The bZIP transcription factor HY5 plays an important role in the UV-B photoreceptor-regulated signaling pathway. This work shows that UV-B enhances the binding of HY5 to the promoters of UV-B-responsive genes and that HY5, as well as its homolog HYH, interact directly with the cis-regulatory T/G-box of the HY5 promoter to mediate UV-B-induced transcriptional activation of HY5.
Abstract
In plants subjected to UV-B radiation, responses are activated that minimize damage caused by UV-B. The bZIP transcription factor ELONGATED HYPOCOTYL5 (HY5) acts downstream of the UV-B photoreceptor UV RESISTANCE LOCUS8 (UVR8) and promotes UV-B-induced photomorphogenesis and acclimation. Expression of HY5 is induced by UV-B; however, the transcription factor(s) that regulate HY5 transcription in response to UV-B and the impact of UV-B on the association of HY5 with its target promoters are currently unclear. Here, we show that HY5 binding to the promoters of UV-B-responsive genes is enhanced by UV-B in a UVR8-dependent manner in Arabidopsis thaliana. In agreement, overexpression of REPRESSOR OF UV-B PHOTOMORPHOGENESIS2, a negative regulator of UVR8 function, blocks UV-B-responsive HY5 enrichment at target promoters. Moreover, we have identified a T/G-box in the HY5 promoter that is required for its UV-B responsiveness. We show that HY5 and its homolog HYH bind to the T/GHY5-box cis-acting element and that they act redundantly in the induction of HY5 expression upon UV-B exposure. Therefore, HY5 is enriched at target promoters in response to UV-B in a UVR8 photoreceptor-dependent manner, and HY5 and HYH interact directly with a T/G-box cis-acting element of the HY5 promoter, mediating the transcriptional activation of HY5 in response to UV-B.
INTRODUCTION
UV-B radiation (UV-B; 280 to 315 nm) is a critical regulatory signal that induces photomorphogenic responses in plants (Heijde and Ulm, 2012; Li et al., 2013; Jenkins, 2014). These UV-B-induced responses are mediated by the photoreceptor UV RESISTANCE LOCUS8 (UVR8) in Arabidopsis thaliana (Rizzini et al., 2011) and include hypocotyl growth inhibition (Ballare et al., 1995; Kim et al., 1998; Favory et al., 2009), altered leaf morphogenesis (Hectors et al., 2007; Wargent et al., 2009), stomatal closure (Tossi et al., 2014), and the biosynthesis of UV light-absorbing “sunscreen” compounds (Beggs and Wellmann, 1994; Kliebenstein et al., 2002; Stracke et al., 2010). Thus, UVR8 regulates the expression of a broad panel of genes that underlie UV-B-dependent photomorphogenic responses and acclimation (Brown et al., 2005; Favory et al., 2009). The acclimation response helps to prevent or repair UV-B damage, and uvr8 mutants are hypersensitive to chronic levels of UV-B (Kliebenstein et al., 2002; Brown et al., 2005; Favory et al., 2009). Such uvr8 mutants are specifically impaired in UV-B acclimation and not in the response to acute UV-B stress (González Besteiro et al., 2011).
UVR8 exists as a homodimer in plants and rapidly monomerizes in response to UV-B (Rizzini et al., 2011; Christie et al., 2012; Wu et al., 2012). Photoactivated UVR8 then interacts with the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1) (Favory et al., 2009; Rizzini et al., 2011; Cloix et al., 2012; Huang et al., 2014), which is a well-known repressor of photomorphogenesis (Lau and Deng, 2012) and also plays an important role in UV-B signaling (Oravecz et al., 2006). As part of the UVR8 photocycle, regeneration of reactive UVR8 occurs by rapid reversion from the monomer to the dimer (Heijde and Ulm, 2013; Heilmann and Jenkins, 2013). The UVR8-interacting and negative feedback regulators REPRESSOR OF UV-B PHOTOMORPHOGENESIS1 (RUP1) and RUP2 (Gruber et al., 2010) facilitate UVR8 redimerization in planta that consequently disrupts the UVR8-COP1 interaction and halts signaling (Heijde and Ulm, 2013).
An important, largely unresolved issue is how UV-B photoreception by UVR8 leads to transcriptional changes. It has been shown that UVR8 itself binds to chromatin in the vicinity of putative target genes via an interaction with histone H2B (Brown et al., 2005; Cloix and Jenkins, 2008). It was suggested subsequently that UVR8 may mediate the recruitment or activation of transcription factors and/or chromatin remodelers. However, the molecular events and the identity of the components mediating the transcriptional regulation of target genes by UVR8 remained elusive. It is known that the bZIP transcription factor ELONGATED HYPOCOTYL5 (HY5) mediates UV-B-induced gene expression changes downstream of UVR8, in partial redundancy with its homolog HYH (Ulm et al., 2004; Brown et al., 2005; Oravecz et al., 2006; Brown and Jenkins, 2008; Stracke et al., 2010; Fehér et al., 2011; Huang et al., 2012). Indeed, HY5 and HYH are thought to govern the majority of the UVR8-mediated UV-B transcriptional responses (Tilbrook et al., 2013; Jenkins, 2014). HY5 is implicated in a positive feedback loop promoting COP1 expression by binding to a specific UV-B-responsive ACGT-containing element within the COP1 promoter (Huang et al., 2012). HY5 itself, as well as HYH, is one of the UVR8- and COP1-regulated genes (Brown et al., 2005; Oravecz et al., 2006; Favory et al., 2009), but the transcription factor(s) and cis-regulatory element(s) mediating its transcription are not known. HY5 is also known to be posttranslationally stabilized by UV-B (Favory et al., 2009; Huang et al., 2013).
HY5 is a target of the ubiquitin ligase activity of COP1 (Osterlund et al., 2000; Lau and Deng, 2012); thus, its UV-B-sensitive stabilization is likely a consequence of the UVR8-COP1 interaction (Favory et al., 2009). Supporting an important role for HY5 in the UV-B acclimation response, hy5 mutants are UV-B stress hypersensitive (Brown et al., 2005; Oravecz et al., 2006; Huang et al., 2012). HY5 is known to be a critical positive regulator of light responses, and chromatin immunoprecipitation (ChIP) combined with microarray analysis has demonstrated its association with the promoter region of over 9000 potential target genes (Zhang et al., 2011). However, HY5 is abundant mainly in young seedlings and declines during later developmental stages, in agreement with its main activity at early stages of photomorphogenesis in visible light (Hardtke et al., 2000). Thus, UV-B responses in older seedlings and adult plants depend upon the reengagement of HY5 through UV-B-induced HY5 expression and protein stabilization (Ulm et al., 2004; Oravecz et al., 2006). Accordingly, ChIP experiments have shown that HY5-yellow fluorescent protein associates with HY5-dependent UV-B-induced genes (Stracke et al., 2010). However, the dynamics of HY5 chromatin association in response to environmental cues, including exposure to UV-B, have been defined to a much lesser extent (Lee et al., 2007; Stracke et al., 2010; Zhang et al., 2011).
To further our understanding of UV-B-induced gene expression changes, we have investigated the UV-B-responsive HY5 association with chromatin. We report here (1) that HY5 chromatin association is enhanced by UV-B in a UVR8 photoreceptor-dependent manner and (2) that this process is impaired in plants overexpressing RUP2, a negative feedback regulator of UVR8. We also identify two cis-regulatory elements, designated T/G- and E-boxes, which mediate the transcriptional activation of HY5 in response to UV-B. Furthermore, we show that HY5 and HYH bind to the T/GHY5-box and that they are both required for UV-B-activated HY5 gene expression.
