Abstract
Staphylococcal enterotoxin A (SEA) is a bacterial superantigen that induces pronounced T cell expansion and cytokine production. In addition, SEA activates the HPA axis and forebrain regions relevant to cognitive functions. Since learning-related cognitive changes have not been assessed in response to SEA, spatial learning in the morris water maze (MWM) was determined in male C57BL/6J mice subjected to acute or repeated injections of 5 μg SEA or Saline. Injections were given 2 hrs prior to 4–5 days of hidden platform sessions. Animals were then rested for 1 month and given retraining without further injections. In addition, splenic IL-1β, IL-2 and TNFα, plasma corticosterone, and hippocampal IL-1β and TNFα were measured after the regimen of treatment used in the behavioral experiments. The results showed no learning impairment following acute or repeated SEA challenge. Moreover, when retested one month later, and without further injections, the SEA group showed more rapid relearning of the MWM. This suggested that coincidental superantigenic T cell activation and training served to promote long-term improvement in recovery of learning. Furthermore, repeated SEA challenge continued to drive increases in plasma corticosterone, but with a compensatory reduction in hippocampal IL-1β. However, while hippocampal TNFα was reduced after acute and repeated SEA treatment, this was not statistically significant. In view of the importance of modest glucocorticoid elevations and hippocampal IL-1β in promoting contextual learning, the data point to the hypothesis that SEA promotes long-term plasticity by restraining disruptive increases in hippocampal IL-1β, and possibly TNFα, during learning.
Keywords: Staphylococcal enterotoxin A, cytokines, corticosterone, interleukin-1β, tumor necrosis factor, interleukin-2, morris water maze, learning, hippocampus, T cells
INTRODUCTION
There is increasing evidence that T cells are actively involved in the modulation of neural and behavioral functions, with possible relevance to psychiatric conditions (Kipnis et al., 2004; Kipnis et al., 2008; Miller, 2010). In a model of in vivo activation of T cells with the superantigens staphylococcal enterotoxins A and B (SEA and SEB), it was shown that there is activation of selective areas of the brain involved in cognition and stress regulation (reviewed by Urbach-Ross & Kusnecov, 2009). The staphylococcal enteroxins have become recognized as strong activators of CD4+ and CD8+ T cells via binding of external regions of the Vβ chain of the TCR (Sundberg et al., 2007). These SEs are referred to as “superantigens” by virtue of their ability to cause pronounced oligoclonal T cell expansion, with an attendant high output of cytokines, including classic T cell cytokines, such as IL-2, IL-4 and IFNγ, as well as IL-1β, TNFα, IL-6 and IL-10 (Rosendahl et al., 1997; Urbach-Ross et al., 2008). The relative appearance and concentration of these cytokines in the circulation varies with the number of superantigenic exposures. For example, whereas a single acute injection with SEA induces measureable and high plasma concentrations of TNFα and IL-2, increasing numbers of injections (given 5 days apart) can result in a reduction of TNFα and IL-2, with a corresponding increased appearance of other cytokines, such as IFNγ, IL-6 and IL-10 (Urbach-Ross et al., 2008).
The high cytokine response to superantigens, such as SEA and staphylococcal enteroxin B (SEB), is associated with neural activation (Urbach-Ross & Kusnecov, 2009). Challenge with SEA and SEB increased expression of the immediate early gene (IEG), c-fos, in the prefrontal cortex, hippocampus, hypothalamus, amygdala, and hindbrain regions involved in central autonomic regulation, such as the nucleus tractus solitaries and locus coeruleus (Goehler et al., 2001; Wang et al., 2004; Rossi-George et al., 2005; Serrats & Sawchenko, 2006). Indeed, challenge with the T cell superantigen, SEB, produces central IEG expression in a vagus-dependent manner (Wang et al., 2004; Serrats & Sawchenko, 2009). Interestingly, the vagus provides afferent input into the NTS, one of the areas activated by SEB, and which contains receptors for TNFα (Hermann & Rogers, 2008), a cytokine necessary for HPA axis activation and food neophobia in mice challenged with SEA (Rossi-George et al., 2005). Finally, the neuroendocrine effects of SEA and SEB, involve the influence of the neuropeptides CRH and orphaninFQ/nociceptin (Kaneta & Kusnecov, 2005; Mallimo et al., 2010), which are well recognized in the modulation of anxiety and arousal (Heinrichs & Koob, 2004).
The CNS impact of immune activation results in significant changes across multiple domains of behavior. The most common categorical label for this effect is sickness behavior, which is applied when immunologically challenged animals display indices of impaired motivation, such as reduced locomotion due to lethargy and/or fatigue, reduced food intake due to anorexia and/or malaise and inhibited exploratory behavior (eg., investigation of social conspecifics) (Dantzer et al., 2008). However, although these overt indices of illness may reflect adaptive reactions to somatic sensations and/or signals pursuant to the elaboration of systemic inflammatory responses, other less obvious alterations may be involved. For example, the basis for anorexia may be anxiety and/or anhedonia (Anisman & Merali, 1999), as opposed to malaise. With regard to the behavioral sequelae of T cell activation with superantigens, there is little evidence that neurally active doses of SEA (or SEB) are associated with frank malaise, loss of body weight, and reduced food intake (Kusnecov et al., 1999; Kawashima & Kusnecov, 2002). However, subtle manipulations of stimulus characteristics suggest that superantigen challenge does not disrupt adaptational cognitive processes. For example, previously experienced foods are not rejected in SEA or SEB challenged mice, but new foods provided in a new environment result in significant reduction of intake (Kusnecov et al., 1999; Kawashima & Kusnecov, 2002; Rossi-George et al., 2005). Centrally, this was shown to be CRH-dependent (Kaneta & Kusnecov, 2005), and linked to TNFα production (Rossi-George et al, 2005), a finding consistent with evidence in rats that TNFα administration reduces food intake if the food is novel (Bernstein et al., 1991). Finally, due to the involvement of CRH and HPA axis activation, it was hypothesized that these behaviors might have involved increased levels of anxiety after SEA challenge. However, challenge with SEA failed to reduce open arm entries in the elevated plus maze, but rather, increased emergence into the distal portions of the open arms (Rossi-George et al., 2004). The significance of this is not clear, although as with the inhibited approach to unique or novel attributes of gustatory and non-gustatory stimuli (Kawashima & Kusnecov, 2002), it is possible that SEA administration orients animals to novel features of the environment. For example, SEA modulates CRH mRNA in the amygdala (Kohman et al., 2009), wherein CRH may be more important for attention to novel and biologically meaningful gustatory and contextual stimuli (Merali et al., 2004).
