Abstract
The majority of causative variants in familial breast cancer remain unknown. Of the known risk variants, most are tumor cell autonomous and little attention has been paid yet to germline variants that may affect the tumor microenvironment. In this study, we developed a system called the Consomic Xenograft Model (CXM) to map germline variants that impact only the tumor microenvironment. In CXM, human breast cancer cells are orthotopically implanted into immunodeficient consomic strains and tumor metrics are quantified (e.g., growth, vasculogenesis, and metastasis). Because the strain backgrounds vary, whereas the malignant tumor cells do not, any observed changes in tumor progression are due to genetic differences in the non-malignant microenvironment. Using CXM, we defined genetic variant(s) on rat chromosome 3 that reduced relative tumor growth and hematogenous metastasis in the SS.BN3IL2Rγ consomic model compared to the SSIL2Rγ parental strain. Paradoxically, these effects occurred despite an increase in the density of tumor-associated blood vessels. In contrast, lymphatic vasculature and lymphogenous metastasis were unaffected by the SS.BN3IL2Rγ background. Through comparative mapping and whole genome sequence analysis, we narrowed candidate variants on rat chromosome 3 to six genes with a priority for future analysis. Collectively, our results establish the utility of CXM to localize genetic variants affecting the tumor microenvironment which underlie differences in breast cancer risk.
Keywords: Breast Cancer, Tumor Microenvironment, Angiogenesis, Genetic, Consomic
Introduction
Breast cancer is the most prevalent female malignancy (http://apps.nccd.cdc.gov/uscs/) and is highly heritable (1–4), yet ~72% of breast cancer risk remains undefined (5). Heritable factors underlie most aspects of breast cancer risk [e.g., incidence (5), age-of-onset (6), metastatic progression(7), and disease-free survival(8)]. In addition to variants that impact tumor cells directly (i.e., tumorigenicity), heritability is implicated in multiple components of the tumor microenvironment [e.g., tissue remodeling(9), angiogenesis (10, 11), and immunity (12)], which also impact tumorigenesis and progression. However, the genetic variant(s) underlying differences in the tumor microenvironment have rarely been the focus of genetic mapping studies and as such, remain poorly defined.
We have developed a new model of breast cancer (termed the Consomic Xenograft Model - CXM) that focuses on genetic mapping of strain-specific variant(s) that impact tumor progression through the tumor microenvironment. A consomic rat is one in which an entire chromosome is introgressed into the isogenic background of another inbred strain by selective breeding (13). Thus, observed phenotypes can be linked to single chromosomes and then further elucidated by comparative sequence analysis and/or selective backcrossing to yield smaller congenics(13). In CXM, the consomic and parental strains are converted to SCID (severe combined immunodeficiency), so that orthotopically xenografted human breast cancer cells can be tested in vivo. Because the human breast cancer cells are not varied between strains, any differences in breast cancer progression (e.g., primary site growth, vasculogenesis, and distal metastasis) are due solely to genetic differences in the tumor microenvironment, not the malignant cancer cells. CXM utilizes transgenically tagged human cancer cells with defined properties (e.g., triple-negative, pro-metastatic, etc.). Thus, it enables testing of clinically relevant cancer models in strain backgrounds with varying genetic predispositions to breast cancer. Finally, we have developed CXM in the rat, but postulate that this technique could also be applied to the mouse and other model species.
For purposes of illustration, the basic concept of CXM is outlined in Figure 1. In brief, CXM utilizes TALENs (14) to generate SCID on any strain background by mutating the IL2Rγ gene (15). We first generated and characterized an IL2Rγ mutant parental SS strain (SSIL2Rγ), which was then selectively bred onto the SS.BN3 consomic background to generate the SS.BN3IL2Rγ consomic. The parental SS (SS/JrHsdMcwi) rat strain is susceptible to mammary tumors, whereas the BN (BN/NHsdMcwi) rat strain is highly resistant to mammary tumors (16). Our rationale for choosing SS (parental) and SS.BN3 consomic (i.e., BN chromosome 3 introgressed onto the isogenic SS background) was based on prior data demonstrating a 64% reduction in breast cancer incidence in the SS.BN3 consomic compared with the SS (16) and other evidence that multiple overlapping protective loci exist on rat chromosome 3 (16–19). We hypothesized that the protective effects of BN chromosome 3 could be in part due to changes in the tumor microenvironment. To test this hypothesis, luciferase-tagged human MDA-MB-231 breast cancer cells (231Luc+) were orthotopically implanted and tracked for tumor growth, vasculogenesis, and distal metastasis. Using CXM, we demonstrate that genetic variant(s) on rat chromosome 3 impact the tumor microenvironment by causing blood vessel-specific defects that attenuate tumor growth and decrease hematogenous metastasis; whereas, lymphatic vasculature and lymphogenous metastasis were unaffected. Finally, we used comparative analysis and whole genome sequencing (WGS) of five rat strains (BN/JrHsdMcwi, Cop/Crl, F344/N, ACI/Eur, and SS/JrHsdMcwi) to prioritize cosegregating candidate genes on rat chromosome 3 for future analysis by gene editing or congenic mapping.
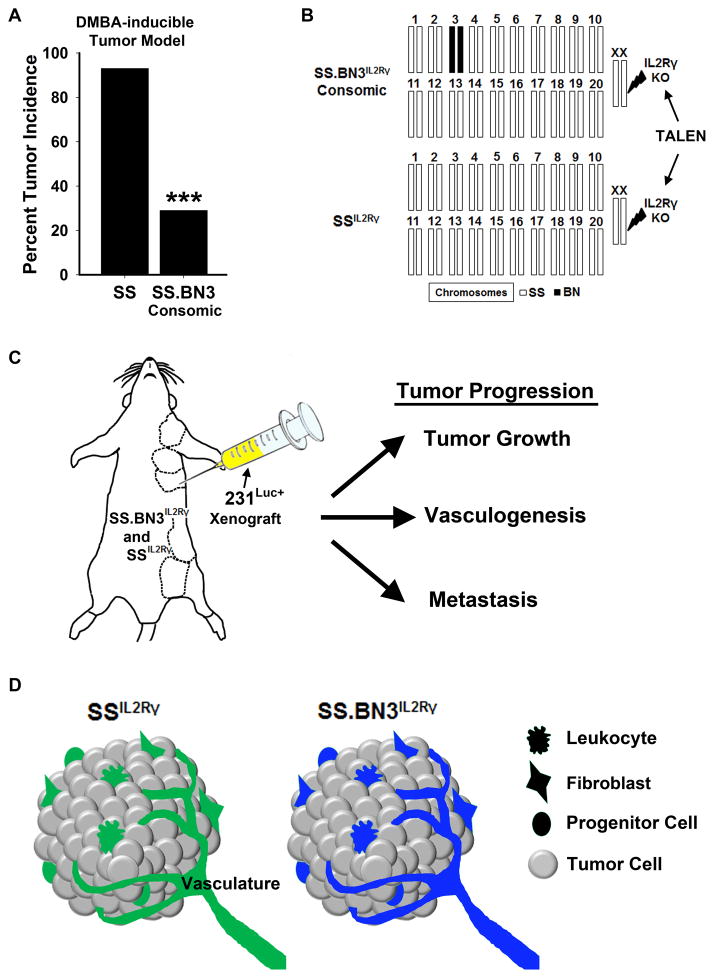
Figure 1.
