This work demonstrates that TGN-located AP1 complex mediates dileucine motif-directed vacuolar targeting via the interaction with this conserved sorting signal in plant cells. The deficiency of the AP1 gamma adaptins, as well as the mutations of the dileucine residues, resulted in the mislocalization of tonoplast proteins containing the dileucine motif.
Abstract
Membrane proteins on the tonoplast are indispensible for vacuolar functions in plants. However, how these proteins are transported to the vacuole and how they become separated from plasma membrane proteins remain largely unknown. In this study, we used Arabidopsis thaliana vacuolar ion transporter1 (VIT1) as a reporter to study the mechanisms of tonoplast targeting. We showed that VIT1 reached the tonoplast through a pathway involving the endoplasmic reticulum (ER), Golgi, trans-Golgi network (TGN), prevacuolar compartment, and tonoplast. VIT1 contains a putative N-terminal dihydrophobic type ER export signal, and its N terminus has a conserved dileucine motif (EKQTLL), which is responsible for tonoplast targeting. In vitro peptide binding assays with synthetic VIT1 N terminus identified adaptor protein complex-1 (AP1) subunits that interacted with the dileucine motif. A deficiency of AP1 gamma adaptins in Arabidopsis cells caused relocation of tonoplast proteins containing the dileucine motif, such as VIT1 and inositol transporter1, to the plasma membrane. The dileucine motif also effectively rerouted the plasma membrane protein SCAMP1 to the tonoplast. Together with subcellular localization studies showing that AP1 gamma adaptins localize to the TGN, we propose that the AP1 complex on the TGN mediates tonoplast targeting of membrane proteins with the dileucine motif.
INTRODUCTION
In eukaryotic cells, protein delivery to their destinations in the endomembrane system relies on various sorting signals (Trowbridge et al., 1993; Bonifacino and Traub, 2003; Bassham et al., 2008; Robinson et al., 2008; Gao et al., 2012). Numerous tonoplast-localized transporters and channels are vital to fulfill the functions of the vacuole, such as maintaining the optimal turgor of the cells, storing nutrients for metabolism, maintaining cellular pH homeostasis, and serving as a location of protein turnover (Martinoia et al., 2007; Isayenkov et al., 2010). Thus, a large amount of research has been performed to illuminate the proper regulation of vacuolar membrane transport systems, especially in yeast and mammalian cells (Jiang and Rogers, 1998a; Bonifacino and Traub, 2003; Cui et al., 2014).
As a first step in transport to the vacuole, membrane proteins are synthesized at the endoplasmic reticulum (ER), sequestered into coat protein complex II (COPII) transport vesicles, via the interaction between coat proteins and specific ER export signals, and delivered to the Golgi apparatus (Bi et al., 2002; Mancias and Goldberg, 2008). Many kinds of ER export signal have been documented in yeast and mammalian cells, including the diacidic motif, dihydrophobic motif, and diaromatic motif (Barlowe, 2003; Mikosch and Homann, 2009).
Beyond the Golgi, several endosomes have been identified, such as the trans-Golgi network (TGN; early endosome) and the prevacuolar compartment (PVC; late endosome) (Jiang et al., 2002; Tse et al., 2004; Lam et al., 2007). In post-Golgi traffic, adaptor protein (AP) complexes select cargo proteins into transport vesicles (Cowles et al., 1997; Bonifacino and Traub, 2003; Robinson, 2004; Robinson and Pimpl, 2014). In the past decade, five types of adaptor protein complexes (AP1 to AP5) have been identified with conserved heterotetramer structural composition, containing two large subunits (γ/α/δ/ε/ζ- and β-adaptin), one medium subunit (μ-adaptin), and one small subunit (σ-adaptin), but with distinct subcellular localizations and functions (Boehm and Bonifacino, 2001; Nakatsu and Ohno, 2003). AP1 localizes to the TGN and functions in multiple trafficking pathways to endosome, lysosome/vacuole, and basolateral domain in epithelial cells (Icking et al., 2007; Kametaka et al., 2010; Robinson et al., 2010; Kang and Fölsch, 2011; Guo et al., 2013b). AP2 mediates clathrin-dependent endocytosis at the plasma membrane (PM) (Jackson et al., 2010; Chen et al., 2011). AP3 functions in the trafficking pathway from TGN to lysosome and lysosome-related organelles (Cowles et al., 1997; Dell’Angelica, 2009). AP4 has been poorly studied, but appears to be involved in trafficking of amyloid precursor protein from the TGN to endosomes (Burgos et al., 2010). Recently, a fifth AP complex, AP5, was discovered and localized to the late endosome in HeLa cells (Hirst et al., 2011). Studies have been extensively performed to identify specific sorting signals of these AP complexes and their recognition mechanisms, among which a tyrosine-based sorting signal (YXXØ; Y is tyrosine; X is any amino acid; Ø is an amino acid with a bulky hydrophobic group) and a dileucine sorting signal ([D/E]XXXL[L/I]; D is aspartic acid; E is glutamic acid; L is leucine) are the best characterized (Bonifacino and Traub, 2003). YXXØ signals are widely involved in protein sorting in both the endocytic and secretory pathways and are recognized by the μ-adaptins of AP1, AP2, AP3, and AP4 (Boll et al., 1996; Bonifacino and Dell’Angelica, 1999; Theos et al., 2005; Chapuy et al., 2008; Guo et al., 2013a). Similarly, dileucine signals also interact with multiple AP complexes (AP1, AP2, and AP3), whereas they show specific affinity with a distinct set of cargoes (Doray et al., 2007; Mattera et al., 2011; Sitaram et al., 2012). Other signals have also been recorded as NPXY-type signals and acidic cluster dileucine signals (Canfield et al., 1991; Misra et al., 2002; Shiba et al., 2002).
In plants, studies designed to uncover the sorting mechanism of membrane proteins are relatively few in number. ER export signals similar to those in mammals have been identified in different proteins (Hanton et al., 2005; Schoberer et al., 2009; Zelazny et al., 2009). Most recently a study with EMP12 showed that its C-terminal ER export signal is composed of a dihydrophobic motif (FV) and a tyrosine residue (Y) (Gao et al., 2012). In the last several years, studies on tonoplast targeting mechanisms have started to emerge (Pedrazzini et al., 2013; Rojas-Pierce, 2013). The C terminus of tonoplast two-pore K+ channels has been proved to contain targeting information for different types of vacuoles both in rice (Oryza sativa) and Arabidopsis thaliana (Isayenkov et al., 2011; Maîtrejean et al., 2011). Another study with Arabidopsis β-Fructosidase 4 has identified EEE and LCPYTRL sequence motifs as sorting signals on the way from the TGN to the central vacuole (Jung et al., 2011). Similar to cases in mammalian cells and yeast, a conserved dileucine motif is also involved in the targeting of tonoplast membrane proteins in plant cells, as recorded for several proteins like the monosaccharide transporter ERD SIX-LIKE1 (ESL1), the molybdate transporter2 (MOT2), two pore channel1 (TPC1), peptide transporter1 (PTR1), and inositol transporter1 (INT1) (Yamada et al., 2010; Gasber et al., 2011; Komarova et al., 2012; Larisch et al., 2012; Wolfenstetter et al., 2012). However, more precise information on the underlying mechanism is lacking. AP3 was shown to mediate vacuole biogenesis and vacuolar sorting in Arabidopsis but appears to have no relation to the trafficking of INT1, indicating a yet unknown transport mechanism (Lee et al., 2007; Niihama et al., 2009; Feraru et al., 2010; Zwiewka et al., 2011). Very recently, AP1 and AP2 were identified in Arabidopsis. AP1 is essential for growth and multiple trafficking pathways, while AP2 mediates endocytosis and is involved in floral organ development (Di Rubbo et al., 2013; Kim et al., 2013; Park et al., 2013; Teh et al., 2013; Wang et al., 2013b; Yamaoka et al., 2013).
Arabidopsis VIT1, a homolog of the yeast iron transporter CCC1 (Ca[2+]-sensitive Cross Complementer 1), has been reported to localize to the tonoplast and be important for vacuolar iron storage in seed (Li et al., 2001; Kim et al., 2006). However, little is known about its trafficking pathway and targeting mechanism. Here, we used VIT1 as a marker to study the sorting mechanism of a tonoplast protein with multiple transmembrane domains (TMDs). Using transient expression, we showed that VIT1 exited the ER via a putative dihydrophobic ER export signal and reached the tonoplast through an ER-Golgi-TGN-PVC-tonoplast pathway. Mutagenesis analysis uncovered a conserved dileucine motif (EKQTLL) at the N terminus of VIT1 for its tonoplast targeting. In addition, we demonstrated that this dileucine tonoplast targeting signal was recognized via interaction with AP1 complex. Finally, deprivation of AP-1 gamma adaptins resulted in mistargeting of vacuole proteins containing the dileucine motif from the tonoplast to the plasma membrane. We believe that this article makes a significant contribution to the current controversy concerning the role(s) of clathrin-coated vesicles in vacuolar protein transport and uncovers the transport mechanism of membrane proteins to the vacuole, which is likely to be very different with that of soluble cargoes, especially in terms of transport vectors (Bottanelli et al., 2011; De Marcos Lousa et al., 2012; Robinson and Pimpl, 2014).
