Abstract
Background:
Intrinsic variance of the urine proteome limits the discriminative power of proteomic analysis and complicates potential biomarker detection in the context of pediatric sleep disorders.
Methods and Results:
Using a rigorous workflow for proteomic analysis of urine, we demonstrate that gender and diurnal effects constitute two important sources of variability in healthy children. In the context of disease, complex pathophysiological perturbations magnify these proteomic differences and therefore require contextualized biomarker analysis. Indeed, by performing biomarker discovery in a gender and diurnal-dependent manner, we identified ~30-fold more candidate biomarkers of pediatric obstructive sleep apnea (OSA), a highly prevalent condition in children characterized by repetitive episodes of intermittent hypoxia and hypercapnia, and sleep fragmentation in the context of recurrent upper airway obstructive events during sleep. Remarkably, biomarkers were highly specific for gender and sampling time since poor overlap (~3%) was observed in the proteins identified in boys and girls across morning and bedtime samples.
Conclusions:
Since no clinical basis to explain gender-specific effects in OSA or healthy children is apparent, we propose that implementation of contextualized biomarker strategies will be applicable to a broad range of human diseases, and may be specifically applicable to pediatric OSA.
Keywords: Urine proteomics, obstructive sleep apnea, biomarkers, pediatric, diagnosis, gender
INTRODUCTION
Obstructive sleep apnea (OSA) is a highly prevalent disorder in children (2-3%) characterized by repeated events of partial or complete obstruction of the upper airway during sleep. This frequent condition, which results in recurring episodes of hypercapnia, hypoxemia, and arousal throughout the night, leads to substantial risk for the development of cardiovascular, metabolic, and neurobehavioral and cognitive problems (1-6).
The gold standard diagnostic approach for OSA is an overnight sleep study, or polysomnography. While this approach is reliable, it suffers from problems associated with its implementation in the clinical setting. Indeed, polysomnography is labor intensive, inconvenient, and expensive resulting in long waiting periods and unnecessary delays in diagnosis and treatment. From this perspective, the identification of surrogate biomarkers that reliably diagnose OSA would substantially overcome these problems and enable early detection and intervention for this important medical problem.
Urine is a highly desirable biological fluid for diagnostic testing because of its ease of collection and widespread use in clinical laboratories. Biomarker discovery strategies in urine, however, have been hindered by problems associated with reproducibility and inadequate standardization of proteomic protocols. Despite these concerns, urinary proteomics analyses have been leveraged to identify numerous candidate biomarkers of a broad range of human disorders that have included, but are not limited to renal disease, diabetes, atherosclerosis, Alzheimer’s disease, and cancer (7-14). Furthermore, our previous studies identified 12 candidate urinary biomarkers capable of reliably detecting OSA in a restricted cohort of children (15).
The litmus test for any given biomarker involves validation in large patient populations, a requirement that few biomarkers pass. Indeed, our preliminary attempts to validate our previously identified biomarkers of OSA in a new patient cohort have highlighted the difficult nature of this endeavor. Genetic and environmental variability impose extraordinary heterogeneity on patient populations, and people in general. These factors introduce significant “instability” into the urinary proteome making the identification of biomarkers with universal application a daunting challenge (16).
In an attempt to circumvent these problems, we interrogated two important likely sources of variability (gender and diurnal effects) on both the urine proteome and biomarker discovery process of pediatric OSA. To facilitate this process, we developed a rigorous and reproducible proteomics workflow for biomarker discovery based on liquid chromatography tandem mass spectrometry (LC-MS/MS), an approach that allows for deeper proteome coverage and interrogation of lower abundance proteins. Our findings demonstrate the presence of dramatic gender and diurnal effects on biomarkers of OSA, suggesting that discovery-based proteomics approaches aimed at identifying biomarkers in a contextualized manner may greatly facilitate our ability to reliably detect human disease.
MATERIALS AND METHODS
Ethics statement
The research protocol was approved by the University of Chicago (protocol 10-708-A-AM011) human research ethics committee. Written informed consent was obtained from the parents and age appropriate written assent from the children.
Patient information
Children (ages 2-12 years) clinically referred for evaluation of OSA underwent an overnight polysomnographic evaluation at the University of Chicago Pediatric Sleep Laboratory. Healthy children were recruited from schools or well-child clinics. Exclusion criteria for all subjects included the presence of significant genetic or craniofacial syndromes, diabetes, cystic fibrosis, cancer, or treatment with oral corticosteroids, antibiotics, or anti-inflammatory medications. All parents completed a detailed intake clinical questionnaire. Height, weight and vital signs were recorded for each child, and body mass index (BMI) z-score was calculated on the basis of CDC 2000 growth standards (www.cdc.gov/growthcharts) and using online software (www.cdc.gov/epiinfo). A BMI z-score exceeding 1.65 (.95th percentile) was considered as fulfilling criteria for obesity.
Overnight Polysomnography
All subjects underwent an overnight polysomnography using standard methods (17). The PSG studies were scored as per the 2007 American Association of Sleep Medicine guidelines for the scoring of sleep and associated events (18). The obstructive apnea–hypopnea index (AHI) was defined as the number of obstructive apneas and hypopneas per hour of total sleep time.
Urine collection
Mid-stream urine specimens were collected in the evening just before bedtime and as the first void in the morning after awakening. Samples (20 ml) were collected into tubes containing phenylmethylsulfonyl fluoride (PMSF, 2mM final concentration), and immediately stored at −80°C until analysis.
Study Design
Morning and bedtime urine samples from healthy children (N=13; 7 boys and 6 girls) and children with OSA (N=14; 7 boys and 7 girls) were collected. Samples were analyzed by mass spectrometry (single injection) and ELISA (in duplicate). Analyses of the resultant proteomics data (Table S1-S3) were used to assess the reproducibility of the proteomics workflow, identify diurnal and gender-dependent effects on the urine proteome of healthy children, and detect candidate biomarkers of pediatric OSA. See below for details.