In view of these findings, the aim of the current study was to determine whether SEA-challenge influences performance in a learning environment requiring attention to spatial cues. The impact of immune processes on learning and memory has already been addressed using LPS and various cytokines as summarized in a recent review (Cunningham & Sanderson, 2008). Many of the studies reviewed examined Morris Water Maze (MWM) learning. Although relatively less information exists on the role of T cells in learning, it does appear that T cell deficiencies are associated with impaired learning in the MWM (Kipnis et al., 2004; Wolf et al., 2009; Derecki et al., 2010). This suggests that T cells are required for optimal neural functioning under conditions of behavioral adaptation.
Therefore, the current study investigated whether activation with T cell superantigens has a modulatory effect on learning. Only one previous study has reported a learned effect of a bacterial superantigen, showing the presence of intact taste aversion learning of rats challenged with SEB, which resulted in a conditioned elevation of IL-2 production (Pacheco-Lopez et al., 2004). Interestingly, chronic IL-2 administration to mice impaired MWM learning, (Lacosta et al., 1999), although the impact of endogenous elevations of IL-2 remain to be determined. Moreover, the neuroendocrine and behavioral effects of SEA are dependent on TNFα (Rossi-George et al., 2005), and TNFα was shown to influence long term potentiation in the hippocampus (Butler et al., 2004). Consequently, in the current study MWM learning was assessed in animals given a single or multiple challenges with SEA. Unlike previous studies using the MWM, the present study also determined long-term effects of SEA challenge on the ability of mice to relearn the MWM. Finally, previous findings of multiple SEA exposures given at 5 day intervals were extended by determining the impact of daily consecutive SEA injections on hippocampal IL-1β and TNFα production, and plasma corticosterone. Since the MWM experiments involved repeated daily SEA injections, this latter experiment was necessary to confirm the continued impact of SEA on CNS function.
METHODS
Animals
Normal C57BL6/J mice were purchased from Jackson Laboratories and housed 4/cage with ad lib access to food and water under a 12:12 hr light:dark cycle (Lights on 0600 hr). Mice were undisturbed for one month prior to experimental manipulation, and at the time of commencing experimentation, were aged 4–6 months. All procedures were conducted according to NIH guidelines and approved by the Institutional Animal Care and Use Committee of Rutgers University.
General Experimental Procedure
Animals were injected IP with a volume of 0.2 ml Staphylococcal enterotoxin A (1 μg/ml in saline; Toxin Technology, Florida) or 0.9% saline. Two hours after injection, animals were subjected to behavioral testing. Depending on the experiment, animals were injected only once (Experiment 1: Acute SEA) or daily for four days (Experiment 3: Repeated SEA). A final experiment was conducted to determine the impact of four daily injections of SEA or saline on cytokine production. No behavioral testing was conducted in this experiment.
Behavioral Testing Apparatus
The apparatus for the Morris Water Maze (MWM) consisted of a steel circular tub (diameter: 110 cm; height: 59 cm). The apparatus was filled with regular tap water and maintained at a temperature of approximately 22°C; water opacity was achieved using non-toxic Crayola washable paint. The platform consisted of a clear plexiglas disc [diameter: 9 cm], perforated with small holes to provide stability once mice mounted the platform. The platform itself rested at a height of 48 cm, and was mounted on a steel rod affixed to a heavy metal base. During acquisition trials, the platform was hidden 1 cm below the water surface. A white canvass curtain completely surrounded the pool from floor to ceiling, and was immediately adjacent to the rim of the apparatus; the opening in the curtain used to place and retrieve animals from the pool was closed during testing. The interior of the curtain was affixed with cues of various shapes and sizes (a constellation of stars near the ‘east’ quadrant, a large cross near the ‘north’ quadrant, and a rectangle of black and white stripes near the ‘south’ quadrant); the lower edge of each set of cues was located 5–7 cm above the rim of the apparatus. The cues were present at all times of testing.
Experiment 1: Effects of Acute SEA Challenge
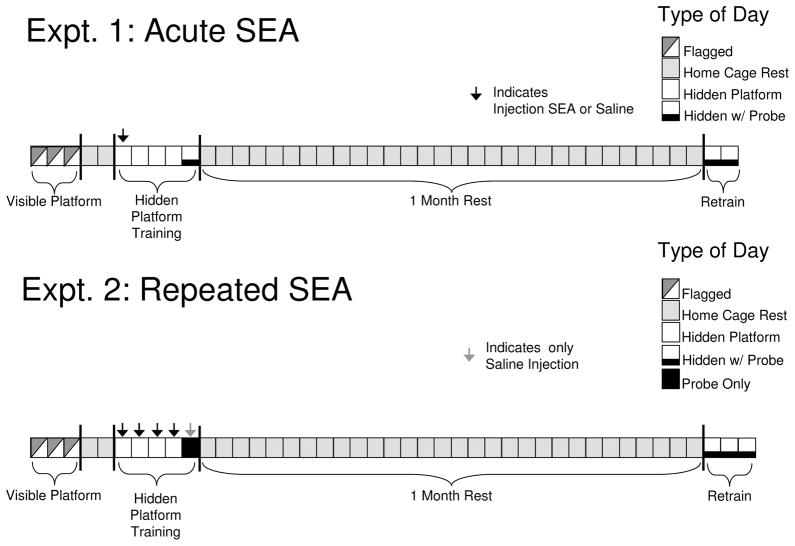
In Experiment 1, two groups of animals (N=8/group) were injected once with SEA or Saline and subjected to hidden platform training in the MWM as detailed below. There were two phases to this experiment: In phase 1, animals were introduced and trained for the first time in the pool; in phase 2, animals were retrained 1 month after the probe trial given in phase 1; in phase 3, animals were tested one week after the end of phase 2. Phase 1 consisted of three stages: visible platform testing (flagged training), hidden platform testing (acquisition), and a short-term memory retention test (probe trial) 30 minutes after the fourth trial on the final day of acquisition). The injection of SEA or Saline noted above was given two hours prior to the first acquisition trial of hidden platform testing (see Figure 1A).
