Summary
The prevention and treatment of cancers with Kras mutations represents a critical clinical need. We show that resveratrol can suppress oncogenic Kras through post-transcriptional regulation and may provide a novel strategy for preventing tumor development or treating tumors with activated Kras mutations
Abstract
Sporadic and non-hereditary mutations account for the majority of colorectal cancers (CRC). After the loss of adenomatous polyposis coli (APC) function and activation of the β-catenin/LEF signaling pathway, activating mutations in Kras are major drivers of sporadic CRC. Preventing the outgrowth of cells that develop sporadic mutations will decrease CRC. Resveratrol, a naturally occurring polyphenolic compound has anti-inflammatory, anti-oxidant and anti-cancer activities. We used a genetically engineered mouse model for sporadic CRC where the APC locus is knocked out and Kras is activated specifically in the distal colon to determine the effects of resveratrol on preventing and treating CRC. Feeding mice a diet supplemented with 150 or 300 ppm resveratrol (105 and 210mg daily human equivalent dose, respectively) before tumors were visible by colonoscopy resulted in a 60% inhibition of tumor production. In the 40% of mice that did develop tumors Kras expression was lost in the tumors. In a therapeutic assay where tumors were allowed to develop prior to treatment, feeding tumor bearing mice with resveratrol resulted in a complete remission in 33% of the mice and a 97% decrease in tumor size in the remaining mice. Analysis of miRNA expression in non-tumoral and tumoral colonic tissue of resveratrol treated mice showed an increased expression of miR-96, a miRNA previously shown to regulate Kras translation. These data indicate that resveratrol can prevent the formation and growth of colorectal tumors by downregulating Kras expression.
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related death in the USA and affects men and women equally (1). The majority of CRC cases are sporadic and non-hereditary accounting for ~75% of all cases (2).
While many somatic mutations occur during the pathogenesis of CRC, among the earliest and most common is the loss of the adenomatous polyposis coli (APC) tumor suppressor gene (3). APC acts as a tumor suppressor by negatively regulating the Wnt signaling pathway, which maintains stem cell pluripotency and intestinal epithelial cell homeostasis (4). APC exists as part of an elaborate proteolytic destruction complex that regulates the degradation of β-catenin, a transcriptional coactivator of the Wnt signaling pathway. Loss of APC stabilizes β-catenin allowing its subsequent translocation to the nucleus and activation of Wnt target genes by associating with LEF-1/TCF proteins (4,5). Sustained activation of Wnt signaling can lead to the accumulation of undifferentiated cells and the formation of a benign adenoma (6,7).
The transition from a benign adenoma to malignant carcinoma often requires additional molecular events, such as activation of proto-oncogenes (7). The Kras oncogene is commonly mutated in multiple cancers, including sporadic CRC. Kras mutations in CRC have been associated with poor survival, tumor aggressiveness and chemoresistance (7). The most common mutation involves a glycine (G) to aspartic acid (D) substitution at residue 12 of the Ras protein (G12D), which results in a constitutively active protein (8). Ras family members are able to regulate many downstream pathways involved in cancer, including the Mitogen-activated protein kinases (MAPK) and phosphatidylinositol 3-kinase (PI3K) signaling pathways (9,10). In addition, Kras can act synergistically with loss of APC to enhance Wnt signaling promoting tumor growth and aggressiveness (11). CRC cases with Kras mutations are difficult to treat and enormous effort has been made to generate Kras inhibitors for clinical use.
Resveratrol is a naturally occurring polyphenolic compound found in various plants including grapes, berries and red wine, and has been found to have broad-spectrum beneficial health effects including, anti-inflammatory, anti-oxidant and anti-cancer activities (12). In vitro resveratrol has been shown to induce cellular apoptosis (13) and inhibit proliferation (14,15) in CRC cell lines. In vivo resveratrol suppressed colon tumor formation in APCMin mice which are genetically predisposed to intestinal tumors (16), and in the azoxymethane (AOM)/dextran sodium sulfate (DSS) colitis-driven animal model of CRC (17). Recent data suggests that the chemopreventive effects of resveratrol and other bioactive food components are in part due to epigenetic regulation and post-transcriptional modifications (18,19). In a human lung cancer cell line resveratrol increased the expression of miRNA-622 and suppressed Kras expression (20).
The current study investigated the efficacy of resveratrol in a sporadic model of CRC that has a conditional knock out of both copies of APC combined with a latent activated gain of function in the KrasG12D mutation, [APCCKO/Krasmut (21)]. This pre-clinical model mimics the human sporadic CRC axis, with one primary tumor that initiates as an adenoma with the potential to develop into a carcinoma and metastasize to the liver. We found that orally administered resveratrol at a human equivalent dose (HED) of ~210mg/day prevented initial tumor formation and retarded the growth of established APCCKO/Krasmut tumors. Resveratrol suppressed the expression of Kras both in vivo and in vitro. In addition, resveratrol treatment resulted in elevated in vivo expression of miR-96, a microRNA that has previously been shown to target Kras. These results indicate that resveratrol can suppress oncogenic Kras activity, possibly by increasing the expression of miRNA-96.
Materials and methods
Animals
All mouse experiments were agreed to and regulated by the Animal Care and Use Committee (ACUC) of the National Cancer Institute (Frederick, MD). Pathogen-free male and female mice were acquired from the NCI-Frederick mouse repository and were bred and maintained in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle. The APCCKO/Krasmut mice were generated by crossing mice homozygous for the APC CKO allele (22) to mice heterozygous for the LSL-Kras allele (23) with both strains developed as Cre-lox recombination models. The APC CKO/LSL-Kras mice were then crossed to restore the homozygous APC CKO allele and the heterozygous LSL-Kras. Mice were genotyped prior to experiment using primers and conditions described previously (22,23). Colonies were expanded to generate 12 mice per experimental group.
Generation of sporadic colon specific APCCKO/Krasmut tumors
APCCKO/Krasmut tumors were generated using methods described previously (21). Briefly, 8- to 12-week old mice were fasted overnight and anesthetized using 2% isoflurane. A midline incision was performed to expose the distal colon, which was then pulled forward and clamped 3cm from the anus. The colon was then flushed with PBS (phosphate-buffered saline) using a flexible 2.5cm plastic animal feeding tube attached to a 1ml syringe. The lining of the distal colon was then mechanically abraded using a plastic dental brush. The plastic animal feeding tube was re-inserted and a second clamp placed 1cm above the rectum with the feeding tube between clamps. Next, 100 μl of trypsin was injected into the colon and incubated for 10min. Following a PBS rinse, 109 PFU of adenovirus expressing Cre recombinase (Gene Transfer Vector Core Facility, University of Iowa) was injected into the colon and incubated for 30min. After the incubation period, both clamps were removed and the abdominal wall was sutured closed.
