Abstract
Aims/Hypothesis
Obstructive sleep apnea (OSA) is a common health problem in children. African American (AA) and obese children have higher prevalence of OSA, and are also at higher risk for reduced vitamin D levels. We hypothesized that OSA would be associated with lower plasma 25-hydroxyvitamin D (25(OH)D), and increase the risk for metabolic dysfunction and systemic inflammation.
Methods
In this observational cross-sectional study, 176 prospectively recruited children (mean age: 6.8±0.8 years) underwent overnight polysomnographic evaluation and a fasting blood draw the morning after the sleep study. In addition to lipid profile, HOMA-IR and hsCRP assays, plasma 25(OH)D levels were assessed using ELISA kits.
Results
AA children, obese children, and children with OSA had significantly lower 25(OH)D levels. Linear associations emerged between 25(OH)D plasma levels and BMI z score, hsCRP, and HOMA-IR, as well as with AHI and SpO2 nadir, the latter 2 associations remaining statistically significant even when controlling for all other potential confounders, and independently accounting for 17.7% of the variance in 25(OH)D (p<0.01).
Conclusions
25(OH)D levels are reduced in pediatric OSA, in AA children and in obesity, particularly when all are present, and may play a role in modulating the degree of insulin resistance and systemic inflammation. The short-term and long-term significance of reduced 25(OH)D in pediatric OSA remains undefined.
Keywords: vitamin D, sleep apnea, children, metabolism, insulin resistance, systemic inflammation
Introduction
Obstructive sleep apnea (OSA) is a prevalent health problem in children affecting up to 3-4% of all children, with African American and obese children being at particularly higher risk. 1 It has become apparent that OSA is strongly associated with the presence of insulin resistance and altered lipid homeostasis, as well as with systemic inflammation and endothelial dysfunction, and an increased prevalence of respiratory illnesses that lead to higher healthcare utilization costs.2-5
Similar to OSA, low vitamin D levels, as indicated by concentrations of serum 25-hydroxyvitamin D (25(OH)D), have been linked to increases in the frequency and severity of metabolic dysfunction, cardiovascular disease risk factors, as well as with an increased incidence of upper respiratory tract infections.6-15 Interventions aimed at increased 25(OH)D concentrations have resulted in improvements in these outcomes. 15 Furthermore, a recent preliminary study suggested that children at risk for adenotonsillectomy may exhibit lower serum 25(OH)D concentrations,16 even if such findings were not replicated in another small cohort.17
We hypothesized that OSA in children would be associated with reduced plasma levels of 25(OH)D, particularly in obese children and in African American children. Furthermore, we postulated that significant associations between sleep measures and 25(OH)D would emerge and be independent from confounding factors such as BMI z score, serum lipids, high sensitivity CRP (hsCRP) and a measure of insulin resistance (i.e., the homeostatic model of insulin resistance – HOMA-IR).
Materials and Methods
The research protocol was approved by the University of Chicago (protocol 09-115-B) human research ethics committee. Informed consent was obtained from the parents, and age appropriate assent was also obtained from the children. Children were recruited from the Sleep and ENT clinics at Comer Children’s Hospital, as well as by advertisement in the community. Those children who had genetic or craniofacial syndromes and chronic diseases such as cardiac disease, diabetes, cerebral palsy and chronic lung disease of prematurity were excluded.