Figure 1.
Experimental design for testing the effects of a single (Experiment 1) or repeated (Experiment 2) injections of SEA on morris water maze learning. Injections were given during the initial week of hidden platform learning.
Phase 1: Visible platform training
Visible (flagged) platform testing was performed for three consecutive days and was carried out to identify any visual or motor deficits that could impair performance during hidden platform testing. Daily sessions of visible platform testing consisted of placing mice into the maze for four separate trials, thereby being approximately 2–3 minutes between trials. In each session the escape platform remained submerged but its location was indicated through the use of a circular, three dimensional, high contrast (patterned) flag. Prior to the start of each succeeding trial the platform was placed into a novel location. Mice were run in squads of 4 subjects at a time and were towel dried and placed in heated cages between trials.
Phase 2: hidden platform trials (week 1)
Hidden platform trials were begun two days after completion of visible platform trials. Two hours prior to the beginning of hidden platform testing, animals were injected with SEA or Saline as stated above; there were no further injections for the remainder of the experiment. Hidden platform testing consisted of 4 trials per day for 5 consecutive days. While for each animal platform location remained stable during hidden platform testing, a novel start location was given for each trial. Start locations were designated as north (N), south (S), east (E), and west (W), which represented the four separate quadrants of the maze. Although platform location was always the same for individual animals, the location of the platform varied across animals, such that two animals in each group had the platform in the N, S, E or W quandrant. The location of the platform was always in the center of a quadrant. Furthermore, start locations were counter balanced within each four-animal squad tested, with each squad containing two SEA and two Saline-treated animals. On Day 5, a probe trial was conducted 30 minutes after the final hidden platform trial. For the probe trial, the platform was removed from the pool and mice were given 60 seconds to search for the platform.
Phase 3: Hidden platform retraining after 1 month rest (week 2)
After completion of the probe trial in Phase 2, mice remained in the colony room for 1 month, after which they were returned to the MWM and subjected for two days to retraining for hidden platform testing. This procedure was similar to that described above, with the exception that a probe trial was given 30 minutes after the final trial on each day.
Behavioral Scoring in the Morris Water Maze
All sessions were video-recorded with an overhead camera. An experimenter observed the animals on a video monitor, timing the latency of animals to find the platform using a stop watch. Subsequent to completion of each session, the video tapes were digitally processed by videotracking software (SMART-Spontaneous Motor Activity Recording & Tracking; San Diego Instruments, San Diego) that similarly determined a range of parameters that included (i) distance travelled, (ii) latency to reach the platform, (iii) percent of time spent in each quadrant, and (iv) velocity of movement.
Experiment 2: Effects of Repeated SEA Treatment
The MWM testing in this experiment was similar to that of Experiment 1, with some exceptions. Two groups of animals (SEA or Saline: N=8/group) were run through phase 1 (flagged) trials, then two days after the final trial introduced to Phase 2 (hidden platform trials). There were a total of four daily sessions of hidden platform trials (4 trials/session), with animals being injected with SEA or Saline 2 hrs prior to each session on all 4 days. On Day 5 (24 hrs after the fourth acquisition session), animals were not subjected to a single probe trial (i.e. there were no hidden platform trials). Subsequent to this probe trial, animals were rested for 1 month in the colony room, after which they were introduced to phase 2. In phase 2, animals received three daily sessions of hidden platform trials (4/session), with a probe trial being given 30 minutes after the final trial in each session. Animals did not receive any injections during phase 2, and were not given any further testing after completion of this phase.
Effect of SEA on the Cytokine and Endocrine Response
A experiment was conducted to determine whether repeated SEA injections resulted in altered cytokine and corticosterone production in blood, spleen and brain. Animals were placed into one of three groups: (i) four daily injections of Saline, (ii) three daily injections of Saline, followed by a fourth day on which animals were given an injection 5 μg SEA, and (iii) four daily injections of SEA (5μg/animal). All injections were given on consecutive days, and 2 hours after the final injection on day 4, animals were sacrificed by decapitation. Trunk blood, brains and spleens were collected. The 2 hr time point was chosen, since it is optimal for increased HPA axis and cytokine production. Trunk blood was collected by rapid decapitation into heparin-treated vacutainer tubes (Becton Dickinson, Rutherford, NJ), centrifuged immediately at 2000 rpm for 15 min, and the plasma stored at −70°C. Spleens and brains were removed and flash frozen in 2-methylbutane and stored at −70°C until protein extraction and quantitation as previously described (Urbach-Ross et al., 2008).