RESULTS
HY5 Association with Target Genes Is Regulated by UV-B
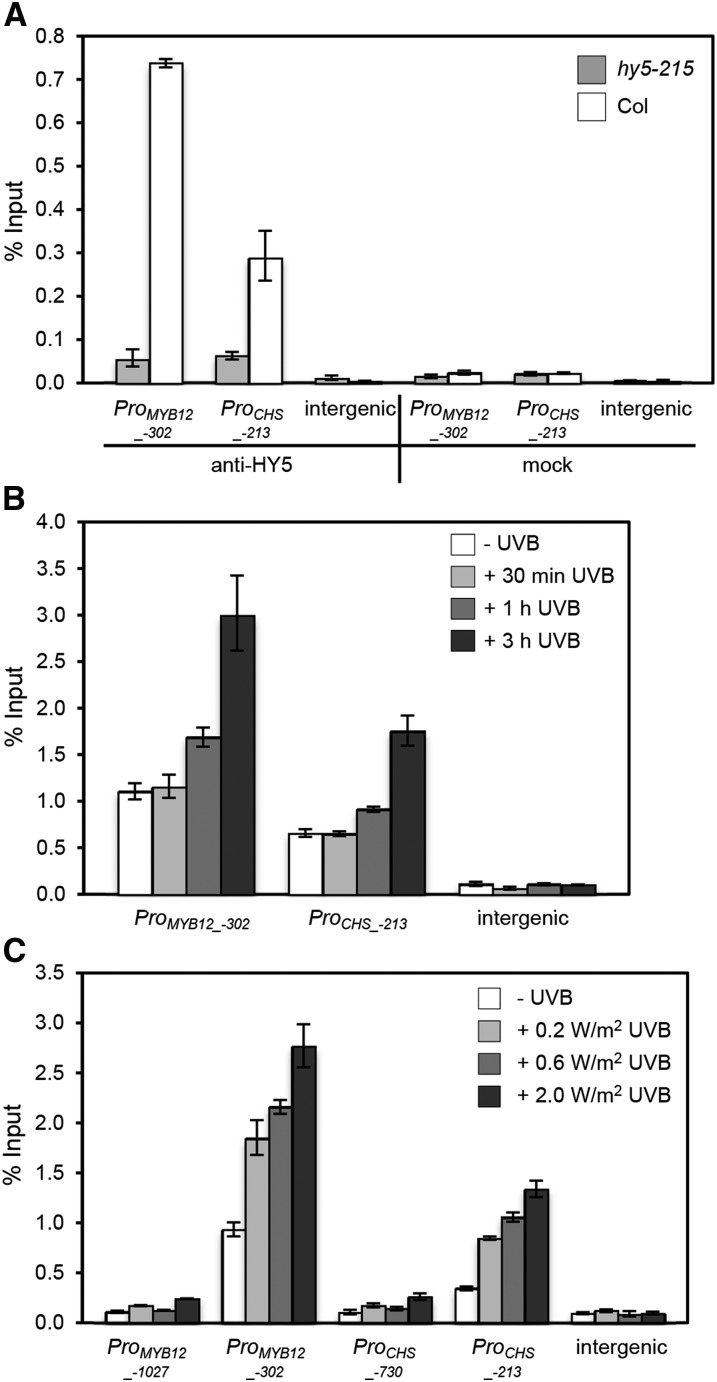
HY5 has been shown previously to associate with the promoters of its UV-B-responsive target genes CHALCONE SYNTHASE (CHS) and MYB12 (Stracke et al., 2010; Zhang et al., 2011); however, the dynamics of this process in response to UV-B are unclear. In order to test whether UV-B specifically affects the association of endogenous HY5 with its target genes, we performed ChIP experiments using anti-HY5 antibodies. First, we analyzed the chromatin immunoprecipitated DNA for specific enrichment of previously established HY5 target genes, such as CHS and MYB12. Indeed, endogenous HY5 specifically associated with CHS and MYB12 promoter fragments in wild-type seedlings (Figure 1A). The specificity of the ChIP data was demonstrated by the negative controls provided by the hy5 mutant and a mock immunoprecipitation without primary antibody as well as by an intergenic region not bound by HY5 (Figure 1A). Together, the data confirm that HY5 ChIP is specific for DNA immunoprecipitated with endogenous HY5 protein and that HY5 associates specifically with its target genes.
Figure 1.
UV-B Enhances HY5 Binding to the Promoters of Its Target Genes MYB12 and CHS.
(A) ChIP of DNA associated with HY5. ChIP-qPCR was performed for the MYB12 and CHS promoters and an intergenic region between the At4g26900 and At4g26910 genes using 10-d-old wild-type plants (Col) or hy5-215 null mutants grown in white light (no UV-B). ChIP was performed with an anti-HY5 antibody or without addition of the antibody (mock). Data shown are representative of five independent experiments.
(B) HY5 ChIP-qPCR using 7-d-old wild-type seedlings grown in weak light and treated with narrowband UV-B for 0.5, 1, and 3 h compared with a −UV-B control.
(C) HY5 ChIP-qPCR using 7-d-old wild-type seedlings grown in weak light and treated for 2 h with different intensities of narrowband UV-B compared with an untreated control (−UV-B).
The numbers of the analyzed DNA fragments indicate the positions of the 5′ base pair of the amplicon relative to the translation start site (referred to as position +1). ChIP efficiency of DNA associated with HY5 is presented as the percentage recovered from the total input DNA (% Input). Error bars represent sd of three technical replicates.
We further tested whether UV-B affects the association of HY5 with target promoters. Our data show that HY5 binding to CHS and MYB12 target promoters increased in response to UV-B, detectable as early as 1 h after UV-B exposure (Figure 1B), and that its accumulation at target promoters was dose dependent (Figure 1C). As expected, HY5 did not associate with the intergenic region under any condition tested (Figures 1A to 1C). The specificity of binding was further supported by the absence of a signal for regions ∼0.5 kb upstream (ProMYB12−1027 and ProCHS−730) of the apparent binding site regions (ProMYB12−302 and ProCHS−213) (Figure 1C). Altogether, we conclude that increased promoter occupancy of HY5 at its target genes is an integral part of the UV-B response.
UV-B-Mediated Enhancement of HY5 Chromatin Association Requires UVR8 and Is Negatively Regulated by RUP2
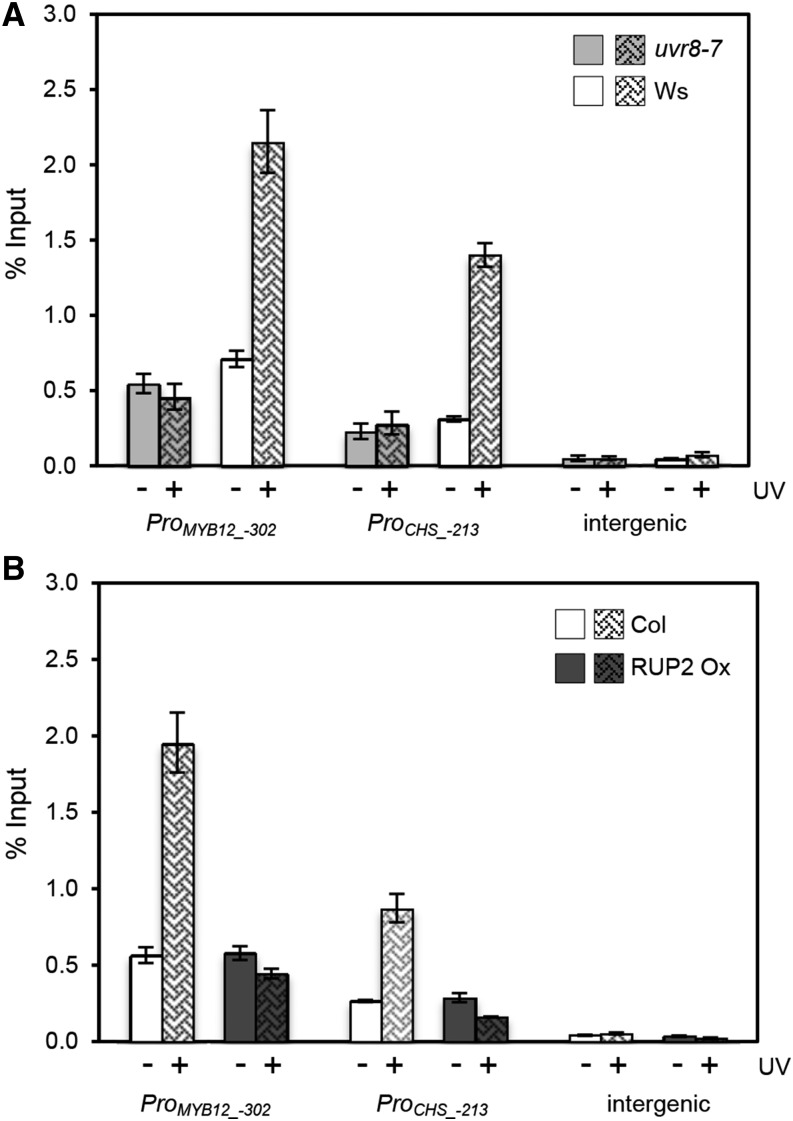
To test whether the UV-B-enhanced association of HY5 with target promoters is regulated by UVR8, we performed HY5 ChIP using the uvr8-7 null mutant in comparison with the wild type. Whereas the association with HY5 target promoters was similar to that in the wild type in white light, UV-B-mediated enhancement was absent in uvr8-7 mutants (Figure 2A).
Figure 2.
HY5 Chromatin Association Is Regulated by the UVR8 Photoreceptor Pathway.
UV-B-responsive HY5 chromatin association in wild-type plants (Ws and Col) was compared with that in the uvr8-7 mutant (A) and a RUP2 overexpression line (RUP2 Ox) (B). Seedlings were grown for 7 d in a weak light field and exposed for 2 h to narrowband UV-B. ChIP-qPCR was performed for the MYB12 and CHS promoters and an intergenic region between the At4g26900 and At4g26910 genes. The numbers of the analyzed DNA fragments indicate the positions of the 5′ base pair of the amplicon relative to the translation start site (referred to as position +1). ChIP of DNA associated with HY5 is presented as the percentage recovered from the total input DNA (% Input). Data shown are representative of three (A) and two (B) independent biological replicates. Error bars represent sd of three technical replicates.