Overnight Polysomnographic Studies
All children underwent standard overnight NPSG evaluation as previously described, 18 with assessment of 8 standard EEG channels, bilateral EOG, EMG, 2-lead ECG, oronasal airflow measurement using thermistor, nasal pressure transducer, and end tidal CO2, chest and abdominal movement by respiratory inductance plethysmography, and pulse oximetry including pulse waveform using a commercially available data acquisition system (Polysmith; Nihon Kohden America Inc, CA, USA). The NPSG studies were scored as per the 2007 American Association of Sleep Medicine guidelines for the scoring of sleep and associated events. 19 The proportion of time spent in each stage of sleep was calculated as a percentage of total sleep time (TST). A respiratory event was scored as an obstructive apnea if it was associated with a >90% fall in signal amplitude for >90% of the entire event compared to the baseline amplitude, the event lasted for at least 2 breaths and there was continued or increased respiratory effort throughout the period of the event. A mixed apnea was scored if there was absent inspiratory effort in the initial part of the event, followed by resumption of inspiratory effort before the end of the event. A central apnea was scored if there was absent respiratory effort throughout the duration of the event, the event lasted for at least 2 missed breaths, and was associated with an arousal/ awakening or a ≥3% desaturation. A hypopnea was scored if the event was associated with a ≥50% fall in amplitude of the nasal pressure transducer, lasted at least for 2 breaths, and was associated with an arousal/ awakening or ≥3% desaturation. The obstructive apnea-hypopnea index (AHI) was calculated as the number of apneas and hypopneas per hour of TST. Arousals were classified as either spontaneous or respiratory, and corresponding indices, namely the total arousal index (TAI) and the respiratory arousal index (RAI) were computed.
The diagnosis of OSA was defined by the presence of an AHI ≥ 2/hour of total sleep time (hrTST). Control children were non-snoring children with a AHI < 2 / hrTST.
Plasma Assays
High sensitivity CRP (hsCRP) was measured within 2 to 3 hours after collection using the Flex reagent Cartridge (Date Behring, Newark, DE), which is based on a particle-enhanced turbidimetric immunoassay technique. Serum levels of lipids, including total cholesterol, high-density lipoprotein (HDL) cholesterol, calculated low-density lipoprotein (LDL) cholesterol, and triglycerides, were also assessed with a Flex reagent cartridge (Date Behring, Newark, DE). Plasma insulin levels were measured using a commercially available radioimmunoassay kit (Coat-A-Count Insulin, Cambridge Diagnostic Products, Inc, Fort Lauderdale, FL). Plasma glucose levels were measured using a commercial kit based on the hexokinase-glucose-6-phosphate dehydrogenase method (Flex Reagent Cartridges, Dade Behring, Newark, DE). Insulin resistance was then assessed using the homeostasis model assessment (HOMA-IR) equation (fasting insulin × fasting glucose ÷ 405).20 In addition, plasma samples were frozen at −80°C till assay.
25(OH)D Assay
25(OH)D plasma levels were assessed using a commercially available kit (Eagle Biosciences; cat # VID31-K01). The assay exhibited a low level detection threshold of 1.6 ng/ml, linearity up to 225 ng/ml, and inter-assay and intra-assay coefficients of variability of 4.9% and 7.1%.
Statistical Analysis
All analyses were conducted using SPSS software (version 19.0; SPPS Inc., Chicago, Ill.), and data are presented as mean ± SD. Since both OSA and obesity are associated with systemic low grade inflammation, children were subdivided into 4 groups, based on the presence or absence of obesity (OB) and OSA (i.e., OSA-NOB, NOSA-NOB, OSA-OB, NOSA-OB). Significant differences within groups were analyzed using ANOVA, followed by post-hoc tests with Bonferroni corrections for multiple comparisons for continuous variables and chi-square tests for categorical variables. If the data were not normally distributed, they were logarithmically transformed. Spearman’s correlation analyses were conducted to examine potential associations between BMI z score, sleep variables, lipid profiles, hsCRP, and HOMA and plasma concentrations of 25(OH)D, followed by stepwise logistic regressions. All p-values reported are 2-tailed with statistical significance set at <0.05.
Results
A total of 176 children fulfilling entry criteria completed the overnight polysomnographic evaluation and provided a fasting blood sample after the sleep study. 18 children refused to participate in the study (3 parents declined to participate altogether and 15 parents were not willing to participate in the blood draw portion of the study). The demographic and polysomnographic characteristics of these 18 children were similar to those of the cohort, which are shown in Table 1 and Table 2.
