Abstract
Following the identification of the male (S-locus Cysteine Rich/S-locus Protein 11) and female (S Receptor kinase [SRK]) factors controlling self-incompatibility in the Brassicaceae, research in this field has focused on understanding the nature of the cellular responses activated by these regulators. We previously identified the ARM Repeat Containing1 (ARC1) E3 ligase as a component of the SRK signaling pathway and demonstrated ARC1’s requirement in the stigma for self-incompatible pollen rejection in Brassica napus, Arabidopsis lyrata, and Arabidopsis thaliana. Here, we discuss our findings on the role of ARC1 in reconstructing a strong and stable A. thaliana self-incompatibility phenotype, in the context of the putative issues outlined in a commentary by Nasrallah and Nasrallah. Additionally, with their proposed standardized strategy for studying self-incompatibility in A. thaliana, we offer our perspective on what constitutes a strong and stable self-incompatibility phenotype in A. thaliana and how this should be investigated and reported to the greater community.
With many angiosperms possessing hermaphroditic flowers, self-incompatibility (SI) systems have evolved to avoid the deleterious effects of inbreeding (Figures 1A and 1B). As defined by Charlesworth et al. (2005), “plant SI systems all prevent self-fertilization through recognition and rejection of pollen by pistils expressing ‘cognate’ allelic specificity.” In Brassicaceae species, the allele specificity is conferred by two well-characterized polymorphic genes encoding the female S Receptor kinase (SRK) and the male S-locus Protein 11/S-locus Cysteine Rich (SP11/SCR; hereby referred to as SCR) (reviewed in Iwano and Takayama, 2012). The major outstanding area in this field is identifying the signaling proteins activated by SRK, determining their function at the cellular level, and investigating whether these signaling proteins have conserved functions across the self-incompatible species in the Brassicaceae. Despite strong interest in finding these potential factors by us and other groups, only the Brassica rapa M Locus Protein Kinase (Murase et al., 2004; Kakita et al., 2007a, 2007b) and the ARM Repeat Containing1 (ARC1) E3 ligase have emerged as direct downstream signaling proteins. We demonstrated a conserved role for ARC1 in self-incompatible Brassica napus (Gu et al., 1998; Stone et al., 1999, 2003), self-incompatible Arabidopsis lyrata (Indriolo et al., 2012), and self-incompatible Arabidopsis thaliana expressing A. lyrata SCRb, SRKb, and ARC1 transgenes (Indriolo et al., 2014). The commentary by Nasrallah and Nasrallah (2014) focuses on our proposed role for ARC1 in reconstituting self-incompatibility in transgenic A. thaliana.
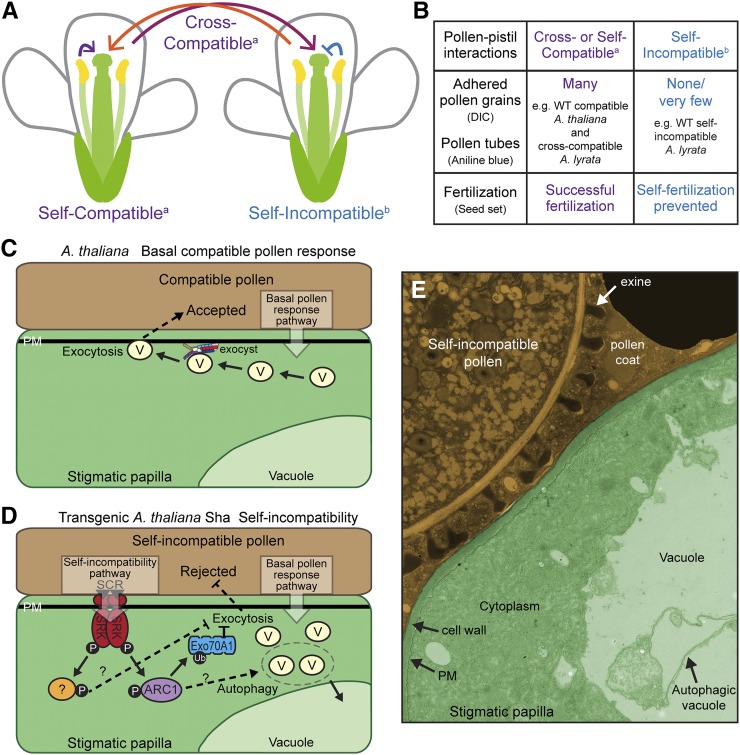
Figure 1.