The WD40-repeat protein RUP2 acts as repressor of the UV-B response by facilitating UVR8 redimerization and thus ground state reversion (Gruber et al., 2010; Heijde and Ulm, 2013). In agreement with this activity, RUP2 overexpression resulted in a reduced UV-B-specific response (Gruber et al., 2010), and this was associated with the absence of UV-B-induced enhancement of HY5 chromatin association at target genes (Figure 2B). Together, the absence of enhanced HY5 association with its target promoters in the uvr8-7 mutant and RUP2 overexpression lines underlines the importance of UVR8-mediated UV-B perception and signaling impinging on HY5 activity.
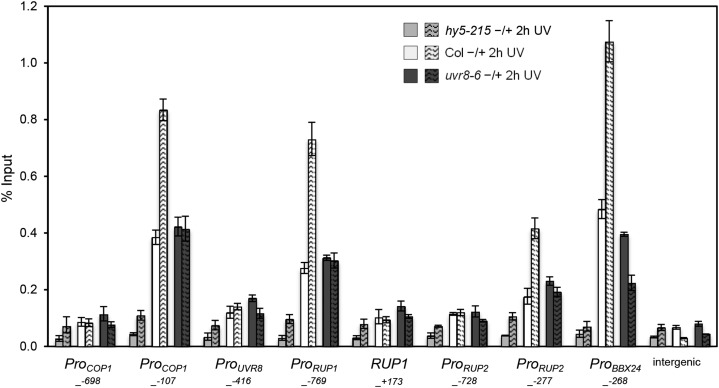
HY5 Binds to Genes Encoding Proteins Involved in UV-B Signaling but Not to UVR8
We tested the capacity of HY5 to bind the genomic regions of key UV-B pathway factors, namely RUP1, RUP2, the B-box family gene BBX24/STO, UVR8, and COP1 (Tilbrook et al., 2013; Jenkins, 2014). Except for UVR8, which is constitutively expressed, these genes are known to be transcriptionally activated by UV-B irradiation (Ulm et al., 2004; Gruber et al., 2010; Huang et al., 2012). In accordance with the UV-B responsiveness, the HY5 association with RUP1, RUP2, COP1, and BBX24 genomic regions was enhanced in response to UV-B in the wild type (Columbia [Col]) but not in uvr8-6 null mutants (Figure 3). In contrast with UV-B-regulated genes, no clear association of HY5 with the constitutively expressed UVR8 gene was detectable (Figure 3). The chromatin association of HY5 with RUP1, RUP2, COP1, and BBX24 is in agreement with the HY5 dependence of their UV-B-induced expression (Gruber et al., 2010; Huang et al., 2012; Jiang et al., 2012) and further underlines the regulatory activity of HY5 chromatin association in UV-B signaling.
Figure 3.
HY5 Associates with RUP1, RUP2, COP1, and BBX24 but Not with the UVR8 Promoter.
hy5-215, uvr8-6, and wild-type (Col) seedlings were grown for 7 d in a standard growth chamber followed by weak light acclimation for 12 h and narrowband UV-B irradiation for 2 h. ChIP was performed with an anti-HY5 antibody, and copurified DNA was analyzed by qPCR for different primer pairs amplifying parts of the COP1, UVR8, RUP1, RUP2, and BBX24 genomic regions and an intergenic region between genes At4g26900 and At4g26910. The numbers of the analyzed DNA fragments indicate the positions of the 5′ base pair of the amplicon relative to the translation start site (referred to as position +1). ChIP of DNA associated with HY5 is presented as the percentage recovered from the total input DNA (% Input). Data shown are representative of two independent experiments. Error bars represent sd of three technical replicates.
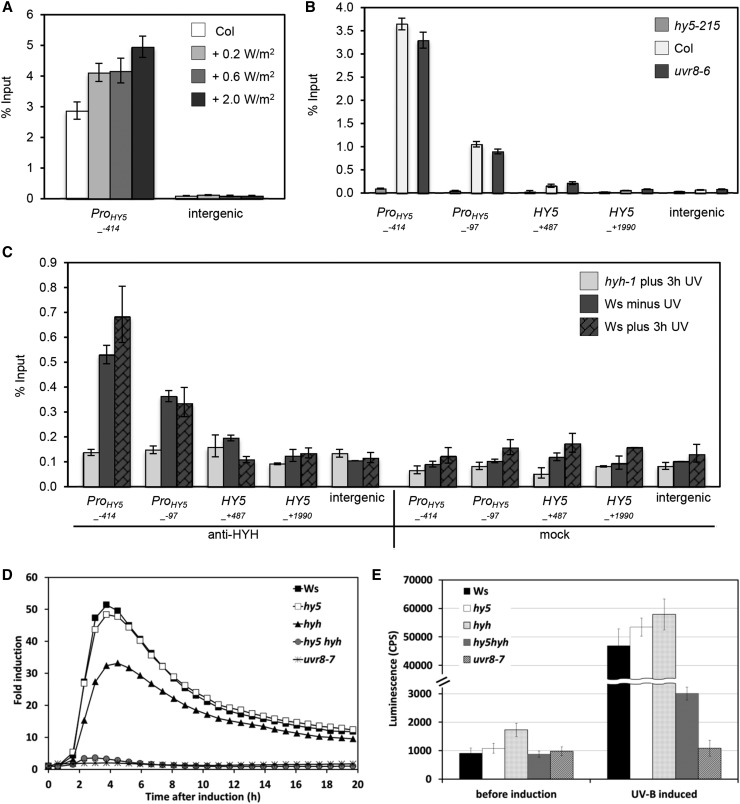
HY5 and HYH Bind to the Promoter of HY5 and Act Redundantly in UV-B-Induced HY5 Gene Activation
We performed two lines of experiments to test our hypothesis that HY5 and/or its homolog HYH regulates HY5 gene induction. First, we tested whether HY5 binds to the promoter region of HY5. ChIP assays demonstrated that HY5 is indeed associated with its own promoter in white light-grown seedlings and UV-B increases binding in a dose-dependent manner (Figure 4A). Binding of HY5 was specific for the promoter region, as no binding to the HY5 coding region was detected (Figure 4B). Furthermore, as with the promoters of other HY5 target genes (Figures 2A and 3), the association of HY5 with its own promoter did not depend on UVR8 in the absence of UV-B (Figure 4B).
Figure 4.
HY5 and HYH Act Redundantly in Inducing HY5 Gene Expression and Associate with Common Target Genes, Including the HY5 Promoter.
(A) and (B) HY5 associates with its own promoter. Seven-day-old wild-type (Col) seedlings grown in weak light were treated for 2 h with different intensities of narrowband UV-B (A), and hy5-215, uvr8-6, and wild-type (Col) seedlings were grown for 7 d in a standard growth chamber (B). ChIP was performed with an anti-HY5 antibody, and copurified DNA was analyzed by qPCR for different primer pairs covering the HY5 genomic locus and an intergenic region between genes At4g26900 and At4g26910. The numbers of the analyzed DNA fragments indicate the positions of the 5′ base pair of the amplicon relative to the translation start site (referred to as position +1). ChIP of DNA associated with HY5 is presented as the percentage recovered from the total input DNA (% Input). Error bars represent sd of three technical replicates.
(C) HYH binds to the HY5 promoter in vivo. Wild-type plants (Ws) and null mutant hyh-1 were grown for 7 d in a weak light field and exposed for 3 h to narrowband UV-B. ChIP was performed with an anti-HYH antibody (left) or without the addition of antibody (mock; right). ChIP-qPCR was performed for different primer pairs covering the HY5 genomic locus and an intergenic region between genes At4g26900 and At4g26910. The numbers of the analyzed DNA fragments indicate the positions of the 5′ base pair of the amplicon relative to the translation start site (referred to as position +1). ChIP of DNA associated with HYH is presented as the percentage recovered from the total input DNA (% Input). Data shown are representative of two independent experiments. Error bars represent sd of three technical replicates.
(D) and (E) HY5 and HYH play redundant but essential roles in mediating the responsiveness of the HY5 promoter to UV-B. Luciferase activity is shown for transgenic wild-type Arabidopsis (Ws), hy5, hyh, hy5 hyh, and uvr8-7 plants carrying the same copy of the full-length HY5 promoter fused to the LUC reporter gene. Thirty-six individual seedlings were assayed for each genotype. Error bars represent se.
Sequence conservation, the ability to form heterodimers, and partial functional redundancy between HY5 and HYH predicted a possible involvement of HYH in regulating the activity of the HY5 promoter (Holm et al., 2002; Brown and Jenkins, 2008; Stracke et al., 2010). Accordingly, ChIP data clearly demonstrated a specific association of endogenous HYH with chromatin in the HY5 promoter region in wild-type seedlings (Figure 4C). In the ChIP assays, the strongest signal for HY5 and HYH binding to the HY5 promoter was obtained with the primer pair amplifying the region −414 to −251 bp of the HY5 promoter (with the first base pair in the cDNA defined as +1) (ProHY5−414 in Figures 4B and 4C). Thus, we concluded that both HY5 and HYH bind to the HY5 promoter in vivo.