Overview of the Consomic Xenograft Model (CXM). (A) Reduced susceptibility to mammary tumors (DMBA-induced) in SS.BN3 consomic rats (n = 14) compared with SS (n = 40), as reported previously by Adamovic et al (16). ***P<0.001 as determined by Fisher’s exact test using Bootstrap.(B) Schematic representation of the SS and SS.BN3 genomes that were modified by TALEN-mediated editing of the IL2Rγ gene. The numbered bars represent chromosomes that are derived from SS (white) or BN (black). Note that the only genetic differences between SSIL2Rγ and SS.BN3IL2Rγ are the inheritance of chromosome 3 from the SS or BN rats. (C) The transgenically labeled human 231Luc+ breast cancer cells were orthotopically implanted in the MFP (indicated by dashed lines) of SSIL2Rγ and SS.BN3IL2Rγ rats. Tumor progression (e.g., growth, vasculogenesis, and metastasis) were tracked over the duration of the experiment. (D) Because the 231Luc+ tumor cells are the same between strains (gray), any differences in tumor progression can be attributed to differences in the SSIL2Rγ (green) and SS.BN3IL2Rγ (blue) microenvironments.
Materials and Methods
Generation of SS.BN3IL2Rγ and SSIL2Rγ rat strains
All procedures and protocols were approved by the MCW IACUC committee. The IL2Rγ gene was targeted in the SS/JrHsdMcwi rat by TALEN injection into single-cell rat embryos, as described previously (20). Once established, a homozygous female rat from the SSIL2Rγ line was intercrossed with a homozygous SS.BN3 male to yield heterozygous SS.BN3IL2Rγ offspring (F1), followed by brother-sister mating to yield homozygous SS.BN3IL2Rγ offspring by the F3 generation.
Tumor Implantation
Luciferase-tagged MDA-MB-231 (231Luc+) cells were orthotopically implanted and measured as described previously (21), with slight modifications. Briefly, 231Luc+ cells (6 × 106) in 50% Matrigel were mammary fat pad (MFP) of 4- to 6-week-old female SSIL2Rg(n = 11) and SS.BN3IL2Rg(n = 19) rats. Tumor volumes were measured weekly by calipers, as described previously(21).
Detection of Lung Metastasis by Ex Vivo Luminescent Imaging
Rats were intraperitoneally injected with 200 μl of d-luciferin (Promega, Madison, WI) diluted in sterile saline (40 mg/ml). The substrate was allowed to circulate for 5 minutes before rats were euthanized and lunges were removed for bioluminescence imaging using the Xenogen Ivis Lumina (Caliper Life Sciences, Hopkinton, MA).
Detection of Metastatic Burden by Luciferase Activity
Ipsilateral axillary lymph nodes and lungs were excised, washed in PBS, and homogenized in 0.2 ml and 2 ml of cold cell culture lysis reagent buffer (Promega, Madison, WI), respectively. Cell debris was removed by centrifugation. Protein concentrations of cleared lysates were determined by Bradford assay (Bio-Rad, Hercules, CA). Fifty microliters of Luciferase Assay Reagent (Promega) was mixed with 10 μl of lysate, and a 10-second average of luminescence was detected using a Varioskan Flash luminometer (ThermoFisher, Waltham, MA). Cell culture lysis reagent buffer without tissue homogenates and LNs or lungs of non–tumor-bearing rats were used to calculate background and subtracted from the results. The net results are expressed as relative light units normalized per milligram of total protein.
Histological Detection of Lung Metastasis
Metastatic lesions in the lungs of tumor-bearing SSIL2Rγ (n = 5) and SS.BN3IL2Rγ (n =5) rats were also assessed by hematoxylin and eosinophil (H&E) staining, as described previously(22). Formalin fixed lung sections were H&E-stained using a Tissue-TekPrisma Automated Stainer (Sakura, Torrance, CA) according to the manufacturer’s protocol. Following H&E staining, three serial sections per lung were examined by two blinded observers.
Matrigel Plug Angiogenesis Assay
Female SS(n = 9) and SS.BN3 (n = 19) rats at 6–8 weeks-of-age were implanted in the MFP with 500 μl of 100% matrigel (cat. CB-40234; BD Biosciences, San Jose, CA) supplemented with 500ng/ml of recombinant rat VEGFA164 purchased (R&D Systems, Minneapolis, MN). At 5 days-post implantation, matrigel plugs were excised, snap-frozen in OCT (Tissue Tek, Torrance, CA) and stained with anti-CD31 as described in the Expanded Materials and Methods.
Blood and Lymphatic Vessel Densities
231Luc+ tumors and matrigel plugs were excised, frozen-sectioned, and immunostained with antibodies against the blood vessel marker, CD31, or the lymphatic vessel marker, LYVE-1, as described previously (23). To quantify tumor BVD and LVD, 3–4 representative images of CD31+ structures (BVD) and LYVE-1+ structures (LVD) per tissue were acquired at 100X magnification using a Nikon E400 microscope equipped with a Spot Insight camera (Nikon Instruments, Melville, NY). BVD and LVD are presented as the average number of vessels per area of the field ± SEM (n = 5–7 rats per group).
Quantification of Blood Vessel Densities (BVD) in DMBA-induced Mammary Tumors
Acquisition of DMBA-induced mammary tumors from the SS.BN3 consomic (n = 9) and SS (n = 9) rats were previously described by Adamovic et al (16). These tumors were previously formalin fixed and paraffin embedded, precluding us from using the traditional anti-CD31 antibody that does not recognize formalin-fixed antigens. To circumvent this issue, DMBA-induced mammary tumors were stained with anti-SH2B3 antibodies (Sigma Aldrich, St. Louis, MO; Cat. HPA005483), which we have demonstrated to specifically recognize CD31+ blood vessels, but not LYVE1+ lymphatic vessels (Supplementary Figure 2). The area of SH2B3+ blood vessels across the entire tumor cross-section was acquired at 100X magnification on a Nikon Eclipse 80i microscope (Nikon Instruments, Melville, NY) equipped with an automated stage and a QImaging Micropublisher camera. Images were analyzed using custom Matlab code (MathWorks, Natick, MA) that stitched together tissue photographs (~100–750 per sample) and segmented the tissue into to differentiate positively stained vessels (Figure 4C). The histology segmentation process included a white background correction and contrast optimization. Static thresholds were applied to each photo for vessel segmentation. Relative vascular density was then calculated for each sample and normalized to the entire cross-sectional tumor area.
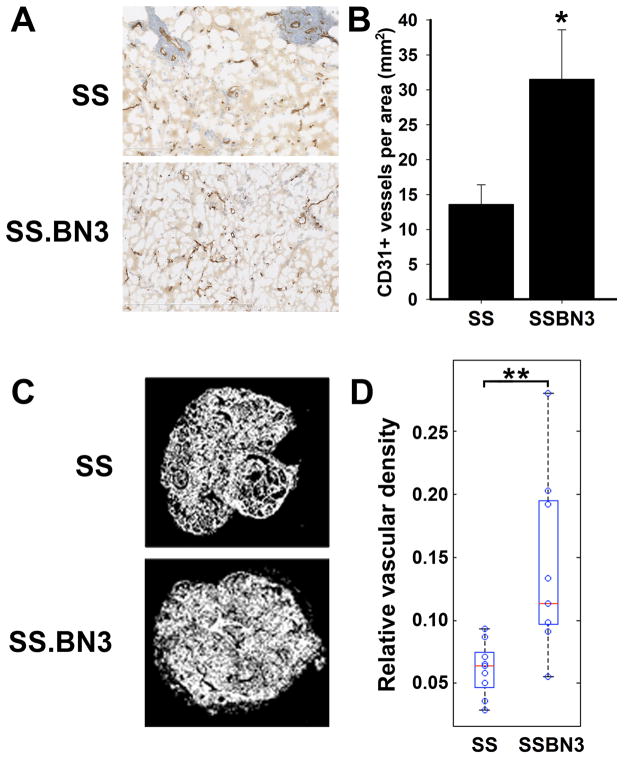
Figure 4.
