bcl-2 apoptotic pathway by promoting Nur77 nuclear export through phosphorylation of Nur77. Whether the activation of JNK leads to cell proliferation or apoptosis depends on stimuli and cellular context (38). Our observation that apaensin activation of JNK led to Nur77 nuclear export suggested that Nur77 expression might be an important prerequisite for JNK to exert its death effect in cancer cells. Whether and how Nur77 expression in cancer cells plays a role in the switch of JNK signaling from survival and death remains to be further investigated.
Another interesting finding reported here is the role of p38 MAPK activation in the regulation of the apoptotic effect of apaensin and the Nur77-Bcl-2 apoptotic pathway. In addition to its activation of JNK, apaensin strongly activated p38 MAPK (Figure 2A). Treatment of cancer cells with the p38 MAPK inhibitor SB203580 inhibited the effect of apaensin on inducing PARP cleavage (Figure 2B) and BAX activation (Figure 4D), similar to the effect of the JNK inhibitor. However, the p38 MAPK inhibitor had no effect on the nuclear export of Nur77 (Figure 3A), suggesting that p38 MAPK might target at the different stage of the Nur77-Bcl-2 apoptotic pathway. Indeed, our confocal microscopy (Figure 4A) and cellular fractionation (Figure 4B) results demonstrated that p38 MAPK inhibitor acted to inhibit the ability of apaensin to induce the association of Nur77 with mitochondria but not its nuclear export. Mitochondrial targeting of Nur77 involves its interaction with Bcl-2 (22). Interestingly, our co-immunoprecipitation assays (Figure 5A) showed that the p38 MAPK inhibitor could abrogate the Nur77-Bcl-2 interaction induced by apaensin. Thus, the activation of p38 MAPK could contribute to the apoptotic effect of apaensin by promoting the interaction of cytoplasmic Nur77 with Bcl-2, leading to its mitochondrial targeting. How p38 MAPK regulates the Nur77-Bcl-2 interaction is currently unknown. There is no apparent p38 MAPK phosphorylation site on Nur77 (not shown). In addition, SB203580 had no effect on the appearance of the high molecular weight Nur77 induced by apaensin (Figure 3C). Thus, it is unlikely that apaensin activation of p38 MAPK acts at Nur77 to achieve its apoptotic effect. However, it is worth noting that p38 MAPK could phosphorylate Bcl-2 at its unstructured loop region, and that the phosphorylation of Bcl-2 by p38 MAPK could inhibit the antiapoptotic effect of Bcl-2, leading to apoptosis (49,50). Interestingly, the unstructured loop region of Bcl-2 mediates Bcl-2 interaction with Nur77 (21,22). Thus, apaensin activation of p38 MAPK could presumably phosphorylate the loop of Bcl-2 by p38 MAPK, which may be responsible for its effect on enhancing the interaction of Bcl-2 with Nur77.
Taken together, the results demonstrate that apaensin promotes apoptosis of cancer cells by targeting the Nur77-Bcl-2 apoptotic pathway through its activation of JNK and p38 MAPK. JNK and p38 MAPK target at different stages of the Nur77-Bcl-2 pathway to confer the death effect of apaensin in cancer cells. Further studies are warranted for the use of apaensin or derivatives as potential chemopreventive and chemotherapeutic agents for cancer.
Funding
U.S. Army Medical Research and Material Command (W81XWH-11-1-0677); National Institutes of Health (CA140980, GM089927, CA179379); California Breast Cancer Research Program (20IB-0138); 985 Project from Xiamen University; the National Natural Science Foundation of China; (NSFC-91129302; NSFC-81370097; NSFC-31271453); Ministry of Education of China, Fundamental Research Funds for the Central Universities (2013121038); Fujian Natural Science Foundation Program (2013J01385).
Acknowledgements
We thank L.Fraser and J.Kang for help preparing the manuscript.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- DAPI
4',6'-diamidino-2-phenylindole
- JNK
Jun N-terminal kinase
- LMB
leptomycin B
- MAPK
mitogen-activated protein kinase
- PARP
poly (ADP ribose) polymerase
- PBS
phosphate-buffered saline
- siRNA
small interfering RNA.