Table 1. Characteristics of 176 obese and non-obese children with and without OSA.
| Non-Obese with OSA (n=57) |
Non-Obese without OSA (n=38) |
Obese with OSA (n=45) |
Obese without OSA (n=36) |
|
|---|---|---|---|---|
| Age (years) | 6.5±0.9 | 7.1±1.4 | 6.8±0.9 >1 | 7.2±1.5 |
| Gender (male, %) | 56.1 | 52.6 | 55.5 | 52.7 |
| Ethnicity (Caucasian, %) | 52.2 | 50.0 | 51.1 | 50.0 |
| BMI-z score | 0.22±1.04 § | 0.16±0.94 § | 2.42±0.40§ | 2.38±0.47 § |
| Total Cholesterol (mg/dl) † | 150.2±21.8**,§ | 139.6±19.2**,§ | 171.6±40.0**,§ | 161.2±32.1**,§ |
| HDL cholesterol (mg/dl) † | 51.7±15.2**,§ | 67.5±12.29*,§ | 49.6±11.9**,§ | 51.9±12.8**,§ |
| LDL cholesterol (mg/dl) † | 92.4±21.3**,§ | 78.3±18.9**,§ | 130.1±28.9**,§ | 96.5±21.3**,§ |
| Tryglycerides (mg/dl) † | 60.7±40.5§ | 53.2±28.8§ | 79.7±3481§ | 71.5±33.8§ |
| HOMA-IR | 1.76±0.92**,§ | 1.02±1.15**,§ | 4.64±1.83**,§ | 3.49±2.95**,§ |
| hsCRP (mg/dl) | 5.08±4.25 | 1.33±2.29**,§ | 6.86±4.40**,§ | 2.61±2.45**,§ |
| 25(OH)D (ng/ml) | 83.4±24.9**,§ | 94.8±24.3**,§ | 67.7±22.4**,§ | 81.1±22.2**,§ |
HDL: high density lipid cholesterol; LDL: low density lipid cholesterol; hsCRP: high sensitivity C-reactive protein.
non-obese vs. obese – p<0.01
- OSA vs. no-OSA
Table 2. Polysomographic data of 176 obese and non-obese children with and without OSA.
| Non-Obese with OSA (n=57) |
Non-Obese without OSA (n=38) |
Obese with OSA (n=45) |
Obese without OSA (n=36) |
|
|---|---|---|---|---|
| Total sleep duration (min) | 478.7±59.3 | 471.9±48.5 | 471.1±56.7 | 470.1±54.9 |
| Stage 1 (%) | 7.5±3.9** | 5.0±4.2** | 8.8±5.7** | 5.6±5.1** |
| Stage 2 (%) | 38.9±9.7 | 35.2±8.5 | 43.9±11.7 | 37.2±9.9 |
| Stage 3 (%) | 36.6±15.1** | 45.4±13.4** | 37.2±16.2 | 44.4±13.4 |
| REM sleep (%) | 18.9±6.9** | 26.1±8.7** | 17.1±9.2** | 21.9±10.5** |
| Sleep latency (min) | 24.1±18.3**,§ | 31.6±17.1**,§ | 11.3±10.9**,§ | 25.8±17.4**,§ |
| REM latency (min) | 118.2±61.1**,§ | 137.3±55.1**,§ | 110.4±58.2**,§ | 141.3±58.7**,§ |
|
Total Arousal Index
(events /hour TST) |
23.3±11.8 ** | 10.6±7.2 ** | 23.8±12.6 ** | 12.6±7.5 ** |
|
Respiratory Arousal Index
(events /hour TST) |
7.2±3.6**,§ | 0.1±0.1**,§ | 8.8±4.5**,§ | 0.7±0.5**,§ |
|
Obstructive Apnea Hypopnea Index
(events/ hour TST) |
8.8±8.4** | 0.6±0.5** | 12.4±13.4** | 0.6±0.6** |
| SpO2 Nadir (%) | 84.6±6.3** | 95.9±0.4** | 83.7±8.4** | 92.1±2.5** |
All data are expressed as mean±SD.
non-obese vs. obese – p<0.02
- OSA vs. no-OSA– p<0.02
In general, AA children had lower 25(OH)D levels when compared to Caucasian children (69.9±21.9 vs. 93.4±22.5 ng/ml; p<0.0001), and obese children had lower levels than non-obese children (83.6±19.2 vs.97.1 ±20.9 ng/ml; p<0.01). However, there were no differences in 25(OH)D according to age or according to gender. Similarly, we did not find any significant differences from mean values for the whole cohort between samples collected during winter season compared to summer season.