Protein and Cytokine Assays
On the day of assay, spleens and brains were removed for protein extraction. The brain was dissected while still frozen and the hippocampus isolated as previously described (Kawashima et al, 2002) (Kawashima et al., 2002). Tissues were placed immediately in 1 ml of 1 mM Phenylmethanesulfonyl fluoride (PMSF) in 0.1 M phosphate buffer to inhibit protease activity, and then homogenized and centrifuged at 4000 RPM for 30 min. The supernatant was quantified for total protein using the BCA protein assay kit (Pierce, Rockford, IL). Absorbance was read at 562 nm and a bovine serum albumin (BSA) standard curve was used to calculate sample protein levels (expressed as μg/ml). To determine the tissue concentration of TNFα and IL-1β, the supernatants assayed for total protein were assayed using OptEIA ELISA kits according to the manufacturer’s instructions (BD Biosciences, San Diego, CA). Lower limits of cytokine detection were in the range of 3 pg/ml (IL-2) and 10 pg/ml (IL-1β and TNFα). For assay of hippocampal homogenates, samples were assayed undiluted. Splenic samples were diluted in assay diluents as follows: 1:40 for IL-2, and 1:4 for IL-1 and TNFα. Well absorbance was read at 450 nm using EL800 universal BioTek microplate reader, and the concentration of cytokine calculated off a standard curve using KC Junior software (Biotek). Given variations in spleen size and cellular numbers, the cytokine concentration was expressed as a ratio of total protein (pg of cytokine/μg of protein).
Corticosterone Radioimmunoassay (RIA)
Corticosterone was measured in plasma using a ImmunoChem™ Double Antibody Corticosterone 125I kit (MP Biomedicals, Irvine, CA), with a lower detection limit of 25 ng/ml. All standards and samples were run in duplicate and counted using a Cobra II Auto Gamma counter. The corticosterone data were expressed as ng/ml plasma.
Data Analysis
Behavioral data collected during flagged or hidden platform training was analyzed using ANOVA with repeated measures. Probe trials were analyzed using either student’s t-test, or ANOVA with repeated measures when days was included as a variable. Cytokine and corticosterone data were analyzed by one-way ANOVA, and either the Neuman-Keuls or Dunnett’s post hoc test where deemed appropriate. Statistical significance was set at p < 0.05.
RESULTS
Morris Water Maze Phase 1: Visible Platform Training
In both Experiments 1 and 2 visible (i.e. flagged) platform training was performed for all animals prior to random allocation to SEA or Saline treatments. As shown in Table 1, over the course of three daily sessions (4 trials/day), all animals displayed rapid learning, achieving average escape latencies of 27–33 seconds, and then progressing to greater than 50% faster latencies on the second day. These data serve as evidence that all animals were visually and cognitively proficient in locating the specific cue (i.e. flag) locating the platform.
Table 1.
Escape Latencies in Seconds During Visible Platform Training
| Experiment | Treatment* | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|
| Expt 1: Acute | Saline | 33.50 ± 3.79 | 17.13 ± 4.56 | 6.99 ± 0.12 |
| SEA | 35.03 ± 2.39 | 10.25 ± 1.38 | 6.12 ± 0.66 | |
| Expt 2: Repeated | Saline | 28.25 ± 2.87 | 7.97 ± 0.86 | 6.59 ± 0.88 |
| SEA | 27.56 ± 5.50 | 7.38 ± 2.10 | 8.5 ± 0.97 |
Morris Water Maze Phase 2: Hidden Platform Training (week 1)
Two days following completion of Phase 1, animals were injected with SEA or Saline and subjected to hidden platform training as described in Materials and Methods. Table 2 summarizes the average escape latency for each group across days of training. Repeated measures ANOVAs were conducted on the escape latency and total distance (data not shown) in each Experiment, with ANOVAs including trials as a variable. For Experiment 1, latency and distance data showed independent effects of Days (latency: F(4,56) = 2.38, p = 0.06; distance: F(4,56) = 4.82, p = 0.002), and Trials (latency: F(3,42) = 4.2, p = 0.01; distance: F(3.42) = 6.35, p = 0.001). However, there was no main effect of treatment, nor interactions between treatment and other variables. Similarly, in Experiment 2, there was no main effect of treatment, but significant changes were found across Days (latency: F(4,56) = 23.3, p = 0.001; distance: F(4,56) = 18.3, p = 0.001), and Trials (latency: F(3,42) = 5.0, p = 0.005; distance: F(3.42) = 2.74, p = 0.056). These analyses revealed that for both experiments learning had taken place across the different days of acquisition training. However, this learning did not appear to be affected by SEA challenge, whether given at one time only (Experiment 1) or prior to each session of training (Experiment 2).