To address the dependency of UV-B-mediated HY5 induction on HY5 and/or HYH proteins, we used transgenic plants expressing a chimeric ProHY5:LUCIFERASE (LUC) reporter (Ulm et al., 2004) in hy5, hyh, and hy5 hyh mutant backgrounds. After applying supplemental UV-B for 30 min to light-grown seedlings, luciferase activity was monitored over several hours. Induction of luminescence peaked around 4 h after UV-B irradiation to a similar extent in hy5, hyh, and wild-type background seedlings (Figures 4D and 4E; note that the slight reduction of hyh in Figure 4D is due to a slightly elevated basal level of luciferase activity; see Figure 4E). By contrast, ProHY5:LUC induction was almost absent in the hy5 hyh double mutant background and completely absent in uvr8 knockout mutants (Figures 4D and 4E). These data demonstrate that HY5 and HYH act redundantly in regulating the UV-B-induced transcription of HY5.
Three cis-Regulatory Elements Mediate the Transcription of HY5, Including a T/G-Box Required for Its UV-B Responsiveness
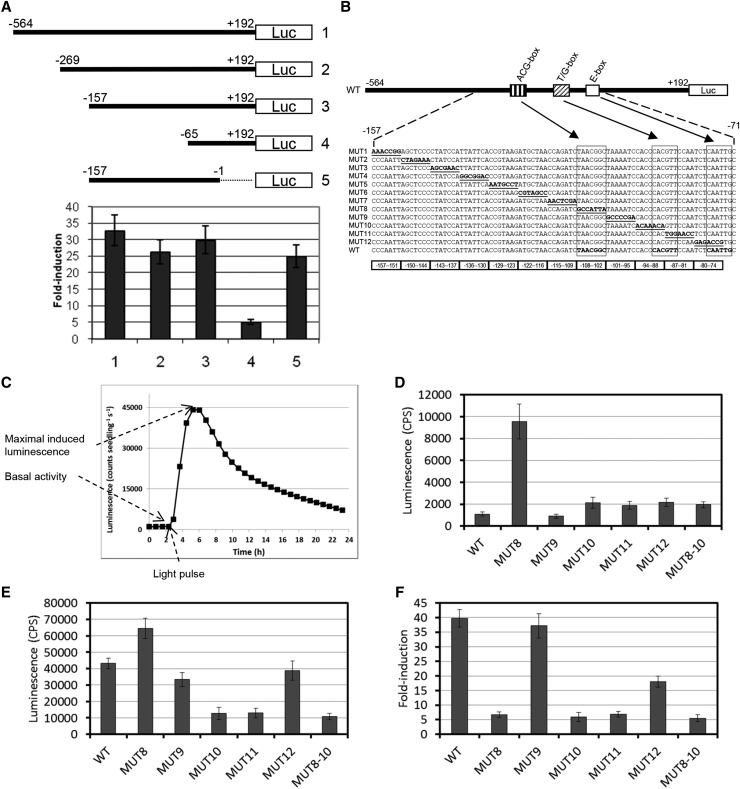
The rapid and transient induction of HY5 upon UV-B exposure requires the binding of HY5 and HYH to the promoter of HY5 (Figures 4A to 4D). To identify the underlying UV-B-specific cis-regulatory sequences, we created stable transgenic Arabidopsis lines containing chimeric gene constructs of different HY5 promoter fragments fused to the LUC reporter gene. The extent of LUC induction upon UV-B treatment in plants harboring the HY5 promoter at −157 to −1 bp (ProHY5[−157/−1]:LUC) (the last base pair before the 5′ untranslated region being defined as −1) was similar to that of plants containing the chimeric construct of the complete HY5 promoter (ProHY5[−565/+192]:LUC) (defined as the sequence between the stop TGA of the upstream gene At5g11270 and the start ATG of HY5/At5g11260), whereas LUC induction was markedly lower in plants containing ProHY5[−65/+192]:LUC (Figure 5A, compare constructs 1 and 5 with construct 4). Therefore, we performed a linker scan analysis examining the UV-B-responsive region between −157 and −74 within the ProHY5[−565/+192]:LUC construct (Figure 5B). To characterize how the mutations modulate HY5 activity with or without UV-B irradiation, we measured the basal activity (the luminescence measured in white light-grown seedlings; Figures 5C and 5D) as well as the UV-B-modulated activity of the promoter (the maximal luminescence measured after UV-B treatment; Figures 5C and 5E) and calculated the fold UV-B induction displayed by the various mutants (Figure 5F). Using this approach, we identified three cis-regulatory domains designated as a previously undescribed ACG-box (−108-TAACGGC-102), a T/G-box (−90-CACGTT-85), and an E-box (−77-CAATTG-72) that appear to be involved in modulating the activity and UV-B induction of HY5 (Figure 5). We found that mutations located in the 5′ proximal region of the HY5 promoter (−157 to −109) did not affect UV-B responsiveness. Mutation of sequences perturbing the ACG-box located between −108 and −102 (MUT8) drastically elevated the basal activity of the mutated promoter in white light (Figure 5D) and marginally increased maximal UV-B-induced fluorescence (Figure 5E). It follows that this mutation significantly lowered the relative UV-B induction of the HY5 promoter compared with the wild type (6-fold versus 40-fold; Figure 5F). Mutation of sequences between −101 and −95 (MUT9) did not modify either the basal or the maximal UV-B-induced activity of the HY5 promoter. Mutations perturbing the T/G-box, including MUT10 (−94 to −88) and MUT11 (−87 to −81), uniformly but only moderately elevated the basal activity of the HY5 promoter in white light (Figure 5D) and reduced maximal UV-B-induced fluorescence (Figure 5E). Accordingly, mutations of the T/G-box strongly reduced the fold UV-B induction of the HY5 promoter (Figure 5F). The activity of the HY5 promoter containing a disrupted E-box (MUT12) but intact ACG- and T/G-boxes was again slightly higher than that of the wild type in white light (Figure 5D), and it produced a maximal UV-B-induced fluorescence similar to that in the wild type (Figure 5E), an ∼20-fold UV-B induction (Figure 5F).
Figure 5.
Identification of Functional cis-Regulatory Elements of the HY5 Promoter.
(A) Deletion analysis of the HY5 promoter. A schematic representation of the 5′ truncated derivatives of the HY5 promoter fused to the LUC reporter gene is shown. Constructs 1 to 4 carry the full 5′ untranslated region of HY5, whereas the 5′ untranslated region was replaced by a 35S minimal promoter in construct 5. Nucleotide positions relative to the first base pair of the 5′ untranslated region (transcriptional start site defined as position +1) are shown. Wild-type Arabidopsis (Ws) seedlings carrying the reporter constructs were grown in a standard growth chamber for 1 week. Plants were transferred to continuous white light at 10 µmol m−2 s−1 fluence rate for 48 h. Luminescence measurements were started 3 to 4 h before application of the light pulse. Plants were irradiated with narrowband UV-B for 30 min and then returned to white light, where luminescence measurement was resumed. Twenty-four to 36 individual seedlings were assayed for each line, and two independent transgenic lines were tested for each construct. Error bars represent se.
(B) to (F) Linker scan mutant derivatives of the HY5 promoter.
(B) Putative cis-elements (ACG-box, T/G-box, and E-box) are indicated as boxes, and corresponding nucleotides are framed in the sequence panel and shown in boldface in the wild-type sequence. Mutations are underlined and printed in boldface and are shown in the corresponding mutant sequence line. All mutations were generated in the −565 to +192 fragment of the HY5 promoter, but only sequences from −157 to −71 are shown. Nucleotide positions relative to the first base pair of the 5′ untranslated region (transcriptional start site defined as position +1) are shown.
(C) Induction profile of ProHY5[−565/+192]:LUC in response to UV-B pulses (plants were treated as described in [A]). Basal activity was calculated from average counts at the three time points just before the light pulse. The highest luminescence value detected after the light pulse is taken as the maximal induced luminescence. Fold induction is the ratio of maximal induced luminescence to basal activity. Data represent averages of 36 individual seedlings for each condition.
(D) to (F) Wild-type Arabidopsis (Ws) seedlings carrying the indicated mutant derivatives of the HY5 promoter fused to the LUC reporter gene were grown and assayed as in (C). Twenty-four to 36 individual seedlings were assayed for each line, and two independent transgenic lines were tested for each construct. Basal activity (D), maximal induced luminescence (E), and fold induction (F) were calculated according to (C). Error bars represent se.