Increased angiogenic potential in SS.BN3 consomic rats. (A) Density of CD31+ blood vessels in matrigel plugs implanted in SS (n = 20) and SS.BN3 (n = 10) consomic rats. (B) Mean CD31+ blood vessel density in matrigel plugs implanted in SS and SS.BN3 consomic rats was calculated from the entire matrigel plug cross-section. Data are presented as the mean vascular density per mm2 ± SEM. *P<0.05 as determined by Student unpaired t test. (C) Density of SH2B3+ blood vessels in DMBA-induced tumors of SS (n = 9) and SS.BN3 (n = 9) consomic rats. (D) Mean density of SH2B3+ blood vessels was calculated from 400X images of the entire tumor cross-section that were acquired using an automated microscope. Data are presented as the mean vascular density per area ± SEM. **P<0.01 as determined by Student unpaired t test.
Peripheral Blood Analysis
Whole blood from anesthetized rats was analyzed by automated complete blood counts (Marshfield Laboratories, Waukesha, WI) and flow cytometry was performed, as described previously (24, 25).
Genomic Sequencing and Analysis
Genomic sequence was accessed from the Rat Genome Database (http://rgd.mcw.edu/) and has been described in detail elsewhere (26–28).
Statistical analysis
Statistical analyses were performed using Sigma Plot 11.0 software. Data are presented as mean ± SEM. All data were analyzed by unpaired Student t test.
Expanded materials and methods are provided in the online-only Data Supplement.
Results
Consomic Xenograft Model(CXM)
Traditional mapping studies localize cancer risk variants(29), but do not differentiate between variants that impact malignant cells directly versus those that affect the tumor microenvironment. Here, our goal was to develop a new genetic model of breast cancer risk (i.e., CXM), whereby genetic variants that specifically impact breast cancer progression through the tumor microenvironment are mapped.
Following strain generation (SSIL2Rγ and SS.BN3IL2Rγ), we first examined gross organ morphologies and peripheral blood phenotypes to confirm SCID status and noted strain-dependent differences. No differences in viability, body weight, organ weight and morphology, and some peripheral blood phenotypes (T-cells, B-cells and NK cells) were observed between SSIL2Rγ(n = 7) and SS.BN3IL2Rγ(n = 11) rats (Supplementary Figure 1). Differences were observed in circulating monocytes of SS.BN3IL2Rγ (0.4 ± 0.01 per μl blood, P< 0.001) compared with SSIL2Rγ rats (0.17 ± 0.02 per μl blood); in neutrophils of SS.BN3IL2Rγ(1.9 ± 0.3 per μl blood, P <0.001) compared with SSIL2Rγ rats (4.3 ± 0.4 per μl blood); and in total WBCs of SS.BN3IL2Rγ (2.4 ± 0.4 per μl blood, P< 0.001) compared with SSIL2Rγ rats (5.6 ± 0.5 per μl blood) (Supplementary Table 1). Compared with the immune competent parental SS and SS.BN3 strains, circulating lymphocytes were reduced 4- to 9-fold (P<0.001) in SSIL2Rγ and SS.BN3IL2Rγ (Supplementary Table 1), whereas neutrophils were increased by 5- to 9-fold (P<0.001) in SS.BN3IL2Rγ and SSIL2Rγ compared with the parental strains (Supplementary Table 1). Similar changes in leukocytes have been reported in other immunodeficient models [e.g., SCID rat(15) and SCID mouse(30)]. Taken together, these data demonstrate that SSIL2Rγ and SS.BN3IL2Rγ are both SCID, with limited strain-dependent differences in immunity.
Tumor Growth
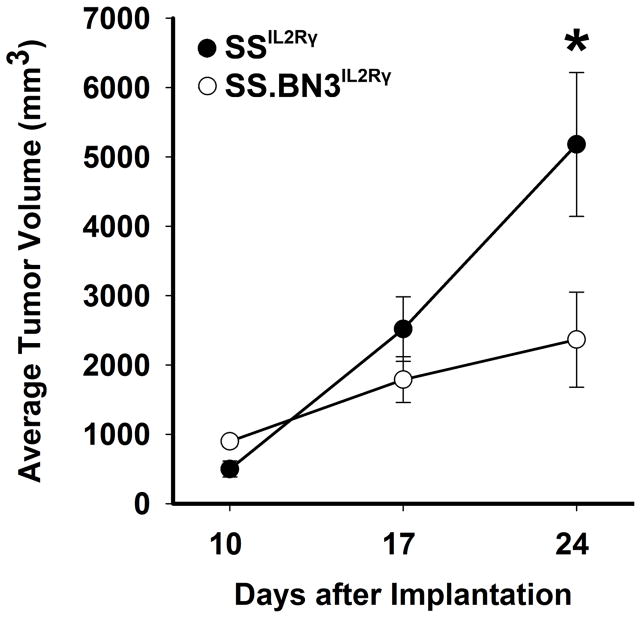
Previously we showed that the SS.BN3 consomic rat has significantly reduced tumorigenicity in a DMBA-inducible model of breast cancer(16). We postulated that this could be at least partially due to genetic differences in the tumor microenvironment, rather than inherent differences in the malignant tumor cells only. To test this possibility, 231Luc+(6x106 cells) were orthotopically implanted in the mammary fat pads (MFP) of SSIL2Rγ and SS.BN3IL2Rγ rats and tumor growth was measured by caliper measurement at 10-, 17-, and 24-days post-implantation. Compared with 231Luc+ tumors in SSIL2Rγ rats at 24 days post-implant (5,117±1,038 mm3, n = 11), tumors grown in SS.BN3IL2Rγ rats (2,616±624 mm3, n = 19) were roughly 2-fold smaller (P<0.05) (Figure 2), suggesting that BN-derived anti-tumor variant(s) or SS-derived pro-tumor variant(s) reside on rat chromosome 3 and function through the tumor microenvironment.
Figure 2.
Growth curve of 231Luc+ breast carcinoma cells implanted in SS.BN3IL2Rγ and SSIL2Rγ rats. The growth of 231Luc+ tumors was monitored by caliper measurement at 10, 17, and 24 days post-implantation in SS.BN3IL2Rγ (n=19) and SSIL2Rγ(n=11) rats. Data are presented as mean tumor volume ± SEM. *P<0.05 as determined by Student unpaired t test.
Tumor Angiogenesis and Hematogenous Metastasis
To assess blood vessel density (BVD), 231Luc+ tumors that were orthotopically implanted in MFPs of SSIL2Rγ (n = 5 rats) and SS.BN3IL2Rγ (n = 6 rats) rats were excised at 24 days post-implantation and stained with antibodies against the blood vessel marker, CD31 (23, 31) (Figure 3A). Compared with tumor BVD in SSIL2Rγ rats (101 ± 11 vessels/field), the BVD in SS.BN3IL2Rγ tumors was significantly increased by 27% (130 ± 18 vessels/field; P<0.05) (Figure 3B). Despite increased BVD in SS.BN3IL2Rγ tumors, we observed fewer blood vessels with open lumens in SS.BN3IL2Rγ tumors (3.2 ± 1.2%; P< 0.05) compared with tumors implanted in SSIL2Rγ rats (6.2 ± 2.0%) (Figure 3C), suggesting that although SS.BN3IL2Rγ had a higher BVD, a greater percentage of these vessels were collapsed and nonfunctional. A similar phenotype (i.e., high density of malformed vessels) was observed by Pandey et al (32) in estrogen-induced pituitary tumors from an overlapping F344.BN-Edpm3BN congenic rat with a 75.7Mb congenic interval (chr3:36.6–112.3Mb), suggesting that the vascular defects might be due to BN-derived variant(s) in a common region of rat chromosome 3.