There were no significant differences in age, gender, or ethnicity across the 4 sub-groups. However, obese children exhibited higher BMI z scores, as well as higher HOMA-IR, serum lipids, and hs-CRP, and reduced HDL cholesterol levels. Similarly, children with OSA had significantly higher HOMA-IR, LDL cholesterol, and hs-CRP concentrations, and lower HDL cholesterol levels (Table 1).
Primary sleep disturbance measures clinically used to characterize the severity of OSA were not significantly different in obese and non-obese children with OSA. Similarly, there were no differences in sleep measures in obese and non-obese children without OSA (Table 2).
Obese children without OSA had lower 25(OH)D levels than non-obese children without OSA (p<0.01; Table 1). Similarly, non-obese children with OSA also exhibited lower 25(OH)D levels compared to non-obese controls (p<0.01; Table 1). However, obese children with OSA demonstrated the lowest 25(OH)D levels (p<0.01; Table 1).
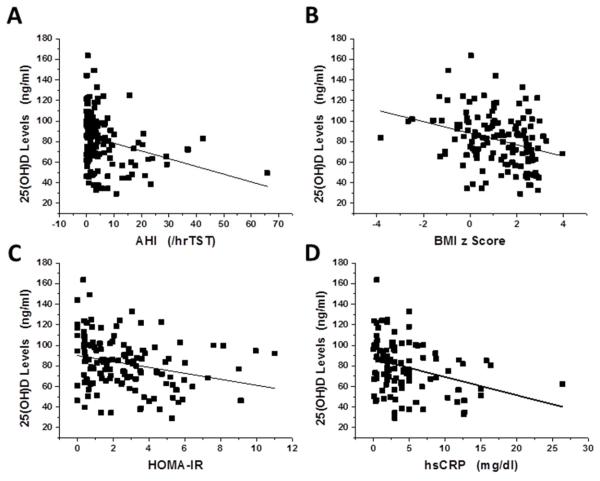
In order to estimate potential associations between 25(OH)D plasma levels, polysomnographic measures and metabolic indices, we initially performed bivariate Spearman correlation analyses (Figure 1).Significant linear correlations emerged between 25(OH)D and AHI (Figure 1A, r=−0.285, p<0.001), nadir SpO2 (r=0.283, p<0.001), BMI z score (Figure 1B; r=−0.3r02, p<0.0001), HOMA-IR (Figure 1C, r=−0.259, p<0.001), and hsCRP (Figure 1D, r=−0.318, p<0.001), but not with respiratory arousal index, total cholesterol, LDL or HDL cholesterol, or triglyceride levels. Furthermore, we compared whether those children with clinically defined low 25(OH)D levels (<60 ng/ml; n=31) differed from those with normal 25(OH)D levels, and found that children with low 25(OH)D levels were of similar age and gender, but more likely to be African-American (RR: 1.76; 95% CI: 1.32-2.45; p<0.03), to have increased BMI (BMI z score: 1.8±0.9 vs. 1.1±1.3; RR for BMI z score >1.65: 1.67; 95% CI: 1.45-3.14; p<0.0001), had more severe OSA (AHI: 11.3±13.7/hrTST vs. 4.75±7.9/hrTST; p<0.0001), had higher hsCRP levels (6.7±5.1 vs. 4.3±3.7 mg/dl; p<0.001), and higher total cholesterol (166.4±40.7 vs. 152.7±27.8; p<0.01) and LDL cholesterol (105.6±35.7 vs. 94.1±24.4 mg/dl; p<0.01) and HOMA-IR (3.8±2.2 vs. 2.4±2.2; p<0.001), as well as lower HDL levels (.49.9±13.5 vs. 57.8±15.1 mg/dl; p<0.01).