References
- 1. Riveiro M.E., et al. (2010). Coumarins: old compounds with novel promising therapeutic perspectives. Curr. Med. Chem., 17, 1325–1338. [DOI] [PubMed] [Google Scholar]
- 2. Venugopala K.N., et al. (2013). Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res. Int., 2013, 963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parrish J.A., et al. (1974). Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N. Engl. J. Med., 291, 1207–1211. [DOI] [PubMed] [Google Scholar]
- 4. Peng Y., et al. (2012). Down regulation of differentiated embryonic chondrocytes 1 (DEC1) is involved in 8-methoxypsoralen-induced apoptosis in HepG2 cells. Toxicology, 301, 58–65. [DOI] [PubMed] [Google Scholar]
- 5. Luo K.W., et al. (2011). Anticancer effects of imperatorin isolated from Angelica dahurica: induction of apoptosis in HepG2 cells through both death-receptor- and mitochondria-mediated pathways. Chemotherapy, 57, 449–459. [DOI] [PubMed] [Google Scholar]
- 6. Lee S.O., et al. (2011). Targeting NR4A1 (TR3) in cancer cells and tumors. Expert Opin. Ther. Targets, 15, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohan H.M., et al. (2012). Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clin. Cancer Res., 18, 3223–3228. [DOI] [PubMed] [Google Scholar]
- 8. Moll U.M., et al. (2006). p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene, 25, 4725–4743. [DOI] [PubMed] [Google Scholar]
- 9. Zhang X.K. (2007). Targeting Nur77 translocation. Expert Opin. Ther. Targets, 11, 69–79. [DOI] [PubMed] [Google Scholar]
- 10. Kolluri S.K., et al. (2003). Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell. Biol., 23, 8651–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S.O., et al. (2010). Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res., 70, 6824–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee S.O., et al. (2012). The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene, 31, 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu H., et al. (2011). Regulation of Nur77 expression by β-catenin and its mitogenic effect in colon cancer cells. FASEB J., 25, 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Q.X., et al. (2006). NR4A1, 2, 3–an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol. Histopathol., 21, 533–540. [DOI] [PubMed] [Google Scholar]
- 15. Ramaswamy S., et al. (2003). A molecular signature of metastasis in primary solid tumors. Nat. Genet., 33, 49–54. [DOI] [PubMed] [Google Scholar]
- 16. Mullican S.E., et al. (2007). Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat. Med., 13, 730–735. [DOI] [PubMed] [Google Scholar]
- 17. Li H., et al. (2000). Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science, 289, 1159–1164. [DOI] [PubMed] [Google Scholar]
- 18. Dawson M.I., et al. (2001). Apoptosis induction in cancer cells by a novel analogue of 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalenecarboxylic acid lacking retinoid receptor transcriptional activation activity. Cancer Res., 61, 4723–4730. [PubMed] [Google Scholar]
- 19. Han Y.H., et al. (2006). Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene, 25, 2974–2986. [DOI] [PubMed] [Google Scholar]
- 20. Holmes W.F., et al. (2003). Early events in the induction of apoptosis in ovarian carcinoma cells by CD437: activation of the p38 MAP kinase signal pathway. Oncogene, 22, 6377–6386. [DOI] [PubMed] [Google Scholar]
- 21. Kolluri S.K., et al. (2008). A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell, 14, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin B., et al. (2004). Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell, 116, 527–540. [DOI] [PubMed] [Google Scholar]
- 23. Liu J., et al. (2008). Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res., 68, 8871–8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maddika S., et al. (2005). Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway. J. Cell Sci., 118(Pt 19), 4485–4493. [DOI] [PubMed] [Google Scholar]
- 25. Thompson J., et al. (2008). During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J. Exp. Med., 205, 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang A., et al. (2009). Phosphorylation of Nur77 by the MEK-ERK-RSK cascade induces mitochondrial translocation and apoptosis in T cells. J. Immunol., 183, 3268–3277. [DOI] [PubMed] [Google Scholar]