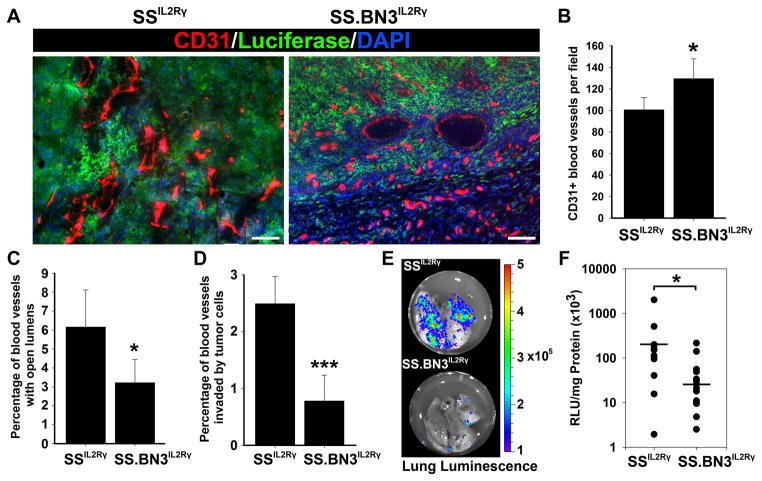
Figure 3.
Characterization of tumor-associated blood vessels and hematogenous metastasis in 231Luc+ tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ rats at 24 days post-implantation. (A) Visualization of tumor-associated blood vessels at using anti-CD31 staining of 231Luc+ tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ rats. Images were acquired at 100X magnification. Scale bar represents 100 μm. (B) Mean blood vessel density in 231Luc+ tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ was calculated from three images per tumor (n= 5–6 tumors per strain) acquired at 100X magnification. Data are presented as the mean vascular density per 100X field ± SEM. *P<0.05 as determined by Student unpaired t test.(C) Percentage of open lumens in 231Luc+ tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ was calculated from three images per tumor (n= 5–6 per strain) acquired at 100X magnification. Data are presented as the percentage of CD31+ vessels with open lumens per 100X field ± SEM. *P<0.05 as determined by Student unpaired t test. (D) Percentage of CD31+ vessels invaded by 231Luc+ cells in tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ was calculated from three images per tumor (n= 5–6 tumors per strain) acquired at 100X magnification. Data are presented as the percentage of CD31+ vessels with open lumens per 100X field ± SEM. ***P<0.001 as determined by Student unpaired t test. (E) Representative luminescent imaging of lungs from tumor-bearing SSIL2Rγ and SS.BN3IL2Rγ rats. (F) Hematogenous metastatic burden in was measured by luciferase activity normalized to total milligrams of protein in lung lysates from SSIL2Rγ (n = 12) and SS.BN3IL2Rγ (n = 16) rats. Metastatic burden of individual rats are represented by the dots and the black bars indicate the average metastatic burden per strain. *P<0.05 as determined by Student unpaired t test.
We also observed a small percentage of CD31+ tumor blood vessels being invaded by 231Luc+ tumor cells in both SSIL2Rγ and to a lesser degree in SS.BN3IL2Rγ rats, demonstrating that tumor cells were actively undergoing hematogenous metastasis. Quantification of CD31+ blood vessels invaded by 231Luc+ cells revealed a significant decrease of vascular invasion in SS.BN3IL2Rγ tumors (0.8 ± 0.5%; P < 0.001) compared with SSIL2Rγ (2.5 ± 0.5%) (Figure 3D), suggesting that 231Luc+ have reduced metastatic potential in the SS.BN3IL2Rγ rat that is blood vessel-dependent. Likewise, upon comparison of metastatic burden in the lungs of tumor-bearing SSIL2Rγ and SS.BN3IL2Rγ rats, we observed decreased metastatic lung nodules (Figure 3E) corresponding to a 7-fold decrease in metastatic burden in SS.BN3IL2Rγ lungs (41 ± 16 RLU/S/mg; P< 0.05) compared with SSIL2Rγ lungs (300 ± 161 RLU/S/mg) (Figure 3F). Moreover, H&E staining revealed histologically detectable metastatic lesions in 80% of SSIL2Rγ lungs, but only 20% of SS.BN3IL2Rγ lungs examined (data not shown). Collectively, these data suggest that genetic variant(s) on BN chromosome 3 increase tumor BVD; however, with a paradoxical decrease in hematogenous metastasis that is potentially due to decreased vascular invasion.
In most cases, BVD is positively correlated with tumor growth and hematogenous metastasis(33), prompting us to validate whether the paradoxical angiogenesis phenotype seen in SS.BN3IL2Rγ (Figure 3B) is present in the unmodified parental SS and SS.BN3 consomic strains using two independent methods: a matrigel plug angiogenesis assay (34) and reanalysis of the DMBA-inducible mammary tumors from Adamovic et al (16). For the matrigel plug angiogenesis assay, female SS and SS.BN3 consomic rats were implanted with 500 μl of matrigel supplemented with 500 ng/ml of recombinant rat VEGF164. At 5 days post-implantation, matrigel plugs were excised and quantified for CD31+ blood vessels across the entire cross-section of the plug. Compared with SS plugs (14 ± 3 vessels/mm2; n = 9 rats), the BVD in SS.BN3 consomic rats was ~2-fold higher (23 ± 4 vessels/mm2; P< 0.05; n = 19 rats) (Figure 4A, B). Likewise, BVD of SS.BN3 mammary tumors (n = 9) from the DMBA-inducible model showed a ~2-fold (P < 0.01) increase relative to SS tumors (n = 9) from the same study (16) (Figure 4C, D), collectively demonstrating that SS.BN3 has increased angiogenic potential (i.e., the ability to form new blood vessels), despite having decreased tumor growth, vascular invasion, and hematogenous metastasis (Figures 2 and 3).
Tumor Lymph angiogenesis and Lymphogenous Metastasis
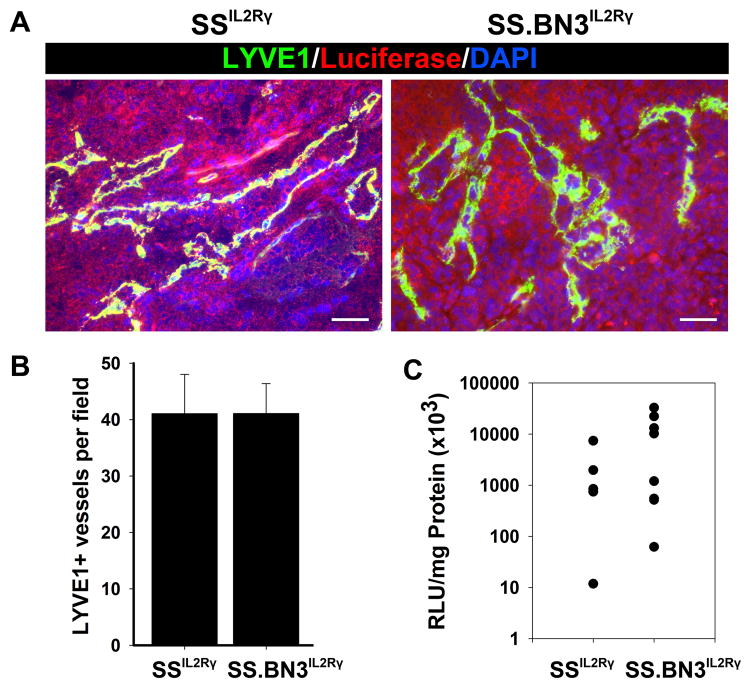
To assess LVD, 231Luc+ tumors that were orthotopically implanted in MFPs of SSIL2Rγ and SS.BN3IL2Rγ rats were excised at 24 days post-implantation and stained with antibodies against the lymphatic vessel marker, LYVE-1 (35)(Figure 4A). In contrast to tumor BVD, we observed no difference in LVD between SSIL2Rγ (41 ± 7 vessels per field; n = 5 rats) and SS.BN3IL2Rγ(41 ± 5 vessels per field; n = 6 rats) tumors (Figure 4B), which coincided with no differences in metastatic burden in the axillary lymph nodes of either strain (Figure 4C). Both strains showed a high incidence of lymphatic vessel invasion by 231Luc+ cells in 80% (SSIL2Rγ) and 67% (SS.BN3IL2Rγ) of tumors examined (data not shown), indicating a similar propensity of 231Luc+ tumors to undergo lymphogenous metastasis in both rat strains. Because no differences were detected in LVD, lymphatic invasion, and LN metastasis (Figure 4), our data suggest that the causative variant(s) on BN chromosome 3 are vascular cell-type specific.
