Abstract
Background:
Primary pancreatic lymphoma (PPL) is a rare pancreatic neoplasm that is difficult to diagnose. PPL has a vastly different prognosis and treatment regimen than other pancreatic tumors; therefore, accurate diagnosis is vital. In this article, we describe the characteristic presentation, endoscopic ultrasound (EUS) features, and the role of fine-needle aspiration (FNA) in the diagnosis of PPL compared with pancreatic adenocarcinoma.
Materials and Methods:
This was a retrospective case-control study of 11 patients diagnosed with PPL via EUS between 2002 and 2011. The clinical and EUS features of the cases were then compared with age-matched controls with adenocarcinoma in a 1:3 ratio.
Results:
There were 11 patients with PPL and 33 with adenocarcinoma. At last follow-up, 7 of 11 PPL patients were alive, and 3 of 33-adenocarcinoma patients were alive (P < 0.001). The most common presenting symptoms for PPL were pain 73%, weight loss 45%, and jaundice 18%, while patients with adenocarcinoma presented with pain 52% (P = 0.3), weight loss 30% (P = 0.47) and jaundice 76% (P = 0.001). The EUS appearance was similar in the two groups in that ultrasound imaging of the pancreas lesions tended to be hypoechoic and heterogenous, but the PPL group was more likely to have peripancreatic lymphadenopathy (LAD) (64% vs. 18%, P = 0.008) and were larger (4.8 cm × 5.3 cm vs. 3.2 cm × 3.1 cm, P < 0.001). The PPL group was less likely to have vascular invasion (18% vs. 55%, P = 0.045) and less likely to be found in the head of the pancreas (36% vs. 85%, P = 0.004). FNA and cytology (without flow cytometry [FC]) made the diagnosis in 28% of PPL patients compared with 91% of adenocarcinoma patients (P = 0.002). In the PPL group, 7 of 11 FNA samples were sent for FC. If FC was added, then the diagnosis of PPL was increased to 100%.
Conclusions:
Compared with adenocarcinoma, pancreatic lymphoma has a better prognosis, is less likely to present with jaundice and less likely to have vascular invasion. PPL is more likely to be located outside the head of the pancreas and to include peripancreatic LAD, and is less likely to be diagnosed with cytology. The diagnostic accuracy of FNA for PPL is improved greatly with the addition of FC.
Keywords: Endoscopic ultrasound, fine needle aspiration, lymphoma, pancreas
INTRODUCTION
Primary pancreatic lymphoma (PPL) is a rare extranodal lymphoma representing approximately 0.5% of all pancreatic neoplasms and <2% of all lymphomas.[1,2] Most PPLs are non-Hodgkin lymphomas (NHLs) that present as mass lesions which have to be differentiated from other primary pancreatic lesions such as adenocarcinoma, neuroendocrine tumors, and chronic pancreatitis. The diagnostic criteria [Table 1] for PPL include:
Table 1.
Diagnostic criteria for primary pancreatic lymphoma
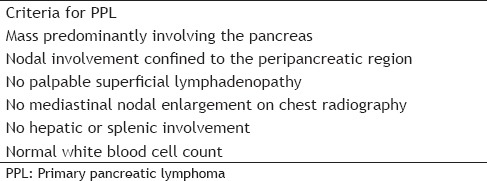
Mass predominantly involving the pancreas,
Nodal involvement confined to peripancreatic region,
No palpable superficial lymphadenopathy (LAD),
No mediastinal nodal enlargement on chest radiography,
No hepatic or splenic involvement, and
Normal white blood cell count.[3]
Making an accurate diagnosis is vital since treatment of PPL typically involves chemotherapy and/or radiation therapy, while pancreatic adenocarcinoma (PA) is managed surgically when possible. Tissue sampling is necessary for an accurate diagnosis and was previously only achieved with percutaneous biopsy or exploratory laparotomy in order to perform detailed analysis of tissue architecture and special stains for subtype classification.[4] Diagnosing NHL with fine needle aspiration (FNA) has, therefore, been difficult. However, recent studies have shown that FNA with combined cytology and flow cytometry (FC) can be used to make the diagnosis of PPL.[3,4,5,6] This advance in knowledge has led to endoscopic ultrasound (EUS)-guided FNA (EUS-FNA) being used as a diagnostic modality for PPL.[7] FC is not routinely sent on all pancreatic masses that undergo EUS-FNA and, therefore, the diagnosis of PPL could be missed if the endoscopist does not keep a high index of suspicion. Identifying factors that would help the endoscopist predict a possible PPL would be helpful in determining, which cases should have FC sent. The aim of this study is to describe the characteristic presentation, EUS features, and role of FNA in the diagnosis of PPL compared to PA.