Preparation of soluble urine proteins for mass spectrometry (MS)
Urine (10mL) was thawed quickly at 37°C, vortexed for 90s, and centrifuged (500×g, 4°C) for 5min. Supernatants were centrifuged at 12,000×g, 4°C for 20min to remove urinary sediment, and incubated with 1mL ProteinG magnetic beads (Millipore) for 30min at 20°C. Depletion of IgG was performed according to the manufacturer’s protocol. IgG-depleted urine samples were precipitated using TCA/DOC as previously described (19, 20). Briefly, urine was supplemented with 0.02% sodium deoxycholate and 20% trichloroacetic acid, and incubated overnight with rocking at 4°C. Proteins were harvested by centrifugation (18,000×g for 60 min at 4°C). The protein pellet was washed twice with ice-cold acetone, and reconstituted in 0.1% RapiGest (Waters Corp.), 250 mM ammonium bicarbonate, pH 8.8. Protein concentration was determined by the Bradford assay with albumin as a standard. Samples (90μg) were incubated with α-human albumin-coupled magnetic beads (90μL, Millipore) and depletion was performed according to the manufacturer’s protocol. Samples were reduced, alkylated, and digested overnight at 37°C with sequencing-grade trypsin (1:50, w/w, trypsin/protein; Promega). Tryptic digests were mixed with acetic acid (1:1, v/v) and subjected to solid-phase extraction on a C18 column (HLB, 1 mL; Waters Corp.) according to the manufacturer’s protocol. Fractions containing peptides were dried under vacuum and resuspended in 0.3% formic acid, 5% acetonitrile (0.4 mg/mL) for LC-MS/MS analysis.
Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS)
Tryptic digests (1.5 μg) were loaded directly onto 2 cm C18 trap column (packed in-house), washed with 10 μl of solvent A (5% acetonitrile, 0.1% formic acid), and eluted on a 15 cm long, 75 μM reverse phase capillary column (ProteoPep™ II C18, 300 Å, 5 μm 1 size, New Objective, Woburn MA). Peptides were separated at 300 nL/min over a 180 minute linear gradient from 5% to 35% buffer B (95% acetonitrile, 0.1% formic acid) on a Proxeon Easy n-LC II (Thermo Scientific, San Jose, CA). Mass spectra were acquired in the positive ion mode, using electrospray ionization and a linear ion trap mass spectrometer (LTQ Orbitrap Velos®, Thermo Scientific, San Jose, CA). The mass spectrometer was operated in data dependent mode, and for each MS1 precursor ion scan, the ten most intense ions were selected from fragmentation by CID (collision induced dissociation). Other parameters for mass spectrometry analysis included: resolution of MS1 was set at 60,000, normalized collision energy 35%, activation time 10ms, isolation width 1.5, and the +1 and +4 and higher charge states were rejected.
Peptide and protein identification
MS/MS spectra were searched against the International Protein Index human (v3.87, 91464 entries) primary sequence database (21) using Sorcerer™-SEQUEST® (version v. 3.5,) (Sage-N Research, Milpitas, CA). Search parameters included trypsin specificity (after Arg orLys) with up to 2 missed cleavages. SEQUEST® was searched with a parent ion tolerance of 50 ppm and a fragment ion mass tolerance of 1 amu with fixed Cys alkylation, and variable Met oxidation. SEQUEST results were further validated with PeptideProphet (22) and ProteinProphet (23) using an adjusted probability of ≥0.90 for peptides and ≥0.96 for proteins. Search results were further processed by the Computational Proteomics Analysis System (CPAS) (24) prior to statistical analysis (see below). Proteins considered for analysis had to be identified in at least 70% of individuals in at least one patient group (eg. healthy girls, or boys with OSA). When MS/MS spectra could not differentiate between protein isoforms, all were included in the analysis.
Protein quantification and statistical analysis
Proteins detected by LC-MS/MS were quantified by spectral counting (the total number of MS/MS spectra detected for a protein (25)). Differences in relative protein abundance were assessed with the t-test and G-test (19, 26, 27). Permutation analysis was used to empirically estimate the FDR (28). Significance 1 cutoff values for the G-statistic and t-test were determined using PepC (29), a software package that maximizes the number of differentially abundant proteins identified for a given FDR.
ELISA
Urine samples were thawed rapidly at 37°C and clarified by centrifugation at 500×g for 10min. Protein levels in resultant supernatants were quantified using commercially available ELISAs for DPP4 (Abnova; KA0141), AZGP1 (Abnova; KA1689), CP (Assaypro; EC4101-1), HPX (Innovative Research, Inc.; IRKTAH2562), and creatinine (Abcam; ab65340) according to the manufacturer’s protocols. All protein levels were standardized to urine creatinine levels (30) and statistical significance between the groups was assessed by a two-tailed, Student’s t-test.
Functional annotation
Functional enrichments in Gene Ontology annotations in the urine proteome or differentially abundant putative urine biomarkers (relative to the entire human genome) were identified using the Bingo 2.0 plugin in Cytoscape (V2.8.2) (31). Statistical significance was assessed using the hypergeometric test (p<0.05) with Benjamini-Hochberg correction (28) and functional categories with ≥ 5 proteins were considered.
RESULTS
Proteomics workflow for urine biomarker discovery
Biomarker discovery strategies based on proteomics are complicated by low protein concentrations and high levels of interfering substances (e.g., salts and nitrogenous bases) in urine. To develop a rigororous and reproducible workflow for proteomics analysis of urine, we developed a 4-step procedure involving: i) centrifugation to remove particulate material and urinary sediment, ii) depletion of immunoglobulin (IgG) and albumin (ALB) to facilitate deeper proteome coverage, iii) protein precipitation to concentrate urine proteins and remove interfering substances, and iv) mass spectrometric analysis by LC-MS/MS (Fig. 1a).
Fig. 1. Pipeline for urine biomarker discovery by LC-MS/MS.