Intervention and treatment studies
One-week prior to initiation of studies all mice were placed on a synthetic AIN-93M control diet (Teklad, Harlan Laboratories, Madison, WI). For the experimental diet AIN-93M was supplemented with 150 or 300 ppm of resveratrol (Sigma, St. Louis, MO). Interventions were started 4-weeks post-infection with virus expressing Cre recombination to allow for pre-adenoma neoplastic lesions to form. After 4-weeks mice were maintained on the control diet (Ct) or started on the resveratrol-supplemented diet (Rv). Mouse colonoscopy [Karl Storz Veterinary Endoscopy-America, El Segundo, CA (24)] was used to monitor the development of tumors weekly and tumor incidence was scored as presence or absence of tumor visualized by colonoscopy by day 91.
The treatment study began on day 91. Mice from the control group were subdivided into two groups, one continued on the Ct and the other was placed on the 300 ppm Rv (Ct + Rv). In addition, on day 91, the tumor-bearing mice that were fed the resveratrol diet during the prevention arm were maintained on the resveratrol diet, while the tumor-free (TuF) mice from the resveratrol treated group were switched to the control diet (TuF + Ct)
Resveratrol HED and tolerance
The daily HED was calculated using a representative surface area to weight ratios (km) normalization method (25) in which the animal dose is converted to a dose based on surface area for humans by the following formula. HED (mg/kg) = animal dose (mg/kg) × (mouse Km factor/human Km factor). A 60kg person was used to calculate human supplemented dose (mg). The daily animal dose = diet concentration (mg/kg) × animal mass (kg)/food consumption [kg/week (mean)]/7 days. Km factor for mouse and human is 3 and 37, respectively. Dietary resveratrol was well tolerated at this dose with no obvious toxicity, consistent with that of a previous study using the same dose (17).
Tumor growth
Tumor growth was visualized by colonoscopy and calculated as percent luminal occlusion. Percent luminal occlusion is a visual semi quantitative index for assessing tumor size using captured colonoscopy images. Using Image-J software a 2D contour is visually drawn to determine the area of the colon lumen, and a second contour is placed to determine the area of the tumor. The percent occlusion is the ratio between the two areas. This measurement was found to be a good predictor of tumor size when we compared visual calculations to the actual standard caliper tumor measurement at the end of the study.
DNA, RNA, microRNA and protein extraction from colon tissue
Fresh colon tissue was homogenized using 1.0mm glass beads in RLT-plus buffer. Genomic DNA, RNA and microRNA were extracted using Allprep Universal Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. Protein was precipitated from RLT buffer using five volumes of an 8:1 acetone: methanol solution overnight at −20°C and re-suspended in radio immunoprecipitation assay (RIPA) buffer (Santa Cruz, Santa Cruz, CA).
Detection of Cre-mediated recombination of APC and Kras allele
PCR was performed on genomic DNA extracted from colon tissue to verify the Cre-mediated recombination of the APC and LSL-Kras allele. The APC truncated transcript was detected using methods described previously (22). The LSL-Kras recombination was detected using a multiplex PCR with specific primers flanking the Lox-Stop-Lox cassette. The primers used in this study were KrasG12D1 5′-GTC TTT CCC Cag CAC AgT gC-3′, and KrasG12D2 5′-CTC TTg CCT ACg CCA CCA gCT C-3′ that detected both the wild-type Kras allele (622bp) and the mutant allele with one remaining lox site after lox recombination (650bp). The third primer KrasG12D3 5′-AgC Tag CCA CCA Tgg CTT gAg TAA gTC TgC A-3′ was added to detect the presence of the intact LSL-cassette (500bp). We used 5 μM of each primer (Integrated DNA technologies, San Jose, CA) with a Go Taq Hot start Green Master mix (Promega, Madison, WI) using the following PCR conditions: 95°C 2min; 34 cycles of 95°C 0:30 s, 61°C 0:30 s, 72°C 0:45 s; 72°C 10min.
Histopathology
Colonic tissues and livers were harvested from animals and fixed overnight in 4% neutral buffered formalin and stored in 70% ethanol. Fixed tissue was embedded in paraffin and cut in 5 μm sections and placed on glass microscope slides (Histoserv, Germantown, MD). For histological analysis all colon and liver sections were stained with Hematoxylin and Eosin (H&E) or 1% Alcian Blue in PBS (Sigma). Colon tissues were evaluated by a board certified pathologist (MSM) and diagnosed applying the criteria of a consensus report on murine colon tumors (26). Briefly, tumors were characterized as an adenoma or carcinoma and scored on a number of criteria including; number of tumoral foci, grade of dysplasia, and presence of goblet cells. Liver was examined by macroscopy and microscopy to determine the presence or absence of metastasis.
Immunohistochemistry
Immunohistochemical analysis was performed using methods described previously (27). The primary antibodies and dilutions were as follows: β-catenin, 1:50 (Cell Signaling Technology, Danvers, MA), Ki-67, 1:100, pBrafser445 1:100, Kras 1:500, 1:50 (Abcam, Cambridge, MA), LGR5, 1: 250 (Origene, Rockville, MD). All slides were digitized using Aperio Scanscope CS2 (Leica biosystems, Vista, CA) and evaluated using the Aperio analysis package, which includes highly advanced algorithms for quantifying percentage of nuclear staining, and staining intensity quantification ranging from 0 (negative) to 4 (strongly positive).
Microarray analysis
Sample preparation, cDNA labeling, hybridization to Affymetrix Mouse Genome 430 2.0 Array, and scanning was conducted at the Laboratory of Molecular Technology (Frederick, MD) using the Affymetrix GeneChip System. The raw data files were normalized to expression values estimated using GC-RMA with Partek Genomics suite. ANOVA was applied to derive a list of statistically significant genes. Unsupervised clustering heat map was constructed based on differentially expressed genes with a P value of <0.001 with an FDR of 0.10. The expression of genes was standardized to mean 0 and standard deviation of 1. Genes which are unchanged are displayed as a value of zero. Up-regulated genes have positive values and down-regulated genes have negative values. Genes were uploaded into Ingenuity pathway analysis (Qiagen) and placed into networks based on biological function.
miRNA analysis
Sample preparation, hybridization, fluorochrome labeling and counting using NanoString nCounter technology were conducted according to the manufacturer’s instructions for the nCounter mouse miRNA expression assay kit (NanoString, Seattle, WA) at the Laboratory of Molecular Technology. Raw data was normalized using internal negative and positive controls, adjusted for background and process variability. The biological normalization was performed to correct for differences in sample abundances and is normalized to the geometric mean of the top 100 most highly expressed miRNA using a confidence interval of 99% (P < 0.01) using nSolver software. Differentially expressed miRNA was identified using Partek genomics suite applying ANOVA to calculate statistical significance.