Sequence Analysis of Rat Chromosome 3
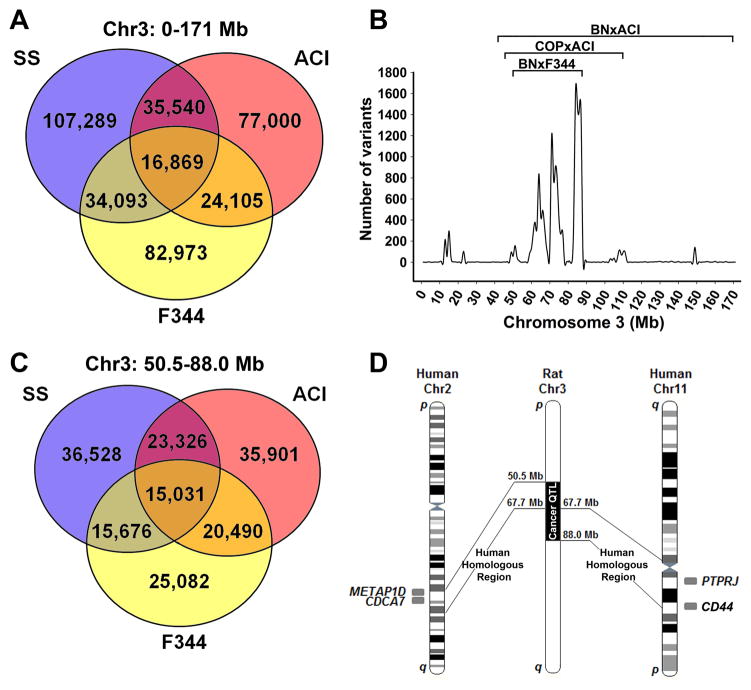
Our data demonstrate that genetic element(s) on BN chromosome 3 affect tumor growth, vascularity, vascular invasion, and metastasis (Figures 2–5). Overlapping cancer QTLs on rat chromosome 3 were previously reported in independent F2 crosses using cancer resistant (BN/SsNHsd and COP/CrCrl) and cancer susceptible (F344/NHsd and ACI/SegHsd) strains, including the Epdm3 QTL [chr3:50.5–88.6 Mb, BN/SsNHsd x F344/NHsd (17)], the Emca5 QTL[chr3:41.0–171.0 Mb, BN/SsNHsd x ACI/SegHsd (18)], and the Ept2 QTL[chr3:44.6–110.2 Mb, ACI/SegHsd x COP/CrCrl (19)] (Figure 6). Analysis of the F344.BN3 congenic rat (F344.BN-Edpm3BN) showed decreased tumor susceptibility and increased density of malformed tumor blood vessels (32), which closely resembles the SS.BN3IL2Rγ consomic phenotypes (Figures 2 and 3). Since the protective allele(s) in the F344.BN-Edpm3BN QTL (17, 32) and the SS.BN3IL2Rγ consomic are BN-derived, the causative alternative allele(s) in this region are likely shared by SS and F344. Because the causative allele(s) in the Emca5 QTL (18) and Ept2 QTL (19) are ACI-derived, the protective alternative allele(s) in this region are likely shared by BN and Cop. Combining all data, we extended the cosegregating sequence analysis to: BN and Cop (protective) vs. SS, F344, and ACI (susceptible).
Figure 5.
Characterization of tumor-associated lymphatic vessels and lymphogenous metastasis in 231Luc+ tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ rats at 24 days post-implantation.(A) Visualization of tumor-associated lymphatic vessels at using anti-LYVE-1 staining of 231Luc+ tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ rats. Scale bar represents 100 μm. (B) Mean lymphatic vessel density in 231Luc+ tumors implanted in SS.BN3IL2Rγ and SSIL2Rγ was calculated from three images per tumor (n= 5–6 per strain) acquired at 100X magnification. Data are presented as the mean vascular density per 100X field ± SEM. (C) Lymphogenous metastatic burden was measured by luciferase activity normalized to total milligrams of protein in axillary LN lysates from SS.BN3IL2Rγ (n = 5) and SSIL2Rγ (n = 8) rats. Metastatic burden of individual rats are represented by the dots and the black bars indicate the average metastatic burden per strain. For (B) and (C), statistical analysis of data was performed by Student unpaired t test.
Figure 6.
Sequence analysis of cosegregating alleles and comparative mapping of rat chromosome 3. (A) Venn diagram of sequence comparison the entire rat chromosome 3 between variants shared by the cancer-resistant BN/NHsdMcwi and Cop/Crl strains versus the cancer-susceptible strains ACI/Eur, F344/N, and SS/JrHsdMcwi. (B) Density mapping of variants shared by the cancer-resistant BN/NHsdMcwi and Cop/Crl strains versus the cancer-susceptible strains ACI/Eur, F344/N, and SS/JrHsdMcwi. Data are presented as the number of variants per 1 Mb bin. Note the highest density of cosegregating variants overlap with three breast cancer QTLs (labeled brackets). (C) Venn diagram of sequence comparison the shared QTL region of chromosome 3 (50.5–88.0 Mb) between variants shared by the cancer-resistant BN/NHsdMcwi and Cop/Crl strains versus the cancer-susceptible strains ACI/Eur, F344/N, and SS/JrHsdMcwi. (D) Schematic representation of the overlapping cancer QTLs on rat chromosome 3 and the orthologous regions in human. The overlapping human breast cancer loci indicated on the figure.
We analyzed the distribution of alleles on rat chromosome 3 using WGS of BN/NHsdMcwi, ACI/Eur, Cop/Crl, F344/N, and SS/JrHsdMcwi that was recently generated in collaboration with Atanur et al (27)(Figure 6A). To identify cosegregating and unique genomic regions that could influence cancer susceptibility, we plotted the total unique and common variants per Mb that cosegregated between two groups: BN/NHsdMcwi and Cop/Crl(tumor-resistant) versus F344/NHsd, ACI/SegHsd, and SS/JrHsdMcwi(tumor-susceptible)(Figure 6B). The overlapping QTL regions (chr3:50.5–88.0 Mb) were significantly enriched by 30-fold (P <0.001) for cosegregating alleles compared to the average of the rest of chromosome 3 (Figure 6A–C), indicating that the genetic mechanism(s) underlying these similar QTLs are likely shared in these regions. Combined, these data suggest that common heritable elements are most likely enriched in the overlapping cancer QTLs (16–19).
Using the cosegregating variant analysis we preliminarily reduced the list of candidate variants from 455,272 total variants on rat chromosome 3 to 15,031 variants (3.3% of total chromosome 3 variants) in the cosegregating region (chr3:50.5–88.0 Mb) that overlapped with the three cancer QTLs (17–19). Of the 15,031 cosegregating variants, 46 lie within coding regions of conserved genes, 10 are expected to cause nonsynonymous amino acid changes, and 2 of these were predicted to be damaging by Provean (http://provean.jcvi.org/seq_submit.php; Table 1 and Supplementary Table 3).