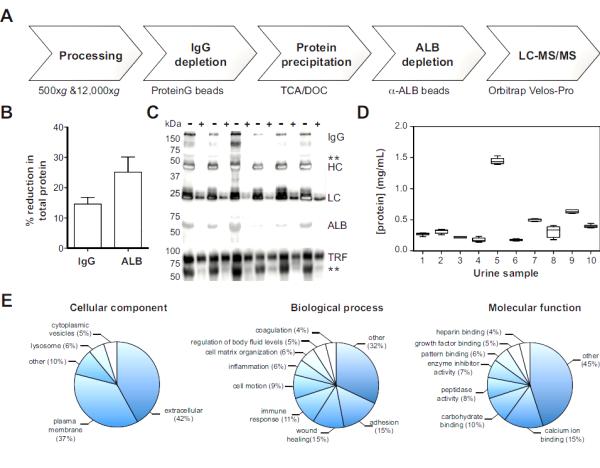
Panel A: A workflow for proteomic analysis of urine. Panels B-C Immunoglobulin (IgG) and albumin (ALB) depletion. The extent of depletion of total protein (% reduction) was quantified by Bradford (Panel B) and visualized by SDS-PAGE (Panel C). Specificity of IgG and ALB removal was assessed by comparing serotransferrin (TRF) levels in depleted (+) and non-depleted (-) samples (Panel C). IgG, whole antibody; HC, heavy chain; LC, light chain; **, non-specific detection of ALB. Panel D: Urine samples were precipitated with TCA/DOC and protein levels were determined for 10 subjects. Results (N=6/subject) are displayed as box-and-Whisker plots (5-95% confidence intervals). Panel E: Gene ontology analysis of all urine proteins detected by mass spectrometry. All functional annotations presented are statistically significant (p<0.05) based on the hypergeometric test with Benjamini-Hochberg correction.
ALB and IgG are highly abundant urine proteins (40-60% of total urinary protein) that interfere with detection of low abundance species and complicate quantification in label-free proteomic approaches (32). Magnetic beads were carefully titrated to maximize depletion of ALB and IgG (Fig. 1b,c) and minimize non-specific loss of unrelated proteins, as assessed by loss of serotransferrin (TRF) levels (Fig. 1c). Since proteins are more efficiently precipitated in concentrated solutions (due to molecular crowding), we depleted ALB after protein precipitation. However, IgG depletion was incompatible with the buffer (0.1% RapiGest) used to solubilize protein pellets, and was therefore performed prior to precipitation.
Protein precipitation is a crucial step in urine proteomic studies, that concentrates urinary proteins and removes interfering substances that prohibit direct analysis by mass spectrometry. For this purpose, we incorporated a method involving tricholoroaceteic acid and deoxycholate (TCA/DOC (19, 20)) because it is well suited for precipitating proteins out of dilute solutions. The reproducibility of this method within and across samples was interrogated by precipitating 6 aliquots of the same urine sample collected from each of 10 subjects. This approach yielded highly reproducible results (6% CV, intra-sample) over a wide range of urinary protein concentrations (Fig. 1d).
To test the reproducibility of our proteomics workflow, urine samples from 27 children (Table 1) were processed and subjected to LC-MS/MS analysis (Table S1). Based on a minimum of 2 unique peptide identifications per protein, our approach reliably identified 505±10 proteins per sample. Moreover, variation in sample depth, the number of high quality peptide identifications per run, was minimal (10,053±237 peptides) indicating that our method was robust and reproducible. Indeed, gene ontology analysis of the detected urinary proteome identified significant enrichments in functional annotations (Fig. 1e) consistent with previous proteomics analyses of urine (33, 34).
Table 1.
Demographic and polysomnographic characteristics of subjects.
| Control (N=13) | 0SA (N=14) | t-test (p-value) | |
|---|---|---|---|
| Age (years) | 7.5 ± 0.8 | 5.9 ± 0.6 | 0.11 |
| Gender (boy,girl) | 7,6 | 7,7 | N/A |
| BMI, z-score | 0.6 ± 0.3 | 1.2 ± 0.5 | 0.27 |
| AHI (events/hr/total sleep time) |
0.4 ± 0.1 | 23.3 ± 5.3 | 0.002 |
Abbreviations: BMI = body mass index; AHI = obstructive apnea-lnypopnea index; OSA = obstructive sleep apnea. Where applicable, results are presented as means ±SEM.
Gender and diurnal effects introduce variability into the urine proteome of healthy children
Identifying biomarkers of disease is complicated by the inherent variability in the urine proteome. Gender and diurnal effects represent two important factors that can potentially introduce noise into the measurement. To investigate their impact on the urine proteome under physiological conditions, we collected morning and bedtime samples from healthy boys (N=7) and girls (N=6). Healthy children (ages 2-12 years) were selected by a priori excluding participants with genetic or craniofacial syndromes, diabetes, cystic fibrosis, or cancer. Additional exclusion criteria included chronic use of medications, steroids, or immunotherapy drugs.
Samples were processed through our proteomics workflow (see Fig. 1), and subjected to LC-MS/MS analysis. Proteins were quantified by spectral counting (25), and statistically significant changes in protein levels were identified using a combination of the t-test and G-test (19, 26, 27) using cutoffs that minimized the false discovery rate (28, 29). A representative analysis is provided in Fig. 2a, which demonstrates the detection of gender-regulated proteins in morning urine samples upon application of our stringent statistical criteria (G-test: G≥1.5 or G≤−1.5; t-test: α=0.05; FDR<0.05).
Fig. 2. Gender and diurnal effects on the urinary proteome of healthy children.
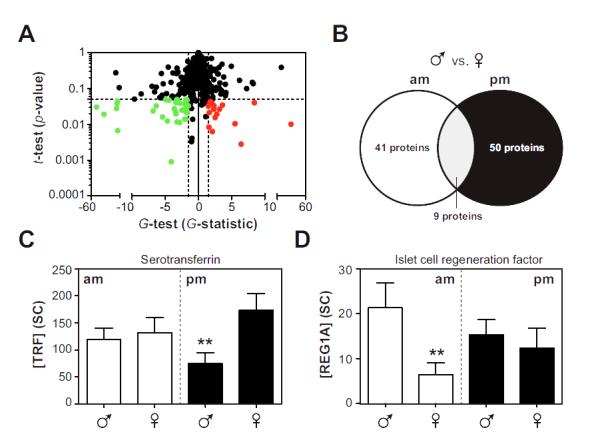
Morning (am) and bedtime (pm) urine samples were collected from healthy boys (N=7) and girls (N=6) and subjected to LC-ESI-MS/MS analysis. Proteins were quantified by spectral counting and differentially abundant proteins were detected using the t-test and G-test. Panel A: A representative statistical analysis demonstrating proteomic differences in morning samples between boys and girls. Red, elevated in boys; Green, reduced in boys. Confidence intervals (dashed lines; G>1.5 or G<−1.5 and α=0.05) and the FDR (<5%) were established by permutation analysis. Proteins that were elevated in boys were assigned negative values in the G-test. Panel B: A comparison of differentially abundant proteins in boys (relative to girls) in morning and bedtime samples. Panels C-D: Examples of proteins (TRF and REG1A) that are subjected to both gender and diurnal regulation. Results are means ±SEMs, statistical significance (**) was assessed by a combination of the t-test and G-test.