Pathways for Compatible and Self-Incompatible Pollen Responses in A. thaliana.
(A) Compatible (arrow) and self-incompatible (bar) pollen-pistil interactions.
(B) Criteria for assessing compatible versus self-incompatible pollinations.
(C) Model for the basal compatible pollen response. An unknown basal pollen response pathway is activated in the stigmatic papilla under the compatible pollen grain leading to the activation of vesicle secretion. Our research on Brassica and Arabidopsis Exo70A1 revealed a putative role for the exocyst complex in docking secretory vesicles at the stigmatic papillae plasma membrane (Samuel et al., 2009; Safavian and Goring, 2013; Safavian et al., 2014). Exo70A1 is proposed to assemble with the remaining subunits of the exocyst complex to dock secretory vesicles (reviewed in Zárský et al., 2013). SNARE proteins mediate vesicle fusion to the plasma membrane, and unknown cargo (ACA13 as one candidate; Iwano et al., 2014) are released to enable pollen hydration pollen tube entry through the stigmatic papillar cell wall (compatible pollen is accepted).
(D) Model for the reconstituted self-incompatibility signaling pathway in the Sha ecotype. Following self-pollination in transgenic SCR-SRK+ARC1 Sha ecotype flowers, the pollen SCR ligand binds to SRK at the stigmatic papillar plasma membrane, resulting in the activation of the downstream signaling pathway. The ARC1 E3 ligase is recruited by SRK and targets Exo70A1 for ubiquitination. Even though the basal compatible pollen response pathway has been also activated, ubiquitinated Exo70A1 is somehow inhibited so that exocyst-mediated vesicle secretion to the self-incompatible pollen grain is blocked. In addition, secretory vesicles are degraded in the vacuole through autophagy. An unknown signaling protein (yellow) also has activity in the Sha ecotype in blocking exocytosis (see Samuel et al. [2009], Safavian and Goring [2013], and Indriolo et al. [2014] for further details and references therein).
(E) Transmission electron microscopy image of a self-incompatible pollen-stigmatic papillar interaction at 10 min postpollination from the transgenic SCRb-SRKb+ARC1 Sha ecotype. Pseudocoloring has been added to distinguish the pollen (brown) from the stigmatic papilla (green). Autophagy is detected with the autophagic vacuole in the vacuole (see Rose et al. [2006] and Indriolo et al. [2014] for details). (Figures 1C to 1E adapted from Indriolo et al. [2014], Figures 9 and 10.)
ARC1’S FUNCTION IN THE SI RESPONSE
There is a widespread loss of the ARC1 gene from the A. thaliana genome across 357 ecotypes tested, including the Columbia-0 (Col-0) and Sha ecotypes discussed here, and other ecotypes that still carry an intact SCR or SRK gene (Indriolo et al., 2012 and references therein). Therefore, we proposed that loss of the ARC1 gene likely predated the loss of SCR and SRK genes in A. thaliana, during the transition to selfing from the ancestral self-incompatible state (reviewed in Castric et al., 2014). The requirement of ARC1 for self-pollen rejection in naturally occurring self-incompatible A. lyrata was validated through transgenic ARC1 RNA interference knockdown plants (Indriolo et al., 2012). In the follow-up study, we examined how transforming ARC1 combined with A. lyrata SCRb and SRKb genes into A. thaliana contributed to recapitulating the A. lyrata SI response and demonstrated that the presence of all three transgenes (SCRb-SRKb+ARC1) was correlated with the presence of a strong and stable SI response in A. thaliana (Indriolo et al., 2014).
In our experimental setup, the Nasrallah group’s A. lyrata SCRb-SRKb construct alone or in combination with the A. lyrata ARC1 or B. napus ARC1 transgenes were transformed into A. thaliana Col-0 and Sha ecotypes, and 20 independent primary transformants were selected for each transgene combination. This approach was selected to avoid differences and biases due to expression variation, transgene position effects, or any potential phenotypic plasticity in the ecotypes tested. An initial survey was conducted on these randomly selected primary transformants, and then detailed analyses were conducted on T2 generation plants for three independent lines from each transgenic combination in the Col-0 and Sha ecotypes (Indriolo et al., 2014). All self-incompatible T2 plants were found to carry both the SCRb-SRKb and ARC1 T-DNAs (Indriolo et al., 2014). Transgene expression levels were quantified by quantitative RT-PCR (qRT-PCR) using RNA samples extracted from anther or stigma tissue (harvested from T2 flowers) at the correct developmental stage for all samples (flower buds just starting to open; before anther dehiscence). The transgene qRT-PCR expression analyses included an A. lyrata control for comparison, and the overall conclusion was that ARC1’s impact on the SI response was not due to variable transgene expression levels (see Indriolo et al. [2014] for a detailed discussion).