Cell culture
Human colon cancer HCT116 and SW480 cell lines have been extensively characterized and were obtained from American Type Culture Collection (Manassas, VA). The cell lines were tested and authenticated by supplier using identifiler® STR genotyping prior to distribution. Cells were cultured and stored according to the supplier’s instructions and used at passage 5–10. Once resuscitated, cell lines were cultured in DMEM with 10% FBS, 100U/ml penicillin and 100mg/ml streptomycin at 37°C in humidified air with 5% CO2 never passaged over a period exceeding 2 months to ensure authenticity.
Western blot analysis
Approximately 30–80mg of protein isolated from normal colon tissue, colonic tumor tissue, HCT116, or SW480 CRC cells was loaded and separated by SDS–PAGE, transferred to nitrocellulose membrane, and probed with the following antibodies overnight at indicated dilutions. Kras, 1:1000 (Abcam), pERKThr202/Tyr204, 1:1000, pBrafSer445, 1:1000, β-catenin, 1:2000, pAKTSer473, 1:1000 (Cell Signaling Technology); each membrane was probed with β-actin, 1:5000 (Sigma) to ensure consistent loading. To assess nuclear localization of the β-catenin protein in HCT116 and SW480 cells, a separate isolation of nuclear and cytoplasmic protein was performed using NE-PER nuclear and cytoplasmic extraction kit (Thermo Scientific, Waltham, MA). Each membrane was probed with P84 (Gene Tex, Irvine, CA) to ensure consistent loading of nuclear protein.
Statistical analyses
All data is presented as the mean ± standard deviation. The significance of the difference between groups was evaluated by one-way analysis of variance (ANOVA) or Student’s t-test, and multiple comparisons with Prism 5.0 software. P < 0.05 were considered to be statistically significant.
Results
KrasG12D mutation increased tumor incidence and accelerated tumor growth
To study resveratrol’s ability to suppress tumor formation in a mouse model of spontaneous CRC, we utilized a genetically engineered mouse model where a localized infection of colonic tissue with adenovirus expressing Cre recombinase results in a tumor driven by either loss of both copies of APC with wild-type Kras (APCCKO/KrasWT) or a tumor driven by loss of APC with activated Kras harboring a gain in function mutation KrasG12D (APCCKO/Krasmut) (21). Consistent with previous studies (21), we found that Kras mutational activation was not required for tumor initiation; however, activation of Kras significantly increased tumor incidence and accelerated tumor growth (Figure 1). APCCKO/Krasmut tumors were visualized as early as 21 days post-adenovirus-Cre infection, with 100% incidence by day 91, whereas, APCCKO/KrasWT tumors were visualized at day 51, with only a 15% incidence by day 91 (Figure 1a). In addition, APCCKO/Krasmut tumors showed accelerated growth compared with APCCKO/KrasWT tumors and were significantly larger on day 91 (Figure 1b). The decreased incidence of tumor development in the APCCKO/KrasWT model limited our ability to measure the chemo-prevention activity of resveratrol in these mice. As a result, we investigated resveratrol’s ability to prevent sporadic tumorigenesis only in the APCCKO/Krasmut mouse model.
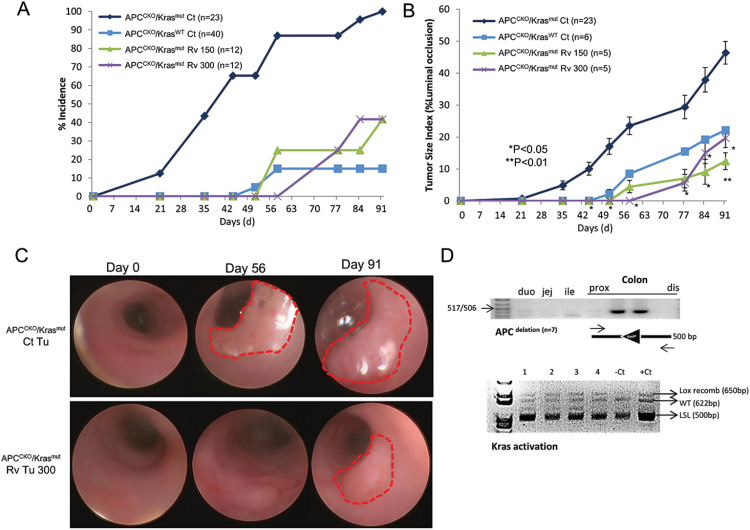
Fig. 1.
Resveratrol prevented tumor formation and tumor growth in APCCKO/Krasmut mouse model of sporadic CRC as measured by endoscopy. (A) Tumor incidence and (B) tumor size index of APCCKO/KrasWT and APCCKO/Krasmut GEM mice. Mice were fed a control diet (APCCKO/KrasWT Ct and APCCKO/Krasmut Ct) or a diet supplemented with 150 or 300 ppm resveratrol diet (APCCKO/Krasmut Rv 150 and APCCKO/Krasmut Rv 300). (C) Representative endoscopic images of visualized APCCKO/Krasmut tumors taken from mice on control diet (Ct Tu) and mice on diet supplemented with 300 ppm of resveratrol (Rv Tu 300). (D) Polymerase chain reaction results show that mice treated with resveratrol that are TuF have successful adenovirus-Cre recombination of APC and Kras alleles. Top panel shows PCR results of recombinant APC allele. DNA from mouse duodenum (duo), jejunum (jej), ileum (ile) and proximal to distal colon were analyzed. Bottom panel shows PCR results from four resveratrol tumors (1–4), 1 control tumor (Ct) and 1 NAT (−Ct). PCR products are LSL-Kras allele [LSL (500bp)], the Cre induced recombinant Lox-Kras allele [Lox recomb (650bp)], and the wt Kras allele [WT (622)]. (*P < 0.05, **P < 0.01 versus control).