RESULTS
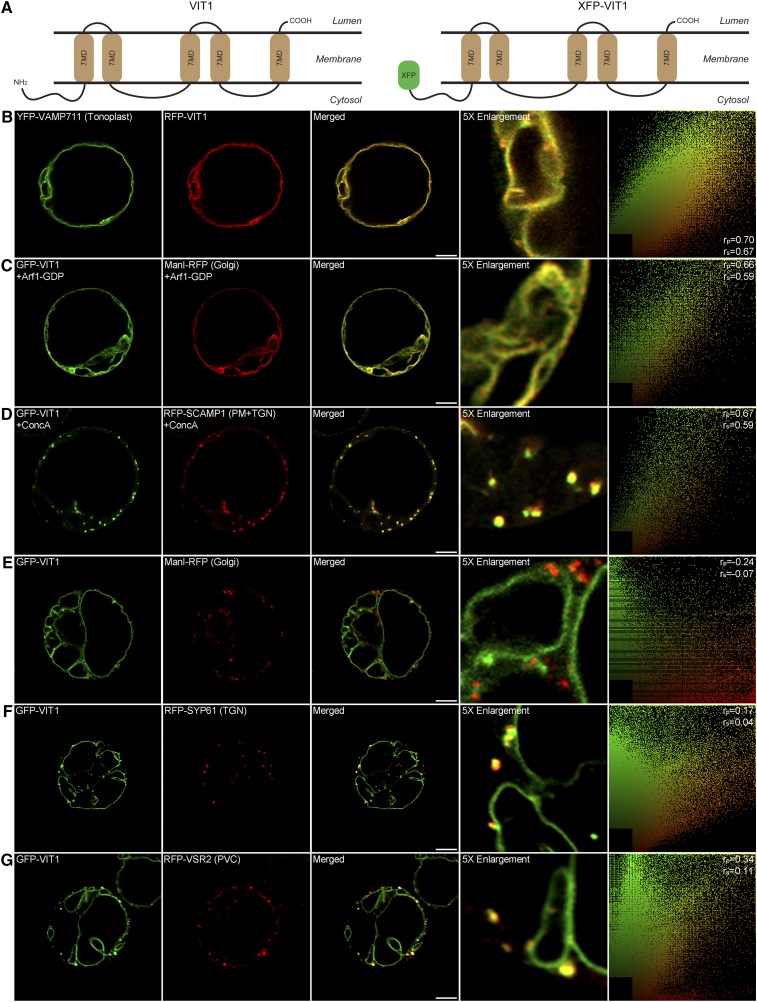
Arabidopsis VIT1 Traffics through the Secretory Pathway to the Tonoplast
VIT1 has been reported to be a tonoplast transporter in Arabidopsis (Kim et al., 2006). From the predicted topology using the online tool TMHMM server 2.0, it was shown to be a membrane protein containing a large cytosolic N terminus, five transmembrane domains (TMDs), and a very short luminal C terminus (Figure 1A). To study the targeting mechanism of VIT1, we first tested its localization and trafficking route in Arabidopsis protoplasts, a well-established transient expression system (Miao and Jiang, 2007). We used an XFP fusion approach to generate a green fluorescent protein (GFP)/red fluorescent protein (RFP)-VIT1 construct. As shown in Figure 1B, the signals of RFP-VIT1 colocalized with the tonoplast SNARE protein VAMP711, which is consistent with a previous report (Kim et al., 2006). To dissect the trafficking pathway of VIT1, we used the GDP-fixed mutant form of ADP-ribosylation factor1 (ARF1), which blocks ER-Golgi transport and induces the relocation of Golgi proteins to the ER (Takeuchi et al., 2002; Robinson et al., 2011). As shown in Figure 1C, GFP-VIT1 was redistributed into the ER together with the Golgi marker ManI-RFP, indicating that GFP-VIT1 was transported from ER to Golgi. To further test the post-Golgi trafficking route of VIT1, we treated the protoplasts with Concanamycin A (ConcA), an inhibitor of vacuolar (H+)-ATPase that disrupts the TGN and causes accumulation of the marker molecule SCAMP1 (Dettmer et al., 2006; Lam et al., 2007; Wang et al., 2010). Upon ConcA treatment, GFP-VIT1 formed aggregations and colocalized with the TGN marker RFP-SCAMP1 (Figure 1D), indicating that TGN lies on the route of VIT1 trafficking. Notably, GFP-VIT1 also showed punctate fluorescent signals in addition to the tonoplast. To verify the identity of these punctae, we coexpressed GFP-VIT1 with known organelle markers in Arabidopsis protoplasts, such as the Golgi marker ManI-RFP, the TGN marker RFP-SYP61, and the PVC marker RFP-VSR2 (Shen et al., 2013; Cai et al., 2014). The punctate signal of GFP-VIT1 was clearly separate from ManI-RFP (Figure 1E), while it showed partially colocalization with the TGN marker RFP-SYP61 (Figure 1F) and with the PVC marker RFP-VSR2 (Figure 1G). In addition, GFP-VIT1 has been shown to colocalize with RFP-ARA7(Q69L) on the membrane of enlarged PVC in a previous study (Cai et al., 2014). Taken together, these results showed that GFP-VIT1 was delivered from the ER to the tonoplast along the secretory pathway, passing through the Golgi, TGN, and PVC.
Figure 1.
Predicted Topology and Subcellular Localization of GFP-VIT1 in Arabidopsis Protoplasts.
(A) Predicted topology of Arabidopsis VIT1 as determined using the TMHMM server 2.0 and diagram of construct GFP-VIT1.
(B) to (G) GFP-VIT1 was coexpressed with known organelle markers into Arabidopsis protoplasts as indicated ([B] and [E] to [G]). Images were collected by confocal microscopy after 12 to 14 h incubation. Vesicle trafficking from Golgi and TGN was inhibited by overexpression of Arf1 mutant (C) and ConcA treatment (D). The right column shows the scatterplot images obtained from ImageJ with the PSC colocalization plug-in. The linear Pearson correlation coefficient (rp) and the nonlinear Spearman correlation coefficient (rs) indicate the extent of colocalization with the value of +1.0 for complete colocalization. Bars = 10 μm.
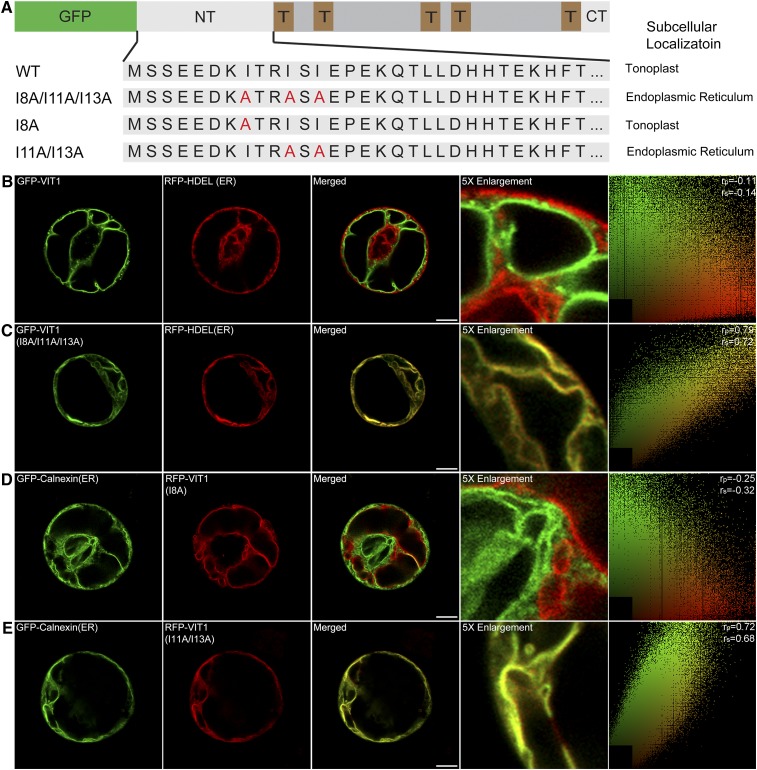
The N Terminus of Arabidopsis VIT1 Contains a Putative ER Export Signal
In transmembrane spanning proteins, most sorting signals are found in the cytosolic region. Thus, we first tested whether the large cytosolic N terminus of VIT1 contributed to its trafficking. We generated the N-terminal deletion mutant construct GFP-VIT1ΔNT and transiently expressed it in Arabidopsis protoplasts. As shown in Supplemental Figure 1A, GFP-VIT1ΔNT was retained in the ER and fully colocalized with the ER marker RFP-HDEL, indicating that N-terminal of VIT1 was essential for exiting from the ER and a potential ER export signal might exist in the N-terminal.
Up to now, several kinds of ER export signals have been well documented, such as diacidic and dihydrophobic motif (Barlowe, 2003). The amino acid sequence of the VIT1 N terminus revealed two potential diacidic motifs (EED and EPE). To verify whether they were functional signals, we next deleted the first six residues after the first diacidic motif (VIT1ΔN6) and the following seven residues just before the second motif (VIT1ΔN13). Surprisingly, GFP-VIT1ΔN6 separated from the ER, but showed a clear tonoplast pattern and punctate signals that colocalized with RFP-VSR2 (Supplemental Figure 1B). The pattern was similar with the full-length construct, indicating that the first six residues containing one diacidic motif was not necessary for the trafficking of VIT1. By contrast, the deletion of the following seven amino acids led to the retention in the ER, suggesting that these seven residues (KITRISI) functioned as the putative ER export signal of VIT1, but not the second diacidic motif. After checking the sequence carefully, we found that one hydrophobic amino acid isoleucine (I) appeared frequently, indicating that this hydrophobic motif acted as the sorting signal of VIT1. To confirm this hypothesis, we further performed a site-directed mutagenesis analysis (Figure 2A). Mutation of the three isoleucines to alanines gave the construct GFP-VIT1 (I8A/I11A/I13A), which showed fully colocalization with the ER in Arabidopsis protoplasts, proving that this motif was functional for the ER export of VIT1 (Figure 2C). Interestingly, further studies showed that single mutation of one isoleucine (I8A) had no effect on the localization of VIT1 (Figure 2D), while double mutation of the other two isoleucines (I11A/I13A) totally blocked the ER export of VIT1, leading to colocalization with the ER marker GFP-Calnexin (Figure 2E). Therefore, the dihydrophobic motif (ISI) at the N terminus of VIT1 functions as a putative ER export signal.
Figure 2.
The N Terminus of Arabidopsis VIT1 Contains an ER Export Signal.
(A) Scheme of VIT1 N terminus mutation constructs and their subcellular localization in Arabidopsis protoplasts. NT, N terminus; T, TMD; CT, C terminus.
(B) to (E) Confocal images showing the subcellular localizations of VIT1 N terminus point mutation constructs. The right column shows the scatterplot images indicating the extent of colocalization with the linear Pearson correlation coefficient (rp) and the nonlinear Spearman correlation coefficient (rs). Bars = 10 μm.