Using this approach, we detected substantial differences in the urinary proteome of healthy boys and girls, both in morning (~7%; 50 of 750 proteins) and bedtime (8%; 41 of 750) samples (Fig. 2a,b, Table S2). Interestingly, we observed poor overlap (<10%) between differentially abundant proteins in morning and bedtime samples, suggesting that gender-related differences were also highly sensitive to diurnal effects (Fig. 2b). For example, TRF levels were elevated in girls at bedtime, while islet cell regeneration factor (REG1A) was specifically increased in morning urine samples collected from boys (Fig. 2c).
In general, urine protein composition was more substantially influenced by gender (69 proteins: G-test, G≥1.5 or G≤−1.5; t-test, p<0.05; FDR<0.05) over diurnal effects (8 proteins: G-test, G≥1.5 or G≤−1.5; t-test, p<0.05; FDR<0.05), an observation that is quite remarkable given that our analysis focused on pre-pubescent children. Consistent with this finding, gene ontology analysis of the gender-regulated urinary proteome in healthy children revealed significant enrichments in functional annotations that are not classically associated with gender (cell adhesion, p=6.0×10−7; pattern binding, p=7.0×10−3; complement and coagulation cascades p=4.2×10−3). In sharp contrast, this approach failed to identify significance in more intuitive modules such as female pregnancy (p=0.11) or embryo implantation (p=0.11).
Gender and diurnal dependence of urine biomarker discovery of pediatric OSA
Having demonstrated that gender and diurnal factors substantially regulate the urinary proteome of children under physiological conditions, we sought to investigate how these differences would impact the process of biomarker discovery among children with OSA. Children (ages 2-12 years) with moderate to severe OSA, as assessed by the polysomnography-derived criterion of apnea hypopnea index (AHI>5 events/hour total sleep time), were recruited along with age- and gender-matched controls. Their demographic characteristics were such that no statistically significant differences in age, gender, ethnicity, or BMI distribution were present (Table 1).
Using stringent criteria for quality and reproducibility of protein detection (see Materials and Methods), our mass spectrometric analyses of urine samples identified 742 urine proteins across all patient samples (Table S1). To investigate the impact of gender and diurnal variation on biomarker discovery, we performed statistical analysis (using the t-test and G-test (19, 27, 29)) in three ways (Fig. 3a). In level 1 analysis, protein levels were averaged across morning and bedtime samples and groups were not differentiated according to gender. Level 2 analysis investigated morning and bedtime samples independently, while level 3 analysis treated samples in a collection time- and gender-dependent fashion (Fig. 3a).
Fig. 3. Identification of candidate biomarkers of pediatric OSA.
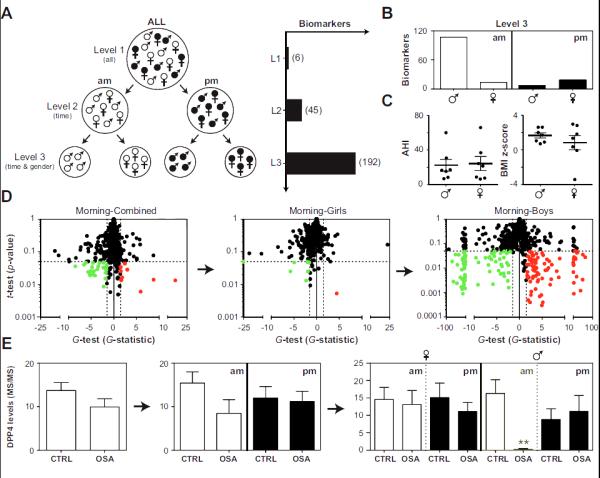
Morning (am) and bedtime (pm) samples were collected from children with and without OSA and subjected to LC-MS/MS. Panel A: Analysis of proteomic data was performed as follows: Level 1 (L1), morning and bedtime measurements were averaged and boys and girls were pooled; Level 2 (L2), analyses for morning and bedtime samples were conducted independently; Level 3 (L3) analyses for morning and bedtime samples were conducted independently in both boys and girls. The number of candidate biomarkers identified at each level is shown in parentheses. Panel B: Biomarkers detected in level 3 were split according to collection time and gender. Panel C: AHI and BMI z-scores in boys and girls with OSA. Panel D: A demonstration of the “gender effect” on global proteomic analysis (based on the t-test and G-test) of morning urine samples. Red, up-regulated in OSA; green, down-regulated in OSA; dashed lines confidence intervals (FDR < 5%). Panel E: Dipeptidyl peptidase 4 (DPP4) as an example of a specific biomarker for OSA in the morning samples of boys. Protein levels (mean ±SEMs) were determined by spectral counting. **, statistically significant based on the t-test and G-test.
Six candidate biomarkers of pediatric OSA were identified in level 1 analysis (Table S3). Notably, orosomucoid 1 (ORM1), a protein that was initially identified in our previous OSA biomarker screen (15), was also detected in this analysis. The statistical significance level for ORM1, however, barely cleared statistical thresholds, and subsequent ELISA measurements failed to validate this finding (Control: 42.8±14.3 μg/mg creatinine, OSA: 68.5±29.5 μg/mg creatinine, p=0.37, N=14). A substantial increase in the number of biomarkers detected was evident when morning and bedtime samples were treated independently (level 2, 45 proteins) and a further, more dramatic, increase was visualized when gender was also accounted for in the analysis (level 3, 192 proteins) (Fig. 3a, Table S3). Accounting for gender and sampling time also increased the statistical significance of the candidate biomarkers identified. In this regard, 26% of the candidate biomarkers exhibited p<0.005 (t-test) in level 3 analysis, while no proteins satisfied this criterion in level 2 or level 1 analysis. Moreover, fatty acid binding protein 3 (FABP3), a candidate biomarker that cleared statistical thresholds in all levels of analysis, demonstrated substantially improved statistics from level 1 (G-test: G = 2.4; t-test, p=0.022) to level 2 (G-test: G = 6.0; t-test, p=0.006) to level 3 analysis (G-test: G = 12.8; t-test, p=0.0009).