Figure 1. Scatterplots of 25(OH)D plasma levels vs. BMI z score, obstructive apnea hypopnea index (AHI), nadir SpO2, hsCRP, and HOMA-IR expression in a cohort of 176 children with and without obesity or OSA.
Panel A - r=−0.285, p<0.001
Panel B - r=−0.302, p<0.0001
Panel C - r=−0.259, p<0.001
Panel D - r=−0.318, p<0.001
Therefore, to further explore whether AHI was an independent predictor of 25(OH)D levels, we performed stepwise multiple regression analyses with age, gender, ethnicity, BMI z score, HOMA-IR, and hsCRP included as potential confounders in the model. In the stepwise multiple regression model, AHI or SpO2 nadir were independently associated with 25(OH)D levels, and accounted for 17.7% of the variance in 25(OH)D after controlling for race, BMI z-score, HOMA-IR, and hsCRP (Table 3; p<0.01),.
Table 3. Multivariate regression analyses between anthropometric, demographic and polysomnogrpahic measures, HOMA-IR, hs-CRP, lipid profile, and 25(OH)D levels.
| Variables | 25(OH)D Plasma Levels | |
|---|---|---|
| Standardized Coefficients |
P-value | |
| Age | 0.008 | 0.976 |
| Gender | 0.004 | 0.981 |
| Race | 0.432 | <0.0001 |
| BMI-z score * | −0.167 | <0.003 |
| HOMA-IR ** | −0.136 | <0.01 |
| LDL cholesterol | 0.013 | 0.923 |
| HDL cholesterol | 0.079 | 0.897 |
| hsCRP† | 0.017 | 0.945 |
| AHI† *** | −0.178 | <0.01 |
| SpO2 nadir *** | 0.177 | <0.01 |
| Respiratory arousal index† | 0.007 | 0.968 |
Data were log-transformed; Data for age, gender and race are not adjusted.
- Data for BMI z score are shown after adjusting for age, race and gender only.
- All other data are shown after controlling for age, gender, race, and BMI z score. ;
HDL: high density lipid cholesterol; LDL: low density lipid cholesterol; hsCRP: high sensitivity C-reactive protein; AHI – obstructive apnea-hypopnea index; HOMA-IR – homeostatic model of insulin resistance
Discussion
This study shows that both obese children and children with OSA exhibit significantly lower 25(OH)D plasma levels when compared to healthy controls, even when adjusted for ethnicity. Indeed, AA consistently demonstrate markedly lower 25(OH)D plasma concentrations, a finding that has been corroborated in several previous studies, and linked to insulin resistance and obesity.11, 21-24 Furthermore, we found that if both obesity and OSA are concurrently present, 25(OH)D levels are further reduced. Linear associations emerged between 25(OH)D plasma levels and BMI z score, as well as with the 2 of the major polysomnographic measures traditionally used to characterize OSA, namely AHI and SpO2 nadir. Similarly, both HOMA-IR and hsCRP, as surrogate reporters of reduced insulin sensitivity and of low-grade inflammation respectively, were significantly associated with circulating 25(OH)D levels, but this was not the case for any of the serum lipid measurements, even if such levels were different among children with clinically-defined low 25(OH)D levels. These findings suggest that assessment of 25(OH)D plasma levels may provide a potential biochemical indicator for children with OSA at risk for end-organ morbidities. 3, 25, 26
Before we explore the potential implications of our findings, several methodological issues deserve mention. First, the prospective recruitment approach enabled careful standardization of blood collection procedures, which coincided with a very narrow time window, i.e., immediately after the overnight sleep study. Therefore, not only were fasting conditions strictly enforced, but any circadian variance in any of the plasma measures was avoided by the uniformity of sampling times. In addition, the duration of sleep during the night preceding the blood draw was available as derived from the polysomnogram, and did not significantly differ among the 4 subgroups. It remains thus far unknown whether sleep restriction, either acute or chronic, will alter 25(OH)D levels. Based on current findings, sleep fragmentation, as indicated by the respiratory arousal index, was not significantly associated with 25(OH)D concentrations. Thus, it is unlikely that perturbations of sleep will lead to altered vitamin D bioavailability. Secondly, all of the 25(OH)D assays were conducted using same batch commercial assays and were performed concomitantly, thereby reducing additional sources of assay variability. Thirdly, we should indicate that the sample collection was evenly distributed along the calendar year without seasonal preference, such that the effect of season did not emerge among our findings. Finally, we did not thoroughly investigate for the presence of asthma in our cohort. In this context, vitamin D deficiency appears to be associated with a greater risk for asthma, 27,28 even if the 25(OH)D levels do not correlate with the severity of asthma. 29 Of note, we and others have previously reported on the potential association between asthma and OSA in children. 30,31