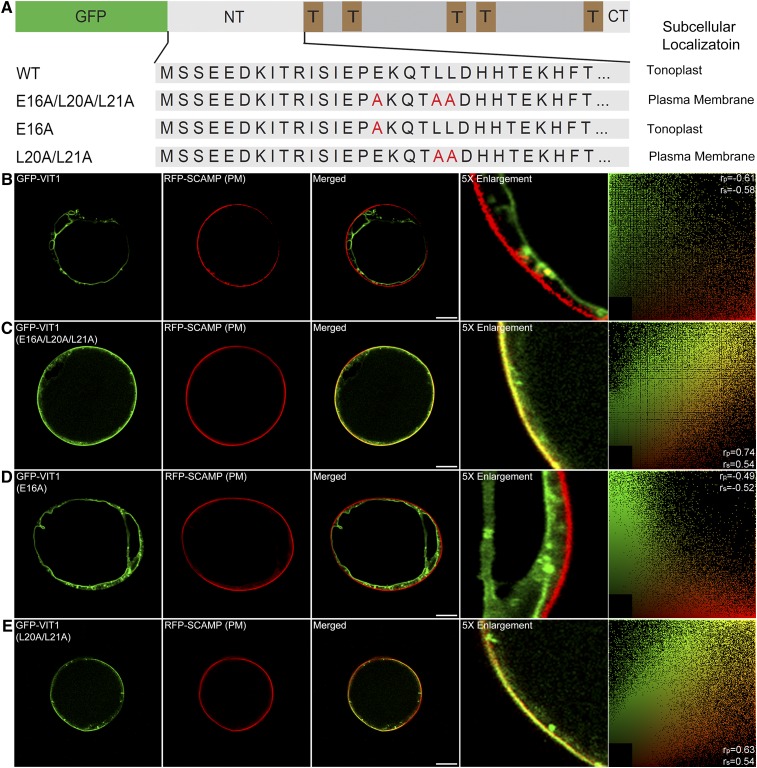
The N Terminus of VIT1 Contains a Dileucine Motif for Tonoplast Targeting
Several checkpoints for protein delivery exist along the secretory pathway. Beyond the Golgi apparatus, proteins need additional sorting signals for correct tonoplast targeting. Recently, several studies reported on the dileucine motif in plant, which was also found in the N terminus of VIT1 (EKQTLL) (Komarova et al., 2012; Larisch et al., 2012; Wolfenstetter et al., 2012). To test whether this putative motif is functional, we further mutated the conserved amino acids (glutamic acid and leucine) to alanine in this motif (Figure 3A). As shown in Figures 3B and 3C, the construct with three point mutations colocalized with the PM marker RFP-SCAMP1 and was different from the original pattern of GFP-VIT1, indicating that mutation of this motif resulted in protein relocation from the tonoplast to the PM. This result proved that EKQTLL in the N terminus was the tonoplast sorting signal of VIT1. The PM pattern was also consistent with previous reports, indicating that the PM might be a default destination for proteins without specific signals (Wolfenstetter et al., 2012). We also noticed that some punctate signals could be seen, which might represent leakage from this checkpoint, or internalized signals from the PM. In the following study, single mutation of glutamic acid (E) did not cause a dominant effect on the expression pattern, which still showed a clear tonoplast pattern (Figure 3D), while double mutation of the dileucine (LL) resulted in PM localization, thereby efficiently blocking the tonoplast trafficking of VIT1 (Figure 3E). These results together demonstrate that the dileucine motif at the N-terminal is a tonoplast sorting signal for VIT1.
Figure 3.
The N Terminus of Arabidopsis VIT1 Contains a Tonoplast Sorting Signal.
(A) Scheme of VIT1 N terminus mutation constructs and their subcellular localization in Arabidopsis protoplasts.
(B) to (E) Confocal images showing the subcellular localizations of VIT1 N terminus point mutation constructs. The right column shows the scatterplot images indicating the extent of colocalization with the linear Pearson correlation coefficient (rp) and the nonlinear Spearman correlation coefficient (rs). Bars = 10 μm.
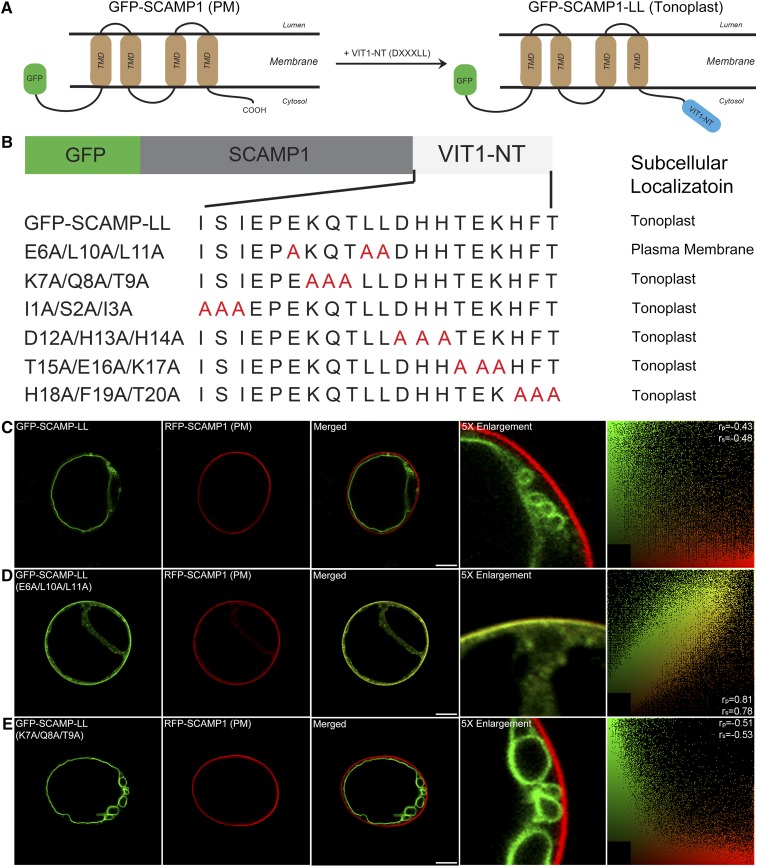
The N Terminus of VIT1 Can Reroute PM Proteins to the Tonoplast
To test whether the dileucine motif of VIT1 functions similarly for other membrane proteins, we generated a fusion construct by adding the N terminus of VIT1 to one terminus of another membrane protein, GFP-SCAMP1 (Figures 4A and 4B), which also has four transmembrane domains with both its N terminus and C terminus facing to the cytosol (Lam et al., 2007; Cai et al., 2011). The fusion protein GFP-SCAMP1-LL localized to the tonoplast (Figure 4C), clearly different from its original PM localization, indicating that the VIT1 N terminus acted as a functional tonoplast sorting signal in the unrelated protein SCAMP1 and changed its trafficking route. Since a total 20 amino acids were added to the fusion construct, to rule out the possible effect of residues other than the dileucine motif, triple point mutants based on the fusion construct were produced and expressed in Arabidopsis protoplasts (Figure 4B). When the three conserved amino acids were mutated to alanine (E6A/L10A/L11A), the N terminus of VIT1 lost its ability to relocate the GFP-SCAMP1 protein, which returned to the PM (Figure 4D). By contrast, all the mutations on the other amino acids had no effect on the location of the fusion protein (Figure 4E; Supplemental Figure 2). Taken together, our results show that addition of the dileucine motif resulted in the relocation of SCAMP1 from the PM to the tonoplast, confirming its function as tonoplast sorting signal.
Figure 4.
Dileucine Motif of Arabidopsis VIT1 Targets SCAMP1 to the Tonoplast.
(A) Topology of GFP-SCAMP1 and fusion protein with N terminus of VIT1.
(B) Scheme of SCAMP1 fusion constructs with wild-type or mutated N terminus of VIT1 and their subcellular localization in Arabidopsis protoplasts.
(C) to (E) Confocal images showing the subcellular localizations of three selective fusion constructs in Arabidopsis protoplasts. The right column shows the scatterplot images indicating the extent of colocalization with the linear Pearson correlation coefficient (rp) and the nonlinear Spearman correlation coefficient (rs). Bars = 10 μm.
The Dileucine Motif Interacts with the AP1 Complex
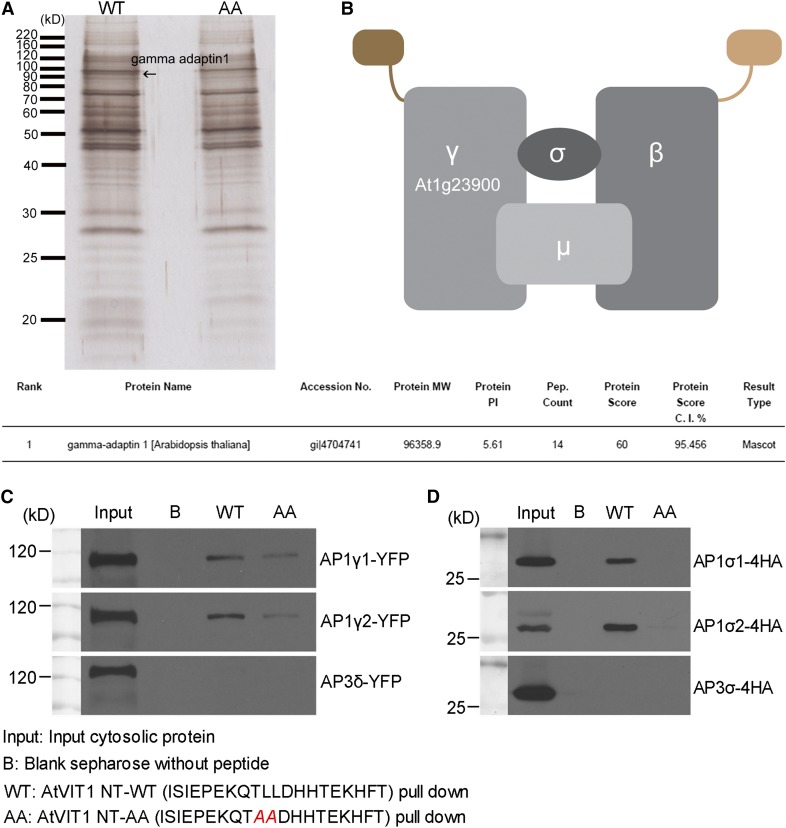
In mammalian and yeast cells, the dileucine motif is recognized by multiple AP complexes, including AP1, AP2, and AP3 (Bonifacino and Traub, 2003). However, in plants, such a recognition mechanism is still obscure. Recently, studies with other tonoplast proteins showed that the AP3 complex was not involved in this process (Wolfenstetter et al., 2012). In order to better understand the molecular mechanism of dileucine motif-mediated tonoplast sorting, we used the synthetic peptides as bait to pull down potential interacting molecules (Gao et al., 2012). The peptides used in this study were exactly the same with the sequence that could reroute SCAMP1 from the PM to the tonoplast, while the dileucine amino acids were mutated to alanine as a control. Sepharose beads conjugated with peptides were incubated with total proteins extracted from wild-type Arabidopsis PSBD suspension cell cultures. Eluted proteins were further subjected to SDS-PAGE and silver staining. In duplicate experiments, one band was clearly labeled around 100 kD only in the lane with the wild-type VIT1 N terminus (Figure 5A, lane WT), but not in the lane with the mutated peptide (lane AA). Using tandem mass spectrometry analysis, the protein was identified as gamma adaptin 1, which is one of the large subunits of the AP1 complex (Figure 5B).