Table 2.
Escape Latencies in Seconds During Week 1 Hidden Platform Training
| Experiment | Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|---|
| Expt 1: Acute | Saline | 23.70±4.17 | 19.45±4.64 | 16.01±4.31 | 17.20±4.39 | 15.98±3.01 |
| SEA | 27.04±5.64 | 16.29±3.21 | 21.33±5.46 | 17.98±5.79 | 16.36±4.36 | |
| Expt 2: Repeated | Saline | 22.97±4.34 | 10.78±2.28 | 9.50±3.00 | 4.84±0.67 | n.d. |
| SEA | 18.13±2.68 | 13.06±1.98 | 9.75±2.37 | 4.72±1.15 | n.d. |
Morris Water Maze Phase 3: Retraining One Month After Hidden Platform Training
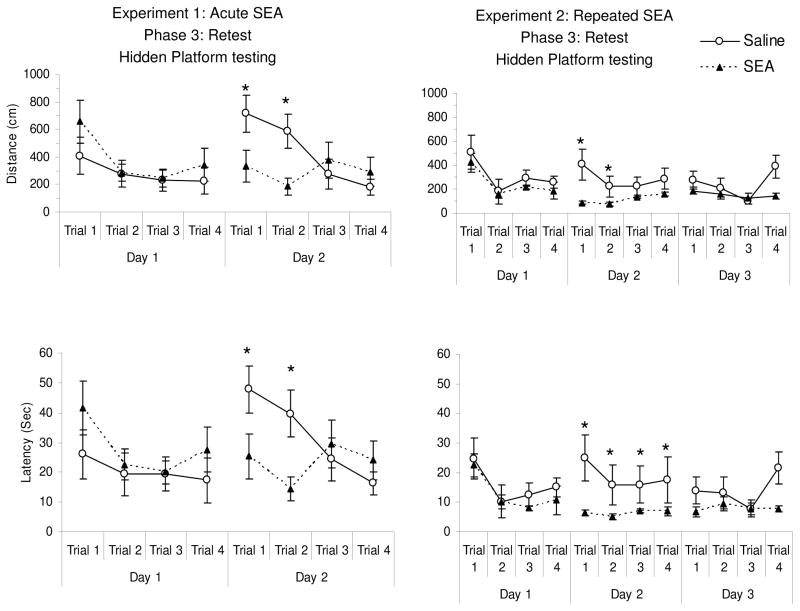
As described in Materials and Methods (also see Figure 1), animals in both Experiments 1 and 2 were rested for 1 month before being returned to the MWM and subjected to hidden platform trials. Figure 2 provides the latency and distance data. One animal (from the saline group in Experiment 1) was excluded from retesting due to a technical error with recording. For Experiment 1, a three-way repeated measures ANOVA of latency and distance showed a three-way treatment x days x trials interaction (latency: F(3,39) = 2.713, p = 0.05; distance: F(3,39) = 3.0, p = 0.04). For both latency and distance, this three-way interaction was evident as a marked difference in performance between SEA and saline groups on day 2 of testing (see Figure 2). A separate two-way ANOVA conducted for the Day 2 data confirmed that there was a significant treatment x trials interaction (latency: F(3,39) = 2.713, p = 0.05; distance: F(3,39) = 3.0, p = 0.04). Surprisingly, the saline group displayed increased latency on trials 1 and 2 of the second day, in spite of having had lower latencies on day 1. Such effects have been noted by others, and are described as “saw-tooth” phenomena whereby performance on the first and/or second trials of each session is less than the final trial on the previous day (Vorhees & Williams, 2006). While this was evident here, it was not observed for the SEA group. Similar data were obtained in Experiment 2 (see Figure 5), whereby Day 2 distance achieved significance for treatment (F(1,42) = 4.6, p = 0.05), but showed a marginal influence of treatment for latency (F(1,42) = 3.85, p = 0.07).
Figure 2.
Distance and latency to reach the platform one month after initial training (as shown in Table 2) for animals in Experiments 1 and 2. The SEA groups showed reduced distance and latency (* p < 0.05, as based on a significant trials x treatment interaction, see Results) in both Experiments on Day 2 of relearning. Each point represents the mean (+/− standard error of the mean) of N= 8 animals per group.
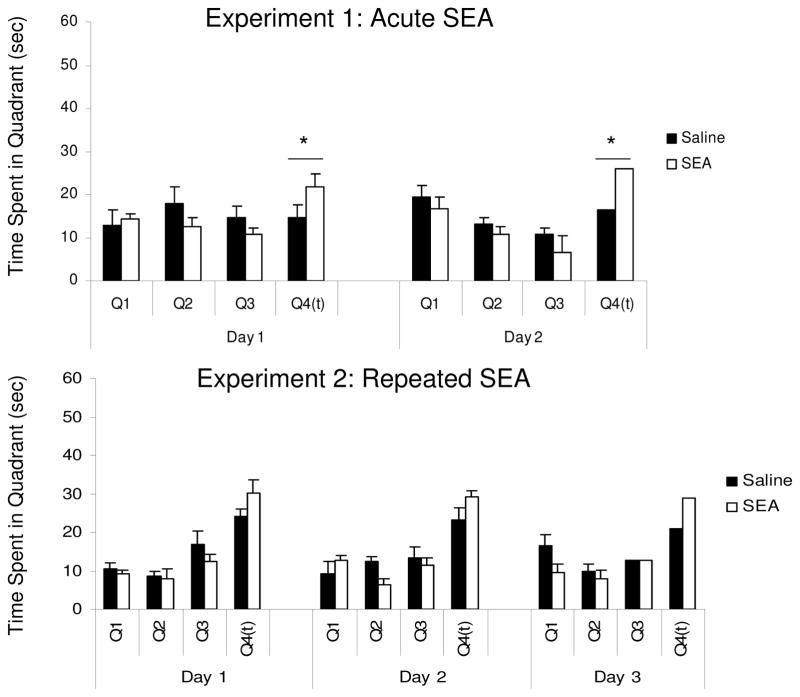
The results of Probe testing during the retraining sessions are shown in Figure 3, which presents the time spent in each quadrant (see Figure 3). For Experiment 1, ANOVA revealed a significant Quadrant x Treatment interaction (F(1,39) = 2.8, p = 0.05). Simple effects analyses for each quadrant revealed only a significant effect of Treatment for the trained quadrant (F(1,13) = 6.28, p = 0.026), indicating greater time spent in the target quadrant by the SEA group. Alternatively, for Experiment 2, the Quadrant x Treatment interaction was marginal (F(3,42) = 2.44, p = 0.07). Given that this interaction was consistent with that for Experiment 1, a simple effects analysis was conducted for each quadrant. This revealed a treatment effect only for the trained quadrant (F(1,28) = 5.67, p = 0.03), which is evident across all days as longer time spent in the target quadrant for animals in the SEA group (see Figure 3).
Figure 3.
Relative amounts of time (in seconds) spent in the four quadrants of the morris water maze during probe trials given during the relearning phase one month after initial training. Each probe trial was given for 60 seconds, and Q4(t) indicates the quadrant where the platform was normally present. Each bar represents the mean (+/− standard error of the mean) of N= 8 animals per group. In both experiments, the SEA group showed a statistically significant higher preference (* p < 0.05) for the target quadrant (as determined by a ANOVA conducted on the Q4(t) time data, see Results).