MATERIALS AND METHODS
This was a retrospective case-control study of patients diagnosed with PPL via EUS and was approved by the Institutional Review Board at the University of Wisconsin Hospital and Clinics. A prospectively gathered EUS database from 2002 to 2011 was searched for patients who had undergone EUS-FNA and who were subsequently found to have a confirmed diagnosis of lymphoma. The diagnostic criteria for PPL [Table 1] were then applied to each case to ensure the accuracy in the diagnosis (as opposed to a widespread lymphoma which involves the pancreas). After identification of the patients with PPL, the same database was used to identify age-matched controls with PA in a 1:3 ratio. The medical records of all patients were then reviewed to collect data were on demographics, presenting symptoms, labs, and EUS characteristics.
All patients gave written informed consent prior to the EUS. All procedures were performed by an experienced endosonographer, and FNA was performed in all patients in this series. There was on-site cytology available for all cases to determine specimen adequacy. FC was ordered at the discretion of the endosonographer performing the procedure.
Statistical analysis comparing the two groups was done using the Student's t-test for continuous outcomes and a Chi-square analysis for categorical outcomes. Statistical significance was considered at a two-sided P < 0.05.
RESULTS
There were 11 patients with PPL and 33 age-matched controls with PA [Table 2]. At last follow-up, 7 of 11 (64%) of the PPL patients were alive, and 3 of 33 (9%) of the adenocarcinoma patients were alive (P < 0.001). The average age of the patients was 67 years old (range: 30-89). There were 8 men and 3 women in the PPL group and 18 men and 15 women in the adenocarcinoma group. There was no difference between the two groups in risk factors for malignancy, including smoking, alcohol consumption, or history of pancreatitis. The most common presenting symptoms for PPL were pain 73%, weight loss 45%, and jaundice 18%, while patients with adenocarcinoma presented with pain 52% (P = 0.3), weight loss 30% (P = 0.47), and jaundice 76% (P = 0.001).
Table 2.
Comparison of patients with adenocarcinoma to patients with primary pancreatic lymphoma
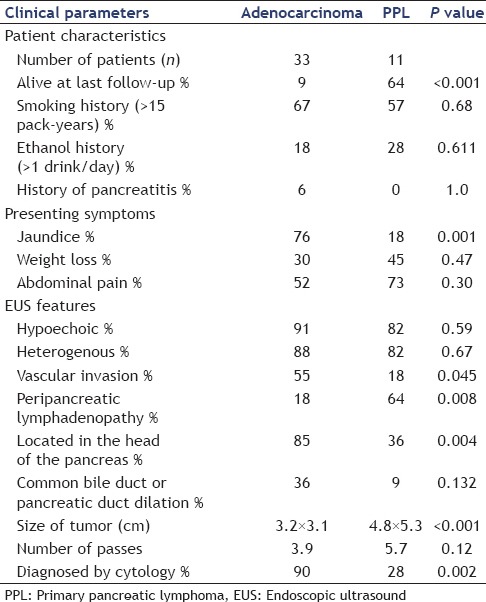
The EUS appearance was similar in the two groups in that both PPL and PA tended to be hypoechoic and heterogeneous. However, the PPL group was more likely to have peripancreatic LAD (64% vs. 18%, P = 0.008) and had larger masses (4.8 cm × 5.3 cm vs. 3.2 cm × 3.1 cm, P < 0.001). The PPL group was less likely to have vascular invasion (18% vs. 55%, P = 0.045) and less likely to be found in the head of the pancreas (36% vs. 85%, P = 0.004) [Figure 1].