In general, morning urine samples were overrepresented in differentially abundant proteins, a result largely based on the overwhelming effect of OSA on the urinary proteome of boys (Fig. 3b). This observation is not surprising given that OSA is a sleep disorder characterized by repetitive respiratory events at night that should therefore be more likely to manifest in morning urine; however, the opposite results emerged among girls, in whom bedtime urine samples yielded a higher number of candidate biomarkers (Fig. 3b). Moreover, differentially abundant proteins were highly specific for gender and sampling time, since poor overlap (~3%) was observed in the candidate biomarkers identified in boys and girls across morning and bedtime samples (Table S3). Importantly, gender differences in the biomarkers detected could not be accounted for by differences in age, disease severity, or obesity (BMI z-score) since these parameters were not significantly different between the groups (Fig. 3c).
Taken together, our results suggest that failing to account for sampling time and gender substantially masks significant differences in protein abundance associated with a disease state such as OSA. This concept is clearly illustrated by global proteomic analysis of morning urine samples with the t-test and G-test, which shows dramatic improvements in both number and statistical significance of biomarkers identified (Fig. 3d). Similar conclusions emerge at the individual protein level using dipeptidyl peptidase 4 (DPP4) as an example (Fig. 3e).
Validation of candidate biomarkers identified by proteomic analysis
To validate our findings, we used commercially available ELISA assays to measure urinary levels of 4 candidate biomarkers. Since protein levels in urine are highly variable, and influenced by body fluid volume, all measurements were standardized against corresponding urinary creatinine levels (30). ELISA measurements generally correlated well with label-free quantification by MS/MS (eg. HPX, p<0.0001, R2=0.52; Fig. 4a) and provided strong validation for gender and diurnal regulation of protein levels (e.g., DPP4; compare Figs. 3d and 4b). Gender and diurnal effects on protein levels were not due to systematic biases in creatinine levels, which were insensitive to gender and time of collection (Fig. S1). In total, ELISA assays provided independent confirmations of changes in protein levels for 4 candidate biomarkers detected in our proteomic analyses: DPP4 (p=0.02), HPX (p=0.02), and CP (p=0.01) emerged as reliable indicators of OSA in boys, and AZGP1 (p=0.07) was identified in girls (Fig. 4b,c). Moreover, because ELISA assays involved minimal processing of urinary samples (centrifugation), while proteomic analyses required substantial processing efforts (centrifugation, IgG and ALB depletion, protein precipitation, sample digestion, etc.) the strong concordance between these two approaches further suggests that our proteomic workflow for urine biomarker discovery is robust.
Fig. 4. Validation of mass spectrometry data by ELISA.
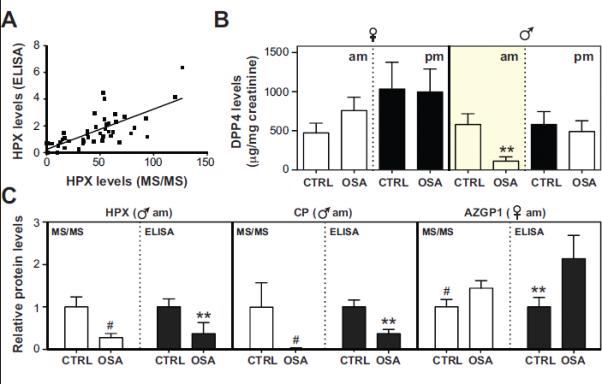
Differentially abundant proteins identified by proteomic analysis were validated in morning (am) and bedtime (pm) samples using commercially available ELISA assays. Panel A: Comparison of hemopexin (HPX) level quantified by mass spectrometry (MS/MS) and ELISA (ng/mg creatinine). Linear regression analysis (line) detected a strong positive correlation (R2 = 0.52, p<0.0001) between both techniques. Panel B: Measurement of DPP4 levels by ELISA demonstrating specific reduction of dipeptidyl peptidase 4 (DPP4) in morning urine samples (compare to Fig. 3d). Panel C: Comparison of HPX (ng/mg creatinine), ceruloplasmin (CP; ng/mg creatinine), and zinc-α-2-glycoprotein (AZGP1; ng/mg creatinine) levels quantified by MS/MS and ELISA. Measurements were normalized relative to control samples. Where applicable results are means ±SEMs. #, statistically significant based on the t-test (p<0.05) and G-test (G>1.5). **, statistically significant based on the t-test (p<0.05).
Biomarkers of pediatric OSA map to pathophysiological functional modules
Having identified a wide range of candidate biomarkers in urine collected from children with OSA, we next sought to determine whether those proteins mapped to specific functional pathways. To this end, we used gene ontology analysis to organize the 192 proteins into functional modules based on biological processes and molecular function (Fig. 5). This strategy identified significant enrichment (relative to the entire human genome) in a number of functional annotations including acute phase proteins (p=8.4×10−5), angiogenesis (p=2.7×10−3), hemostasis (p=4.2×10−8), leukocyte immunity (p=2.4×10−2), and lipid binding (p=2.3×10−4). Previous studies provide evidence that all of these pathways are affected in OSA. For example, disruption in inflammatory/immune, lipid, angiogenic, and hemostatic pathways have all been reported in patients with OSA (35-38), and are proposed as the mechanistic basis for the heightened prevalence of associated co-morbidities in OSA, such as obesity, diabetes, and atherosclerosis. Taken together, these findings suggest that aside from providing an abundant repository of disease biomarkers, the urinary proteome also comprises a wealth of information concerning disease-related pathological processes.
Fig. 5. Biomarkers of pediatric OSA map to pathophysiological modules.