Interestingly, the HY5 promoter containing both mutant ACG- and T/G-boxes (MUT8-10) behaved similarly to that of the single T/G-box mutants (MUT10 and MUT11) (Figures 5D to 5F; Supplemental Figure 1A). This suggests (1) that the T/G-box is essential for the full activity of HY5 in response to UV-B, (2) that the unleashed activity of MUT8 (mutated repressive ACG-box) in visible light also requires a functional T/G-box, and (3) that the ACG element itself is likely to be superfluous for UV-B induction. It is important to note that luminescence levels in the various transgenic seedlings kept in light for 48 h after the UV-B treatment were almost identical to the levels before treatment (Supplemental Figure 1B). Thus, the results above also indicate that perturbation of the ACG-box (1) elevates the activity of the HY5 promoter in seedlings grown in visible light but (2) does not interfere with deactivation after UV-B irradiation. To test whether the ACG-box is required in the regulation of HY5 promoter activity in the dark, we determined LUC mRNA abundance in wild-type and MUT8 ProHY5:LUC plants grown in darkness and in light. Supplemental Figure 1C shows that MUT8 did not significantly affect the activity of the HY5 promoter in etiolated material but drastically elevated LUC mRNA levels, as expected, in light-grown material compared with wild-type HY5. We note that Abbas et al. (2014) recently reported the involvement of T/G- and E-boxes in regulating the activity of the HY5 promoter in visible light. Thus, we also analyzed the induction of the corresponding mutated promoter versions (T/G-box mutated MUT10 and MUT11 as well as E-box mutated MUT12; Figure 5B) and hy5, hyh, and hy5 hyh mutants after illuminating etiolated seedlings with continuous white light for 72 h. Our data illustrate that the induction of HY5 is transient, it reaches a maximum level 1 to 4 h after the onset of light, and it declines to low levels afterward (Supplemental Figures 2A to 2D). This figure also demonstrates that (1) mutations of the T/G-box eliminate the induction of HY5 transcription, (2) mutation of the E-box has a much less pronounced effect, and (3) activation of HY5 transcription by white light is below the detection level in the hy5 hyh double mutant. Taken together, we conclude that the T/G-box positively regulates HY5 activity in visible light, whereas the T/G-box and to a minor extent the E-box, but not the ACG-box, are essential for full induction by UV-B light.
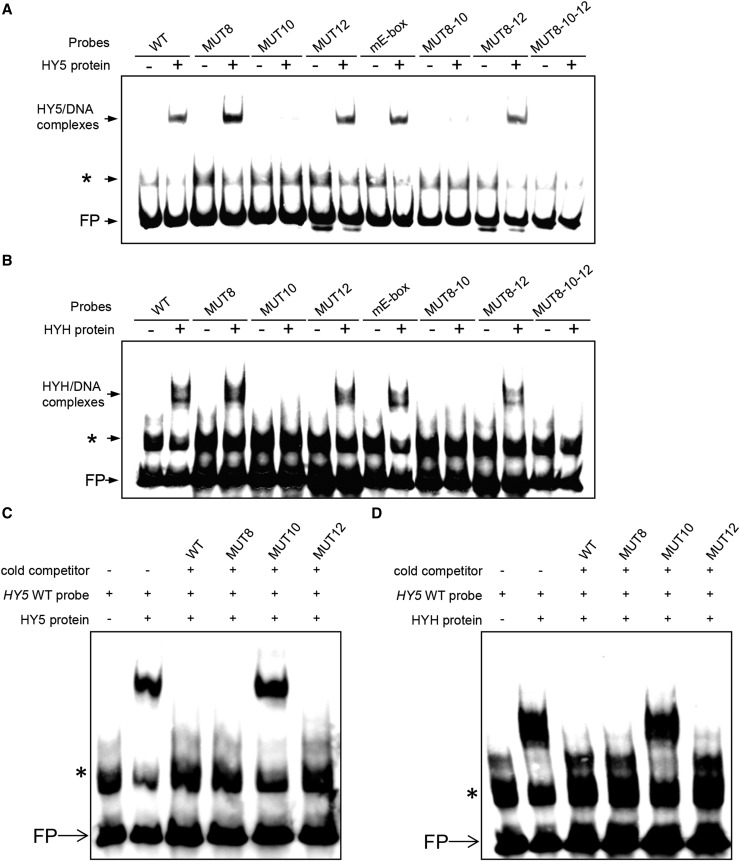
HY5 and HYH Bind to the cis-Regulatory T/G-Box in Vitro
We showed above that the activity of the HY5 promoter in transgenic plants is regulated by three cis-regulatory domains, the ACG-, T/G-, and E-boxes, located between −108 and −72. To test which of these binds HY5 and/or HYH, we performed electrophoretic mobility shift assays (EMSAs) using recombinant HY5 and HYH proteins. The target sequence spanned the promoter region of HY5 between −113 and −62 and thus contained all identified cis-regulatory domains. The design of the mutations facilitated the identification of regulatory elements that can bind HY5 and HYH independently of the other two. The EMSA results clearly demonstrated (1) that the wild-type T/G-box efficiently binds HY5 (Figure 6A) as well as HYH (Figure 6B), even when the ACG- and E-box sequences (MUT8-12) are perturbed, and (2) that mutation of the T/G-box (MUT10) eliminates the binding of HY5 and HYH to the probe containing intact ACG- and E-box elements (Figures 6A and 6B). The results in Figures 6C and 6D demonstrate that binding of HY5 and HYH to the T/G-box cannot be competed off with ACG- and E-box sequences but only with an excess amount of cold probe including an intact T/G-box and, therefore, is sequence-specific. Taken together, we conclude that the T/G-box binds HY5 and HYH and thus plays a critical role in regulating the induction of HY5 by UV-B irradiation.
Figure 6.
HY5 and HYH Bind to the cis-Regulatory Element T/GHY5-Box.
Biotin-labeled double-stranded probes (40 fmol) were incubated with (+) or without (−) expressed and purified HY5 ([A] and [C]) or HYH ([B] and [D]) protein (400 ng). Binding reactions were resolved on 6% native polyacrylamide gels. WT corresponds to the −117 to −62 fragment of the HY5 promoter, whereas MUT8, MUT10, MUT12, MUT8-10, and MUT8-10-12 carry the corresponding single, double, and triple linker scan mutations in this context. The mE-box probe has mutations identical to those described by Abbas et al. (2014). In (C), reactions were performed as in (A) using HY5 wild-type probe and HY5 protein, but unlabeled HY5 MUT8 or HY5 MUT10 fragments were used as cold competitors at a 200-fold molar excess. In (D), reactions were performed as in (B) using HY5 wild-type probe and HYH protein, but unlabeled HY5 MUT8 or HY5 MUT10 fragments were used as cold competitors at a 200-fold molar excess. Sequences of all probes are provided in Supplemental Table 2. FP indicates free (nonbound) probe. Asterisks mark a nonspecific band that appears independent of the presence of HY5 or HYH protein.
DISCUSSION
UV-B-activated UVR8 photoreceptor signaling alters gene expression, which also underpins induced UV-B acclimation and subsequently elevated UV-B tolerance. However, despite their physiological importance, the processes involved in UV-B-mediated transcriptional regulation are poorly understood. HY5 is the major transcription factor required downstream of UVR8. In addition, the HY5 gene is an excellent and widely used marker for UVR8-mediated gene expression changes (Ulm et al., 2004; Brown et al., 2005, 2009; Oravecz et al., 2006; Gruber et al., 2010; Huang et al., 2012; Jiang et al., 2012). Thus, it is thought that HY5 is required for UV-B-responsive gene activation and that its own transcriptional induction as well as posttranslational stabilization further enforce the UV-B response (Li et al., 2013; Tilbrook et al., 2013; Jenkins, 2014). However, the impact of UV-B on HY5 chromatin association as well as the identity of the cis-regulatory elements and transcriptional regulators required for UV-B-mediated HY5 gene activation were unclear. Our analyses reveal that the HY5 association with target gene promoters is enhanced by UV-B in a UVR8-dependent manner. Furthermore, HY5 itself, together with HYH, is required for transcriptional activation of its own gene by binding the T/GHY5-box, a cis-regulatory element required for HY5 gene activation by UV-B. Thus, our study provides increased understanding of the transcriptional response to UV-B. In particular, it reveals HY5 as a transcriptional regulator of its own gene expression in a positive feedback loop and identifies a UV-B-responsive cis-element targeted by HY5.