Figure 5.
The N Terminus of Arabidopsis VIT1 Interacts with the AP1 Complex via a Dileucine Motif.
(A) Synthetic peptides were conjugated to CnBr-activated Sepharose and incubated with proteins extracted from Arabidopsis PSBD suspension cells. Eluted proteins were subjected to silver staining and identified by tandem mass spectrometry analysis. Results are listed in the table, showing that the interacting protein was AP-1 gamma adaptin. Arrow indicates the identified protein band.
(B) Schematic drawing of the AP-1 complex.
(C) and (D) Components of AP-1 complex with YFP/HA tag were expressed in Arabidopsis protoplasts. Proteins were extracted and incubated with peptides conjugated to Sepharose. Eluted proteins were subjected to immunoblot analysis using GFP/HA antibody. Peptide with intact dileucine motif showed obvious higher affinity than the mutated one. Blank Sepharose was used as control.
[See online article for color version of this figure.]
To confirm the relation between AP1 gamma adaptin and the dileucine motif, we further performed the in vitro peptide binding experiment as described previously (Contreras et al., 2004; Gao et al., 2012). For the two homologs identified as AP1 gamma adaptins (AP1γ1 and AP1γ2) (Bassham et al., 2008; Teh et al., 2013), we prepared the yellow fluorescent protein (YFP)-tagged constructs and transiently expressed them in Arabidopsis protoplasts. Proteins were extracted and incubated with peptides conjugated to Sepharose. Isolated proteins were examined by immunoblot analysis using a GFP antibody. As shown in Figure 5C, AP1γ1 with the YFP tag could be efficiently pulled out by the peptide with the dileucine motif (WT), whereas the binding ability was significantly reduced by the mutations from leucine to alanine, verifying the importance of the dileucine motif in the interaction with AP1 gamma adaptin. Another AP1γ2 showed similar binding ability with the peptides, indicating that the two homologs of AP1 gamma adaptin might function redundantly in the sorting of VIT1. As a control, we also included the equivalent delta subunit of AP3 adaptor complex, AP3δ. Interestingly, AP3δ showed no binding ability with the peptides, indicating the specificity of the interaction between AP-1 complex and the dileucine motif of VIT1. AP complexes consist of four different subunits. To make it clear that the gamma adaptin represented the functional subunit of the AP1 complex, we performed similar experiments with the sigma adaptin of both AP1 and AP3 (AP1σ1, AP1σ2, and AP3σ) (Bassham et al., 2008). Essentially, as shown in Figure 5D, both AP1σ1 and AP1σ2 could be detected in the eluted sample, while AP3σ was not, and the binding was blocked by the mutation of dileucine. These results further demonstrated that the AP-1 complex had the ability to interact with dileucine motif of VIT1.
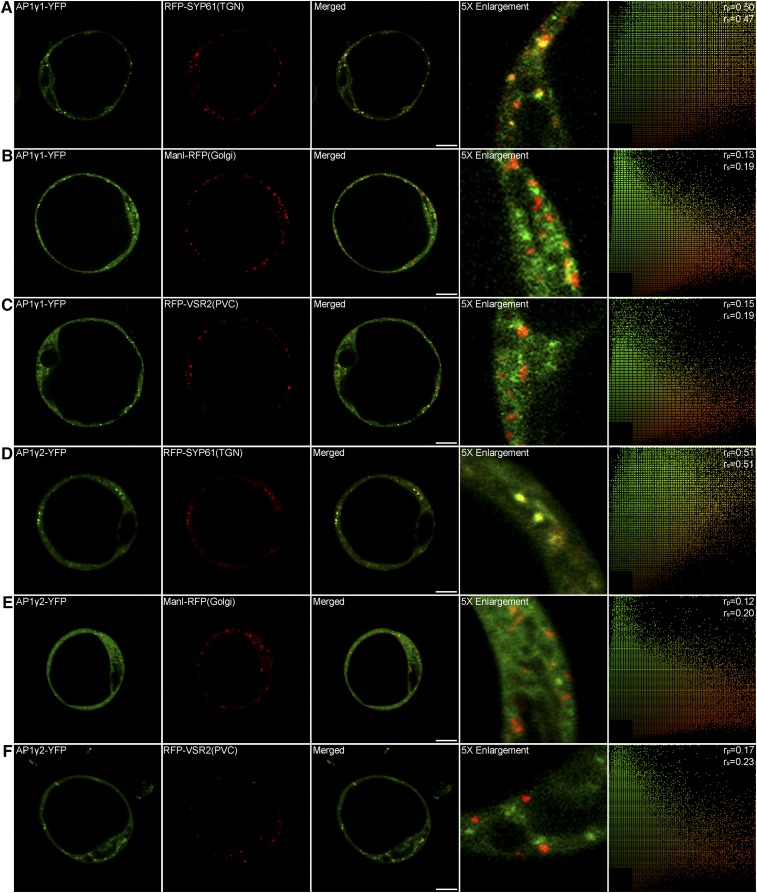
AP-1 Gamma Adaptins Localize to the TGN
To learn more about the AP1 gamma adaptins, we expressed the YFP-tagged fusion proteins together with known organelle markers in Arabidopsis protoplasts. As shown in Figure 6, both YFP-tagged AP1γ1 and AP1γ2 showed cytosolic and punctate fluorescent signals. These punctae partially colocalized with the TGN marker RFP-SYP61 (Figures 6A and 6D) but were clearly separate from the Golgi marker ManI-RFP (Figures 6B and 6E) and the PVC marker RFP-VSR2 (Figures 6C and 6F). This result showed that AP1 gamma adaptins preferentially localized to the TGN in Arabidopsis protoplasts, consistent with previous reports about the AP1 mu subunit (AP1μ) as a TGN-localized adaptin in plant (Park et al., 2013; Teh et al., 2013).
Figure 6.
Subcellular Localization of AP1 Gamma Adaptin.
AP1γ1-YFP ([A] to [C]) and AP1γ2-YFP ([D] to [F]) partially colocalized with the TGN marker RFP-SYP61, while they separated from the Golgi marker ManI-RFP and the PVC marker RFP-VSR2. The right column shows the scatterplot images indicating the extent of colocalization with the linear Pearson correlation coefficient (rp) and the nonlinear Spearman correlation coefficient (rs). Bars = 10 μm.
Tonoplast Targeting of Dileucine Motif-Containing Proteins Is Impaired in AP1γ Deficient Cells
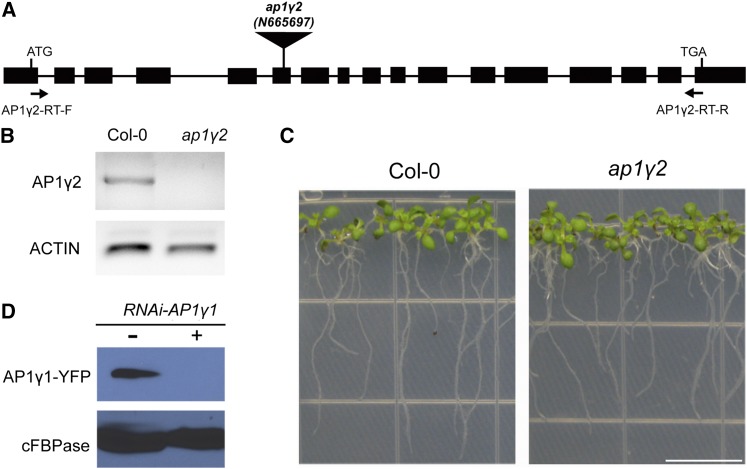
The biochemical interaction between the AP1 complex and the dileucine motif of VIT1 gave rise to the hypothesis that the AP1 complex participates in the tonoplast sorting of VIT1. To test this hypothesis, we ordered T-DNA insertion mutants of AP1 gamma adaptins. As shown in Figures 7A to 7C, a single mutant of AP1γ2 showed a normal growth phenotype, indicating that two homologs of AP1 gamma adaptin might function redundantly. Indeed, we failed to obtain a double knockout mutant of AP1γ1 and AP1γ2 (data not shown). Several groups have reported that AP1μ2 is essential for the generation of the haploid gametophyte (Park et al., 2013; Teh et al., 2013). Thus, it is possible that the case is similar for the double mutant of AP1 gamma adaptins. Alternatively, we used the hairpin RNA interference (RNAi) method to generate a loss-of-function effect. An RNAi construct was prepared against AP1γ1 under the control of a 35S promoter and tested transiently in Arabidopsis protoplasts (Figure 7D). It was further delivered to the homozygous mutant plant of AP1γ2 with a transient expression system using particle bombardment (Wang et al., 2013a). The GFP-VIT1 signal was clearly separated from the PM marker RFP-SCAMP1 and showed a nice tonoplast pattern in Arabidopsis root cells that were successfully targeted by the gold particles (Figure 8A). However, when the RNAi construct was added, the signal of GFP-VIT1 was partially redirected to the PM, showing colocalization with RFP-SCAMP1, indicating that deficiency of AP1 gamma adaptins partially blocked the tonoplast sorting of VIT1 and resulted in relocation of VIT1 to the PM (Figures 8B and 8C). The diagrams in the right column show green and red fluorescence densities along the white arrow as indicated on the merged channel, with the overlapping of the peaks indicating colocalization (Figure 8). As a control, we also compared the localization of GFP-VIT1 in AP3-defective cell. The localization of GFP-VIT1 was not affected in the isolated mesophyll cell of the ap3δ mutant compared with the wild type, further showing that AP3 is not involved in dileucine containing tonoplast protein sorting (Supplemental Figure 3).