We found ARC1 to be absolutely required, along with SCRb and SRKb, for generating a stable and robust self-incompatible response in Col-0 plants, but the presence of ARC1 had less of an additive effect in the Sha ecotype (Indriolo et al., 2014). To make this conclusion, we use the widely accepted definition of self-incompatibility: the rejection of self-pollen to prevent self-fertilization (Figures 1A and 1B). This is required to avoid inbreeding and to promote outcrossing: the end goal of self-incompatibility systems (reviewed in Charlesworth et al., 2005; Iwano and Takayama, 2012; Castric et al., 2014). For Col-0, Figure 2 from Nasrallah and Nasrallah (2014) presents data illustrating a “transient self-incompatibility” response for Col-0:AlSCRb-SRKb transformants, following a 2-h pollination at stage 14E [described as “young flowers (early stage 14), before anthers had extended above the stigma and deposited their pollen on the stigma epidermis”; Nasrallah et al., 2002]. These Col-0:AlSCRb-SRKb transformants were described as follows: “Unlike Brassica, however, older SRKb/SCRb flowers regained a measure of receptivity to self pollen” (Nasrallah et al., 2002); are “compatible to self-pollination in mature flowers,” and positive for “open-pollinated seed set” (Table 1 in Nasrallah et al., 2004); and “set as much seed as untransformed plants” (Table 1 in Boggs et al., 2009). The Col-0:AlSCRb-SRKb transformants in Kitashiba et al. (2011) and Yamamoto and Nasrallah (2013) are also fully self-fertile. Based on these published data, the Col-0:AlSCRb-SRKb transformants fail to meet the criteria of preventing self-fertilization and therefore are not fully self-incompatible. In our study, the Col-0:AlSCRb-SRKb transformants produced seed levels (49.1 seeds/silique) comparable to wild-type Col-0 (50.3 seeds/silique). With the addition of ARC1 (Col-0:AlSCRb-SRKb+ARC1), however, these transformants displayed a statistically significant decrease in seed set following self-pollination (5.6 seeds/silique in the strongest line). As expected for an SI response, self-pollinated pistils from these transformants displayed reduced self-pollen attachment (differential interference contrast) and pollen tube growth (aniline blue). Following cross-compatible pollinations between the transformants and wild-type Col-0 flowers, many attached pollen grains and growing pollen tubes were observed as predicted (Figures 1A and 1B). Thus, ARC1 is required for the Col-0:AlSCRb-SRKb+ARC1 transformants to meet the criteria of a self-incompatible plant (Figures 1A and 1B). The effect of ARC1 was not due to changes in A. lyrata SRKb expression levels as shown by qRT-PCR analyses (Indriolo et al., 2014). In addition, the Indriolo et al. (2014) study was conducted in a true wild-type Col-0 background and not in the RNA-dependent RNA polymerase6 mutant background described as a “modifier” by Nasrallah and Nasrallah (2014) and Tantikanjana et al. (2009).
Because A. thaliana Sha:AlSCRb-SRKb transformants were previously reported by the Nasrallah group to have a self-incompatibility phenotype in open flowers with reduced seed production (∼219 seeds/plant; Table 1 in Boggs et al., 2009), we conducted the same transgenic analyses in the Sha ecotype as a comparison. Consistent with Boggs et al. (2009), Indriolo et al. (2014) identified A. thaliana Sha:AlSCRb-SRKb transformants that met the definition of self-incompatibility with statistically significant reductions in seed set in the absence of ARC1 (16.6 seeds/silique in the strongest line). Therefore, both studies show that Sha:AlSCRb-SRKb transformants have self-incompatibility responses preventing self-fertilization. However, we found that adding the ARC1 transgene with SCRb and SRKb significantly enhanced the self-incompatibility phenotype in the Sha ecotype to cause a stronger pollen rejection response resulting in an even greater decrease in seed set (0.7 seeds/silique in the strongest line). All of these transformants also displayed a corresponding decrease in attached pollen grains and pollen tube growth on self-pollinated pistils. Nasrallah and Nasrallah (2014) argue that ARC1 is not needed as the reduced amount of seed set that they observed is lower than what we reported. In our qRT-PCR analyses, equivalent SCRb and SRKb expression levels were observed in the self-incompatible Sha:AlSCRb-SRKb transformants versus the Sha:AlSCRb-SRKb+ARC1 transformants (Indriolo et al., 2014). Since the Nasrallah group publications did not provide quantitative SCRb and SRKb transgene expression data (Nasrallah et al., 2002, 2004; Boggs et al., 2009; Kitashiba et al., 2011; Yamamoto and Nasrallah, 2013), any potential expression level differences could not be determined. Environmental conditions can also influence the self-incompatibility trait (Horisaki and Niikura, 2008), so this will also need to be explored further.