Cytokine and Corticosterone Responses
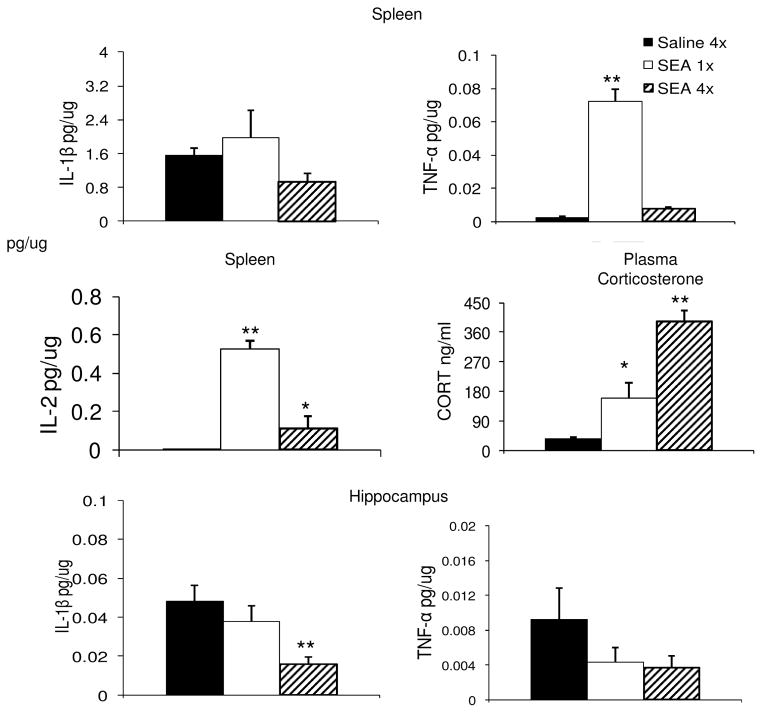
We have consistently demonstrated that acute SEA challenge increases the production of TNFα, as well as elevating plasma corticosterone (Urbach-Ross & Kusnecov, 2008). Since Experiment 2 involved daily injections of SEA, a separate experiment was conducted to determine whether daily treatment with SEA continues to produce these effects. The results showed that by the fourth injection of SEA, splenic levels of TNFα were substantially reduced from the high amount induced by a single SEA injection (F(2,13) = 129.3, p < 0.001), which was also observed for IL-2 (See Figure 4). In addition, we also measured spleen IL-1β, but this was not found to be significantly altered by SEA (F(2,13) = 2.47, p = 0.12). Coincident with these splenic cytokine levels, measures of plasma corticosterone concentrations showed a significant increase in both the single and repeatedly injected SEA groups (F(2,13) =43.0, p < 0.0001). Interestingly, while both SEA groups had higher plasma corticosterone than the Saline group (p < 0.05), animals given four injections of SEA were higher than those given a single SEA challenge (p < 0.05) (see Figure 4). Finally, since previous research had identified hippocampal IL-1β and TNFα as potential influences on learning and memory (Yirmiya et al., 2002), including neurophysiological correlates of learning, such as long-term potentiation (Butler et al., 2004), whole hippocampi were assayed for concentrations of murine IL-1β and TNFα. Analysis by one-way ANOVA revealed a significant main effect only for IL-1β (F(2,13) = 6.83, p < 0.01), but not TNFα (F(2,13) = 1. 4, p = 0.28). Inspection of Figure 4 shows that hippocampal IL-1β was lowest for the group that received four injections of SEA, and this was significantly different from both the Saline and 1x SEA groups (p < 0.05, Dunnett’s Test).
Figure 4.
Effects of acute (N=4) or repeated (N=6) SEA treatment on splenic IL-1β, TNFα and IL-2, plasma corticosterone, and hippocampal IL-1β and TNFα. The saline group consisted of N=6 animals. Each bar represents the mean +/− standard error of the mean. All animals were sacrificed 2 hrs after the final injection. * p < 0.05, ** P < 0.01 relative to Saline.
DISCUSSION
Staphylococcal enterotoxins A and B (SEA and SEB) are regarded as T cell superantigens, and have previously been shown to exert significant endocrine and neural effects in mice and rats (Wang et al., 2004; Serrats & Sawchenko, 2006; Kohman et al., 2009; Urbach-Ross & Kusnecov, 2009). While most evidence has pointed to SEA-induced alterations in gustatory and anxiety-like behaviors (Urbach-Ross & Kusnecov, 2009), cognitive functions have not yet been investigated. In the current study, results from two experiments involving MWM learning, failed to show significant retardation of acquisition after varying numbers of SEA exposure. Interestingly, in other studies that involved LPS, there was little disruption of learning during the early sessions of the MWM (Shaw et al., 2001), although other studies have observed early reduction of latency, increased swimming distance, and deficits in the use of working memory in the MWM (Arai et al., 2001; Sparkman et al., 2005; Sparkman et al., 2006). Nonetheless, in some of these studies (Sparkman et al, 2005), it was evident that swimming speed was reduced, and that this may have accounted for delayed location of the hidden platform (Cunningham & Sanderson, 2008). In regard to the present study, latency to find the platform was not increased by SEA treatment, nor was swimming speed. While this is not inconsistent with findings that LPS and low doses of IL-1 do not impair learning (Cunningham & Sanderson, 2008)), varying the difficulty of the water maze may confirm out whether SEA is without effect on acquisition of escape performance. For example, altering platform location to engage working memory and/or reconsolidation differentiates between aging and stressor effects in mice (Lacosta et al., 1999; Richwine et al., 2009), as well as the presence or absence of CD4 T cells (Wolf et al., 2009). Whether such approaches reveal underlying deficits in SEA challenged mice remains to be determined.
An important finding of the present study was that immune activation combined with training, may promote long-term plasticity and strengthen future learning. Whether animals received acute or repeated challenge with SEA during initial training (week 1, phase 2), this was found to benefit MWM relearning one month later. This is not inconsistent with evidence that T cell-mediated responses, including those to the superantigen, SEB, increase neurogenesis in the dentate gyrus of the hippocampus (Baron et al., 2008), and that neurogenesis improves MWM learning (Wolf et al., 2009). Furthermore, superantigenic challenge with SEB results in persistent sympathetic changes lasting 10 days (del Rey et al., 2002), as well as a 17-fold increase in activated T cells infiltrating the choroid plexus (Petito & Adkins, 2005). Although similar studies have not been conducted using SEA, these studies suggest potential mechanisms to explore in determining the reasons for the improved long-term learning of SEA-challenged mice. Moreover, whether this is dependent on initial training coinciding with immune activation, as opposed to a long-term effect predicated on an isolated instance of immunologic challenge, warrants further attention.