Figure 7.
T-DNA Insertion Mutant of AP1γ2 Displays Normal Growth.
(A) Diagram of the T-DNA insertion and primers used in RT-PCR.
(B) RT-PCR characterization of ap1γ2. The transcript of AP1γ2 was not detected in the mutant. ACTIN was used as loading control.
(C) ap1γ2 single mutant showed normal growth compared with wild-type Arabidopsis, ecotype Columbia-0 (Col-0). Bar = 1 cm.
(D) Characterization of hairpin RNAi construct against AP1γ1 in Arabidopsis protoplasts. cFBPase was used as loading control.
[See online article for color version of this figure.]
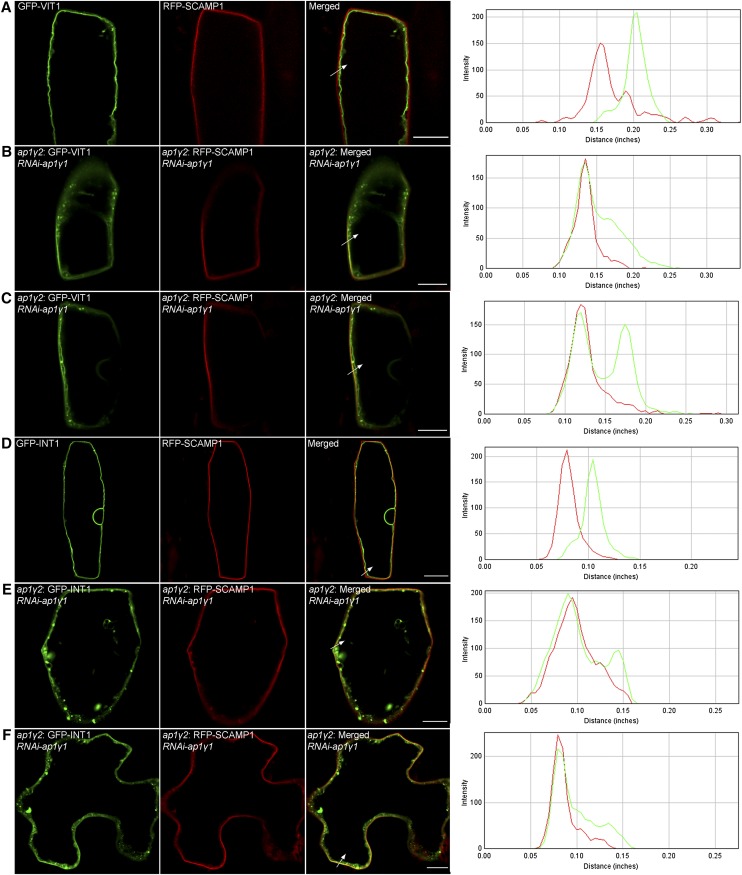
Figure 8.
Dileucine Mediated Tonoplast Trafficking Is Affected When Both AP1 Gamma Adaptins Were Knocked Down.
Tonoplast proteins containing dileucine motif were coexpressed with PM marker RFP-SCAMP1 through particle bombardment. GFP-VIT1 (A) and GFP-INT1 (D) showed tonoplast pattern which separated from RFP-SCAMP1 in wild-type Arabidopsis plants. When RNAi construct against AP1γ1 was coexpressed, GFP-VIT1 ([B] and [C]) and GFP-INT1 ([E] and [F]) were partially targeted to PM showing colocalization with RFP-SCAMP1. The fluorescence intensity profiles along the direction of white arrows were obtained using ImageJ. Bars = 10 μm.
INT1 was reported to be a tonoplast transporter with a dileucine motif at its C terminus (Komarova et al., 2012; Larisch et al., 2012; Wolfenstetter et al., 2012). Indeed, GFP-INT1 labeled the tonoplast in the wild-type plant (Figure 8D). However, similar to the case with VIT1, it was partially colocalized with RFP-SCAMP1 when expressed together with the RNAi construct, showing that sorting of GFP-INT1 was also affected in the AP1γ-deficient cells (Figures 8E and 8F). Notably, besides the tonoplast and PM pattern, abnormal punctate and aggregated fluorescent signals were clearly seen in the recorded cells (Figures 8B, 8C, 8E, and 8F), indicating the delayed delivery and accumulation of VIT1 and INT1. Taking all these results together, the AP1 gamma adaptins were essentially involved in the sorting of dileucine motif-containing tonoplast proteins like VIT1 and INT1. In addition, we also noticed that only a part of the expressed cells showed relocation of GFP-VIT1 and GFP-INT1, which might be due to variation in the expression level and resulting knockdown effect of RNAi construct in different cells. Thus, it would be helpful to generate stable materials expressing different tonoplast proteins in the AP1γ-deficient plant for further studies.
DISCUSSION
The N Terminus of VIT1 Contains Both a Putative ER Export Signal and a Tonoplast Sorting Signal
The targeting mechanism of integral membrane proteins has been studied extensively in plant cells. For the type I integral membrane protein binding protein 80 kD (BP-80), its TMD and C terminus are essential and sufficient to target the protein to the PVC (Jiang and Rogers, 1998a). Another study showed that the length of the TMDs played important roles in determining the subcellular localization of single transmembrane proteins, with shorter TMDs resulting in localization to the ER and longer TMDs resulting in localization to the PM (Brandizzi et al., 2002). A recent study demonstrated that VSR exits the ER via bulk flow without any specific export signal (Gershlick et al., 2014). However, it remains elusive if the roles of TMD length in targeting would be applied to multiple spanning membrane proteins. The demonstration of various sorting signals in multiple TMD proteins such as SCAMP1 further indicated the complexity of the targeting mechanism of proteins with multiple TMDs in plant cells (Cai et al., 2011; Jung et al., 2011).
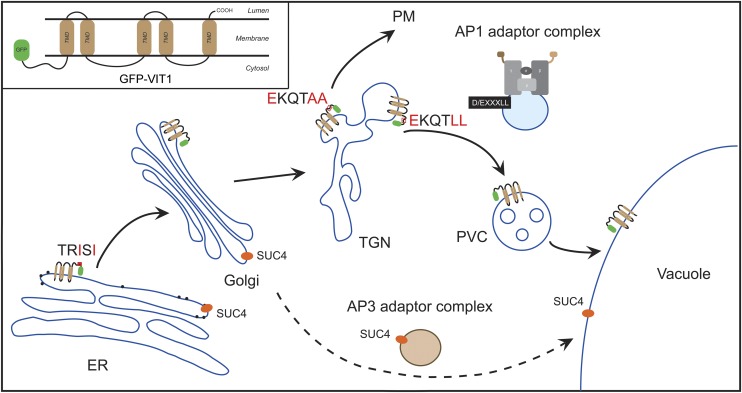
In this study, we used a tonoplast-localized ion transporter VIT1, trafficking along the secretory pathway, to study the sorting mechanism of a multiple membrane-spanning protein (Kim et al., 2006). Our results demonstrated that the N terminus of VIT1 contained a putative ER export signal of dihydrophobic type (ISI), as well as a dileucine motif (EKQTLL) as a tonoplast-sorting signal (Figure 9).
Figure 9.
Working Model of VIT1 Trafficking in Arabidopsis.
VIT1 reaches the tonoplast through the ER, Golgi, and TGN. N terminus of VIT1 contains a dihydrophobic ER export motif (IXI) and a tonoplast sorting signal D/EXXXLL. The AP1 complex can interact with the dileucine motif and mediate tonoplast trafficking. By contrast, SUC4 was delivered from Golgi to the tonoplast through AP3-mediated vesicles.
[See online article for color version of this figure.]
As the first step of protein quality control, ER export of transmembrane proteins usually depends on the interaction between sorting signals at the cytosolic regions and the coat proteins of COPII vesicle (Contreras et al., 2004). So far, various ER export signals have been identified in yeast, mammalian, and plant cells, such as diacidic motif ([D/E]X[E/D]) and dihydrophobic motif (FF, FY) (Otte and Barlowe, 2002; Hanton et al., 2005). In this study, truncation of the N terminus of Arabidopsis VIT1 resulted in the ER retention, indicating the existence of a putative ER export signal in this domain. The amino acid sequence revealed several diacid motifs at the N terminus (MSSEEDKITRISIEPE), which is most widely found in plant transporters. However, further mutagenesis analysis showed that it was not these two putative diacidic motifs, but the fragment between these two motifs (KITRISI) that had an effect on VIT1 localization, indicating that the protein background provides important information for valid sorting signals. Subsequent studies identified the dihydrophobic tripeptide ISI motif as the putative ER export signal. In addition, several studies have reported the involvement of COPII coat protein Sec24 in the process of ER export, which was not included in our study (Gao et al., 2012). In future experiments, it would be interesting to see whether this ISI motif from VIT1 can interact with Sec24.