Resveratrol prevented sporadic tumor formation in APCCKO/Krasmut mice
Previous studies have shown that resveratrol can prevent colon adenomatous polyp formation in mice harboring a genomic APC mutation (APCmin) and in mice with inflammatory CRC induced by AOM and gut irritant DSS (16,17). While both pre-clinical models involve mutations in the APC-β-catenin axis, an early aberration in CRC, they do not model the genetic alterations associated with spontaneous CRC, including Kras, Braf and p53 mutations (28). To test the chemopreventive activity of resveratrol on spontaneous CRC, APCCKO/Krasmut mice were fed a control diet or one supplemented with 150 or 300 ppm resveratrol starting 4 weeks after adenoviral-Cre infection. Supplementation of either concentration of resveratrol resulted in 60% reduction in tumor incidence 91 days after adenovirus infection (P < 0.01; Figure 1a). The tumors that did develop in the remaining resveratrol fed mice had a delayed onset. The APCCKO/Krasmut tumors in mice fed the control diet were first visible by colonoscopy on day 21, whereas in the resveratrol fed mice, tumors were not visible until day 58 (150 ppm) and day 77 (300 ppm; Figure 1a–c). Furthermore, the tumors were significantly smaller as measured by colonoscopy for tumor size index on day 91 (Figure 1b and c).
To ensure Cre-recombination occurred in the infected cells and that the recombined alleles were not selected against by the resveratrol diet, we performed PCR on genomic DNA isolated from the colons of TuF mice. The Cre-mediated recombinant APC allele was only detected in the region of the colon infected by the adenovirus-Cre and not in the proximal or most distal colon tissue (Figure 1d top panel). The Cre recombinant Lox-Krasmut [Lox-recomb (650bp)] and the Kraswt (622bp) allele were also detected in virus infected tissue but not in the control tissue (Figure 1d bottom panel). The LSL sequence [LSL (500bp)] was also detected, indicating heterogeneous tissues were assayed. The detection of both the APC and LSL-Kras alleles that underwent Cre-recombination in TuF APCCKO/Krasmut tissue confirmed the initial adenoviral-Cre infection of the stem cells of the crypts. Thus, the activity of resveratrol to prevent tumorigenesis occurs after tumor initiation by mutational activation.
Resveratrol inhibited growth of established APCCKO/Krasmut tumors
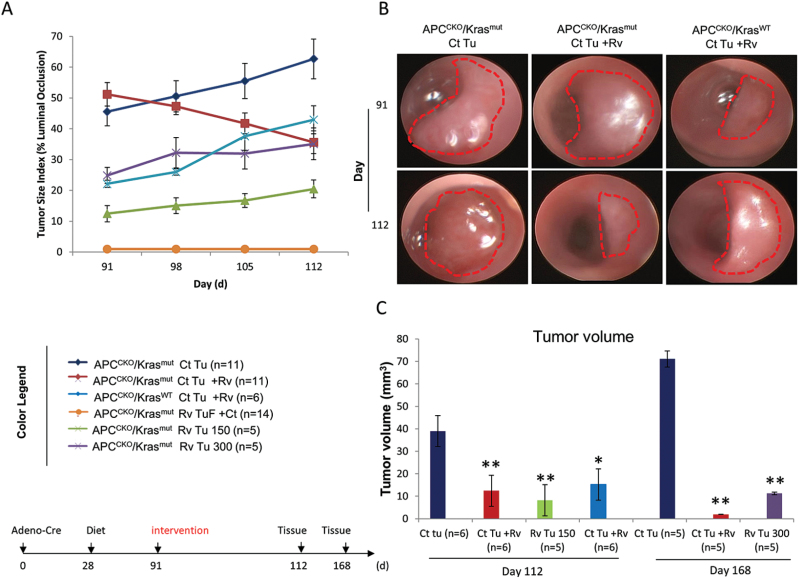
Resveratrol showed chemopreventive activity against APC deleted Kras-activated tumorigenesis when administered 28 days after adenoviral-Cre infection. On day 91, 100% of the mice in the control group had visible tumors. To determine if resveratrol has chemotherapeutic activity against established APCCKO/Krasmut tumors, the mice from the control diet group were subdivided and a subset of these mice were placed on a diet supplemented with 300 ppm resveratrol for either 21 or 77 days, while the other group was maintained on the control diet. In addition, all of the APCCKO/KrasWT mice with established tumors (15% of total) were also switched to the resveratrol diet.
As expected, the APCCKO/Krasmut tumors in animals maintained on the control diet continued to grow, whereas feeding tumor bearing mice resveratrol resulted in regression of tumor growth. After 21 days, the resveratrol treated APCCKO/Krasmut tumors were 56% smaller as measured by colonoscopy tumor size index (Figure 2a), which correlated with a 68% (P < 0.05) smaller tumor volume measured by standard caliper measurements (Figure 2c). Treatment with resveratrol for 77 days resulted in complete remission of 33% of APCCKO/Krasmut tumors (Supplementary Figure 1 is available at Carcinogenesis Online), and an average tumor volume that was 97% (P < 0.01) smaller than that of control tumors, as measured by standard caliper measurements (Figure 2c). Interestingly, unlike the APCCKO/Krasmut tumors, resveratrol treatment had no effect on established APCCKO/KrasWT tumors (Figure 2a and b), suggesting that in this model; resveratrol’s chemotherapeutic activities correlates with Kras mutational status. Furthermore, the resveratrol treated mice that were TuF at day 91 remained TuF even after being switched to the control diet (Figure 2a Rv TuF +Ct), suggesting epigenetic regulation of the mutated alleles in the crypts of these mice.
Fig. 2.
Resveratrol inhibited tumor growth of established APCCKO/Krasmut tumors with no effect on established APCCKO/KrasWT tumors. (A) Tumor growth curve starting on day 91 when a subset of mice that were originally maintained on the control diet with visualized APCCKO/Krasmut or APCCKO/KrasWT tumors were switched to the 300 ppm resveratrol supplemented diet (Ct Tu + Rv) or maintained on control diet (Ct Tu). TuF mice from resveratrol group were switch to control diet (Rv TuF+Ct). Resveratrol APCCKO/Krasmut tumor bearing mice were maintained on respective 150 ppm (Rv Tu 150) and 300 ppm supplemented diets (Rv Tu 300). (B) Representative endoscopic images of APCCKO/Krasmut tumors from mice on control diet (Ct Tu), mice originally maintained on the control diet and switched to resveratrol diet (Ct Tu + Rv) and APCCKO/KrasWT tumors from mice originally maintained on control diet and switched to resveratrol diet (Ct Tu + Rv) taken on day 91 and 112. (C) Standard caliper measurements of tumors collected on day 112 and 168. (*P < 0.05, **P < 0.01 versus control).