- 27. Yang H., et al. (2011). ERK1/2 deactivation enhances cytoplasmic Nur77 expression level and improves the apoptotic effect of fenretinide in human liver cancer cells. Biochem. Pharmacol., 81, 910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferlini C., et al. (2009). Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res., 69, 6906–6914. [DOI] [PubMed] [Google Scholar]
- 29. Thompson J., et al. (2010). Protein kinase C regulates mitochondrial targeting of Nur77 and its family member Nor-1 in thymocytes undergoing apoptosis. Eur. J. Immunol., 40, 2041–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chintharlapalli S., et al. (2005). Activation of Nur77 by selected 1,1-Bis(3’-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J. Biol. Chem., 280, 24903–24914. [DOI] [PubMed] [Google Scholar]
- 31. Zhan Y., et al. (2008). Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol., 4, 548–556. [DOI] [PubMed] [Google Scholar]
- 32. Wilson A.J., et al. (2003). TR3/Nur77 in colon cancer cell apoptosis. Cancer Res., 63, 5401–5407. [PubMed] [Google Scholar]
- 33. Jeong J.H., et al. (2003). Orphan nuclear receptor Nur77 translocates to mitochondria in the early phase of apoptosis induced by synthetic chenodeoxycholic acid derivatives in human stomach cancer cell line SNU-1. Ann. N. Y. Acad. Sci., 1010, 171–177. [DOI] [PubMed] [Google Scholar]
- 34. Chinnaiyan P., et al. (2006). Enhancing the antitumor activity of ErbB blockade with histone deacetylase (HDAC) inhibition. Int. J. Cancer, 118, 1041–1050. [DOI] [PubMed] [Google Scholar]
- 35. Chen Y.L., et al. (2008). The induction of orphan nuclear receptor Nur77 expression by n-butylenephthalide as pharmaceuticals on hepatocellular carcinoma cell therapy. Mol. Pharmacol., 74, 1046–1058. [DOI] [PubMed] [Google Scholar]
- 36. Liu D.P., et al. (2011). Furocoumarin derivatives from radix Angelicae dahuricae and their effects on RXRα transcriptional regulation. Molecules, 16, 6339–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Finn G., et al. (2003). Modulation of mitogen-activated protein kinases by 6-nitro-7-hydroxycoumarin mediates apoptosis in renal carcinoma cells. Eur. J. Pharmacol., 481, 159–167. [DOI] [PubMed] [Google Scholar]
- 38. Dhanasekaran D.N., et al. (2008). JNK signaling in apoptosis. Oncogene, 27, 6245–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J., et al. (2005). Role of JNK activation in apoptosis: a double-edged sword. Cell Res., 15, 36–42. [DOI] [PubMed] [Google Scholar]
- 40. Brady H., et al. (2002). Effects of SP500263, a novel, potent antiestrogen, on breast cancer cells and in xenograft models. Cancer Res., 62, 1439–1442. [PubMed] [Google Scholar]
- 41. Suzuki N., et al. (2011). Anti-breast cancer potential of SS5020, a novel benzopyran antiestrogen. Int. J. Cancer, 128, 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shukla S., et al. (2012). Synthesis, characterization, biological evaluation and docking of coumarin coupled thiazolidinedione derivatives and its bioisosteres as PPARγ agonists. Med. Chem., 8, 834–845. [DOI] [PubMed] [Google Scholar]
- 43. Kasaian J., et al. (2013). Synthesis, biosynthesis and biological activities of galbanic acid - A review. Pharm. Biol. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44. Hirano T. (2013). Development of functional molecules for elucidation of the physiological roles of several nuclear receptors and their endogenous ligands. Chem. Pharm. Bull. (Tokyo)., 61, 111–120. [DOI] [PubMed] [Google Scholar]
- 45. Sakai H., et al. (2011). 6-Arylcoumarins as novel nonsteroidal type progesterone antagonists: an example with receptor-binding-dependent fluorescence. J. Med. Chem., 54, 7055–7065. [DOI] [PubMed] [Google Scholar]
- 46. Rulcova A., et al. (2010). Stereoselective interactions of warfarin enantiomers with the pregnane X nuclear receptor in gene regulation of major drug-metabolizing cytochrome P450 enzymes. J. Thromb. Haemost., 8, 2708–2717. [DOI] [PubMed] [Google Scholar]
- 47. Yao R., et al. (2011). Regulatory effect of daphnetin, a coumarin extracted from Daphne odora, on the balance of Treg and Th17 in collagen-induced arthritis. Eur. J. Pharmacol., 670, 286–294. [DOI] [PubMed] [Google Scholar]
- 48. Bradham C., et al. (2006). p38 MAPK in development and cancer. Cell Cycle, 5, 824–828. [DOI] [PubMed] [Google Scholar]
- 49. De Chiara G., et al. (2006). Bcl-2 Phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J. Biol. Chem., 281, 21353–21361. [DOI] [PubMed] [Google Scholar]
- 50. Torcia M., et al. (2001). Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation, and cytochrome c release. J. Biol. Chem., 276, 39027–39036. [DOI] [PubMed] [Google Scholar]