We are unaware of published studies in children with OSA examining whether alterations are present in 25(OH)D levels. As mentioned above, 2 small studies assessing vitamin D levels in children undergoing surgical adenotonsillectomy that did not incorporate polysomnographic recordings yielded conflictive results. 16,17 In a large cohort of >800 adult patients with OSA, an inverse association of 25(OH)D with diabetes and metabolic syndrome was reported by Barceló and collaborators. 32 Similarly, another study involving 190 adult patients with OSA showed that those patients with more severe OSA polysomnographic indices were more likely to exhibit reduced vitamin D levels that were in turn linearly correlated to the presence of insulin resistance.33 Thus, the inverse linear relationships identified herein between vitamin D levels and metabolic and inflammatory markers as well as with AHI and nadir SpO2 would potentially suggest the possibility that administration of supplemental vitamin D could ameliorate aspects of the pediatric OSA clinical phenotypic spectrum. 1,34 If current findings are confirmed in future studies, implementation of a randomized clinical trial involving pharmacological administration of exogenous vitamin D might be warranted.35
In the context of the recently uncovered functional roles of 25(OH)D, 36 it was expected that the declines in 25(OH)D levels in both obese and OSA children would not only be associated with correlates of insulin resistance (i.e., HOMA-IR), but also with previously described alterations in serum lipid profiles and hCRP, the latter serving as a reporter of low-grade systemic inflammation. 6-14,37 Indeed, both HOMA-IR and hsCRP were strongly and independently associated with 25(OH)D levels, suggesting the intriguing possibility that reduced biological availability and activity of 25(OH)D in clinical settings such as obesity or OSA may facilitate the emergence of insulin resistance, and other OSA-induced morbidities. The potentiation of the effect of obesity and OSA when jointly present on 25(OH)D plasma concentrations was also confirmed in the present study. Although it remains unclear whether obesity and OSA exert their effects via similar and overlapping pathways or not, it is possible that these chronic systemic inflammatory states may ultimately result in reduced bioavailability of 25(OH)D, the latter then potentiating some of the adverse effects of the 2 underlying disorders. Conversely, it cannot be excluded that specific extraneous factors leading to lower 25(OH)D concentrations may promote pro-inflammatory states that exaggerate the proliferation of upper airway lymphadenoid tissues thereby favoring more severe OSA among susceptible individuals. Furthermore, obese children with OSA are more likely to consume a skewed and unbalanced diet primarily composed by energy dense food items, 38,39 that may lead to reduced intake of essential vitamins such as vitamin D.
In summary, we have shown that the presence of obesity and the presence of sleep-disordered breathing in children are associated with reduced plasma levels of 25(OH)D, and that 25(OH)D levels are strongly associated with measures of insulin resistance and hsCRP, but not with dyslipidemia. Improved understanding of the causal pathways underlying these associations may offer not only clinical diagnostic opportunities, but also enable delineation of therapeutic interventions targeting vitamin D and aiming for example to reduce the magnitude and risk for development of some of the morbid consequences of pediatric OSA.
Highlights.
-
-
Low vitamin D levels are associated with adverse outcomes in systemic inflammatory diseases.
-
-
Obstructive sleep apnea in children is a low-grade systemic inflammatory disease.
-
-
We found that vitamin D levels are reduced in pediatric OSA, particularly in obese children.
-
-
Vitamin D levels account for a proportion of the variance in insulin resistance pediatric OSA.