How immunologic stimuli affect learning and memory is not known. Although there may be fundamental changes in neuronal encoding that are initiated at the transcriptional level by cytokines and/or chemokines (Lehmann et al., 2010), just as plausible, are indirect changes induced by the immune response. A prominent effect of endogenous cytokine elevations is HPA axis activation, and glucocorticoids contribute to the retention of learned information (Oitzl et al., 1998; Sandi, 1998; Conboy & Sandi, 2010). For example, mice carrying an NFkB mutation, were found to learn better in the MWM as a function of greater glucocorticoid production (Lehmann et al., 2010), supporting the hypothesis that moderate glucocorticoid increases facilitate learning. However, excessive or prolonged glucocorticoid elevations may also impair learning and memory, revealing in principle an inverted U-shaped effect of glucocorticoids on spatial learning (Dumas et al., 2010). In the current study, corticosterone elevations in response to MWM were not measured, although it is commonly observed and well documented (Conboy & Sandi, 2010). However, SEA elevates activates the HPA axis, making it likely that mice were introduced to the MWM with a pre-existing elevation in circulating corticosterone. Morover, repeatedly challenged mice were required to learn under conditions of persistent HPA axis activation by SEA, as revealed by a separate experiment showing that after a fourth SEA injection plasma corticosterone was still highly elevated. Given that in the repeated SEA experiment there was no impairment of learning, this suggests that immunologically induced glucocorticoid elevations were minimally disruptive.
The cytokines IL-1β, TNFα and IL-2 were measured to monitor the magnitude of the cytokine response after four consecutive injections of SEA, with both TNFα and IL-2 production being significantly attenuated. This is consistent with other evidence where the spacing of repeated SEA injections was every 5 days (Urbach-Ross et al., 2008), rather than daily. Previously we showed that the corticosterone response to SEA was dependent on TNFα, and more recently that this requires TNFRI (Urbach-Ross, in preparation). Therefore, it was surprising that plasma corticosterone was still significantly elevated in spite of a dramatic reduction in TNFα output. This could not be accounted for by a compensatory increase in IL-1β production, which as previously reported, achieves negligible circulating levels in response to SEA (Urbach-Ross et al, 2008). Therefore, while an acute injection of SEA may increase corticosterone in a TNF-dependent manner, prolonged closely spaced exposure to SEA may recruit alternative mechanisms.
Hippocampal IL-1β and TNFα were measured given their role in spatial and contextual learning (Goshen et al., 2007), and hippocampal LTP (Butler et al., 2004). Interestingly, although hippocampal TNFα appeared markedly reduced in both acute and repeated SEA experiments, this did not achieve statistical significance. However, hippocampal IL-1β was significantly reduced only after repeated SEA challenge, and was inversely related to the concentration of plasma corticosterone. Although this suggests possible inhibitory glucocorticoid feedback on IL-1β production, others have shown glucocorticoids to promote the central inflammatory response to neurological insults, as well as systemic LPS challenge (Sorrells et al., 2009; Frank et al., 2010). Still, there is good evidence in favor of moderate levels of endogenous hippocampal IL-1β being important for promoting contextual learning, while excessive concentrations of IL-1β may impair learning (Goshen et al., 2007). Therefore, if repeated exposures to SEA ultimately reduce hippocampal IL-1β, this may serve as a compensatory change to retain adequate adaptational function. This question will need to be addressed in future studies.
With regard to the cognitive influence of classic T cell derived cytokines, chronic IL-2 treatment was shown to impair MWM learning (Lacosta et al., 1999). However, this does not predict that endogenous IL-2 elevations are associated with deficits in learning, as reported here. Indeed, IL-2 deficiency impairs MWM learning (Petitto et al., 1999), suggesting that IL-2 may be a necessary biological element during normal learning. Similarly, IL-4 producing T cells are necessary to promote learning in the MWM, a requirement operating in the meninges surrounding the brain and possibly working through an anti-inflammatory mechanism (Derecki et al., 2010). A similar anti-inflammatory role may occur for IL-10, which is necessary to protect cognitive function in response to inflammatory insults, such as LPS (Richwine et al., 2009). These and other studies showing that IFNγ promotes neurogenesis and spatial learning (Baron et al., 2008), raise important questions about the role of T cell-derived and anti-inflammatory cytokines in the regulation of cognitive activity. Future research can determine whether these factors are relevant in the promotion of long-term learning after challenge with T cell superantigens.