Regulation of the HY5 Association with Chromatin
Even though initial data suggested that binding of HY5 in vivo is not affected by light (Lee et al., 2007), more recent results suggest that the HY5 association with target genes is enhanced in response to white light (Zhang et al., 2011). However, the photoreceptor(s) mediating this enhanced binding in response to visible light have remained unclear. Here, we provide evidence for UV-B-responsive enrichment of promoter-associated HY5 at target genes, dependent on the UVR8 UV-B photoreceptor. In agreement, the UV-B-responsive increased binding is also affected by overexpression of the negative regulatory RUP2 gene. In RUP2 overexpression lines, UVR8 remains in its inactive homodimeric form, which blocks signaling (Gruber et al., 2010; Heijde and Ulm, 2013). Thus, enhanced chromatin association of HY5 is a response closely associated with UVR8-mediated gene expression changes. However, it is still unclear whether the detected UV-B-enhanced binding is due to stronger binding per individual target promoter (e.g., multiple cis-elements) or to binding of previously unoccupied promoters in the same or additional cells. This is further complicated by the fact that HYH binds to the same cis-element in the HY5 promoter; thus, the relative abundance of the competing transcription factors can further distort the actual data. These dilemmas are not specific to the results for HY5 but represent general uncertainties when interpreting ChIP data. In addition, it is yet uncertain whether the UV-B-induced HY5 association with chromatin of target genes primarily reflects the stabilization of the HY5 protein (Favory et al., 2009; Huang et al., 2013) or the activity of the HY5 protein is affected otherwise by UV-B. It was previously reported that phosphorylation of HY5 within its COP1 binding domain may regulate HY5 stability and activity (Hardtke et al., 2000). However, any potential impact of UV-B on modifying the phosphorylation of HY5 remains to be demonstrated.
There are several genes among the direct targets of HY5 that encode components of the UV-B signaling pathway. For example, HY5 binds and regulates COP1 as well as RUP1 and RUP2. This transcriptional modulation appears to increase the abundance of active UVR8-COP1-HY5 core pathway components but, in parallel, also to induce the negative feedback regulators RUP1 and RUP2 (Oravecz et al., 2006; Favory et al., 2009; Gruber et al., 2010; Heijde and Ulm, 2013). The induction of COP1 by UV-B was shown to be modulated by combinatorial regulation of FHY3 and HY5 (Huang et al., 2012). Interestingly, HY5 and FHY3 physically interact with each other, and this is influenced by UV-B. FHY3 binds to an FHY3 binding site and HY5 to an ACGT-containing element in the COP1 promoter, both of which seem to be required for the UV-B responsiveness of COP1 (Huang et al., 2012). Combinatorial regulation exerted by the interaction of other transcription factors with HY5/HYH is widely present, with negative or positive regulatory effects (Shin et al., 2007; Andronis et al., 2008; Holtan et al., 2011; Huang et al., 2012; Jiang et al., 2012; Singh et al., 2012; Gangappa et al., 2013a, 2013b; Jing et al., 2013; Abbas et al., 2014). Moreover, HY5 interacts with the chromatin-remodeling factor PICKLE (PKL), recruiting PKL to promoters of target genes repressing the H3K27me3-repressive histone mark (Jing et al., 2013). Thus, an exact understanding of HY5/HYH function in response to UV-B will also require systematic analysis of known HY5-interacting transcriptional regulators and an unbiased approach to identify those that are specifically involved.
Regulation of the HY5 Promoter
Our ChIP experiments clearly support the binding of HY5 to its own promoter, suggesting that HY5 is one of the missing transcription factors for HY5 gene activation by UV-B. However, our previous results showed that HY5 is superfluous for transcriptional activation of itself (Ulm et al., 2004). Here, we also demonstrate that HY5 or its homolog HYH can individually activate HY5 transcription in the absence of the other. This redundancy is clearly demonstrated by the similar UV-B-mediated activation of ProHY5:LUC in the wild type and the hy5 and hyh single mutants but the very strongly reduced activation in hy5 hyh double mutants. Notably, whereas ProHY5:LUC activation was not found in uvr8, an approximately 3-fold induction was still apparent in hy5 hyh (compared with >50-fold in the wild type). This indicates the possible involvement of additional transcriptional regulators of likely minor importance. Next to transcriptional regulation, the expression of HY5 was recently shown to involve light-regulated posttranscriptional regulation by the small regulatory microRNA miR157d (Tsai et al., 2014); however, whether UV-B affects miR157d expression remains unknown. This notwithstanding, HY5 and HYH both associate with the HY5 promoter and are required in vivo for mediation of the early event of HY5 gene induction, which strengthens the concept of their central regulatory role in rapid UV-B responses downstream of UVR8.
Regulatory cis-Elements Contributing to HY5 Gene Expression
In a linker scan analysis, we identified three cis-regulatory elements that mediate the transcriptional activity of HY5, namely an ACG-box, a T/G-box, and an E-box. Mutation of the ACG-box resulted in elevated basal expression of the luciferase reporter after extended white light treatment (24 to 72 h) but not in darkness, indicating that this box functions as a light-induced HY5 repression element. In contrast, mutation of the T/G or E element did not significantly affect the expression of ProHY5:LUC after 24 to 72 h of illumination with white light. It was reported recently that HY5 and CALMODULIN7 (CAM7) bind to T/G and E in vitro and interact in vivo; the expression of a ProHY5:GUS reporter is downregulated in hy5 and cam7 mutants after irradiation with visible light (Abbas et al., 2014). We found that the activity of the HY5 promoter is not strongly responsive to extended irradiation with visible light. It is notable, however, that after transfer from darkness to white light, the induction of luminescence (ProHY5:LUC) peaked around 4 h to a similar extent in hy5, hyh, and wild-type seedlings, and mutation of the E-box did not significantly affect this response (Supplemental Figures 2C and 2D). By contrast, this acute induction by white light was eliminated by the mutation of the T/G-box as well as in the hy5 hyh double mutant background (Supplemental Figures 2C and 2D). These data indicate that other transcription regulators, including CAM7, may play a minor role in the absence of HY5 and HYH for acute white light induction of HY5 expression during the dark-to-light transition. Similarly, mutation of the T/G-box or the lack of HY5/HYH in hy5 hyh double mutants dramatically reduced UV-B-induced transcription of the ProHY5:LUC reporter (Figures 4D and 4E). HY5 and HYH bind specifically to the T/G-box, and mutations disrupting this binding in vitro also block UV-B-mediated activation of the HY5 promoter in planta, even in the presence of functional HY5/HYH. Together, these data suggest that HY5 and HYH are major and critical factors for regulating the UV-B and visible light responsiveness of the HY5 promoter via binding to the T/GHY5 motif. Mutation of the E-box also reduced UV-B-induced HY5 transcription in planta, albeit much less effectively than T/G-box mutation. HY5 and HYH do not bind to the E-box, and its mutation does not affect the binding of HY5/HYH to the T/GHY5-box (Figure 6). By contrast, Abbas et al. (2014) found that binding of HY5 and CAM7 to the HY5 promoter required the presence of intact T/G and E elements. The apparent contradiction between our data and those of Abbas et al. (2014) may be due to the use of differentially tagged HY5 proteins (HY5-6xHis versus HY5-GST) in the in vitro binding assays.
Independent of this, our data clearly demonstrate (1) that full induction of the HY5 promoter by UV-B also requires an intact E-box (Figure 5F) but (2) that the functional significance of this cis-element is low in the absence of HYH/HY5 (Figure 4D) or an intact T/GHY5-box (Figures 6E and 6F). Mutation of the ACG-box identified in this study also appears to compromise UV-B-induced activity of the HY5 promoter in planta, but the molecular mechanism underlying this phenomenon is not understood. The drastically (50-fold) elevated expression of the HY5 promoter carrying a mutant ACG-box in white light clearly demonstrates that this cis-regulatory element mediates the binding of an as yet unknown transcription factor, downregulating the activity of HY5 in continuous visible light. Upon exposure to UV-B, this repressor may be degraded or competed off by an activating transcription factor with a higher affinity for the ACG motif. Accordingly, mutation of the ACG-box might not only interfere with binding of the repressor but also of an activator. The high activity of the mutant HY5 promoter in white light, however, could deplete one or more components and, thus, limit further induction by UV-B.
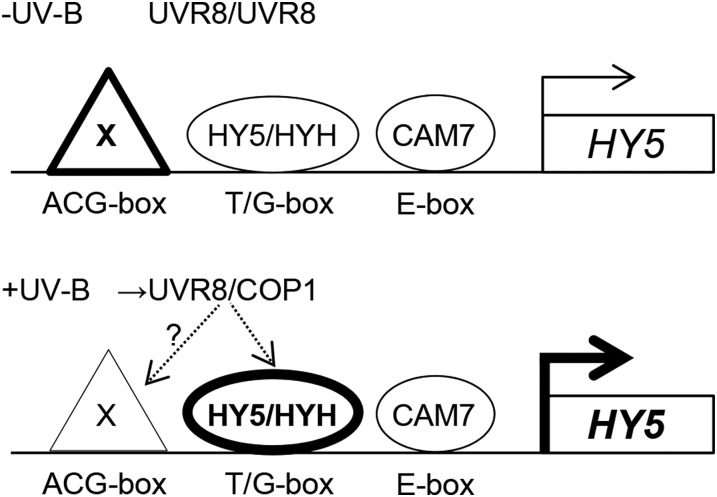
Taken together, our data support a model in which HY5 and HYH act as major regulators of the UV-B-enhanced transcription of HY5 and other target genes (Figure 7). In broader terms, by demonstrating the impact of UV-B via UVR8 on the dynamics of chromatin occupancy by HY5 and HYH and revealing cis-elements and transcription factors regulating HY5 transcription in response to UV-B, these results provide a conceptual framework for further experiments towards a better understanding of UV-B-induced transcriptional responses.