The second question that we investigated was whether ARC1 was required for reconstituting a self-incompatibility response in A. thaliana that produced similar cellular responses to wild-type self-incompatible A. lyrata (Safavian and Goring, 2013). Based on our work and others (see Samuel et al., 2009; Safavian and Goring, 2013; Indriolo et al., 2014 and references therein), our working models for the acceptance of compatible pollen versus the rejection of self-incompatible pollen by the stigmatic papillae are shown in Figures 1C and 1D. Compatible pollen is accepted through a basal signaling pathway that leads to vesicle secretion at the pollen contact site in the stigmatic papilla, which in turn promotes pollen hydration and pollen tube entry into the stigma (Figure 1C). Self-incompatible pollen is rejected by the activation of allele-specific SCR-SRK interactions, and the downstream SRK signaling pathway disrupts vesicle secretion from the simultaneously activated basal pollen response pathway (Figure 1D). ARC1 functions downstream of activated SRK to inhibit Exo70A1 and prevent exocyst-mediated vesicle secretion, resulting in rejection of self-pollen (Figure 1D). In the A. lyrata SI response, we discovered that autophagy is induced by 10 min postpollination, sending vesicles to the vacuole for degradation (Figure 1D; Safavian and Goring, 2013). Similar to A. lyrata, autophagic organelles were detected in the vacuoles of A. thaliana SCRb-SRKb+ARC1 transgenic plants but not in the AlSCRb-SRKb transformants at 10 min postpollination. In addition, the Sha:AlSCRb-SRKb+ARC1 transformants were most similar to A. lyrata with the highest frequency of autophagic organelles in the vacuole at 10 min postpollination (Figure 1E; Indriolo et al., 2014). This suggested that ARC1 is directly or indirectly linked to the autophagy pathway during the Arabidopsis sp self-incompatibility response. Therefore, we concluded that ARC1 is required (along with A. lyrata SCR and SRK genes) to reconstitute an A. lyrata-type self-incompatibility cellular response in A. thaliana.
Finally, the fact that the Sha:AlSCRb-SRKb transformants (in the absence of ARC1) can produce a self-incompatibility response preventing self-fertilization indicates the presence of another downstream signaling protein in the Sha ecotype (Figure 1D). In our B. napus and A. lyrata ARC1 knockdown studies (Stone et al., 1999; Indriolo et al., 2012), we typically observed a partial breakdown of self-incompatibility, and while this phenotype could be due to residual ARC1 activity, it is equally likely to result from the activity of other unknown downstream signaling proteins. Plant receptor kinase signaling pathways are complex (reviewed in Osakabe et al., 2013; Macho and Zipfel, 2014), and so it would be reasonable to expect multiple signaling proteins functioning downstream of SRK. The activity of this putative unknown signaling protein(s) appears to be attenuated in Col-0 (see Indriolo et al. [2014] for an in-depth discussion), and the differences between the Sha and Col-0 ecotypes for this activity will only be fully explained once it has been identified. Whether this unknown signaling protein(s) is another member of the PUB E3 ligase family that ARC1 belongs to (Mudgil et al., 2004; Samuel et al., 2008), and whether it targets Exo70A1, another exocyst subunit, or acts elsewhere in the secretory system to disrupt exocytosis will need to be investigated (Figure 1D).
ISSUES TO CONSIDER IN A STANDARDIZED STRATEGY FOR STUDYING SI IN A. THALIANA
In terms of the recommendations for the adoption of a standardized strategy for functional studies of self-incompatibility in A. thaliana, Nasrallah and Nasrallah (2014) recommended the use of “well-defined true breeding SCR-SRK lines.” We feel that in presenting new data, multiple independently transformed plants should be studied in both the T1 and T2 generations to ensure that the reported phenotypes are reproducible and segregate with the introduced transgenes in progeny of the primary transformants. After this has been established, then working in more detail with well-defined homozygous transgenic self-incompatible line is reasonable. It is also important to work with transgenic lines showing similar transgene expression levels to A. lyrata self-incompatibility genes (i.e., qRT-PCR analyses with A. lyrata controls for comparison). Relative humidity levels should also be tracked during these analyses as pollinations on Brassicaceae dry stigmas can be affected (Safavian et al., 2014 and references therein).