Resveratrol inhibited tumor progression and proliferation
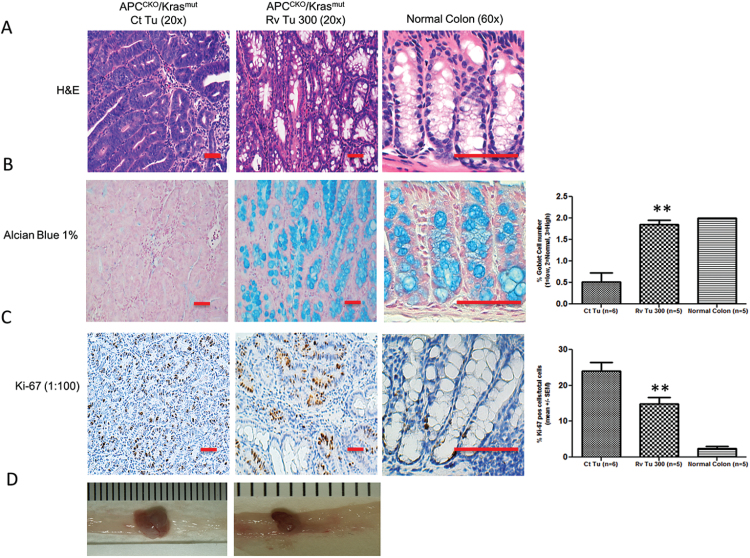
Previous studies with the APCCKO/ Krasmut mice revealed that ~50% of tumors progress into carcinomas and 20% of those tumors metastasize to the liver, measured at day 140 and 168, respectively (21). In the present study, most of our analyses were performed on day 112 with only a small subset of animals assessed at day 168. Histopathology revealed that all the tumors were tubular adenomas with low- or high-grade dysplasia regardless of treatment and that no metastasis could be detected at the time analyzed (day 168). Further examination revealed subtle differences in histopathology between the control tumors and resveratrol treated tumors. Control adenomas showed loss of goblet cells, whereas the resveratrol treated adenomas were more mucinous with a greater number of goblet cells, as shown by H&E and Alcian blue staining (Figure 3a and b). The severity of colonic mucosal dysplasia is associated with enlarged nuclei, loss of nuclear polarity, increased mitosis and markedly reduced or complete loss of goblet cells (29). In the resveratrol-treated tumors there were a higher number of goblet cells suggesting that although they may have regions of high-grade dysplasia; these tumors are predominately less progressed compared with that of the control tumors. Consistent with less progressed tumors, the resveratrol-treated tumors had a significant reduction in cellular proliferation, as shown by Ki-67 IHC staining, compared with the control tumors (Figure 3c).
Fig. 3.
Resveratrol inhibited APCCKO/Krasmut tumor progression and proliferation. On day 168 normal colon or APCCKO/Krasmut tumors were collected from mice on control diet (Ct Tu) or 300 ppm supplemented resveratrol diet (Rv Tu 300). A representative image stained for (A) Haematoxylin & Eosin, or (B) Alcian blue, which detects the presence of mucous is shown. (C) Representative immunohistochemistry for proliferation marker Ki-67 is shown. Far right histograms in (B) and (C) show percentage of positive cells for Alcian blue or Ki-67 within total epithelial cells as quantitated by Aperio Scanscope. (D) Macroscopic view of colonic tumors, scale bar mm. Tumor tissues are shown at 20×. Normal colon is shown at 60× magnification. Scale bar, 100 μM, Quantitation was normalized to % total cells. (*P < 0.05, **P < 0.01 versus control).
Resveratrol tumors expressed less Kras, LGR5 and nuclear β-catenin
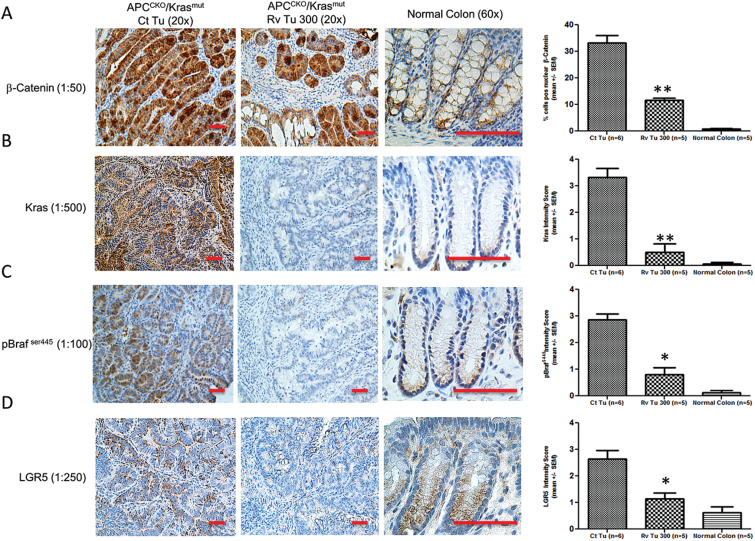
Loss of APC leads to activation of the Wnt/β-catenin pathway, an early event during tumorigenesis which can give rise to adenomas. Activated Kras accelerates the growth of adenomas and is associated with changes in the expression of proteins involved in cell proliferation and cell survival. Consistent with deletion of the APC alleles and expression of KrasG12D, we observed elevated levels of β-catenin and Kras in the control APCCKO/Krasmut tumors compared with that of normal colon tissue (Figure 4a and b). In addition, phosphorylated Braf (pBraf S445), an indicator of increased Ras activity, was elevated in control tumors. LGR5, an intestinal stem cell marker, was also elevated in these tumors, which is consistent with hyperplastic cell growth [(30); Figure 4c and d]. While β-catenin levels were also elevated in the resveratrol APCCKO/Krasmut tumors, the nuclear localized protein was significantly lower than the levels seen in the control APCCKO/Krasmut tumors (Figure 4a). The levels were however, similar to those seen in APCCKO/KrasWT tumors (Supplementary Figure 2a is available at Carcinogenesis Online). Interestingly, the levels of Kras and pBraf were barely detectable in the resveratrol tumors, indicating a loss of Kras expression in these tumors (Figure 4b and c). The level of LGR5 was also significantly lower in the resveratrol-treated tumors compared with the control tumors (Figure 4d), consistent with less progressed tumors. Resveratrol appears to be suppressing the growth and progression of these tumors accompanied by a reduction of nuclear β-catenin, expression of LGR5, and expression of Kras.
Fig. 4.
Resveratrol APCCKO/Krasmut tumors expressed less nuclear β-catenin, Kras, pBraf and LGR5. Normal colon or APCCKO/Krasmut tumors collected on day 168 from mice on control diet (Ct Tu) or 300 ppm supplemented diets (Rv Tu 300) were subject to immunohistochemical analysis of (A) β-catenin, (B) Kras, (C) pBraf ser445 and (D) LGR5. A representative image is shown. Left panels are IHC, right panels are quantitation of staining. Nuclear β-catenin was determined using Aperio Scanscope nuclear staining program. Scale bar, 100 μM.