Table 1.
Cosegregating nonsynonymous variants in the overlapping cancer QTL on rat chromosome 3.
| Gene Symbol | Gene ID | Reference nucleotide | Variant nucleotide | Position | aa | Provean Prediction |
|---|---|---|---|---|---|---|
| Lrp2 | 68407 | G | A | 51,642,105 | S1443L | −2.026 |
| Fsip2 | 1593013 | A | T | 66,068,203 | E4965D | −2.625 |
| Pramel7 | 1565990 | T | A | 71,166,422 | D435V | −5.107 |
| Pramel6 | 1565946 | T | G | 71,183,838 | F462C | 1.346 |
| Pacsin3 | 1307327 | G | T | 75,614,920 | G13V | −0.473 |
| Rag2 | 1305588 | T | C | 86,771,280 | I387T | 0.584 |
| Rag1 | 619790 | A | G | 86,785,697 | V503A | 1.105 |
| Rag1 | 619790 | G | C | 86,786,211 | Q334E | 0.357 |
| Pamr1 | 1308745 | A | C | 87,845,730 | E299A | 0.105 |
| Slc1a2 | 3697 | A | G | 87,988,333 | I515V | −0.020 |
Provean prediction of −2.5 or higher (emboldened) indicates that the amino acid change is likely damaging to protein function. aa, amino acid
Comparative Genomics
Our data suggest that cosegregating genetic element(s) on rat chromosome 3 affect tumor growth, angiogenesis, vascular invasion, and metastasis (Figures 2–5A). The 37.5 Mb region (chr3:50.5–88.0 Mb) of overlapping cancer QTLs contains 184 conserved and validated genes (Supplementary Table 2) and is syntenic to two regions on human chromosomes 2 and 11 (Figure 6D) and one region on mouse chromosome 2 (data not show). A total of four genes [METAP1D(5), CDCA7(5), PTPRJ(36), and CD44 (37–40)] in syntenic regions of the human genome have been associated with breast cancer risk (Figure 6D), whereas no mouse studies have reported associations for breast cancer in the syntenic mouse region. Of the human risk genes, METAP1D and CDCA7 were associated with breast cancer incidence by genome wide association study (GWAS) (5). CD44 has been associated with multiple aspects of human breast cancer risk: incidence(39), age-of-onset (37, 40), angiogenesis (41), metastasis(42), and survival (39, 43, 44). PTPRJ mutations were associated with breast cancer risk by loss of heterozygosity (LOH) analysis (36) and PTPRJ has also been implicated in angiogenesis (45). Strikingly, PTPRJ mutation caused disorganized vascular plexus formation in mouse embryos(46), which was marked by increased density of poorly formed blood vessels that are reminiscent of the vascular phenotypes associated with the SS.BN3IL2Rγ consomic (Figure 3) and F344.BN-Edpm3BN congenic (32) models.
Discussion
The heritability of breast cancer is well established (1–3), yet the majority of causative variants remain unknown (5). Of the few known variants, most are considered tumor cell-autonomous (47–49), with far less emphasis placed on germline variants that might impact breast cancer progression through the tumor microenvironment. We developed an experimental model that specifically focused on mapping genetic variant(s) underlying differences in the tumor microenvironment. CXM utilizes genetically “fixed” tumor cells that are xenografted into rat strains with different genetic backgrounds, so that any observed differences in tumor progression (e.g., growth, vasculogenesis, and metastasis) would be attributable to the tumor microenvironment, including interaction between the tumor and host. From a clinical perspective, these data show that germline variants in the tumor microenvironment impact breast cancer risk and should be taken into account in future studies. As a technical advancement, this work demonstrates that CXM can be used to directly map germline variants in the tumor microenvironment for the first time.
Using CXM, we demonstrated that genetic variant(s) on BN chromosome 3 inhibited tumor growth and lung metastasis of 231Luc+ breast cancer cells (Figures 2 and 3), without affecting LN metastasis(Figure 5). Sequence analysis by cross-strain comparisons and comparative analysis prioritized a list of 6 candidate genes for future study(Figure 6 and Table 1). These 6 candidate genes were prioritized using functional predictions of nonsynonymous variants (Fsip2 and Pramel7) and shared association with human breast cancer [METAP1D(5), CDCA7(5), PTPRJ(36), and CD44 (37–40)]. Within the region, it is also possible that other nonsynonymous variants and intergenic variants have functional consequences beyond the limitations of prediction software. Thus, validating these candidates genes will likely require further narrowing of the QTL by congenics (26, 50, 51), gene expression analysis, and/or directly testing candidates by gene-editing (20, 52, 53), all of which we have done previously for other disease loci (see below for description).
Breast Cancer Risk QTL on Rat Chromosome 3
Previously, we(16) and others (17–19) identified overlapping cancer QTL on rat chromosome 3 (50.5–88.0 Mb). A congenic of the Edpm3 QTL (F344.BN-Edpm3BN) indicated that the protective cancer QTL is likely due to malformed immature blood vasculature, despite the tumors having a higher BVD(32). Similarly, we observed significant tumor growth inhibition in the SS.BN3IL2Rγ consomic compared with SSIL2Rγ(Figure 2); with a paradoxical increase in tumor-associated blood vessels(Figure 3A–B), suggesting that the vascular phenotypes underlying the rat chromosome 3 QTL are likely derived from the tumor microenvironment. Tumor metastasis in the previous rat QTL studies was not reported (17–19, 32), as most inducible rat mammary tumors are poorly metastatic. By CXM, we were able to show that variant(s) on BN chromosome 3 also impact vascular invasion and distal metastasis of 231Luc+ cells (Figures 3 and 5). Despite the increased BVD in SS.BN3IL2Rγ, both vascular invasion of tumor cells and hematogenous metastatic burden were significantly lower in SS.BN3IL2Rγ(Figure 3C–E), suggesting a potential mechanism that attenuates metastasis by limiting invasion or transport of tumor cells. Collectively, CXM extended the previous findings (17–19) by demonstrating that(1) genetic variant(s) in the rat chromosome 3 QTL likely act through the tumor microenvironment; (2) the genetic mechanism(s) are blood vessel specific; and (3) tumor growth and hematogenous metastasis are also attenuated by the genetic variant(s).
Blood vasculature is essential for tumor growth and metastatic progression (54). Our data suggest that germline variant(s) impacting tumor-associated blood vessels can significantly affect cancer risk (Figures 2 and 3). Vascular defects also decrease risk of solid tumors in Down’s syndrome patients with trisomy 21, due to increased gene-dosage of DSCR1 (55). The important distinction is that Down’s syndrome is a non-mendelian genetic disease, whereas our findings suggest that common germline variant(s) could have similar effects in a much broader spectrum of patients. Our data also suggest that “looks can be deceiving” – i.e., metastatic potential and tumor growth are frequently correlated with vascular density(33), but based on our findings this might not always be the case. Instead, we propose that variants affecting vascular function and maturity will similarly impact breast cancer progression, which might be overlooked as a prognostic indicator by relying on vascular density only. Finally, from a translational viewpoint, the findings of this study indicate that some germline variant(s) could potentially function as “genetic switches” between hematogenous and lymphogenous metastasis. Thus, a better understanding of germline variant(s) that impact tumor metastasis will likely yield better patient stratification for vascular-specific adjuvant therapies [e.g., bevacizumab(56), TKi(57), etc.].