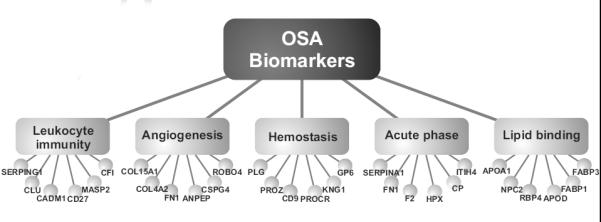
Gene ontology analysis of the 192 candidate biomarkers identified numerous functional modules enriched in children with OSA (p<0.05, hypergeometric test with Benjamini-Hochberg correction). Six representative proteins in each functional module are presented as examples.
DISCUSSION
Using a rigorous and reproducible workflow for proteomic analysis, we here demonstrate that gender and diurnal effects introduce significant variability into the urinary proteome of healthy children. By incorporating these constitutive determinants of variance into our analyses, 192 candidate biomarkers were a priori identified in urine collected from children with OSA. Moreover, we show that most if not all (~97%) of these biomarkers retained their predictive ability only if their use was implemented in the contextual setting of their collection (i.e., morning in boys, or bedtime in girls), a result that was validated by ELISA measurements. These results highlight the complexity of the biomarker discovery process, and suggest that carefully contextualized biomarker discovery strategies will be obligatorily needed to effectively detect human disease across broad populations.
Genetic and environmental perturbations impose dramatic variability on protein expression patterns in individuals. Epigenetic, transcriptomic, metabolomic, and proteomic studies have highlighted the dynamics of regulation of gene expression within healthy populations (37, 39). For example, DNA methylation patterns in healthy human tissues were highly sensitive to age and environmental factors (39). Similarly, metabolites relating to mitochondrial energy metabolism were found to differentiate gender and age in healthy adults (37). Current findings demonstrating that diurnal and gender-related effects operate as powerful modulators of the urinary proteome in healthy children are highly consistent with these other studies.
Given the inherent variability in healthy populations, how does this influence how we detect disease? Traditional approaches to biomarker discovery are based on the premise that introducing a complex pathophysiological process will overwhelm the substantial underlying heterogeneity, and produce common factors or signatures that can be leveraged to detect disease in broad populations. Our findings suggest an alternative scenario, whereby disease predictably exaggerates the underlying variability, resulting in the identification of biomarkers that are effective only in a context-specific manner. Indeed, we show that gender and diurnal differences in urinary proteome are more robust in children with OSA than in healthy controls.
Importantly, our identification of gender-related biomarkers of OSA cannot be attributed to disease etiology. To our knowledge, there are no previous studies documenting distinct pathophysiological responses or clinical outcomes of OSA in boys and girls. Although a diurnal effect in OSA is predictable, since OSA is a sleep disorder, this rationale cannot explain why such strong diurnal regulation was observed only in boys, or why diurnal factors determined the gender-related effects on urine composition in healthy children. Based on these arguments, we believe that our findings can be extrapolated to a broad range of diseases, and may further provide the basis for retrospectively incorporating these factors in the context of a systematic reassessment of pre-existing biomarker discovery datasets.
Rigorous matching of cases and controls is a precaution often utilized such as to minimize the contribution of “noise-related confounders” that could affect urine protein composition. Although somewhat counterintuitive, our data suggest that such noise-reducing strategies may actually have the opposite effect. Pooling urine samples within an individual patient, or binning information across genders substantially interfered with our ability to reliably detect disease. Accordingly, we demonstrate dramatic improvements in the statistical significance and quantity (~30-fold increase) of OSA biomarkers identified when the “noise-related factors” are incorporated into the biomarker discovery process.
It is important to stress that the conceptual innovations introduced herein were obtained in children, a population that is substantially protected from additional environmental and pathological confounders (e.g., puberty, smoking, chronic illness, medications, etc.) whose incidence and heterogeneity increase exponentially with age. Accordingly, extrapolating these concepts to the detection of disease biomarkers in adult populations will likely require the incorporation of more complex contextualized schema into the biomarker discovery process. In general, these methodological approaches underlie the foundation for implementation of personalized medicine, which could potentially revolutionize the way we diagnose and treat disease. Although the conceptual framework of personalized biomarkers for disease detection has only been recently advanced, it is rapidly gaining traction (40-42). For example, a recent study leveraged massive parallel sequencing to develop personalized biomarkers capable of identifying translocations in solid tumors (43).
At this time, the physiological and pathophysiological mechanisms mediating gender and diurnal regulation of the urinary proteome in children with OSA remain elusive. However, based on our functional assessment of the proteomic data by gene ontology analysis, it becomes apparent that urine adequately reflects pathological processes of the disease. Inflammatory, immune, lipid, hemostatic, and angiogenic pathways are well-recognized clinical sequelae of OSA and its associated co-morbidities (35-38). It should be noted that bioinformatic analysis of urine proteins should be interpreted with caution since changes in urine protein levels are not necessarily correlated to blood levels.
A comparison of our list of candidate biomarkers identified in children with OSA to those previously reported for adults with diabetes (7), coronary artery disease (8), and chronic obstructive pulmonary disease (44) revealed intriguing overlaps. These findings suggest that by targeting similar proteins and/or biological pathways, OSA may predispose children to develop these associated disorders later on in life. Given the large changes observed in the morning urine proteome of boys with OSA, we would further predict that boys might be at a greater risk to develop such co-morbidities. However, future validation of such hypotheses in large-scale cohorts appears warranted. OSA is a highly prevalent health problem in children, particularly among obese children. Considering the escalating problem of childhood obesity in the United States and around the world, properly and timely diagnosing and treating OSA will continue to pose a significant challenge due to the onerous nature of polysomnography and the scarce availability of pediatric sleep experts. Our identification of candidate urinary biomarkers of OSA in children may enable simplified and cost-effective diagnostic algorithms (45), and perhaps lay the foundation for new approaches to monitoring treatment efficacy for this important medical problem.
Supplementary Material
Table S1. Urinary proteomics analysis by LC-MS/MS. A list of the 742 proteins identified by LC-MS/MS analysis of urine and quantification by spectral counting.
Table S2. Gender and diurnal effects on the urinary proteome of healthy children. A list of the statistically significant, gender-regulated proteins detected in morning and bedtime urine samples of healthy children. Results of the t-test and G-test are presented.