Figure 1.
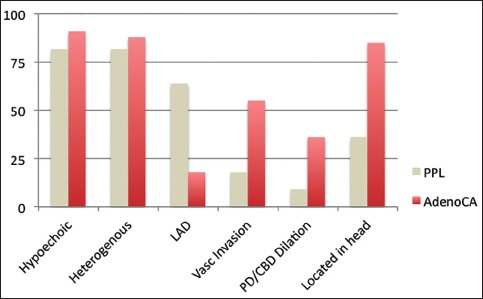
Endoscopic ultrasound characteristics of primary pancreatic lymphoma compared to pancreatic adenocarcinoma. LAD: Lymphadenopathy, Vasc: Vascular, PD/CBD: Pancreatic duct/ common bile duct, PPL: Primary pancreatic lymphoma, AdenoCA: Pancreatic adenocarcinoma
Diagnostically, FNA and cytology (without FC) made the diagnosis in 28% of PPL patients compared with 91% of adenocarcinoma patients (P = 0.002). In the PPL group, 7 of 11 FNA samples were sent for FC. If FC was added, then the diagnosis of PPL was increased to 100%.
DISCUSSION
We present a study describing the risk factors, clinical presentation, EUS characteristics, and diagnosis of PPL in 11 patients and then compare these findings to age-matched controls with adenocarcinoma. Though PPL is a rare diagnosis, distinguishing it from PA is critical because the prognosis and treatment approach are radically different between these two malignancies. For curative intent, treatment of adenocarcinoma is surgical resection, while the treatment of choice for PPL is chemotherapy.[8] The role of radiation therapy in the management of PPL has not yet been fully defined, but has been beneficial when combined with chemotherapy in some studies.[8,9] In our study, 64% of the patients with PPL were alive at follow-up, while only 9% of the PA patients were alive. In other studies, cure rates of up to 30% are seen in PPL while the 5-year survival of PA is dismal at <5%.[8,10]
The present study demonstrates some differences in clinical presentation between PPL and PA. The presence of jaundice is the most significant difference between the two groups with jaundice being present in 76% of patients with PA, while only 18% of the PPL patients had jaundice at presentation. This is somewhat surprising given the larger size of the tumors at diagnosis in the PPL group, however the difference is likely explained by the fact that fewer PPL tumors were found in the head of the pancreas (36% vs. 85%), where obstruction of the bile duct would be expected. In a study by Khashab et al., jaundice was present in 37% of patients with PPL and in a Japanese case series jaundice was the presenting symptom in only 1 of 19 cases.[3,11] In this study, pain was the most common presenting symptom for PPL at 76%, again similar to previous case series, however there was no statistically significant difference in pain as a presenting symptoms between the two groups.[3,11,12] “B-type symptoms” such as fever, and night sweats are uncommon among patients presenting with PPL and their lack of presence should, therefore, not dissuade PPL as a possible diagnosis.
