Abstract
Titi monkeys (Callicebus cupreus) are a monogamous, New World primate. Adult pair-mates form a bidirectional social bond and offspring form a selective unidirectional bond to their father. Some of the neurobiology involved in social bonds and maternal behavior is similar to the neural circuitry involved in nonsocial reward. Due to these overlapping mechanisms, social states may affect responses to external rewarding stimuli. We sought to determine whether having a social attachment, and/or being in the presence of that attachment figure, can affect an individual’s response to a rewarding stimulus. In addition, we compared affiliative bonds between pair-mates to those between offspring and fathers. Eighteen adult male titi monkeys were either living alone (Lone), with a female pair-mate (Paired), or with the natal group (Natal) (N=6/condition). Each individual went through eight 30-min preference tests for a sweet substance, Tang. For Paired and Natal males, half of the test sessions were with their attachment figure and half were alone. Lone males were always tested alone. Preference scores for Tang, time spent drinking, affiliative, and arousal behaviors were measured. Paired and Natal males emitted significantly more isolation peeps and locomoted more when tested alone compared to when tested with their attachment figure, and paired males engaged in more affiliative behavior than Natal males. Lone males engaged in significantly more behaviors indicative of behavioral arousal such as locomotion and piloerection compared to Paired and Natal males. Finally, paired males drank significantly more Tang and had a significantly greater preference for Tang compared to Lone and Natal males. These results indicate that offspring undergo a behavioral separation response upon separation from their father which persists into adulthood, Lone males are more behaviorally reactive, and that living with an attachment figure and the type of attachment relationship result in different responses to a rewarding sweet stimulus.
Keywords: Callicebus, reward, attachment, Tang, social bond
INTRODUCTION
Recent research has started to elucidate the neurobiology of monogamous bonds. Many of the studies on pair-bonding have focused on the prairie vole, a socially monogamous rodent in which adults form strong heterosexual bonds [Aragona and Wang 2004; Carter et al. 1995]. Studies on the neurobiological components of social bonds have focused primarily on neuropeptides, specifically oxytocin and vasopressin. There is strong evidence that these two hormones play a crucial role in pair-bond formation and maintenance in prairie voles [Carter 1998; Donaldson and Young 2008; Williams et al. 1994; Winslow et al. 1993], as well as monogamous primates [Jarcho et al. 2011; Seltzer and Ziegler 2007; Smith et al. 2010a; Snowdon et al. 2010]. The use of non-human primates as models for the neurobiology of social bonding is advantageous due to neuroanatomy considerably closer to that of humans.
The titi monkey (Callicebus cupreus) is a monogamous monkey and acts as an excellent animal model for pair-bonding. Titi monkeys are a New World monkey whose social structure consists of a bonded heterosexual pair living with up to four offspring [Mason 1966]. In the wild, offspring within the family group can consist of infants, juveniles, adolescents, and sexually mature adults of both sexes [Mason 1966]. As in many monogamous species, fathers invest a significant portion of time caring for offspring [Kleiman 1977]. Titi monkey males are the primary carrier of infants with infants transferring to the mother primarily for nursing [Mason 1966; Mendoza and Mason 1986b]. However, despite this large amount of paternal investment, studies have shown that titi monkey fathers lack a selective bond towards their offspring [Hoffman et al. 1995; Mendoza and Mason 1986b]. Separating an infant from its father results in neither a behavioral nor physiological separation distress response in the father. In contrast, the infant shows an increase in vocalizations and plasma cortisol. This effect is seen even when the mother is present [Hoffman et al. 1995; Mendoza and Mason 1986b].
Adult titi males do respond to involuntary separation from their pair-mate. Upon separation, both the male and female pair-mate display a behavioral and physiological distress response indicated by an increase in vocalizations, locomotor behavior, heart rate and plasma cortisol [Cubicciotti and Mason 1975; Mendoza and Mason 1986a]. These data suggest that there are qualitative differences in the types of social bonds found within titi monkey social groups; one is a unidirectional bond from offspring to father and another is a bidirectional bond between the male and female. Titi monkey social structure thus provides an opportunity to examine the interactions between behavior, neurobiology, and the response to sweet reward in the two different types of bond.
The neurobiology of social behavior shares some substrates with the neurobiology of other rewarding behaviors. Data from prairie voles has provided strong evidence that the dopaminergic system, specifically dopamine (DA) in the nucleus accumbens (NAcc), plays a fundamental role in the formation and maintenance of pair-bonds [Aragona et al. 2003; Aragona et al. 2006; Aragona and Wang 2007; Curtis et al. 2006; Gingrich et al. 2000; Liu et al. 2010; Liu et al. 2011; Young et al. 2011a; Young et al. 2011b]. The NAcc is a key neuroanatomical structure involved in various rewarding behaviors. There is an increase in dopamine (DA) in the NAcc in response to feeding behavior [rat: Hajnal et al. 2004; review: Salamone et al. 2003; rat: Smith et al. 2010b], sexual behavior [review: Paredes and Agmo 2004; rat: Pfaus et al. 1995], rat pup exposure to rat dams [Hansen et al. 1993; Young et al. 2011a], and consumption of drugs of abuse [rhesus macaque: Bradberry et al. 2000; review: Di Chiara and Imperato 1988]. DA and the NAcc are also implicated in the rewarding components of sweet stimuli [Martinez-Hernandez et al. 2006]. In rats, DA antagonists decrease a preference for a sucrose solution [Hajnal and Norgren 2001; Muscat and Willner 1989; Towell et al. 1987] and ingestion of a sucrose solution increases DA and its metabolites in the NAcc [Avena et al. 2006; Hajnal and Norgren 2001].