We agree with Nasrallah and Nasrallah (2014) that the pollination analyses should include aniline blue staining for pollen tubes (Figure 1B). Given that self-pollen rejection occurs rapidly at the stigmatic surface, we additionally would like to see corresponding differential interference contrast images of these aniline blue-stained pollinated pistils to view the elongated stigmatic papillae for correct staging and the number of adhered pollen grains. However, this cannot be a stand-alone assay and should be done in conjunction with seed counts. Seed set data are an important measure since self-incompatibility systems exist to prevent self-fertilization (reviewed in Charlesworth et al., 2005; Iwano and Takayama, 2012; Castric et al., 2014). This would certainly help to clear the extensive confusion in the field by identifying transgenic A. thaliana lines that are genuinely self-incompatible. This type of analysis would certainly be expected for any practical application of this breeding system. A simple experiment to confirm that the lack of seed set is not due to other factors is to simultaneously perform reciprocal hand pollinations with a cross-compatible plant (Figure 1A) and quantify seed set (full seed set would be expected in both directions). In our experience, seed set is a very reliable measure in A. thaliana plants grown in growth chambers under standard growth conditions. Finally, it will be important to examine the stigmatic papillar cellular responses and extend these studies to other self-incompatible Brassicaceae species to fully understand the contributions of a candidate signaling protein in the SRK-activated signaling pathway.
Acknowledgments
We thank members of the Goring lab for critically reading this commentary. Research in these laboratories is supported by grants from the Natural Sciences and Engineering Research Council of Canada to D.R.G. and M.A.S. and university start-up grants to M.A.S. and E.I.
AUTHOR CONTRIBUTIONS
D.R.G., E.I., and M.A.S. discussed the ideas and wrote the article.
Footnotes
Articles can be viewed online without a subscription.
References
- Boggs N.A., Nasrallah J.B., and Nasrallah M.E. (2009). Independent S-locus mutations caused self-fertility in Arabidopsis thaliana. PLoS Genet. 5: e1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castric V., Billiard S., and Vekemans X. (2014). Trait transitions in explicit ecological and genomic contexts: plant mating systems as case studies. Adv. Exp. Med. Biol. 781: 7–36. [DOI] [PubMed] [Google Scholar]
- Charlesworth D., Vekemans X., Castric V., and Glémin S. (2005). Plant self-incompatibility systems: a molecular evolutionary perspective. New Phytol. 168: 61–69. [DOI] [PubMed] [Google Scholar]
- Gu T., Mazzurco M., Sulaman W., Matias D.D., and Goring D.R. (1998). Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proc. Natl. Acad. Sci. USA 95: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisaki A., and Niikura S. (2008). Developmental and environmental factors affecting level of self-incompatibility response in Brassica rapa L. Sex. Plant Reprod. 21: 123–132. [Google Scholar]
- Indriolo E., Tharmapalan P., Wright S.I., and Goring D.R. (2012). The ARC1 E3 ligase gene is frequently deleted in self-compatible Brassicaceae species and has a conserved role in Arabidopsis lyrata self-pollen rejection. Plant Cell 24: 4607–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo E., Safavian D., and Goring D.R. (2014). The ARC1 E3 ligase promotes two different self-pollen avoidance traits in Arabidopsis. Plant Cell 26: 1525–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M., and Takayama S. (2012). Self/non-self discrimination in angiosperm self-incompatibility. Curr. Opin. Plant Biol. 15: 78–83. [DOI] [PubMed] [Google Scholar]
- Iwano M., Igarashi M., Tarutani Y., Kaothien-Nakayama P., Nakayama H., Moriyama H., Yakabe R., Entani T., Shimosato-Asano H., Ueki M., Tamiya G., and Takayama S. (2014). A pollen coat-inducible autoinhibited Ca2+-ATPase expressed in stigmatic papilla cells is required for compatible pollination in the Brassicaceae. Plant Cell 26: 636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakita M., Shimosato H., Murase K., Isogai A., and Takayama S. (2007a). Direct interaction between the S-locus receptor kinase and M-locus protein kinase involved in Brassica self-incompatibility signaling. Plant Biotechnol. 24: 185–190. [Google Scholar]