Acknowledgments
LKG provided conceptual initiative and design for the project, recruited subjects, analyzed sleep data and metabolic data, drafted components of the manuscript and analyzed data. EP performed experiments and analyzed data. DG provided the conceptual design of the project, analyzed data, drafted the manuscript, and is responsible for the financial support of the project and the manuscript content. Drs. Gozal are the guarantors of this work, had full access to all the data, and take full responsibility for the integrity of data and the accuracy of data analysis. All authors have reviewed and approved the final version of the manuscript.
Funding: DG is supported by National Institutes of Health grants HL-65270, HL-086662, and HL-107160.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Kim J. C-Reactive Protein and obstructive sleep apnea syndrome in children. Frontiers in Bioscience. 2012;4:2410–22. doi: 10.2741/e553. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Sans Capdevila O, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among non-obese and obese pre-pubertal children. Am J Resp Crit Care Med. 2008;177(10):1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: A tale of inflammatory cascades. Pediatr. Pulmonol. 2011;46(4):313–23. doi: 10.1002/ppul.21370. [DOI] [PubMed] [Google Scholar]
- 5.Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, et al. Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2007;175(1):55–61. doi: 10.1164/rccm.200604-577OC. [DOI] [PubMed] [Google Scholar]
- 6.Kelly A, Brooks LJ, Dougherty S, Carlow DC, Zemel BS. A cross-sectional study of vitamin D and insulin resistance in children. Arch Dis Child. 2011;96(5):447–52. doi: 10.1136/adc.2010.187591. [DOI] [PubMed] [Google Scholar]
- 7.Pacifico L, Anania C, Osborn JF, et al. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol. 2011;165(4):603–11. doi: 10.1530/EJE-11-0545. [DOI] [PubMed] [Google Scholar]
- 8.Olson ML, Maalouf NM, Oden JD, White PC, Hutchison MR. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab. 2012;97(1):279–85. doi: 10.1210/jc.2011-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salo A, Logomarsino JV. Relationship of vitamin D status and cardiometabolic risk factors in children and adolescents. Pediatr Endocrinol Rev. 2011;9(1):456–62. [PubMed] [Google Scholar]
- 10.Codoñer-Franch P, Tavárez-Alonso S, Simó-Jordá R, Laporta-Martín P, Carratalá-Calvo A, Alonso-Iglesias E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr. 2012;161(5):848–54. doi: 10.1016/j.jpeds.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 11.Sulistyoningrum DC, Green TJ, Lear SA, Devlin AM. Ethnic-specific differences in vitamin D status is associated with adiposity. PLoS One. 2012;7(8):e43159. doi: 10.1371/journal.pone.0043159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyman M, Verrijn Stuart AA, van Summeren M, et al. Vitamin D deficiency in childhood obesity is associated with high levels of circulating inflammatory mediators, and low insulin sensitivity. Int J Obes (Lond) 2013 May 20; doi: 10.1038/ijo.2013.75. doi: 10.1038/ijo.2013.75. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013;131(1):e152–61. doi: 10.1542/peds.2012-1711. [DOI] [PubMed] [Google Scholar]
- 14.Science M, Maguire JL, Russell ML, Smieja M, Walter SD, Loeb M. Low Serum 25-Hydroxyvitamin D Level and Risk of Upper Respiratory Tract Infection in Children and Adolescents. Clin Infect Dis. 2013 May 23; doi: 10.1093/cid/cit289. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trialof vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91(5):1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 16.Reid D, Morton R, Salkeld L, Bartley J. Vitamin D and tonsil disease--preliminary observations. Int J Pediatr Otorhinolaryngol. 2011;75(2):261–4. doi: 10.1016/j.ijporl.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Esteitie R, Naclerio RM, Baroody FM. Vitamin D levels in children undergoing adenotonsillectomies. Int J Pediatr Otorhinolaryngol. 2010;74(9):1075–7. doi: 10.1016/j.ijporl.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Chesson A, Quan S for the American Academy of Sleep Medicine . The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 2007. [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–429. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Rajakumar K, Fernstrom JD, Holick MF, Janosky JE, Greenspan SL. Vitamin D status and response to Vitamin D(3) in obese vs. non-obese African American children. Obesity (Silver Spring) 2008;16(1):90–5. doi: 10.1038/oby.2007.23. [DOI] [PubMed] [Google Scholar]