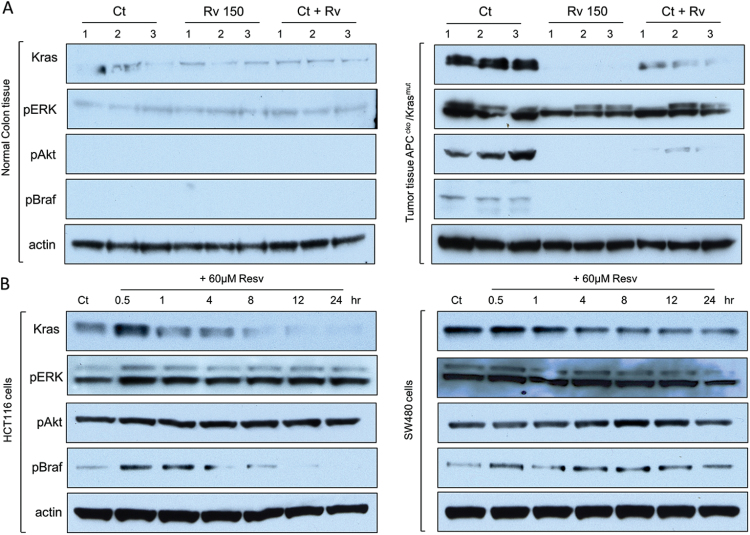
Resveratrol suppressed Kras expression in vivo and in vitro
To better characterize resveratrol effects on Kras expression, we measured Kras protein as well as other known Kras target proteins by western blot. Kras expression was significantly reduced in established APCCKO/Krasmut tumors that were treated with resveratrol for 21 days and in HCT116 and SW480 human carcinoma cell lines that were treated with resveratrol for 24h (Figure 5a and b, and Supplementary Figure 3 available at Carcinogenesis Online) compared with controls that were not exposed to resveratrol. Activation of the Ras kinase pathway leads to activation of the Braf-extracellular signal-regulated kinase (ERK), and PI3K/Akt pathways (31–33). In order to determine if resveratrol associated loss of Kras expression correlated with a loss of activation of the Ras pathway, we measured the phosphorylation status of the kinases in these pathways. In APCCKO/Krasmut control tumors, but not in normal colon tissue, pBraf, pERK and pAKT were detected (Figure 5a). As expected from earlier results, resveratrol tumors had undetectable Kras expression. Furthermore, control tumors in mice that were switched to the resveratrol diet for 3 weeks had significantly lower Kras expression (Figure 5a, Ct + Rv) compared with tumors in mice maintained on the control diet. Similarly, phosphorylation of Ras target kinases Braf and Akt was also significantly lower in resveratrol tumors. Interestingly, phosphorylation of ERK was readily detected in resveratrol treated tumors, although at slightly lower levels compared with the control tumors (Figure 5a). Resveratrol treatment of human CRC cell lines HCT116 and SW480 produced a similar reduction of Kras expression and phosphorylation of Braf. In CRC cell lines, however, both ERK and Akt phosphorylation were not affected by resveratrol (Figure 5b), suggesting other non-Ras-dependent pathways are activated in these cell lines. These data are consistent with resveratrol medicated down regulation of Kras expression and a decrease in Kras activated signaling.
Fig. 5.
Resveratrol suppressed Kras expression in vivo and in vitro. (A) Western blot analysis of total protein isolated from three APCCKO/Krasmut tumors (right panels) collected on day 112 from mice on control diet (Ct), 150 ppm supplemented resveratrol diet (Rv 150), and mice with tumors originally maintained on control diet and switched to 300 ppm resveratrol supplemented diet for 3 weeks (Ct + Rv) with corresponding NAT (left panels) probed with primary antibodies against Kras, pERK, pAkt, pBraf and actin. (B) Western blot analysis of total protein isolated from Kras mutant CRC HCT116 and SW480 cell lines treated with 0 (Ct) or 60 μM of resveratrol in DMSO for 0.5 to 24h probed with primary antibodies against Kras, pERK, pAkt, pBraf and actin. Total protein controls for phospho antibodies were not affected by resveratrol treatment (data not shown). A representative of three repeats is shown (for full length gels, see Supplementary Figures 5–9, available at Carcinogenesis Online).
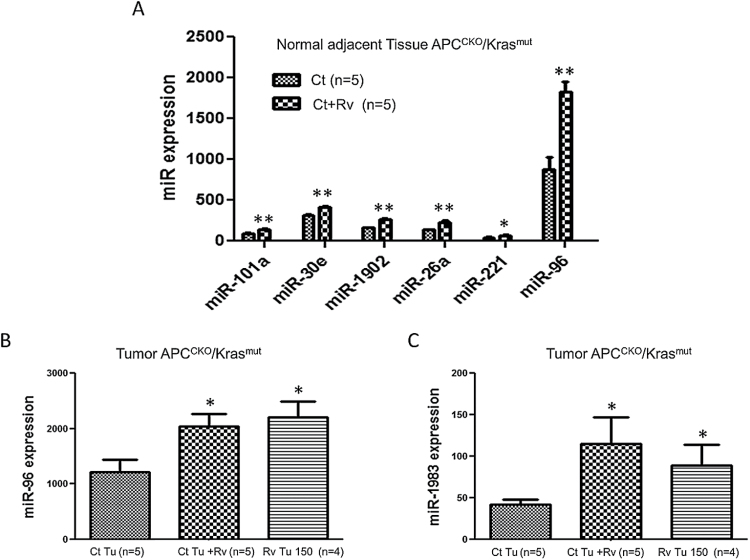
miR-96 expression was upregulated by resveratrol treatment
Our findings indicate that resveratrol can suppress the expression of Kras which results in a reduction in tumor incidence and progression; however, the mechanisms underlying Kras inhibition by resveratrol warranted further elucidation. We first investigated the effects of resveratrol on gene expression using microarray analysis. Resveratrol reduced the expression of many genes associated with inflammation within the tumor microenvironment such as mast cell proteases and genes involved in phagocyte recruitment (Supplementary Figure 4 is available at Carcinogenesis Online). The gene array analysis also showed that Kras mRNA levels were not affected by resveratrol treatment, suggesting that the effects of resveratrol are post-transcriptional. Inhibition of Kras expression in colorectal cell lines by resveratrol was unaffected by treatment with a proteasome inhibitor (data not shown). As a result, we hypothesized that resveratrol may inhibit Kras mRNA translation by upregulating the expression of miRNA.