Using CXM and Similar Strategies to Localize Cancer Risk Variants
From a technical standpoint, we posit that CXM can be tailored to further elucidate causative variants using additional mapping strategies (e.g., F2 intercross, congenic, gene-editing, etc.) or to test interactions with other clinically relevant subsets of breast cancer (e.g., luminal, lobular, inflammatory, etc.). Our rationale for first using a consomic was(1) to develop the general technique and (2) to localize the phenotype to a specific chromosome before proceeding further. Using CXM and comparative mapping, we narrowed the list of potential candidate variants in the rat chromosome 3 QTL by 96.7%. Now, it would be foreseeable to physically narrow the QTL(s), with the ultimate goal of localizing the causative variant(s). Because TALENs and ZFNs do not require specific rat strains (we have now gene-edited on >10 backgrounds; http://rgd.mcw.edu/wg/physgenknockouts), it would be possible to generate SCID rats (or mice) on any background. For example, one could reduce the SS.BN3 cancer QTL by selectively backcrossing SS.BN3IL2Rγ to SSIL2Rγ until the region of BN chromosome 3 has been sufficiently narrowed to resolve the causative variant(s), followed by expression analysis and TALEN- or ZFN-mediated gene-editing of candidate gene(s) on the minimal SS.BN3IL2Rγ congenic background, an approach that we have used previously for other disease loci (53). Finally, although we developed CXM for the rat, we have also demonstrated the utility of TALEN- or ZFN-mediated gene-editing in the mouse (58) and therefore, CXM could be used in mice and potentially other model organisms.
Experimental Considerations of the CXM Strategy
There are several limitations of cancer xenograft models that should be considered when interpreting CXM data. Firstly, like all xenograft models, CXM utilizes SCID rodents that lack fully intact immune systems and thus genetic variants that impact tumor immunity might not be accessible. Secondly, xenotransplantation of human cancer cells into rodent backgrounds might not recapitulate species-specific mechanisms, but rather only mechanisms conserved across species. Finally, some germline variants likely affect both malignant tumor cells and the nonmalignant stromal cells, whereas CXM would only detect effects on the nonmalignant stroma. Despite these limitations, there are also considerable advantages of CXM, including the ability to use etiologically defined and clinically relevant human cell lines that are transgenically-tagged for better tracking in vivo.
Perspective
CXM is the first model for mapping germline variants in the tumor microenvironment. To do so, CXM combines genome-editing (via TALENs), a genetic mapping tool (the consomic), and a widely used model of human breast cancer (the tumor xenograft). As proof-of-concept, we demonstrated that CXM can detect germline variant(s) in an established breast cancer QTL (17–19) (Figures 2 and 3). Using CXM, we also demonstrated that the causative variant(s) on rat chromosome 3 affected the tumor blood vasculature, which potentially disrupted tumor growth and metastasis. Follow-up studies using cell culture systems, localization of candidate gene expression and tissue-specific gene-editing will be required to determine whether the causative variant(s) function directly or indirectly through the blood vasculature. In summary, CXM is a technical advance that localizes cancer phenotypes to a single chromosome and could be used to isolate causative variants/genes by congenic mapping and/or candidate gene-targeting.
Supplementary Material
Acknowledgments
We thank M. Teisch, E. Christenson, C. Duris, M. Tschannen, J. Wendt-Andrae, M. Grzybowski, S. Kalloway, J. Foeckler, and R. Schilling for excellent technical support. M.J.F. is supported by an NHLBI training grant (5T32HL007792). A.M.G is supported by a NIH Director’s New Innovator Award (DP2OD008396). This study was supported by an Interdisciplinary Cancer Research Fellowship from the Medical College of Wisconsin Cancer Center to M.J.F., a Froedtert Hospital Foundation Skin Cancer Center Research Award to Z.L., NCI grant R01CA77876 to J.D.S., and NHLBI grants 5RC2HL101681 and R24HL11447401A1 to H.J.J.
Footnotes
Conflict of Interest: Recombinetics, Inc. is a leader in developing genetically modified livestock and has no commercial interest in the reported rat models that were generated as part of an academic collaboration. As such, the authors declare no financial conflict of interest.
References
- 1.Peto J, Mack TM. High constant incidence in twins and other relatives of women with breast cancer. Nat Genet. 2000;26:411–414. doi: 10.1038/82533. [DOI] [PubMed] [Google Scholar]
- 2.Rosman DS, Kaklamani V, Pasche B. New insights into breast cancer genetics and impact on patient management. Curr Treat Options Oncol. 2007;8:61–73. doi: 10.1007/s11864-007-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pharoah PD, Antoniou A, Bobrow M, Zimmern RL, Easton DF, Ponder BA. Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet. 2002;31:33–36. doi: 10.1038/ng853. [DOI] [PubMed] [Google Scholar]
- 4.Hartman M, Lindstrom L, Dickman PW, Adami HO, Hall P, Czene K. Is breast cancer prognosis inherited? Breast Cancer Res. 2007;9:R39. doi: 10.1186/bcr1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J, Bojesen SE, Bolla MK, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–361. 361e351–352. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafiq S, Tapper W, Collins A, Khan S, Politopoulos I, Gerty S, Blomqvist C, Couch FJ, Nevanlinna H, Liu J, et al. Identification of inherited genetic variations influencing prognosis in early-onset breast cancer. Cancer Res. 2013;73:1883–1891. doi: 10.1158/0008-5472.CAN-12-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park YG, Zhao X, Lesueur F, Lowy DR, Lancaster M, Pharoah P, Qian X, Hunter KW. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat Genet. 2005;37:1055–1062. doi: 10.1038/ng1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu XO, Long J, Lu W, Li C, Chen WY, Delahanty R, Cheng J, Cai H, Zheng Y, Shi J, et al. Novel genetic markers of breast cancer survival identified by a genome-wide association study. Cancer Res. 2012;72:1182–1189. doi: 10.1158/0008-5472.CAN-11-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. Mediators of vascular remodelling coopted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 11.Maae E, Andersen RF, Steffensen KD, Jakobsen EH, Brandslund I, Sorensen FB, Jakobsen A. Prognostic impact of VEGFA germline polymorphisms in patients with HER2-positive primary breast cancer. Anticancer Res. 2012;32:3619–3627. [PubMed] [Google Scholar]
- 12.Smith KC, Bateman AC, Fussell HM, Howell WM. Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur J Immunogenet. 2004;31:167–173. doi: 10.1111/j.1365-2370.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 13.Cowley AW, Jr, Roman RJ, Jacob HJ. Application of chromosomal substitution techniques in gene-function discovery. J Physiol. 2004;554:46–55. doi: 10.1113/jphysiol.2003.052613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson DF, Fahrenkrug SC, Hackett PB. Targeting DNA With Fingers and TALENs. Mol Ther Nucleic Acids. 2012;1:e3. doi: 10.1038/mtna.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mashimo T, Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamovic T, McAllister D, Wang T, Adamovic D, Rowe JJ, Moreno C, Lazar J, Jacob HJ, Sugg SL. Identification of novel carcinogen-mediated mammary tumor susceptibility loci in the rat using the chromosome substitution technique. Genes Chromosomes Cancer. 2010;49:1035–1045. doi: 10.1002/gcc.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendell DL, Gorski J. Quantitative trait loci for estrogen-dependent pituitary tumor growth in the rat. Mamm Genome. 1997;8:823–829. doi: 10.1007/s003359900586. [DOI] [PubMed] [Google Scholar]