In eukaryotic cells, sorting signals are also needed for tonoplast targeting, among which a dileucine motif and a tyrosine-based motif have been studied extensively (Bonifacino and Traub, 2003). Compared with mammalian and yeast systems, the dileucine motif has only recently been identified at the N terminus or C terminus of several plant proteins (Pedrazzini et al., 2013; Rojas-Pierce, 2013). Our study has demonstrated the function of a dileucine motif (EKQTLL) in the trafficking of VIT1, as well as in the fusion construct with the PM protein SCAMP1. In the conserved [D/E]XXXL[L/I] motif, the leucine residues are usually indispensible, while the acidic residue can be replaced by other amino acids in some proteins and the position also varies from −4 from the first leucine to −6 (Gasber et al., 2011; Pedrazzini et al., 2013). In our case, EKQTLL is a strictly defined dileucine motif, but the glutamic acid could be replaced by alanine without abrogating the activity of the motif, proving that it is less important in comparison to the leucine pair (Figure 3C). This dileucine motif showed a specific interaction with the AP1 but not AP3 complex. However, in mammalian cells, both AP1 and AP3 complexes can bind to the dileucine motif, but from distinct sets of cargoes (Bonifacino and Traub, 2003). The preferential binding specificity might be affected by the X residues and other factors as reported (Bonifacino and Traub, 2003). Thus, we cannot exclude the possibility that the dileucine motif of the other proteins might interact with other AP complexes. It would be of great interest to systematically document more tonoplast proteins with dileucine motifs to verify their interaction specificity with distinct AP complexes.
Mutation of Tonoplast Sorting Signal Results in PM Localization
The delivery of newly synthesized proteins diverges at the TGN, followed by further transport to the PM or to the PVC and vacuole (Viotti et al., 2010). The TGN has been shown to be a cluster of tubular and vesicular structures, composed of different functional subdomains with attachment of secretory vesicles and clathrin coated vesicles, but how the cargoes are sorted into different vesicles or domains remains largely unknown (Kang et al., 2011).
In our study, VIT1 was sorted to the tonoplast via an interaction with the AP1 complex. Interestingly, mutation from LL to AA not only blocked the tonoplast trafficking of VIT1, but also diverted the mutated protein to the PM. This result is consistent with previous reports on INT1 and ESL1, providing further evidence that the PM is a default destination for those proteins without specific sorting signals (Yamada et al., 2010; Wolfenstetter et al., 2012). On the other hand, our current studies cannot exclude the possible roles of conformational and structural information. Further exploration will be required to confirm whether the interaction between the dileucine motif and AP1 complex might induce a conformation change in tonoplast proteins, thereby masking the PM targeting information. Consequently, mutation of the dileucine signal will block the interaction further exposing the PM targeting signal to the surface. Actually, a study with the PM protein H+-ATPase in tobacco (Nicotiana tabacum) indicated that one large loop between TMDs is essential for its PM localization, similar to the case of the PM proteins PTR1 and PTR5, whereas the first TMD of SCAMP1 has been shown to be essential for its TGN-PM targeting (Lefebvre et al., 2004; Cai et al., 2011; Komarova et al., 2012). These studies then question whether the default pathway exists for all proteins.
Multiple Routes and Differential Regulation for Tonoplast Protein Targeting, Including AP1- and AP3-Mediated Pathways
The AP3 complex in Arabidopsis targets SUC4 protein directly from Golgi to the tonoplast; SUC4 contains no conserved sorting signals, and its targeting appears to be unrelated to that of another protein INT1 that contains a dileucine motif suggesting a yet unknown machinery (Wolfenstetter et al., 2012). In addition, the tonoplast SNARE protein VAMP713 is mislocalized in an ap3δ mutant (Ebine et al., 2014). A recent study of mu subunits of the AP1 complex in Arabidopsis showed that the trafficking of vacuolar and secretory proteins was affected in an ap1m2 mutant (Park et al., 2013). However, the detailed mechanism of how the AP1 complex selects specific cargoes remains to be elucidated. Our study advances the understanding of tonoplast targeting in plants by providing evidence for an interaction between the dileucine motif and the AP1 complex. In cells with AP1 gamma adaptin deficiency, tonoplast proteins containing the dileucine motif relocated to the PM, further indicating the essential role of the AP1 complex in sorting. Since the AP1 complex functions in multiple pathways and severe defects were observed in ap1 mutant, we cannot exclude the possibility that the observed mistargeting of tonoplast proteins could be due to a secondary effect. It would thus be interesting in future study to directly disrupt the dileucine recognition of AP1 via mutagenesis analysis and test the subsequent effects in transgenic plants.
As shown in Figure 9, both AP1-mediated tonoplast targeting of dileucine motif-containing proteins and AP3-mediated tonoplast targeting of SUC4 pass through the Golgi apparatus. An additional pathway might exist for storage proteins like TPKb, SRC2, and αTIP directly from the ER, bypassing the Golgi (Oufattole et al., 2005). These studies together prove that multiple pathways exist to mediate the sorting of specific cargoes to the vacuole.
Multiple steps of vesicular trafficking increase the complexity of tonoplast targeting, including cargo sorting at the donor membrane as mentioned above, as well as the docking and fusion step at the targeting membrane, which is usually mediated by the Rab family of small GTPases. Recently, studies with Rab5 and Rab7 have revealed that these two molecules and related machinery are involved in different vacuolar trafficking pathways and are responsible for distinct set of cargoes, such as the tonoplast protein SYP22, which is affected by the dysfunction of Rab5 but not Rab7 (Ebine et al., 2014). Another study in tobacco leaf epidermis cells also revealed multiple pathways for membrane proteins involving different Rab GTPases, showing, by contrast, that Rab7 interferes with the trafficking of SYP22 (Bottanelli et al., 2011). The reason for such a difference is still not clear, further indicating the complication of the vacuolar trafficking mechanism in plants. To get a better picture of this process, more studies need to be done considering different pathways and distinct regulation at multiple levels like cargo sorting, vesicle scission, vesicle docking, and vesicle fusion.
METHODS
Plasmid Construction
For GFP/RFP/YFP/HA fusion constructs, full-length cDNAs encoding Arabidopsis thaliana VIT1 and subunits of AP1 (AP1γ1, AP1γ2, AP1σ1, and AP1σ2) and AP3 (AP3δ and AP3σ) complexes were amplified and cloned into pBI221 backbone containing an 35S promoter and the nopaline synthase terminator. All the truncation mutants of VIT1 were amplified from GFP-VIT1 and cloned into the pBI221 vector. All the point mutants of VIT1 were generated using the QuickChange Lightning kit (Agilent Technologies). For the construct of SCAMP1 fused with dileucine motif, reverse primers of SCAMP1 were designed to include the N terminus of VIT1, followed by the normal cloning procedure. All the triple point mutants were further generated from GFP-SCAMP1-LL. For the RNAi construct against AP1γ1 driven by 35S promoter, a 600-bp fragment of AP1γ1 designed using MatchPoint was amplified as two fragments and inserted into the hairpin RNAi vector pHANNIBAL (Helliwell and Waterhouse, 2005). All the constructs were further confirmed by restriction mapping and DNA sequencing. All the primers used in this study are listed in Supplemental Table 1.
Transient Expression in Protoplasts and Confocal Imaging
Transient expression in Arabidopsis PSBD cultures was performed essentially as described previously (Miao and Jiang, 2007). Confocal fluorescence images were taken after 12 to 14 h using an Olympus FV1000 system. For the treatment experiment, 2 mM ConcA (Sigma-Aldrich) in DMSO was applied to the protoplasts for 2 h after overnight incubation (Wang et al., 2010). Images were further processed using Adobe Photoshop software as previously described (Jiang and Rogers, 1998b). Fluorescence density profiles were obtained using the ImageJ program. The colocalization of two fluorescent signals was quantified using the PSC colocalization plug-in in the ImageJ program. Results are presented by scatterplot images. The values of the linear Pearson correlation coefficient (rp) and nonlinear Spearman correlation coefficient (rs) range from +1 for complete colocalization to −1 for negative correlation (French et al., 2008). More than 10 independent images were used for each colocalization study.
In Vitro Peptide Binding Assay
In vitro pull-down analysis and peptide binding assays were performed as described previously (Contreras et al., 2004; Gao et al., 2012). Synthetic peptides were ordered from GeneScript with the sequences corresponding to the N terminus of VIT1. They were conjugated with cyanogen bromide-activated Sepharose 4B according to the standard procedure. The proteins were extracted from the wild-type protoplasts or the protoplasts transfected with YFP/HA-tagged fusion proteins of the AP complexes. The protoplasts were lysed with 2× lysis buffer (50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 0.4% Nonidet P-40, and Complete Protease Inhibitor Cocktail [Roche]) and were then incubated with Sepharose beads coupled with the peptides for 6 h at 4°C in a top to end rotator. After incubation, the beads were washed five times with 1× lysis buffer and then eluted by boiling in SDS sample buffer. Proteins were separated by SDS-PAGE, stained by silver staining, and finally subjected to liquid chromatography-tandem mass spectrometry analysis or immunoblot analysis using appropriate antibodies.
Plant Material and Growth Conditions
T-DNA insertion lines of Arabidopsis were obtained from The European Arabidopsis Stock Centre (N665697 for AP1γ2 and N569881 for AP3δ). The genotype of these lines was confirmed by PCR amplification using the gene-specific primers and the T-DNA-specific primer. Sequences of the primers are listed in Supplemental Table 1. Seeds were surface sterilized and sown on plates containing Murashige and Skoog salts with 0.8% agar. The plates were kept at 4°C for 2 d before being moved to the growth chamber. The plates were incubated at 22°C under a long-day (16 h light/8 h dark) photoperiod. Plants were transferred to soil after 2 weeks. Five-day-old plants were transferred to new plates and used for bombardment experiments.
RT-PCR
Total RNA was isolated from 5-d-old seedlings using RNA extraction kit (Qiagen). The first strand of cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen). RT-PCR was then performed using this template, GoTaq polymerase (Bio-Rad), and the reverse primers in combination with forward primers (Supplemental Table 1). The ACTIN gene was used as a RT-PCR internal control.
Antibodies
GFP antibodies were generated using recombinant GFP purchased from Roche Applied Science as antigen to inject rabbits at the Chinese University of Hong Kong. Antibodies were affinity-purified using CnBr-activated Sepharose (Sigma-Aldrich) conjugated with recombinant GFP. For immunoblot analysis, GFP antibodies were used at a concentration of 4 µg/mL. HA antibody was purchased from Abcam (catalog number ab18181). cFBPase antibody was purchased from Agrisera (catalog number AS04043)
Protein Extraction and Immunoblot Analysis
To prepare proteins from protoplasts, transformed protoplasts were diluted 3-fold with 250 mM NaCl. After that, protoplasts were harvested by centrifugation at 100g for 5 min and resuspended in lysis buffer containing 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% SDS, and Complete Protease Inhibitor Cocktail (Roche). The total extracts were centrifuged at 20,000g for 20 min at 4°C. The supernatant were collected and further subjected to SDS-PAGE and immunoblot analysis.