We utilized the NanoString platform to detect differentially expressed miRNA. Of the top 100 most abundantly expressed miRNA detected we found six miRNAs that were differentially expressed between control and resveratrol treated normal adjacent tissue (NAT) and two differentially expressed miRNAs comparing control tumors and resveratrol treated tumors (Figure 6). Of these miRNAs, only miR-96 was significantly different in both normal and tumor tissue treated with resveratrol. Resveratrol tumors that had minimal detection of Kras expression, had an 80% increase in miR-96 expression (Figure 6b). Similarly, treating mice with established APCCKO/Krasmut with resveratrol for 3 weeks produced a 67% increase in miR-96 expression (Figure 6b) and an almost complete loss of Kras expression (Figures 4a and 5a). Previous studies have shown that miR-96 binds the 3′ untranslated region of Kras mRNA and regulates its translation (34,35). These data support the notion that resveratrol upregulation of miR-96 produces the repression of Kras expression, thus inhibiting tumor growth during Kras activated tumorigenesis.
Fig. 6.
Resveratrol increased expression of miR-96 in vivo. Using nanostring technology global miRNA expression analysis was performed on (A) NAT and (B–C) tumor tissue collected from APCCKO/Krasmut tumor bearing mice on control diet (Ct), originally maintained on control diet and switched to 300 ppm supplemented resveratrol diet for 3 weeks (Ct + Rv), or on resveratrol tumors from mice supplemented with 150 ppm diet (Rv Tu 150). Result show that resveratrol increased the expression of miR-96 in normal tissue and in both tumor groups. (*P < 0.05, **P < 0.01 versus control).
Discussion
We found that feeding Kras activated genetically engineered mouse (GEM) mice a HED of 210mg/day resveratrol inhibited the expression of Kras, prevented the development of tumors and caused a remission of established tumors. In addition, Kras expression was not restored after removal of the resveratrol diet. Expression of miR-96, a microRNA known to regulate Kras expression (34,35), was upregulated in both tumor and non-tumor tissue of resveratrol fed mice. These results indicate that resveratrol supplements can prevent Kras dependent tumor development and can suppress Kras tumor growth, possibly by inducing expression of miR-96 and inhibiting translation of Kras mRNA. The dose of resveratrol administered during this study was well tolerated with no apparent toxicity, consistent with that of a previous report (17) and the equivalent human dose has also been shown to be safe and well tolerated in clinical trials (36).
Inhibition of Kras expression by resveratrol blocked activated Ras signaling. Kras expression and signaling increased in tumor tissue of control mice after adenoviral-Cre infection compared with NAT. Phospho-Braf, phospho-ERK1/2 and phospho-Akt were elevated in these tumors. While resveratrol inhibited Kras and Braf activity, ERK phosphorylation was unaffected. Shin et al. (37) have described a number of feedback loops, including loops driven by the Wnt/β-catenin signaling, that could keep ERK1/2 in the phosphorylated state. Furthermore, the serine/threonine kinase, tumor progression locus-2 (TPL-2, also known as COT and MAPK38), a MEK kinase that is regulated by IκB kinase, can activate ERK1/2 independently of the RAS/Raf/MAPK pathway (38).
The tumors that developed in the APCCKO/Krasmut mice on the resveratrol diet showed similar levels nuclear β-catenin and grew at a similar rate as the APCCKO/KrasWT tumors, indicating that Wnt/β-catenin pathway is driving the resveratrol APCCKO/Krasmut tumors. Unfortunately, the low tumor incidence in the APCCKO/KrasWT mice limited our ability to statistically evaluate the effects of resveratrol on these mice. The increased level of goblet cells along with the decrease in Ki-67 and LGR5 staining in the resveratrol tumors suggest that these tumors are less progressed compared with the control tumors (29,30). Together these results suggest that in the tumors that develop in resveratrol fed APCCKO/Krasmut mice, tumorigenesis is being driven predominantly by the APC deletion and activation of the β-catenin/TCF pathway and, in the absence of Kras expression, the tumors grow more slowly, are smaller and are less progressed.
Gene expression microarray analysis showed elevated levels of genes associated with inflammation within the tumor microenvironment in the control tumors compared with the resveratrol tumors, suggesting that resveratrol inhibits tumor associated inflammation and infiltration of inflammatory cells into the tumor microenvironment. Expression of genes associated with mast cells was significantly increased in the APCCKO/Krasmut tumors and was restored to normal levels by resveratrol. In KrasG12V pancreatic ductal adenocarcinoma, mast cells promoted tumor growth and deletion of the mast cells suppressed tumor growth (39). Dietary resveratrol has been shown to inhibit the growth and development of PanIN lesions in a murine model of pancreatic cancer (40). The anti-inflammatory activity of resveratrol may prevent the recruitment of mast cells to the Krasmut tumors, thus inhibiting tumor growth.
Expression of Kras mRNA was not affected by the resveratrol diet suggesting that resveratrol regulates Kras expression post-transcriptionally. MicroRNAs are small non-coding RNAs that regulate gene expression post-transcriptionally by binding to the 3′ untranslated region and inhibiting mRNA translation. miR-96 was elevated in both non-tumor and tumor tissue of the resveratrol treated mice. miR-96 binds to the 3′UTR of Kras in pancreatic tumors, inhibits Kras expression and suppresses tumorigenesis. Expression of miR-96 was also downregulated in human pancreatic cancer samples with elevated Kras expression (34). These authors suggested that miR-96 is a potent regulator of Kras and increasing miR-96 could provide new methods to treat pancreatic cancer and other Kras-driven cancers. Our observations establish that resveratrol upregulates the expression of miR-96 in mouse colon tissue and inhibits Kras expression in colon tumors. Shankar et al. (40) showed that resveratrol inhibited the growth and development of PanIN lesions of pancreatic cancer in Kras (G12D) mice, indicating that the effects of dietary resveratrol are systemic and are not limited to the microenvironment of the gut and colon cancer. Together these observations suggest that resveratrol can effectively inhibit colon and pancreatic tumorigenesis driven by oncogenic Kras.
Resveratrol is a natural product found in multiple food components shown in preclinical studies to target many pathways. Clinical trials are underway showing that doses of resveratrol effective in targeting glucose metabolism and insulin sensitivity are well tolerated (36,41). Additional clinical studies are needed to determine the beneficial effects of resveratrol on preventing or treating cancer.
Our findings have identified miR-96 as a potent downstream target of resveratrol. Resveratrol induced blockage of Kras expression by upregulation of miR-96 may provide a novel therapeutic strategy for treatment of Kras driven colon and pancreatic cancer.