- Kakita M., Murase K., Iwano M., Matsumoto T., Watanabe M., Shiba H., Isogai A., and Takayama S. (2007b). Two distinct forms of M-locus protein kinase localize to the plasma membrane and interact directly with S-locus receptor kinase to transduce self-incompatibility signaling in Brassica rapa. Plant Cell 19: 3961–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitashiba H., Liu P., Nishio T., Nasrallah J.B., and Nasrallah M.E. (2011). Functional test of Brassica self-incompatibility modifiers in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 108: 18173–18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A.P., and Zipfel C. (2014). Plant PRRs and the activation of innate immune signaling. Mol. Cell 54: 263–272. [DOI] [PubMed] [Google Scholar]
- Mudgil Y., Shiu S.H., Stone S.L., Salt J.N., and Goring D.R. (2004). A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U-box E3 ubiquitin ligase family. Plant Physiol. 134: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Shiba H., Iwano M., Che F.S., Watanabe M., Isogai A., and Takayama S. (2004). A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science 303: 1516–1519. [DOI] [PubMed] [Google Scholar]
- Nasrallah J.B., and Nasrallah M.E. (2014). Robust self-incompatibility in the absence of a functional ARC1 gene in A. thaliana. Plant Cell 26: 10.1105/tpc.114.127712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah M.E., Liu P., and Nasrallah J.B. (2002). Generation of self-incompatible Arabidopsis thaliana by transfer of two S locus genes from A. lyrata. Science 297: 247–249. [DOI] [PubMed] [Google Scholar]
- Nasrallah M.E., Liu P., Sherman-Broyles S., Boggs N.A., and Nasrallah J.B. (2004). Natural variation in expression of self-incompatibility in Arabidopsis thaliana: implications for the evolution of selfing. Proc. Natl. Acad. Sci. USA 101: 16070–16074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y., Yamaguchi-Shinozaki K., Shinozaki K., and Tran L.S. (2013). Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. J. Exp. Bot. 64: 445–458. [DOI] [PubMed] [Google Scholar]
- Rose T.L., Bonneau L., Der C., Marty-Mazars D., and Marty F. (2006). Starvation-induced expression of autophagy-related genes in Arabidopsis. Biol. Cell 98: 53–67. [DOI] [PubMed] [Google Scholar]
- Safavian D., and Goring D.R. (2013). Secretory activity is rapidly induced in stigmatic papillae by compatible pollen, but inhibited for self-incompatible pollen in the Brassicaceae. PLoS One 8: e84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavian D., Jamshed M., Sankaranarayanan S., Indriolo E., Samuel M.A., and Goring D.R. (2014). High humidity partially rescues the Arabidopsis thaliana exo70A1 stigmatic defect for accepting compatible pollen. Plant Reprod. 27: 121–127. [DOI] [PubMed] [Google Scholar]
- Samuel M.A., Mudgil Y., Salt J.N., Delmas F., Ramachandran S., Chilelli A., and Goring D.R. (2008). Interactions between the S-domain receptor kinases and AtPUB-ARM E3 ubiquitin ligases suggest a conserved signaling pathway in Arabidopsis. Plant Physiol. 147: 2084–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Chong Y.T., Haasen K.E., Aldea-Brydges M.G., Stone S.L., and Goring D.R. (2009). Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 21: 2655–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S.L., Anderson E.M., Mullen R.T., and Goring D.R. (2003). ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. Plant Cell 15: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S.L., Arnoldo M., and Goring D.R. (1999). A breakdown of Brassica self-incompatibility in ARC1 antisense transgenic plants. Science 286: 1729–1731. [DOI] [PubMed] [Google Scholar]
- Tantikanjana T., Rizvi N., Nasrallah M.E., and Nasrallah J.B. (2009). A dual role for the S-locus receptor kinase in self-incompatibility and pistil development revealed by an Arabidopsis rdr6 mutation. Plant Cell 21: 2642–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., and Nasrallah J.B. (2013). In planta assessment of the role of thioredoxin h proteins in the regulation of S-locus receptor kinase signaling in transgenic Arabidopsis. Plant Physiol. 163: 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárský V., Kulich I., Fendrych M., and Pečenková T. (2013). Exocyst complexes multiple functions in plant cells secretory pathways. Curr. Opin. Plant Biol. 16: 726–733. [DOI] [PubMed] [Google Scholar]