- 22.Cole CR, Grant FK, Tangpricha V, et al. 25-hydroxyvitamin D status of healthy, low-income, minority children in Atlanta, Georgia. Pediatrics. 2010;125(4):633–9. doi: 10.1542/peds.2009-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab. 2011;96(5):1560–7. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Au LE, Economos CD, Goodman E, Must A, Chomitz VR, Sacheck JM. Vitamin D intake and serum vitamin D in ethnically diverse urban schoolchildren. PublicHealth Nutr. 2012;15(11):2047–53. doi: 10.1017/S1368980012003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176(2):188–93. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Kim J. C-Reactive Protein and obstructive sleep apnea syndrome in children. Frontiers in Bioscience. 2012;4:2410–22. doi: 10.2741/e553. [DOI] [PubMed] [Google Scholar]
- 27.Paul G, Brehm JM, Alcorn JF, Holguín F, Aujla SJ, Celedón JC. Vitamin D and asthma. Am J Respir Crit Care Med. 2012;185(2):124–32. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A, Bush A, Hawrylowicz C, Saglani S. Vitamin D and asthma in children. Paediatr Respir Rev. 2012;13(4):236–43. doi: 10.1016/j.prrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Menon J, Maranda L, Nwosu BU. Serum 25-hydroxyvitamin D levels do not correlate with asthma severity in a case-controlled study of children and adolescents. J Pediatr Endocrinol Metab. 2012;25(7-8):673–9. doi: 10.1515/jpem-2012-0143. [DOI] [PubMed] [Google Scholar]
- 30.Kheirandish-Gozal L, Dayyat EA, Eid NS, Morton RL, Gozal D. Obstructive sleep apnea in poorly controlled asthmatic children: effect of adenotonsillectomy. Pediatr Pulmonol. 2011;46(9):913–8. doi: 10.1002/ppul.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malakasioti G, Gourgoulianis K, Chrousos G, Kaditis A. Interactions of obstructive sleep-disordered breathing with recurrent wheezing or asthma and their effects on sleep quality. Pediatr Pulmonol. 2011;46(11):1047–54. doi: 10.1002/ppul.21497. [DOI] [PubMed] [Google Scholar]
- 32.Barceló A, Esquinas C, Piérola J, et al. Vitamin D Status and Parathyroid Hormone Levels in Patients with Obstructive Sleep Apnea. Respiration. 2012 Nov 15; doi: 10.1159/000342748. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Bozkurt NC, Cakal E, Sahin M, Ozkaya EC, Firat H, Delibasi T. The relation of serum 25-hydroxyvitamin-D levels with severity of obstructive sleep apnea and glucose metabolism abnormalities. Endocrine. 2012;41(3):518–25. doi: 10.1007/s12020-012-9595-1. [DOI] [PubMed] [Google Scholar]
- 34.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5(2):274–82. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97(4):774–81. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 36.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–92. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creo AL, Rosen JS, Ariza AJ, Hidaka KM, Binns HJ. Vitamin D levels, insulin resistance, and cardiovascular risks in very young obese children. J Pediatr Endocrinol Metab. 2013;26(1-2):97–104. doi: 10.1515/jpem-2012-0244. [DOI] [PubMed] [Google Scholar]
- 38.Spruyt K, Sans Capdevila O, Serpero LD, Kheirandish-Gozal L, Gozal D. Dietary and physical activity patterns in children with obstructive sleep apnea. J Pediatr. 2010;156:724–730. 730.e1–730.e3. doi: 10.1016/j.jpeds.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Beebe DW, Miller N, Kirk S, Daniels SR, Amin R. The association between obstructive sleep apnea and dietary choices among obese individuals during middle to late childhood. Sleep Med. 2011;12(8):797–799. doi: 10.1016/j.sleep.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]