Imaging plays a key role in the diagnosis and characterization of pancreatic tumors. Until the advent of EUS, CT was the most common and useful imaging technique to evaluate the pancreas. Certain radiological findings have been found to be beneficial to differential PPL from PA including:
The combination of a bulky localized tumor in the pancreatic head without dilation of the main pancreatic duct,
Enlarged lymph nodes below the level of the renal veins, and
Invasive tumor growth not respecting anatomic boundaries and infiltrating retroperitoneal or upper abdominal organisms.[8,10]
EUS has further advanced the imaging of pancreatic tumors and in this study, found that although both tumors appeared to be hypoechoic and heterogeneous, there were several differences between PPL and PA [Figure 2]. The size of the tumors at diagnosis was large in PPL than in PA (4.8 cm × 5.3 cm vs. 3.2 cm × 3.1 cm). The average size of 4-5 cm was similar to other EUS case series of PPL in the western hemisphere, but significantly smaller than the largest case series in Japan where the average tumor size was 8-9 cm.[3,4,11,12] Despite the larger size at presentation, we found that there was less likely have vascular invasion (18% vs. 55%), and as stated earlier were less likely to have biliary obstruction (18% vs. 76%). If these tumors were incorrectly assumed to be adenocarcinomas, they would be more likely to be deemed surgically respectable which could lead to unnecessary surgeries. Though PA can present with malignant LAD, we found this to be significantly more common in the PPL group.
Figure 2.
Endoscopic ultrasound with fine needle aspiration of a large pancreatic mass
Although EUS has proven to find some morphologic differences between PPL and PA, differentiating the two based solely on imaging morphologic characteristics is very difficult and tissue needs to be obtained to confirm the diagnosis. Lymphoma traditionally required examination of tissue architecture and cytomorphology to make an accurate diagnosis, therefore, limiting the value of FNA as a diagnostic modality. However, the advent of FC and immunocytochemistry has recently been shown in many studies to increase the diagnostic yield with FNA in cases of lymphoma.[6,13] The World Health Organization classification systems of lymphomas have incorporated the use of immunophenotyping and flow cytometric analysis, which are independent of tissue architecture an cytomorphology.[14,15] The diagnosis of lymphoma by EUS-FNA has thus become much more acceptable, and there are several case series specifically for PPL showing the benefits of FC in making a diagnosis.[3,4,5,8,11] The present study shows that cytology alone made the final diagnosis of lymphoma in only 28% of the patients [Figure 3] versus 91% of patients with PA. In the PPL group, if the aspirate was sent for FC, then a diagnosis was made in all of the cases. This also demonstrates the importance of on-site cytopathologic assessment at the time of EUS-FNA to ensure adequate specimens and inform the physician of the presence of abundant lymphocytes or atypical lymphoid cells, which should raise the suspicion of lymphoma, prompting analysis with FC and immunocytochemistry, which may require additional passes.
Figure 3.
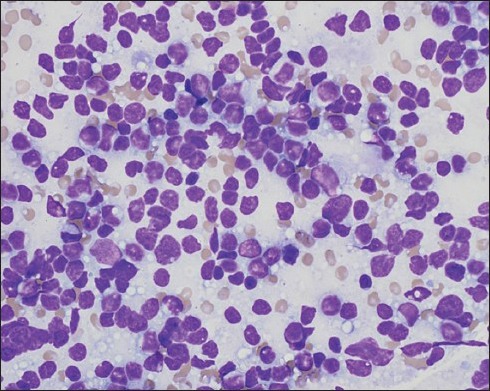
Fine-needle aspiration cytology is consistent with B-cell lymphoma. This Diff-Quik-stained image is highly cellular. The cells are discohesive, and the chromatin is fine. Many nuclei appear to have little or no cytoplasm. A few lymphoglandular bodies (small fragments of cytoplasm often seen when lymphoid tissue is aspirated) are in the background
Though we feel this is an important study, there are some limitations. The use of FC for the diagnosis of lymphoma is more difficult for lymphomas of T-cell origin or Hodgkin's lymphoma. In our study, and in the case series by Khashab et al., the majority of patients were ultimately diagnosed with a type of B-cell lymphoma and the literature suggests the most PPLs are of B-cell origin, however about 20% of the patients in the Japanese case series by Nishimura et al. had T-cell lymphoma.[3,11] Another weakness to our study is the small sample size and retrospective design, however larger studies will likely be difficult to perform due to the rarity of PPL. We did not have access to laboratory evaluations prior to many of the procedures so evaluation of the differences in carbohydrate antigen 19-9, liver function tests, and lactate dehydrogenase could not be completed. Finally, with the recent advent of newer generation of FNB-fine needle biopsy needles (ie. EchoTip® Pro-Core™, High Definition Ultrasound Biopsy needle, COOK Medical, Bloomington, Indiana, USA), more tissue can be obtained than standard FNA, and this will also have to be evaluated to see if the diagnostic yield is further increased with and without FC.