- 18.Schaffer BS, Lachel CM, Pennington KL, Murrin CR, Strecker TE, Tochacek M, Gould KA, Meza JL, McComb RD, Shull JD. Genetic bases of estrogen-induced tumorigenesis in the rat: mapping of loci controlling susceptibility to mammary cancer in a Brown Norway x ACI intercross. Cancer Res. 2006;66:7793–7800. doi: 10.1158/0008-5472.CAN-06-0143. [DOI] [PubMed] [Google Scholar]
- 19.Kurz SG, Hansen KK, McLaughlin MT, Shivaswamy V, Schaffer BS, Gould KA, McComb RD, Meza JL, Shull JD. Tissue-specific actions of the Ept1, Ept2, Ept6, and Ept9 genetic determinants of responsiveness to estrogens in the female rat. Endocrinology. 2008;149:3850–3859. doi: 10.1210/en.2008-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volk LD, Flister MJ, Bivens CM, Stutzman A, Desai N, Trieu V, Ran S. Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia. 2008;10:613–623. doi: 10.1593/neo.08302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faraji F, Hu Y, Wu G, Goldberger NE, Walker RC, Zhang J, Hunter KW. An integrated systems genetics screen reveals the transcriptional structure of inherited predisposition to metastatic disease. Genome Res. 2014;24:227–240. doi: 10.1101/gr.166223.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehurst B, Flister MJ, Bagaitkar J, Volk L, Bivens CM, Pickett B, Castro-Rivera E, Brekken RA, Gerard RD, Ran S. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int J Cancer. 2007;121:2181–2191. doi: 10.1002/ijc.22937. [DOI] [PubMed] [Google Scholar]
- 24.Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One. 2012;7:e31794. doi: 10.1371/journal.pone.0031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flister MJ, Prisco SZ, Sarkis AB, O’Meara CC, Hoffman M, Wendt-Andrae J, Moreno C, Lazar J, Jacob HJ. Identification of hypertension susceptibility loci on rat chromosome 12. Hypertension. 2012;60:942–948. doi: 10.1161/HYPERTENSIONAHA.112.198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atanur SS, Diaz AG, Maratou K, Sarkis A, Rotival M, Game L, Tschannen MR, Kaisaki PJ, Otto GW, Ma MC, et al. Genome sequencing reveals loci under artificial selection that underlie disease phenotypes in the laboratory rat. Cell. 2013;154:691–703. doi: 10.1016/j.cell.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 29.Lifsted T, Le Voyer T, Williams M, Muller W, Klein-Szanto A, Buetow KH, Hunter KW. Identification of inbred mouse strains harboring genetic modifiers of mammary tumor age of onset and metastatic progression. Int J Cancer. 1998;77:640–644. doi: 10.1002/(sici)1097-0215(19980812)77:4<640::aid-ijc26>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katsuki M, Takahashi K, et al. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 31.Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, Pepper MS, Zawieja DC, Ran S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. 2010;115:418–429. doi: 10.1182/blood-2008-12-196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey J, Wendell DL. Angiogenesis and capillary maturation phenotypes associated with the Edpm3 locus on rat chromosome 3. Mamm Genome. 2006;17:49–57. doi: 10.1007/s00335-005-2450-4. [DOI] [PubMed] [Google Scholar]
- 33.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 35.Flister MJ, Volk LD, Ran S. Characterization of Prox1 and VEGFR-3 expression and lymphatic phenotype in normal organs of mice lacking p50 subunit of NF-kappaB. Microcirculation. 2011;18:85–101. doi: 10.1111/j.1549-8719.2010.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruivenkamp CA, van Wezel T, Zanon C, Stassen AP, Vlcek C, Csikos T, Klous AM, Tripodis N, Perrakis A, Boerrigter L, et al. Ptprj is a candidate for the mouse colon-cancer susceptibility locus Scc1 and is frequently deleted in human cancers. Nat Genet. 2002;31:295–300. doi: 10.1038/ng903. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Nagarkatti PS, Zhong Y, Zhang J, Nagarkatti M. Implications of single nucleotide polymorphisms in CD44 exon 2 for risk of breast cancer. Eur J Cancer Prev. 2011;20:396–402. doi: 10.1097/CEJ.0b013e3283463943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Nagarkatti PS, Zhong Y, Creek K, Zhang J, Nagarkatti M. Unique SNP in CD44 intron 1 and its role in breast cancer development. Anticancer Res. 2010;30:1263–1272. [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang L, Deng J, Zhu X, Zheng J, You Y, Li N, Wu H, Lu J, Zhou Y. CD44 rs13347 C>T polymorphism predicts breast cancer risk and prognosis in Chinese populations. Breast Cancer Res. 2012;14:R105. doi: 10.1186/bcr3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tulsyan S, Agarwal G, Lal P, Agrawal S, Mittal RD, Mittal B. CD44 Gene Polymorphisms in Breast Cancer Risk and Prognosis: A Study in North Indian Population. PLoS One. 2013;8:e71073. doi: 10.1371/journal.pone.0071073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao G, Savani RC, Fehrenbach M, Lyons C, Zhang L, Coukos G, Delisser HM. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol. 2006;169:325–336. doi: 10.2353/ajpath.2006.060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 43.Diaz LK, Zhou X, Wright ET, Cristofanilli M, Smith T, Yang Y, Sneige N, Sahin A, Gilcrease MZ. CD44 expression is associated with increased survival in node-negative invasive breast carcinoma. Clin Cancer Res. 2005;11:3309–3314. doi: 10.1158/1078-0432.CCR-04-2184. [DOI] [PubMed] [Google Scholar]
- 44.Berner HS, Suo Z, Risberg B, Villman K, Karlsson MG, Nesland JM. Clinicopathological associations of CD44 mRNA and protein expression in primary breast carcinomas. Histopathology. 2003;42:546–554. doi: 10.1046/j.1365-2559.2003.01622.x. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi T, Takahashi K, Mernaugh RL, Tsuboi N, Liu H, Daniel TO. A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood. 2006;108:1234–1242. doi: 10.1182/blood-2005-10-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Takahashi K, St John PL, Fleming PA, Tomemori T, Watanabe T, Abrahamson DR, Drake CJ, Shirasawa T, Daniel TO. A mutant receptor tyrosine phosphatase, CD148, causes defects in vascular development. Mol Cell Biol. 2003;23:1817–1831. doi: 10.1128/MCB.23.5.1817-1831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 48.Hunter K. Host genetics influence tumour metastasis. Nat Rev Cancer. 2006;6:141–146. doi: 10.1038/nrc1803. [DOI] [PubMed] [Google Scholar]
- 49.Bodenstine TM, Welch DR. Metastasis suppressors and the tumor microenvironment. Cancer Microenviron. 2008;1:1–11. doi: 10.1007/s12307-008-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flister MJ, Hoffman MJ, Reddy P, Jacob HJ, Moreno C. Congenic mapping and sequence analysis of the Renin locus. Hypertension. 2013;61:850–856. doi: 10.1161/HYPERTENSIONAHA.111.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman MJ, Flister MJ, Nunez L, Xiao B, Greene AS, Jacob HJ, Moreno C. Female-specific hypertension Loci on rat chromosome 13. Hypertension. 2013;62:557–563. doi: 10.1161/HYPERTENSIONAHA.113.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flister MJ, Tsaih SW, O’Meara CC, Endres B, Hoffman MJ, Geurts AM, Dwinell MR, Lazar J, Jacob HJ, Moreno C. Identifying multiple causative genes at a single GWAS locus. Genome Res. 2013 doi: 10.1101/gr.160283.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rangel-Filho A, Lazar J, Moreno C, Geurts A, Jacob HJ. Rab38 modulates proteinuria in model of hypertension-associated renal disease. J Am Soc Nephrol. 2013;24:283–292. doi: 10.1681/ASN.2012090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 55.Baek KH, Zaslavsky A, Lynch RC, Britt C, Okada Y, Siarey RJ, Lensch MW, Park IH, Yoon SS, Minami T, et al. Down’s syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459:1126–1130. doi: 10.1038/nature08062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 57.Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18(Suppl 10):x3–10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- 58.Chen YG, Forsberg MH, Khaja S, Ciecko AE, Hessner MJ, Geurts AM. Gene targeting in NOD mouse embryos using zinc-finger nucleases. Diabetes. 2013 doi: 10.2337/db13-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.