Table S3. Identification of urine biomarkers of pediatric OSA. Identification of differentially abundant urinary proteins in OSA relative to control samples. Results of levels 1 (all samples), 2 (corrected for diurnal effects), and 3 (corrected for both diurnal and gender effects) biomarker analysis along with corresponding t-test and G-test values are displayed.
Fig. S1. Urine creatinine levels are insensitive to gender and sampling time. Morning (am) and bedtime (pm) urine samples were collected from children with and without OSA and creatinine levels were determined by ELISA. Results are means ±SEMs and statistical significance was assessed by the t-test.
ACKNOWLEDGEMENTS
We thank Richa Kulkarni for her assistance with subject recruitment. Mass spectrometry experiments were performed at the Northwestern Proteomics Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. American journal of respiratory and critical care medicine. 2008;177(10):1142–9. doi: 10.1164/rccm.200711-1670OC. Epub 2008/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. American journal of respiratory and critical care medicine. 2008;177(4):369–75. doi: 10.1164/rccm.200608-1190PP. Epub 2007/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gozal D, Kheirandish-Gozal L. The multiple challenges of obstructive sleep apnea in children: morbidity and treatment. Current opinion in pediatrics. 2008;20(6):654–8. doi: 10.1097/MOP.0b013e328316ec2d. Epub 2008/11/14. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D, Kheirandish-Gozal L. Childhood obesity and sleep: relatives, partners, or both?-a critical perspective on the evidence. Annals of the New York Academy of Sciences. 2012;1264(1):135–41. doi: 10.1111/j.1749-6632.2012.06723.x. Epub 2012/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010;126(5):e1161–7. doi: 10.1542/peds.2010-0688. Epub 2010/10/20. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Hakim F, Kheirandish-Gozal L, Gozal D. Inflammatory pathways in children with insufficient or disordered sleep. Respiratory physiology & neurobiology. 2011;178(3):465–74. doi: 10.1016/j.resp.2011.04.024. Epub 2011/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soggiu A, Piras C, Bonizzi L, Hussein HA, Pisanu S, Roncada P. A discovery-phase urine proteomics investigation in type 1 diabetes. Acta diabetologica. 2012 doi: 10.1007/s00592-012-0407-0. Epub 2012/06/09. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerli LU, Schiffer E, Zurbig P, Good DM, Kellmann M, Mouls L, et al. Urinary proteomic biomarkers in coronary artery disease. Molecular & cellular proteomics : MCP. 2008;7(2):290–8. doi: 10.1074/mcp.M700394-MCP200. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 9.Riaz S, Alam SS, Srai SK, Skinner V, Riaz A, Akhtar MW. Proteomic identification of human urinary biomarkers in diabetes mellitus type 2. Diabetes technology & therapeutics. 2010;12(12):979–88. doi: 10.1089/dia.2010.0078. Epub 2010/08/26. [DOI] [PubMed] [Google Scholar]
- 10.Zengi O, Karakas A, Ergun U, Senes M, Inan L, Yucel D. Urinary 8-hydroxy-2'-deoxyguanosine level and plasma paraoxonase 1 activity with Alzheimer's disease. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2012;50(3):529–34. doi: 10.1515/CCLM.2011.792. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 11.Huttenhain R, Soste M, Selevsek N, Rost H, Sethi A, Carapito C, et al. Reproducible quantification of cancer-associated proteins in body fluids using targeted proteomics. Science translational medicine. 2012;4(142):142ra94. doi: 10.1126/scitranslmed.3003989. Epub 2012/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoidakis J, Makridakis M, Zerefos PG, Bitsika V, Esteban S, Frantzi M, et al. Profilin 1 is a potential biomarker for bladder cancer aggressiveness. Molecular & cellular proteomics : MCP. 2012;11(4):M111 009449. doi: 10.1074/mcp.M111.009449. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zurbig P, Jerums G, Hovind P, Macisaac R, Mischak H, Nielsen SE, et al. Urinary Proteomics for Early Diagnosis in Diabetic Nephropathy. Diabetes. 2012 doi: 10.2337/db12-0348. Epub 2012/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siwy J, Mullen W, Golovko I, Franke J, Zurbig P. Human urinary peptide database for multiple disease biomarker discovery. Proteomics Clinical applications. 2011;5(5-6):367–74. doi: 10.1002/prca.201000155. Epub 2011/05/19. [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Jortani S, Snow AB, Kheirandish-Gozal L, Bhattacharjee R, Kim J, et al. Two-dimensional differential in-gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. American journal of respiratory and critical care medicine. 2009;180(12):1253–61. doi: 10.1164/rccm.200905-0765OC. Epub 2009/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kentsis A. Challenges and opportunities for discovery of disease biomarkers using urine proteomics. Pediatrics international : official journal of the Japan Pediatric Society. 2011;53(1):1–6. doi: 10.1111/j.1442-200X.2010.03253.x. Epub 2010/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–53. doi: 10.1542/peds.2005-1067. Epub 2006/03/03. [DOI] [PubMed] [Google Scholar]
- 18.Iber SA-I C, Chesson A, Quan S, American Academy of Sleep Medicine The AASM manual for the scoring of sleep and associated events: rules, terminology, and techinical specifications. 2007 [Google Scholar]
- 19.Becker L, Gharib SA, Irwin AD, Wijsman E, Vaisar T, Oram JF, et al. A macrophage sterol-responsive network linked to atherogenesis. Cell metabolism. 2010;11(2):125–35. doi: 10.1016/j.cmet.2010.01.003. Epub 2010/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thongboonkerd V, Chutipongtanate S, Kanlaya R. Systematic evaluation of sample preparation methods for gel-based human urinary proteomics: quantity, quality, and variability. Journal of proteome research. 2006;5(1):183–91. doi: 10.1021/pr0502525. Epub 2006/01/07. [DOI] [PubMed] [Google Scholar]
- 21.Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R. The International Protein Index: an integrated database for proteomics experiments. Proteomics. 2004;4(7):1985–8. doi: 10.1002/pmic.200300721. Epub 2004/06/29. [DOI] [PubMed] [Google Scholar]
- 22.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–92. doi: 10.1021/ac025747h. Epub 2002/10/31. [DOI] [PubMed] [Google Scholar]