Primary pancreatic lymphoma is a rare malignancy which can mimic PA in appearance. Distinguishing between the two is clinically important due to their vastly different prognosis and treatment course and requires a high degree of suspicion by the physician. There are differences between PPL and PA in clinical presentation and EUS characteristics; however, tissue diagnosis is ultimately necessary to make a diagnosis. EUS-FNA can be used to make a diagnosis but should be combined with FC and immunocytochemistry to increase the sensitivity and specificity.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol. 1997;8:727–37. doi: 10.1023/a:1008282818705. [DOI] [PubMed] [Google Scholar]
- 2.Boni L, Benevento A, Dionigi G, Cabrini L, Dionigi R. Primary pancreatic lymphoma. Surg Endosc. 2002;16:1107–8. doi: 10.1007/s00464-001-4247-1. [DOI] [PubMed] [Google Scholar]
- 3.Khashab M, Mokadem M, DeWitt J, et al. Endoscopic ultrasound-guided fine-needle aspiration with or without flow cytometry for the diagnosis of primary pancreatic lymphoma — A case series. Endoscopy. 2010;42:228–31. doi: 10.1055/s-0029-1243859. [DOI] [PubMed] [Google Scholar]
- 4.Rossi ED, Larghi A, Verna EC, et al. Endoscopic ultrasound-guided fine-needle aspiration with liquid-based cytologic preparation in the diagnosis of primary pancreatic lymphoma. Pancreas. 2010;39:1299–302. doi: 10.1097/MPA.0b013e3181dc694e. [DOI] [PubMed] [Google Scholar]
- 5.Nayer H, Weir EG, Sheth S, et al. Primary pancreatic lymphomas: A cytopathologic analysis of a rare malignancy. Cancer. 2004;102:315–21. doi: 10.1002/cncr.20488. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 7.Chang KJ. State of the art lecture: endoscopic ultrasound (EUS) and FNA in pancreatico-biliary tumors. Endoscopy. 2006;38(Suppl 1):S56–60. doi: 10.1055/s-2006-946654. [DOI] [PubMed] [Google Scholar]
- 8.Saif MW. Primary pancreatic lymphomas. JOP. 2006;7:262–73. [PubMed] [Google Scholar]
- 9.Bouvet M, Staerkel GA, Spitz FR, et al. Primary pancreatic lymphoma. Surgery. 1998;123:382–90. [PubMed] [Google Scholar]
- 10.Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: Differential aspects. AJR Am J Roentgenol. 2000;174:671–5. doi: 10.2214/ajr.174.3.1740671. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura R, Takakuwa T, Hoshida Y, et al. Primary pancreatic lymphoma: Clinicopathological analysis of 19 cases from Japan and review of the literature. Oncology. 2001;60:322–9. doi: 10.1159/000058528. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Li SD, Hu XG, et al. Primary pancreatic lymphoma: report of six cases. World J Gastroenterol. 2006;12:5064–7. doi: 10.3748/wjg.v12.i31.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra M, Tamhane A, Eloubeidi MA. Eus-guided FNA combined with flow cytometry in the diagnoses of suspected or recurrent intrathoracic or retroperitoneal lymphoma. Gastrointest Endosc. 2005;62:508–13. doi: 10.1016/j.gie.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting — Airlie House, Virginia, November, 1997. Hematol J. 2000;1:53–66. doi: 10.1038/sj.thj.6200013. [DOI] [PubMed] [Google Scholar]
- 15.Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol. 2000;13:193–207. doi: 10.1038/modpathol.3880035. [DOI] [PubMed] [Google Scholar]