Figure 7.
Working Model of the Transcriptional Regulation of HY5.
HY5 and HYH binding to the T/G-box mediates UV-B responsiveness of the HY5 promoter. An ACG-box is postulated to be bound by an unknown repressor protein X that is potentially removed from the HY5 promoter or overruled by the UV-B responsiveness of the T/G-box in response to UV-B (note that the ACG-box requires the T/G-box for its repressive function in –UV-B). The E-box was previously found to be bound by CAM7 (Abbas et al., 2014) and seems to make only a very minor contribution to UV-B responsiveness. The accumulated HY5 protein (combination of new synthesis and stabilization) then binds to and activates multiple downstream target genes, including genes encoding UV-B signaling components (COP1, BBX24, RUP1, and RUP2).
METHODS
Plant Material
hy5-215 (Oyama et al., 1997), uvr8-6 (Favory et al., 2009), and rup2-1/Pro35S:RUP2 (Gruber et al., 2010) are in the Arabidopsis thaliana Col background, while hy5-ks50 (Oyama et al., 1997), hyh-1 (Holm et al., 2002), hy5-ks50 hyh-1 (Holm et al., 2002), ProHY5:LUC (Ulm et al., 2004), and uvr8-7 (Favory et al., 2009) in the Wassilewskija (Ws) background.
ProHY5:LUC was introduced into hy5-ks50, hyh-1, and hy5-ks50 hyh-1 by genetic crossing. The parental line has a stable single-copy insertion of the ProHY5:LUC transgene and was used previously for a genetic screen identifying cop1 and uvr8 mutants (Ulm et al., 2004; Oravecz et al., 2006; Favory et al., 2009).
Gene Constructs and Transgenic Plants
Linker scan mutagenesis on the HY5 promoter region was performed essentially as described (Gustin and Burk, 1993). Twelve mutant derivatives of ProHY5[−565/+192]:LUC were produced spanning nucleotides −157 to −74 relative to the HY5 transcription initiation site.
Constructs were transformed by the floral dip method into the Arabidopsis Ws accession (Clough and Bent, 1998), and transformants were selected on Murashige and Skoog (MS) medium supplemented with 15 µg/mL hygromycin. Ten to 15 independent transformants for each construct were self-fertilized, and individuals of the T2 or the homozygous T3 progeny were used for luminescence assays.
Growth Conditions and Light Treatments
Arabidopsis seeds were surface-sterilized with sodium hypochlorite and plated on half-strength MS medium (Duchefa) containing 1% sucrose and 0.8% agar. Seeds were stratified for at least 2 d at 4°C and germinated aseptically at 25°C in a standard growth chamber (MLR-350; Sanyo) at 80 μmol m−2 s−1 with a 12-h/12-h light/dark cycle (moderate white light) or under continuous irradiation in a white light field with Osram L18W/30 tubes (3.6 μmol m−2 s−1; measured with an LI-250 Light Meter) (weak white light). UV-B irradiation was performed for the indicated times in a weak white light field supplemented by Philips TL 20W/01 RS narrowband UV-B tubes (0.6 W/m2 or as indicated; measured with a VLX-3W UV Light Meter equipped with a CX-312 sensor; Vilber Lourmat). The UV-B range was modulated using 3-mm transmission cutoff filters of the WG series with half-maximal transmission at the indicated wavelength (WG305 and WG345; Schott Glaswerke). For ChIP assays, seedlings were grown for the indicated times under weak white light conditions or in moderate white light. Seedlings grown in moderate white light were acclimated under weak white light for 12 or 24 h prior to UV-B treatment.
ChIP
ChIP was performed as described previously (Stracke et al., 2010). The chromatin was immunoprecipitated with antibodies against HY5 (Oravecz et al., 2006) and HYH (using a 1:120 dilution of sera). Polyclonal HYH antibodies were raised against recombinant His6-tagged HYH protein (Eurogentec). Quantitative real-time PCR ChIP data were obtained using the ABsolute SYBR Green Rox Mix Kit according to the manufacturer’s instructions (Thermo Scientific). The samples were amplified using a 7900HT real-time PCR system (Applied Biosystems) with the primer pairs listed in Supplemental Table 1. The intergenic region between the At4g26900 and At4g26910 genes was described before (Lee et al., 2007; Stracke et al., 2010). Quantitative PCR (qPCR) data were analyzed according to the percentage of input method (Haring et al., 2007). Technical error bars were calculated according to the Applied Biosystems user manual.
Luminescence Measurements
Surface-sterilized seeds were grown in a 12-h-white-light (50 μmol m−2 s−1)/12-h-dark cycle at 22°C (MLR-350; Sanyo) for 7 d. Plants were grown on MS medium supplemented with 3% sucrose. All experiments were performed at 22°C. Continuous white light was produced by Philips TL-D 18W/33-640 tubes (10 μmol m−2 s−1). UV-B was produced by Philips TL 20W/01 RS tubes and filtered through LP305 cutoff filters (Rapp Optoelectronic), providing a final fluence rate of 1.5 μmol m−2 s−1. Plants were transferred to continuous white light 48 h prior to UV-B induction. Luminescence was monitored with the TopCount NXT luminometer (Perkin-Elmer) at 0.5- to 1.5-h intervals depending on the number of samples, as described previously (Kevei et al., 2006).
EMSAs
Double-stranded probes and nonlabeled fragments (competitors) were produced by annealing complementary oligonucleotides (IDT) in 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, and 50 mM NaCl (sequences are shown in Supplemental Table 2). Equal amounts of complementary oligonucleotides were mixed at a final concentration of 40 µM, heated to 95°C for 5 min in a block heater, and cooled to room temperature overnight. For probes, the 5′ end of the forward oligonucleotide was labeled by biotin (IDT). HY5 and HYH cDNA molecules were PCR-amplified from a size-selected cDNA library (CD4-13; TAIR) and cloned in pET28a vectors (Novagen). Proteins with an N-terminal His6 tag were expressed in Escherichia coli BL21 cells and purified using Ni-NTA agarose matrix (Qiagen). Protein expression and purification were performed according to the manufacturer’s instructions (Qiagen; QIAexpressionist). Binding reactions contained 10 mM Tris-HCl, pH 7.5, 85 mM KCl, 5% (v/v) glycerol, 0.1 µg/μL poly(dI∙dC), 40 fmol of probe, and 100 ng of purified HY5 or HYH protein in a 20-μL volume. Reactions were incubated at room temperature for 20 min and loaded onto native 4% polyacrylamide gels made with 0.5× TBE buffer. Gels were run in 0.5× TBE for 70 min and electroblotted to Hybond N+ (Amersham) nylon membranes in 0.5× TBE for 60 min. Biotin-labeled fragments were detected using the Chemiluminescent Nucleic Acid Detection Module (Thermo Scientific) according to the manufacturer’s protocols. Chemiluminescent signals were captured by a deep-cooled Orca II CCD camera (Hamamatsu).
Accession Numbers
Sequence data from this work can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AT1G06040 (BBX24), AT5G13930 (CHS), AT2G32950 (COP1), AT5G11260 (HY5), AT3G17609 (HYH), AT2G47460 (MYB12), AT5G52250 (RUP1), AT5G23730 (RUP2), AT5G63860 (UVR8), and AT4G26900/AT4G26910 (intergenic region).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Effect of the ACGHY5-Box Mutation on Basal Promoter Activity.
Supplemental Figure 2. T/G-Box Mutation Impairs White Light Induction of HY5 Expression.
Supplemental Table 1. Primer Pairs Used in ChIP-qPCR.
Supplemental Table 2. List of Oligonucleotides Used to Produce Probes for EMSA Experiments.
Supplementary Material
Acknowledgments
We thank Xing-Wang Deng for providing hyh, hy5, and hyh hy5 mutant seeds and Alexander Baumann for generating anti-HYH antibodies. Research performed in Szeged, Hungary was supported by grants from the Hungarian Scientific Research Fund (OTKA-106361 to L.K.-B. and OTKA-108559 to F.N.) and the New Hungary Development Plan (TÁMOP-4.2.2A-11/1/KONV-2012-0035 to F.N.). Research performed in Edinburgh, UK was supported by a Biotechnology and Biological Science Research Council grant (BB/K006975/1) to F.N. and that in Geneva, Switzerland by the State of Geneva as well as grants from the Swiss National Science Foundation (Grant 31003A-132902) and the European Research Council (ERC-StG 310539) to R.U.
AUTHOR CONTRIBUTIONS
The research was designed by M.B., L.K.-B., F.N., and R.U. and performed by M.B., L.K.-B., and K.T. L.D.V. provided new experimental tools. M.B., L.K.-B., F.N., and R.U. analyzed data and wrote the article.