Supplementary material
Supplementary Figures 1–9 can be found at http://carcin. oxfordjournals.org/
Funding
The Office of Dietary Supplements, Office of the Director ; the Division of Cancer Prevention and the Intramural Research Program, National Cancer Institute; Department of Health and Human Services (DHHS), National Institutes of Health, Bethesda, MD (ZIA BC 010025).
Supplementary Material
Acknowledgements
The authors thank Christine Perella and Juanita Mercado from the Laboratory Animal Sciences Program, Leidos Biomedical Research, Inc. for assisting with animal experimental procedures.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- APC
adenoma polyposis coli
- CRC
colorectal cancer
- ERK
extracellular signal-regulated kinase
- HED
human equivalent dose
- PI3K
phosphoinositide 3-kinase.
References
- 1. American C.S. (2013). Cancer Facts and Figures 2013. American Cancer Society, Atlanta, GA. [Google Scholar]
- 2. Haggar F.A., et al. (2009). Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg., 22, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Filippo C., et al. (2002). Mutations of the APC gene in human sporadic colorectal cancers. Scand. J. Gastroenterol., 37, 1048–1053. [DOI] [PubMed] [Google Scholar]
- 4. Clevers H., et al. (2012). Wnt/β-catenin signaling and disease. Cell, 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- 5. Stamos J.L., et al. (2013). The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol., 5, a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cottrell S., et al. (1992). Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas. Lancet, 340, 626–630. [DOI] [PubMed] [Google Scholar]
- 7. Kinzler K.W., et al. (1996). Lessons from hereditary colorectal cancer. Cell, 87, 159–170. [DOI] [PubMed] [Google Scholar]
- 8. Kislitsin D., et al. (2002). K-ras mutations in sporadic colorectal tumors in Israel: unusual high frequency of codon 13 mutations and evidence for nonhomogeneous representation of mutation subtypes. Dig. Dis. Sci., 47, 1073–1079. [DOI] [PubMed] [Google Scholar]
- 9. Campbell S.L., et al. (1998). Increasing complexity of Ras signaling. Oncogene, 17 (11 Reviews), 1395–1413. [DOI] [PubMed] [Google Scholar]
- 10. Zuber J., et al. (2000). A genome-wide survey of RAS transformation targets. Nat. Genet., 24, 144–152. [DOI] [PubMed] [Google Scholar]
- 11. Janssen K.P., et al. (2006). APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology, 131, 1096–1109. [DOI] [PubMed] [Google Scholar]
- 12. Gupta S.C., et al. (2011). Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci., 1215, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang Y.C., et al. (2003). Resveratrol-induced G2 arrest through the inhibition of CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Biochem. Pharmacol., 65, 1053–1060. [DOI] [PubMed] [Google Scholar]
- 14. Juan M.E., et al. (2008). Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J. Agric. Food Chem., 56, 4813–4818. [DOI] [PubMed] [Google Scholar]
- 15. Fuggetta M.P., et al. (2006). Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro . J. Exp. Clin. Cancer Res., 25, 189–193. [PubMed] [Google Scholar]
- 16. Schneider Y., et al. (2001). Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr. Cancer, 39, 102–107. [DOI] [PubMed] [Google Scholar]
- 17. Cui X., et al. (2010). Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev. Res. (Phila)., 3, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross S.A. (2003). Diet and DNA methylation interactions in cancer prevention. Ann. N. Y. Acad. Sci., 983, 197–207. [DOI] [PubMed] [Google Scholar]
- 19. Ross S.A., et al. (2008). Introduction: diet, epigenetic events and cancer prevention. Nutr. Rev., 66 (suppl. 1), S1–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han Z., et al. (2012). MicroRNA-622 functions as a tumor suppressor by targeting K-Ras and enhancing the anticarcinogenic effect of resveratrol. Carcinogenesis, 33, 131–139. [DOI] [PubMed] [Google Scholar]
- 21. Hung K.E., et al. (2010). Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc. Natl. Acad. Sci. U. S. A., 107, 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuraguchi M., et al. (2006). Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet., 2, e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson E.L., et al. (2001). Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev., 15, 3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Becker C., et al. (2006). High resolution colonoscopy in live mice. Nat. Protoc., 1, 2900–2904. [DOI] [PubMed] [Google Scholar]
- 25. Reagan-Shaw S., et al. (2008). Dose translation from animal to human studies revisited. FASEB J., 22, 659–661. [DOI] [PubMed] [Google Scholar]
- 26. Washington M.K., et al. (2013). Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology, 144, 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saud S.M., et al. (2013). Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res., 73, 5473–5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karim B.O., et al. (2013). Mouse models for colorectal cancer. Am. J. Cancer Res., 3, 240–250. [PMC free article] [PubMed] [Google Scholar]
- 29. Pascal R.R. (1994). Dysplasia and early carcinoma in inflammatory bowel disease and colorectal adenomas. Hum. Pathol., 25, 1160–1171. [DOI] [PubMed] [Google Scholar]
- 30. Barker N., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- 31. Fransén K., et al. (2004). Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis, 25, 527–533. [DOI] [PubMed] [Google Scholar]
- 32. Georgieva M., et al. (2008). Analysis of the K-ras/B-raf/Erk signal cascade, p53 and CMAP as markers for tumor progression in colorectal cancer patients. Oncol. Rep., 20, 3–11. [PubMed] [Google Scholar]
- 33. Engelman J.A., et al. (2006). The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet., 7, 606–619. [DOI] [PubMed] [Google Scholar]
- 34. Yu S., et al. (2010). miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res., 70, 6015–6025. [DOI] [PubMed] [Google Scholar]
- 35. Tanaka M., et al. (2014). EVI1 oncogene promotes KRAS pathway through suppression of microRNA-96 in pancreatic carcinogenesis. Oncogene, 33, 2454–2463. [DOI] [PubMed] [Google Scholar]
- 36. Gescher A., et al. (2013). Resveratrol in the management of human cancer: how strong is the clinical evidence? Ann. N. Y. Acad. Sci., 1290, 12–20. [DOI] [PubMed] [Google Scholar]
- 37. Shin S.Y., et al. (2010). Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res., 70, 6715–6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gantke T., et al. (2011). Regulation and function of TPL-2, an IκB kinase-regulated MAP kinase kinase kinase. Cell Res., 21, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang D.Z., et al. (2011). Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin. Cancer Res., 17, 7015–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shankar S., et al. (2011). Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One, 6, e16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poulsen M.M., et al. (2013). Resveratrol in metabolic health: an overview of the current evidence and perspectives. Ann. N. Y. Acad. Sci., 1290, 74–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.