Particle Bombardment of Arabidopsis Seedlings
Five-day-old Arabidopsis seedlings were carefully transferred to a new Murashige and Skoog plate in an ordered arrangement. Gold particles coated with plasmid DNA were prepared and bombarded into the plants as described previously (Wang and Jiang, 2011; Wang et al., 2013a). Plants were kept in dark in the plant growth chamber for 12 to 24 h before observation for fluorescent signals.
Transient Expression in Arabidopsis Mesophyll Protoplasts
Arabidopsis mesophyll protoplasts were isolated and transformed using polyethylene glycol as described previously (Wolfenstetter et al., 2012). Transformed protoplasts were incubated for 12 to 14 h before confocal imaging.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: Arabidopsis VIT1 (At2g01770), INT1 (At2g43330), AP1γ1 (At1g60070), AP1γ2 (At1g23900), AP3δ (At1g48760), AP1σ1 (At2g17380), AP1σ2 (At4g35410), AP3σ (At3g50860), and SCAMP1 (Os07g0564600).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The N terminus of VIT1 Is Essential for Its Trafficking.
Supplemental Figure 2. Subcellular Localization of GFP-SCAMP1 Fused with the Mutated N Terminus of VIT1.
Supplemental Figure 3. Localization of VIT1 Was Not Affected in the ap3δ Mutant.
Supplemental Table 1. Primers Used in This Study.
Supplementary Material
Acknowledgments
This work was supported by grants from the Research Grants Council of Hong Kong (CUHK466011, 465112, 466613, CUHK2/CRF/11G, HKUST10/CRF/12R, HKUST12/CRF/13G, and AoE/M-05/12), from NSFC/RGC (N_CUHK406/12), from NSFC (31270226), and from the Croucher-CAS Joint Lab and Shenzhen Peacock Project (KQTD201101) to L.J.
AUTHOR CONTRIBUTIONS
X.W., Y.C., and L.J. conceived and designed the experiments. X.W., Y.C., H.W., Y.Z., X.Z., and B.L. performed the experiments. X.W. and Y.C. analyzed the data. X.W. and L.J. wrote the article.
Glossary
- ER
endoplasmic reticulum
- TGN
trans-Golgi network
- PVC
prevacuolar compartment
- TMD
transmembrane domain
- ConcA
Concanamycin A
- PM
plasma membrane
- RNAi
RNA interference
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Barlowe C. (2003). Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol. 13: 295–300. [DOI] [PubMed] [Google Scholar]
- Bassham D.C., Brandizzi F., Otegui M.S., Sanderfoot A.A. (2008). The secretory system of Arabidopsis. The Arabidopsis Book 6: e0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X., Corpina R.A., Goldberg J. (2002). Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419: 271–277. [DOI] [PubMed] [Google Scholar]
- Boehm M., Bonifacino J.S. (2001). Adaptins: the final recount. Mol. Biol. Cell 12: 2907–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W., Ohno H., Songyang Z., Rapoport I., Cantley L.C., Bonifacino J.S., Kirchhausen T. (1996). Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15: 5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Dell’Angelica E.C. (1999). Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145: 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Traub L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72: 395–447. [DOI] [PubMed] [Google Scholar]
- Bottanelli F., Foresti O., Hanton S., Denecke J. (2011). Vacuolar transport in tobacco leaf epidermis cells involves a single route for soluble cargo and multiple routes for membrane cargo. Plant Cell 23: 3007–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F., Frangne N., Marc-Martin S., Hawes C., Neuhaus J.M., Paris N. (2002). The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14: 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos P.V., Mardones G.A., Rojas A.L., daSilva L.L., Prabhu Y., Hurley J.H., Bonifacino J.S. (2010). Sorting of the Alzheimer’s disease amyloid precursor protein mediated by the AP-4 complex. Dev. Cell 18: 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Zhuang X., Gao C., Wang X., Jiang L. (2014). The Arabidopsis endosomal sorting complex required for transport III regulates internal vesicle formation of the prevacuolar compartment and is required for plant development. Plant Physiol. 165: 1328–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Jia T., Lam S.K., Ding Y., Gao C., San M.W., Pimpl P., Jiang L. (2011). Multiple cytosolic and transmembrane determinants are required for the trafficking of SCAMP1 via an ER-Golgi-TGN-PM pathway. Plant J. 65: 882–896. [DOI] [PubMed] [Google Scholar]
- Canfield W.M., Johnson K.F., Ye R.D., Gregory W., Kornfeld S. (1991). Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24-29 of the cytoplasmic tail. J. Biol. Chem. 266: 5682–5688. [PubMed] [Google Scholar]
- Chapuy B., Tikkanen R., Mühlhausen C., Wenzel D., von Figura K., Höning S. (2008). AP-1 and AP-3 mediate sorting of melanosomal and lysosomal membrane proteins into distinct post-Golgi trafficking pathways. Traffic 9: 1157–1172. [DOI] [PubMed] [Google Scholar]
- Chen B., Dores M.R., Grimsey N., Canto I., Barker B.L., Trejo J. (2011). Adaptor protein complex-2 (AP-2) and epsin-1 mediate protease-activated receptor-1 internalization via phosphorylation- and ubiquitination-dependent sorting signals. J. Biol. Chem. 286: 40760–40770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I., Yang Y., Robinson D.G., Aniento F. (2004). Sorting signals in the cytosolic tail of plant p24 proteins involved in the interaction with the COPII coat. Plant Cell Physiol. 45: 1779–1786. [DOI] [PubMed] [Google Scholar]
- Cowles C.R., Odorizzi G., Payne G.S., Emr S.D. (1997). The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell 91: 109–118. [DOI] [PubMed] [Google Scholar]
- Cui Y., Zhao Q., Gao C., Ding Y., Zeng Y., Ueda T., Nakano A., Jiang L. (2014). Activation of the Rab7 GTPase by the MON1-CCZ1 complex is essential for PVC-to-vacuole trafficking and plant growth in Arabidopsis. Plant Cell 26: 2080–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica E.C. (2009). AP-3-dependent trafficking and disease: the first decade. Curr. Opin. Cell Biol. 21: 552–559. [DOI] [PubMed] [Google Scholar]
- De Marcos Lousa C., Gershlick D.C., Denecke J. (2012). Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors. Plant Cell 24: 1714–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rubbo S., et al. (2013). The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell 25: 2986–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B., Lee I., Knisely J., Bu G., Kornfeld S. (2007). The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell 18: 1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebine K., Inoue T., Ito J., Ito E., Uemura T., Goh T., Abe H., Sato K., Nakano A., Ueda T. (2014). Plant vacuolar trafficking occurs through distinctly regulated pathways. Curr. Biol. 24: 1375–1382. [DOI] [PubMed] [Google Scholar]
- Feraru E., Paciorek T., Feraru M.I., Zwiewka M., De Groodt R., De Rycke R., Kleine-Vehn J., Friml J. (2010). The AP-3 β adaptin mediates the biogenesis and function of lytic vacuoles in Arabidopsis. Plant Cell 22: 2812–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A.P., Mills S., Swarup R., Bennett M.J., Pridmore T.P. (2008). Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat. Protoc. 3: 619–628. [DOI] [PubMed] [Google Scholar]
- Gao C., Yu C.K., Qu S., San M.W., Li K.Y., Lo S.W., Jiang L. (2012). The Golgi-localized Arabidopsis endomembrane protein12 contains both endoplasmic reticulum export and Golgi retention signals at its C terminus. Plant Cell 24: 2086–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasber A., Klaumann S., Trentmann O., Trampczynska A., Clemens S., Schneider S., Sauer N., Feifer I., Bittner F., Mendel R.R., Neuhaus H.E. (2011). Identification of an Arabidopsis solute carrier critical for intracellular transport and inter-organ allocation of molybdate. Plant Biol (Stuttg) 13: 710–718. [DOI] [PubMed] [Google Scholar]
- Gershlick D.C., Lousa Cde.M., Foresti O., Lee A.J., Pereira E.A., daSilva L.L., Bottanelli F., Denecke J. (2014). Golgi-dependent transport of vacuolar sorting receptors is regulated by COPII, AP1, and AP4 protein complexes in tobacco. Plant Cell 26: 1308–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Mattera R., Ren X., Chen Y., Retamal C., González A., Bonifacino J.S. (2013a). The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev. Cell 27: 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Zanetti G., Schekman R. (2013b). A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. eLife 2: e00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton S.L., Renna L., Bortolotti L.E., Chatre L., Stefano G., Brandizzi F. (2005). Diacidic motifs influence the export of transmembrane proteins from the endoplasmic reticulum in plant cells. Plant Cell 17: 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell C.A., Waterhouse P.M. (2005). Constructs and methods for hairpin RNA-mediated gene silencing in plants. Methods Enzymol. 392: 24–35. [DOI] [PubMed] [Google Scholar]
- Hirst J., Barlow L.D., Francisco G.C., Sahlender D.A., Seaman M.N., Dacks J.B., Robinson M.S. (2011). The fifth adaptor protein complex. PLoS Biol. 9: e1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icking A., Amaddii M., Ruonala M., Höning S., Tikkanen R. (2007). Polarized transport of Alzheimer amyloid precursor protein is mediated by adaptor protein complex AP1-1B. Traffic 8: 285–296. [DOI] [PubMed] [Google Scholar]
- Isayenkov S., Isner J.C., Maathuis F.J. (2010). Vacuolar ion channels: Roles in plant nutrition and signalling. FEBS Lett. 584: 1982–1988. [DOI] [PubMed] [Google Scholar]
- Isayenkov S., Isner J.C., Maathuis F.J. (2011). Rice two-pore K+ channels are expressed in different types of vacuoles. Plant Cell 23: 756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.P., Kelly B.T., McCoy A.J., Gaffry T., James L.C., Collins B.M., Höning S., Evans P.R., Owen D.J. (2010). A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141: 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Rogers J.C. (1998a). Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. J. Cell Biol. 143: 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Rogers J.C. (1998b). Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. J. Cell Biol. 143: 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Erickson A., Rogers J. (2002). Multivesicular bodies: a mechanism to package lytic and storage functions in one organelle? Trends Cell Biol. 12: 362–367. [DOI] [PubMed] [Google Scholar]