- 23.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–58. doi: 10.1021/ac0341261. Epub 2003/11/25. [DOI] [PubMed] [Google Scholar]
- 24.Rauch A, Bellew M, Eng J, Fitzgibbon M, Holzman T, Hussey P, et al. Computational Proteomics Analysis System (CPAS): an extensible, open-source analytic system for evaluating and publishing proteomic data and high throughput biological experiments. Journal of proteome research. 2006;5(1):112–21. doi: 10.1021/pr0503533. Epub 2006/01/07. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76(14):4193–201. doi: 10.1021/ac0498563. Epub 2004/07/16. [DOI] [PubMed] [Google Scholar]
- 26.Becker L, Liu NC, Averill MM, Yuan W, Pamir N, Peng Y, et al. Unique proteomic signatures distinguish macrophages and dendritic cells. PloS one. 2012;7(3):e33297. doi: 10.1371/journal.pone.0033297. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, et al. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Molecular & cellular proteomics : MCP. 2005;4(10):1487–502. doi: 10.1074/mcp.M500084-MCP200. Epub 2005/06/28. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 29.Heinecke NL, Pratt BS, Vaisar T, Becker L. PepC: proteomics software for identifying differentially expressed proteins based on spectral counting. Bioinformatics. 2010;26(12):1574–5. doi: 10.1093/bioinformatics/btq171. Epub 2010/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garde AH, Hansen AM, Kristiansen J, Knudsen LE. Comparison of uncertainties related to standardization of urine samples with volume and creatinine concentration. The Annals of occupational hygiene. 2004;48(2):171–9. doi: 10.1093/annhyg/meh019. Epub 2004/03/03. [DOI] [PubMed] [Google Scholar]
- 31.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21(16):3448–9. doi: 10.1093/bioinformatics/bti551. Epub 2005/06/24. [DOI] [PubMed] [Google Scholar]
- 32.Kushnir MM, Mrozinski P, Rockwood AL, Crockett DK. A depletion strategy for improved detection of human proteins from urine. J Biomol Tech. 2009;20(2):101–8. Epub 2009/06/09. [PMC free article] [PubMed] [Google Scholar]
- 33.Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome biology. 2006;7(9):R80. doi: 10.1186/gb-2006-7-9-r80. Epub 2006/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YT, Tsao CY, Li JM, Tsai CY, Chiu SF, Tseng TL. Large-scale protein identification of human urine proteome by multi-dimensional LC and MS/MS. Proteomics Clinical applications. 2007;1(6):577–87. doi: 10.1002/prca.200600769. Epub 2007/06/01. [DOI] [PubMed] [Google Scholar]
- 35.Adedayo AM, Olafiranye O, Smith D, Hill A, Zizi F, Brown C, et al. Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep & breathing = Schlaf & Atmung. 2012 doi: 10.1007/s11325-012-0760-9. Epub 2012/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chorostowska-Wynimko J, Radomska D, Plywaczewski R, Jonczak L, Stepniewska A, Gorecka D, et al. Disturbed angiogenic activity in sera from obstructive sleep apnea patients. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2005;56(Suppl 4):71–7. Epub 2005/10/06. [PubMed] [Google Scholar]
- 37.Slupsky CM, Rankin KN, Wagner J, Fu H, Chang D, Weljie AM, et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal Chem. 2007;79(18):6995–7004. doi: 10.1021/ac0708588. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 38.von Kanel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131(3):733–9. doi: 10.1378/chest.06-2006. Epub 2007/03/16. [DOI] [PubMed] [Google Scholar]
- 39.Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS genetics. 2009;5(8):e1000602. doi: 10.1371/journal.pgen.1000602. Epub 2009/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Thangue NB, Kerr DJ. Predictive biomarkers: a paradigm shift towards personalized cancer medicine. Nature reviews Clinical oncology. 2011;8(10):587–96. doi: 10.1038/nrclinonc.2011.121. Epub 2011/08/25. [DOI] [PubMed] [Google Scholar]
- 41.Stratz C, Amann M, Berg DD, Morrow DA, Neumann FJ, Hochholzer W. Novel biomarkers in cardiovascular disease: research tools or ready for personalized medicine? Cardiology in review. 2012;20(3):111–7. doi: 10.1097/CRD.0b013e31824394e1. Epub 2012/02/04. [DOI] [PubMed] [Google Scholar]
- 42.Escudero J, Ifeachor E, Zajicek J, Green C, Shearer J, Pearson S. Machine Learning-Based Method for Personalized and Cost-Effective Detection of Alzheimer's Disease. IEEE transactions on bio-medical engineering. 2012 doi: 10.1109/TBME.2012.2212278. Epub 2012/08/16. [DOI] [PubMed] [Google Scholar]
- 43.Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Science translational medicine. 2010;2(20):20ra14. doi: 10.1126/scitranslmed.3000702. Epub 2010/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verrills NM, Irwin JA, He XY, Wood LG, Powell H, Simpson JL, et al. Identification of novel diagnostic biomarkers for asthma and chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2011;183(12):1633–43. doi: 10.1164/rccm.201010-1623OC. Epub 2011/04/08. [DOI] [PubMed] [Google Scholar]
- 45.Gozal D, Kheirandish-Gozal L. New approaches to the diagnosis of sleep-disordered breathing in children. Sleep medicine. 2010;11(7):708–13. doi: 10.1016/j.sleep.2009.12.012. Epub 2010/07/14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Urinary proteomics analysis by LC-MS/MS. A list of the 742 proteins identified by LC-MS/MS analysis of urine and quantification by spectral counting.
Table S2. Gender and diurnal effects on the urinary proteome of healthy children. A list of the statistically significant, gender-regulated proteins detected in morning and bedtime urine samples of healthy children. Results of the t-test and G-test are presented.
Table S3. Identification of urine biomarkers of pediatric OSA. Identification of differentially abundant urinary proteins in OSA relative to control samples. Results of levels 1 (all samples), 2 (corrected for diurnal effects), and 3 (corrected for both diurnal and gender effects) biomarker analysis along with corresponding t-test and G-test values are displayed.
Fig. S1. Urine creatinine levels are insensitive to gender and sampling time. Morning (am) and bedtime (pm) urine samples were collected from children with and without OSA and creatinine levels were determined by ELISA. Results are means ±SEMs and statistical significance was assessed by the t-test.