Acknowledgments
This work was supported by grants MH60706 and NIEHS P30 ES005022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Japanese journal of pharmacology. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- Baron R, Nemirovsky A, Harpaz I, Cohen H, Owens T, Monsonego A. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 2008;22:2843–2852. doi: 10.1096/fj.08-105866. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Taylor EM, Bentson KL. TNF-induced anorexia and learned food aversions are attenuated by area postrema lesions. The American journal of physiology. 1991;260:R906–910. doi: 10.1152/ajpregu.1991.260.5.R906. [DOI] [PubMed] [Google Scholar]
- Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A, Kabiersch A, Petzoldt S, Besedovsky HO. Involvement of noradrenergic nerves in the activation and clonal deletion of T cells stimulated by superantigen in vivo. J Neuroimmunol. 2002;127:44–53. doi: 10.1016/s0165-5728(02)00096-6. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC, Gillette T, Ferguson D, Hamilton K, Sapolsky RM. Anti-glucocorticoid gene therapy reverses the impairing effects of elevated corticosterone on spatial memory, hippocampal neuronal excitability, and synaptic plasticity. J Neurosci. 2010;30:1712–1720. doi: 10.1523/JNEUROSCI.4402-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hansen MK, Kleiner JL, Maier SF, Watkins LR. Staphylococcal enterotoxin B induces fever, brain c-Fos expression, and serum corticosterone in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1434–1439. doi: 10.1152/ajpregu.2001.280.5.R1434. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. The Journal of pharmacology and experimental therapeutics. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNFalpha: a trigger of autonomic dysfunction. Neuroscientist. 2008;14:53–67. doi: 10.1177/1073858407305725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneta T, Kusnecov AW. The role of central corticotropin-releasing hormone in the anorexic and endocrine effects of the bacterial T cell superantigen, Staphylococcal enterotoxin A. Brain Behav Immun. 2005;19:138–146. doi: 10.1016/j.bbi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Fugate J, Kusnecov AW. Immunological challenge modulates brain orphanin FQ/nociceptin and nociceptive behavior. Brain Res. 2002;949:71–78. doi: 10.1016/s0006-8993(02)02966-9. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Kusnecov AW. Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J Neuroimmunol. 2002;123:41–49. doi: 10.1016/s0165-5728(01)00486-6. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and ‘chemo-brain’ have in common? Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Crowell B, Urbach-Ross D, Kusnecov AW. Influence of age on behavioral, immune and endocrine responses to the T-cell superantigen staphylococcal enterotoxin A. Eur J Neurosci. 2009;30:1329–1338. doi: 10.1111/j.1460-9568.2009.06921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnecov AW, Liang R, Shurin G. T-lymphocyte activation increases hypothalamic and amygdaloid expression of CRH mRNA and emotional reactivity to novelty. J Neurosci. 1999;19:4533–4543. doi: 10.1523/JNEUROSCI.19-11-04533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Influence of acute and repeated interleukin-2 administration on spatial learning, locomotor activity, exploratory behaviors, and anxiety. Behav Neurosci. 1999;113:1030–1041. doi: 10.1037//0735-7044.113.5.1030. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Brachman RA, Listwak SJ, Herkenham M. NF-kappaB activity affects learning in aversive tasks: possible actions via modulation of the stress axis. Brain Behav Immun. 2010;24:1008–1017. doi: 10.1016/j.bbi.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallimo EM, Ansonoff MA, Pintar JE, Kusnecov AW. Role of opioid receptor like-1 receptor in modulation of endocrine, immunological, and behavioral responses to the T-cell superantigen staphylococcal enterotoxin A. J Neuroimmunol. 2010;218:48–56. doi: 10.1016/j.jneuroim.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Khan S, Michaud DS, Shippy SA, Anisman H. Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur J Neurosci. 2004;20:229–239. doi: 10.1111/j.1460-9568.2004.03468.x. [DOI] [PubMed] [Google Scholar]
- Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, Sutanto W, de Kloet ER. Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. Eur J Neurosci. 1998;10:3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Pacheco-Lopez G, Niemi MB, Kou W, Harting M, Del Rey A, Besedovsky HO, Schedlowski M. Behavioural endocrine immune-conditioned response is induced by taste and superantigen pairing. Neuroscience. 2004;129:555–562. doi: 10.1016/j.neuroscience.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Petito CK, Adkins B. Choroid plexus selectively accumulates T-lymphocytes in normal controls and after peripheral immune activation. J Neuroimmunol. 2005;162:19–27. doi: 10.1016/j.jneuroim.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Petitto JM, McNamara RK, Gendreau PL, Huang Z, Jackson AJ. Impaired learning and memory and altered hippocampal neurodevelopment resulting from interleukin-2 gene deletion. Journal of neuroscience research. 1999;56:441–446. doi: 10.1002/(SICI)1097-4547(19990515)56:4<441::AID-JNR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behav Immun. 2009;23:794–802. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl A, Hansson J, Antonsson P, Sekaly RP, Kalland T, Dohlsten M. A mutation of F47 to A in staphylococcus enterotoxin A activates the T-cell receptor Vbeta repertoire in vivo. Infect Immun. 1997;65:5118–5124. doi: 10.1128/iai.65.12.5118-5124.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, LeBlanc F, Kaneta T, Urbach D, Kusnecov AW. Effects of bacterial superantigens on behavior of mice in the elevated plus maze and light-dark box. Brain Behav Immun. 2004;18:46–54. doi: 10.1016/s0889-1591(03)00087-4. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C. The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plast. 1998;6:41–52. doi: 10.1155/NP.1998.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J, Sawchenko PE. CNS activational responses to staphylococcal enterotoxin B: T-lymphocyte-dependent immune challenge effects on stress-related circuitry. J Comp Neurol. 2006;495:236–254. doi: 10.1002/cne.20872. [DOI] [PubMed] [Google Scholar]
- Serrats J, Sawchenko PE. How T-cell-dependent and -independent challenges access the brain: vascular and neural responses to bacterial lipopolysaccharide and staphylococcal enterotoxin B. Brain Behav Immun. 2009;23:1038–1052. doi: 10.1016/j.bbi.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behavioural brain research. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Kohman RA, Scott VJ, Boehm GW. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiology & behavior. 2005;86:244–251. doi: 10.1016/j.physbeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Sundberg EJ, Deng L, Mariuzza RA. TCR recognition of peptide/MHC class II complexes and superantigens. Semin Immunol. 2007;19:262–271. doi: 10.1016/j.smim.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach-Ross D, Crowell B, Kusnecov AW. Relationship of varying patterns of cytokine production to the anorexic and neuroendocrine effects of repeated Staphylococcal enterotoxin A exposure. J Neuroimmunol. 2008 doi: 10.1016/j.jneuroim.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach-Ross D, Kusnecov AW. Impact of superantigenic molecules on central nervous system function. Front Biosci. 2009;14:4416–4426. doi: 10.2741/3537. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang BR, Zhang XJ, Duan XL, Guo X, Ju G. Fos expression in the rat brain after intraperitoneal injection of Staphylococcus enterotoxin B and the effect of vagotomy. Neurochem Res. 2004;29:1667–1674. doi: 10.1023/b:nere.0000035801.81825.2a. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiology of learning and memory. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]