- Jung C., Lee G.J., Jang M., Lee M., Lee J., Kang H., Sohn E.J., Hwang I. (2011). Identification of sorting motifs of AtβFruct4 for trafficking from the ER to the vacuole through the Golgi and PVC. Traffic 12: 1774–1792. [DOI] [PubMed] [Google Scholar]
- Kametaka S., Sawada N., Bonifacino J.S., Waguri S. (2010). Functional characterization of protein-sorting machineries at the trans-Golgi network in Drosophila melanogaster. J. Cell Sci. 123: 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H., Nielsen E., Preuss M.L., Mastronarde D., Staehelin L.A. (2011). Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329. [DOI] [PubMed] [Google Scholar]
- Kang R.S., Fölsch H. (2011). ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J. Cell Biol. 193: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.A., Punshon T., Lanzirotti A., Li L., Alonso J.M., Ecker J.R., Kaplan J., Guerinot M.L. (2006). Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314: 1295–1298. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Xu Z.Y., Song K., Kim D.H., Kang H., Reichardt I., Sohn E.J., Friml J., Juergens G., Hwang I. (2013). Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell 25: 2970–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova N.Y., Meier S., Meier A., Grotemeyer M.S., Rentsch D. (2012). Determinants for Arabidopsis peptide transporter targeting to the tonoplast or plasma membrane. Traffic 13: 1090–1105. [DOI] [PubMed] [Google Scholar]
- Lam S.K., Siu C.L., Hillmer S., Jang S., An G., Robinson D.G., Jiang L. (2007). Rice SCAMP1 defines clathrin-coated, trans-golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19: 296–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larisch N., Schulze C., Galione A., Dietrich P. (2012). An N-terminal dileucine motif directs two-pore channels to the tonoplast of plant cells. Traffic 13: 1012–1022. [DOI] [PubMed] [Google Scholar]
- Lee G.J., Kim H., Kang H., Jang M., Lee D.W., Lee S., Hwang I. (2007). EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate. Implications for EpsinR2 function in protein trafficking in plant cells. Plant Physiol. 143: 1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B., Batoko H., Duby G., Boutry M. (2004). Targeting of a Nicotiana plumbaginifolia H+ -ATPase to the plasma membrane is not by default and requires cytosolic structural determinants. Plant Cell 16: 1772–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chen O.S., McVey Ward D., Kaplan J. (2001). CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276: 29515–29519. [DOI] [PubMed] [Google Scholar]
- Maîtrejean M., Wudick M.M., Voelker C., Prinsi B., Mueller-Roeber B., Czempinski K., Pedrazzini E., Vitale A. (2011). Assembly and sorting of the tonoplast potassium channel AtTPK1 and its turnover by internalization into the vacuole. Plant Physiol. 156: 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias J.D., Goldberg J. (2008). Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 27: 2918–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Maeshima M., Neuhaus H.E. (2007). Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 58: 83–102. [DOI] [PubMed] [Google Scholar]
- Mattera R., Boehm M., Chaudhuri R., Prabhu Y., Bonifacino J.S. (2011). Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J. Biol. Chem. 286: 2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y., Jiang L. (2007). Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat. Protoc. 2: 2348–2353. [DOI] [PubMed] [Google Scholar]
- Mikosch M., Homann U. (2009). How do ER export motifs work on ion channel trafficking? Curr. Opin. Plant Biol. 12: 685–689. [DOI] [PubMed] [Google Scholar]
- Misra S., Puertollano R., Kato Y., Bonifacino J.S., Hurley J.H. (2002). Structural basis for acidic-cluster-dileucine sorting-signal recognition by VHS domains. Nature 415: 933–937. [DOI] [PubMed] [Google Scholar]
- Nakatsu F., Ohno H. (2003). Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct. Funct. 28: 419–429. [DOI] [PubMed] [Google Scholar]
- Niihama M., Takemoto N., Hashiguchi Y., Tasaka M., Morita M.T. (2009). ZIP genes encode proteins involved in membrane trafficking of the TGN-PVC/vacuoles. Plant Cell Physiol. 50: 2057–2068. [DOI] [PubMed] [Google Scholar]
- Otte S., Barlowe C. (2002). The Erv41p-Erv46p complex: multiple export signals are required in trans for COPII-dependent transport from the ER. EMBO J. 21: 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oufattole M., Park J.H., Poxleitner M., Jiang L., Rogers J.C. (2005). Selective membrane protein internalization accompanies movement from the endoplasmic reticulum to the protein storage vacuole pathway in Arabidopsis. Plant Cell 17: 3066–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Song K., Reichardt I., Kim H., Mayer U., Stierhof Y.D., Hwang I., Jürgens G. (2013). Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc. Natl. Acad. Sci. USA 110: 10318–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini E., Komarova N.Y., Rentsch D., Vitale A. (2013). Traffic routes and signals for the tonoplast. Traffic 14: 622–628. [DOI] [PubMed] [Google Scholar]
- Robinson D.G., Pimpl P. (2014). Clathrin and post-Golgi trafficking: a very complicated issue. Trends Plant Sci. 19: 134–139. [DOI] [PubMed] [Google Scholar]
- Robinson D.G., Jiang L., Schumacher K. (2008). The endosomal system of plants: charting new and familiar territories. Plant Physiol. 147: 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Scheuring D., Naramoto S., Friml J. (2011). ARF1 localizes to the golgi and the trans-golgi network. Plant Cell 23: 846–849, author reply 849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S. (2004). Adaptable adaptors for coated vesicles. Trends Cell Biol. 14: 167–174. [DOI] [PubMed] [Google Scholar]
- Robinson M.S., Sahlender D.A., Foster S.D. (2010). Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev. Cell 18: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Pierce M. (2013). Targeting of tonoplast proteins to the vacuole. Plant Sci. 211: 132–136. [DOI] [PubMed] [Google Scholar]
- Schoberer J., Vavra U., Stadlmann J., Hawes C., Mach L., Steinkellner H., Strasser R. (2009). Arginine/lysine residues in the cytoplasmic tail promote ER export of plant glycosylation enzymes. Traffic 10: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Suen P.K., Wang X., Lin Y., Lo S.W., Rojo E., Jiang L. (2013). An in vivo expression system for the identification of cargo proteins of vacuolar sorting receptors in Arabidopsis culture cells. Plant J. 75: 1003–1017. [DOI] [PubMed] [Google Scholar]
- Shiba T., Takatsu H., Nogi T., Matsugaki N., Kawasaki M., Igarashi N., Suzuki M., Kato R., Earnest T., Nakayama K., Wakatsuki S. (2002). Structural basis for recognition of acidic-cluster dileucine sequence by GGA1. Nature 415: 937–941. [DOI] [PubMed] [Google Scholar]
- Sitaram A., Dennis M.K., Chaudhuri R., De Jesus-Rojas W., Tenza D., Setty S.R., Wood C.S., Sviderskaya E.V., Bennett D.C., Raposo G., Bonifacino J.S., Marks M.S. (2012). Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol. Biol. Cell 23: 3178–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Ueda T., Yahara N., Nakano A. (2002). Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 31: 499–515. [DOI] [PubMed] [Google Scholar]
- Teh O.K., Shimono Y., Shirakawa M., Fukao Y., Tamura K., Shimada T., Hara-Nishimura I. (2013). The AP-1 μ adaptin is required for KNOLLE localization at the cell plate to mediate cytokinesis in Arabidopsis. Plant Cell Physiol. 54: 838–847. [DOI] [PubMed] [Google Scholar]
- Theos A.C., et al. (2005). Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell 16: 5356–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I.S., Collawn J.F., Hopkins C.R. (1993). Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 9: 129–161. [DOI] [PubMed] [Google Scholar]
- Tse Y.C., Mo B., Hillmer S., Zhao M., Lo S.W., Robinson D.G., Jiang L. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C., et al. (2010). Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Jiang L. (2011). Transient expression and analysis of fluorescent reporter proteins in plant pollen tubes. Nat. Protoc. 6: 419–426. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhuang X., Cai Y., Cheung A.Y., Jiang L. (2013a). Apical F-actin-regulated exocytic targeting of NtPPME1 is essential for construction and rigidity of the pollen tube cell wall. Plant J. 76: 367–379. [DOI] [PubMed] [Google Scholar]
- Wang J., Ding Y., Wang J., Hillmer S., Miao Y., Lo S.W., Wang X., Robinson D.G., Jiang L. (2010). EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell 22: 4009–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.G., Li S., Zhao X.Y., Zhou L.Z., Huang G.Q., Feng C., Zhang Y. (2013b). HAPLESS13, the Arabidopsis μ1 adaptin, is essential for protein sorting at the trans-Golgi network/early endosome. Plant Physiol. 162: 1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenstetter S., Wirsching P., Dotzauer D., Schneider S., Sauer N. (2012). Routes to the tonoplast: the sorting of tonoplast transporters in Arabidopsis mesophyll protoplasts. Plant Cell 24: 215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Osakabe Y., Mizoi J., Nakashima K., Fujita Y., Shinozaki K., Yamaguchi-Shinozaki K. (2010). Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. J. Biol. Chem. 285: 1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka S., Shimono Y., Shirakawa M., Fukao Y., Kawase T., Hatsugai N., Tamura K., Shimada T., Hara-Nishimura I. (2013). Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell 25: 2958–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazny E., Miecielica U., Borst J.W., Hemminga M.A., Chaumont F. (2009). An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J. 57: 346–355. [DOI] [PubMed] [Google Scholar]
- Zwiewka M., Feraru E., Möller B., Hwang I., Feraru M.I., Kleine-Vehn J., Weijers D., Friml J. (2011). The AP-3 adaptor complex is required for vacuolar function in Arabidopsis. Cell Res. 21: 1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.