Glossary
- ChIP
chromatin immunoprecipitation
- Col
Columbia
- EMSA
electrophoretic mobility shift assay
- Ws
Wassilewskija
- MS
Murashige and Skoog
- qPCR
quantitative PCR
Footnotes
Online version contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abbas N., Maurya J.P., Senapati D., Gangappa S.N., Chattopadhyay S. (2014). Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell 26: 1036–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronis C., Barak S., Knowles S.M., Sugano S., Tobin E.M. (2008). The clock protein CCA1 and the bZIP transcription factor HY5 physically interact to regulate gene expression in Arabidopsis. Mol. Plant 1: 58–67. [DOI] [PubMed] [Google Scholar]
- Ballare C.L., Barnes P.W., Flint S.D. (1995). Inhibition of hypocotyl elongation by ultraviolet-B radiation in de-etiolating tomato seedlings. I. The photoreceptor. Physiol. Plant. 93: 584–592. [Google Scholar]
- Beggs C.J., Wellmann E. (1994). Photocontrol of flavonoid biosynthesis. In Photomorphogenesis in Plants, 2nd ed, Kendrick R.E., Kronenberg G.H.M., eds (Dordrecht, The Netherlands: Kluwer Academic Publishers; ), pp. 733–751. [Google Scholar]
- Brown B.A., Jenkins G.I. (2008). UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 146: 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.A., Cloix C., Jiang G.H., Kaiserli E., Herzyk P., Kliebenstein D.J., Jenkins G.I. (2005). A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 102: 18225–18230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.A., Headland L.R., Jenkins G.I. (2009). UV-B action spectrum for UVR8-mediated HY5 transcript accumulation in Arabidopsis. Photochem. Photobiol. 85: 1147–1155. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Arvai A.S., Baxter K.J., Heilmann M., Pratt A.J., O’Hara A., Kelly S.M., Hothorn M., Smith B.O., Hitomi K., Jenkins G.I., Getzoff E.D. (2012). Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloix C., Jenkins G.I. (2008). Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Mol. Plant 1: 118–128. [DOI] [PubMed] [Google Scholar]
- Cloix C., Kaiserli E., Heilmann M., Baxter K.J., Brown B.A., O’Hara A., Smith B.O., Christie J.M., Jenkins G.I. (2012). C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein. Proc. Natl. Acad. Sci. USA 109: 16366–16370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Favory J.J., et al. (2009). Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehér B., Kozma-Bognár L., Kevei E., Hajdu A., Binkert M., Davis S.J., Schäfer E., Ulm R., Nagy F. (2011). Functional interaction of the circadian clock and UV RESISTANCE LOCUS 8-controlled UV-B signaling pathways in Arabidopsis thaliana. Plant J. 67: 37–48. [DOI] [PubMed] [Google Scholar]
- Gangappa S.N., Crocco C.D., Johansson H., Datta S., Hettiarachchi C., Holm M., Botto J.F. (2013a). The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell 25: 1243–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa S.N., Holm M., Botto J.F. (2013b). Molecular interactions of BBX24 and BBX25 with HYH, HY5 HOMOLOG, to modulate Arabidopsis seedling development. Plant Signal. Behav. 8: e25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Besteiro M.A., Bartels S., Albert A., Ulm R. (2011). Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 68: 727–737. [DOI] [PubMed] [Google Scholar]
- Gruber H., Heijde M., Heller W., Albert A., Seidlitz H.K., Ulm R. (2010). Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc. Natl. Acad. Sci. USA 107: 20132–20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin K.E., Burk R.D. (1993). A rapid method for generating linker scanning mutants utilizing PCR. Biotechniques 14: 22, 24. [PubMed] [Google Scholar]
- Hardtke C.S., Gohda K., Osterlund M.T., Oyama T., Okada K., Deng X.W. (2000). HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 19: 4997–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M., Offermann S., Danker T., Horst I., Peterhansel C., Stam M. (2007). Chromatin immunoprecipitation: Optimization, quantitative analysis and data normalization. Plant Methods 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hectors K., Prinsen E., De Coen W., Jansen M.A., Guisez Y. (2007). Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 175: 255–270. [DOI] [PubMed] [Google Scholar]
- Heijde M., Ulm R. (2012). UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 17: 230–237. [DOI] [PubMed] [Google Scholar]
- Heijde M., Ulm R. (2013). Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc. Natl. Acad. Sci. USA 110: 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann M., Jenkins G.I. (2013). Rapid reversion from monomer to dimer regenerates the ultraviolet-B photoreceptor UV RESISTANCE LOCUS8 in intact Arabidopsis plants. Plant Physiol. 161: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M., Ma L.G., Qu L.J., Deng X.W. (2002). Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 16: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtan H.E., et al. (2011). BBX32, an Arabidopsis B-box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol. 156: 2109–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ouyang X., Yang P., Lau O.S., Chen L., Wei N., Deng X.W. (2013). Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc. Natl. Acad. Sci. USA 110: 16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ouyang X., Yang P., Lau O.S., Li G., Li J., Chen H., Deng X.W. (2012). Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 24: 4590–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Yang P., Ouyang X., Chen L., Deng X.W. (2014). Photoactivated UVR8-COP1 module determines photomorphogenic UV-B signaling output in Arabidopsis. PLoS Genet. 10: e1004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G.I. (2014). The UV-B photoreceptor UVR8: From structure to physiology. Plant Cell 26: 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Wang Y., Li Q.F., Björn L.O., He J.X., Li S.S. (2012). Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 22: 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Zhang D., Wang X., Tang W., Wang W., Huai J., Xu G., Chen D., Li Y., Lin R. (2013). Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25: 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevei E., et al. (2006). Forward genetic analysis of the circadian clock separates the multiple functions of ZEITLUPE. Plant Physiol. 140: 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.C., Tennessen D.J., Last R.L. (1998). UV-B-induced photomorphogenesis in Arabidopsis thaliana. Plant J. 15: 667–674. [DOI] [PubMed] [Google Scholar]
- Kliebenstein D.J., Lim J.E., Landry L.G., Last R.L. (2002). Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol. 130: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O.S., Deng X.W. (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17: 584–593. [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yang L., Jin D., Nezames C.D., Terzaghi W., Deng X.W. (2013). UV-B-induced photomorphogenesis in Arabidopsis. Protein Cell 4: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oravecz A., Baumann A., Máté Z., Brzezinska A., Molinier J., Oakeley E.J., Adám E., Schäfer E., Nagy F., Ulm R. (2006). CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466. [DOI] [PubMed] [Google Scholar]
- Oyama T., Shimura Y., Okada K. (1997). The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11: 2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L., Favory J.J., Cloix C., Faggionato D., O’Hara A., Kaiserli E., Baumeister R., Schäfer E., Nagy F., Jenkins G.I., Ulm R. (2011). Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106. [DOI] [PubMed] [Google Scholar]
- Shin J., Park E., Choi G. (2007). PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 49: 981–994. [DOI] [PubMed] [Google Scholar]
- Singh A., Ram H., Abbas N., Chattopadhyay S. (2012). Molecular interactions of GBF1 with HY5 and HYH proteins during light-mediated seedling development in Arabidopsis thaliana. J. Biol. Chem. 287: 25995–26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R., Favory J.J., Gruber H., Bartelniewoehner L., Bartels S., Binkert M., Funk M., Weisshaar B., Ulm R. (2010). The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 33: 88–103. [DOI] [PubMed] [Google Scholar]
- Tilbrook K., Arongaus A.B., Binkert M., Heijde M., Yin R., Ulm R. (2013). The UVR8 UV-B photoreceptor: Perception, signaling and response. The Arabidopsis Book 11: e0164, doi/10.1199/tab.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossi V., Lamattina L., Jenkins G.I., Cassia R.O. (2014). Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol. 164: 2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.-L., Li Y.H., Hsieh W.-P., Lin M.-C., Ahn J.H., Wu S.-H. (2014). HUA ENHANCER1 is involved in posttranscriptional regulation of positive and negative regulators in Arabidopsis photomorphogenesis. Plant Cell 26: 2858–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R., Baumann A., Oravecz A., Máté Z., Adám E., Oakeley E.J., Schäfer E., Nagy F. (2004). Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargent J.J., Gegas V.C., Jenkins G.I., Doonan J.H., Paul N.D. (2009). UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol. 183: 315–326. [DOI] [PubMed] [Google Scholar]
- Wu D., Hu Q., Yan Z., Chen W., Yan C., Huang X., Zhang J., Yang P., Deng H., Wang J., Deng X., Shi Y. (2012). Structural basis of ultraviolet-B perception by UVR8. Nature 484: 214–219. [DOI] [PubMed] [Google Scholar]
- Zhang H., He H., Wang X., Wang X., Yang X., Li L., Deng X.W. (2011). Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 65: 346–358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.