The DAergic activity in the NAcc found in pair-bonding and maternal behavior has been shown to interact with external rewarding stimuli, such as psychostimulants, which also release DA into the NAcc [Ferris et al. 2005; Liu et al. 2010; Liu et al. 2011; Mattson et al. 2001; Young et al. 2011a]. Mattson et al. [2001] compared the rewarding effects of pups to that of cocaine in rat dams through the use of a conditioned place preference (CPP) paradigm. When dams were conditioned to associate their pups and cocaine with two distinct environments, they preferred the environmental cues associated with their pups. The preference of pups over cocaine is in part thought to be due to neural reorganization in lactating dams that can interfere with the rewarding properties of cocaine [Ferris et al. 2005]. Prairie voles find amphetamine rewarding as measured by a CPP [Aragona et al. 2007], however, pair-bonded prairie voles fail to develop a CPP for amphetamine indicating that the development of a pair-bond can impair the rewarding components of amphetamine [Liu et al. 2011]. The data from maternal behavior and pair-bonding suggest that social rewards that involve DAergic activity in the NAcc can reduce the rewarding effects of other stimuli that activate the same pathway (ex. psychostimulants).
To date, the studies that have examined the interaction between social rewards and external rewards have only utilized drugs of abuse and not other rewarding stimuli. In the present study we sought to determine if there is an interaction between a social reward and a sweet reward by utilizing the titi monkey as an animal model. The titi monkey acts an interesting model to test this since there are different types of social bonds in titi monkey society, the male-female bidirectional bond and the offspring-father unidirectional bond. The primary goal of the present study was to investigate whether the state of having an attachment figure, and/or being in the presence of that attachment figure, could reduce an animal’s response to a rewarding nonsocial stimulus as seen in rat dams and prairie voles. Because having an attachment figure may interact with similar neural circuitry activated by a sweet substance and decrease its rewarding properties, we predicted that the state of having an attachment figure would reduce the response to a sweet reward in titi monkeys, We tested this hypothesis through the use of a two bottle preference test with Tang as a sweet stimulus. We hypothesized that the preference for Tang in an animal which has an attachment figure, specifically paired males and adult offspring living with their father, would be less than that of an adult male which is living alone and does not have an attachment figure. Furthermore, we wanted to explore if there was an effect on Tang preference when a paired or natal male was tested with or without his attachment figure present. The second goal of the study was to explore whether an adult offspring underwent a behavioral separation distress response when separated from his father.
METHODS
Subjects
Subjects were 18 male titi monkeys. Animals were housed in cages (1.2 m x 1.2m x 2.1m) and were on a 12:12 light:dark cycle with lights on at 0600 and lights off at 1800. Temperature was maintained at 21 °C. Housing conditions are identical to that in Mendoza [1999]. Animals were fed a diet of monkey chow, banana, marmoset jelly, cottage cheese, apple, and carrot at 0800 and 1300. Subjects were in one of three social conditions (N=6/condition): males living alone (Lone), males living with a female pair-mate (Paired), and males living in their natal group (Natal). Mean (±SEM) ages in months of Lone, Paired, and Natal males at time of testing were 69.3 (±15.07), 79.5 (±4.92), and 36 (±2.50), respectively. Although all males were adults, there was a significant difference in ages between groups, ANOVA (F=5.88, df=2,15, P=0.01). Natal males were significantly younger than Paired, t-test (t=−3.28, df=10, P=0.0051), and Lone males, (t=−2.51, df=10, P=0.0243). There was not a significant difference between Lone and Paired males, (t=0.78, df=10, P=0.4500). Lone males had been living alone for a mean (±SEM) of 9.33 ± 2.28 months, and Paired males had been cohabitating with their female pair-mate for a mean (±SEM) of 21.83 ± 3.22 months. One subject’s pair-mate gave birth to an infant during habituation and another subject’s pair-mate gave birth near the end of testing. All testing occurred at the California National Primate Research Center between March 2009 and December 2009.
Procedures
Prior to testing all subjects were habituated to the test bottles. Habituation sessions involved placing 2 water bottles containing 2.5% (w/v) Tang (Kraft Foods) solution on the front door of their cages. Habituation sessions lasted 20–30 minutes and were filmed. The first habituation session occurred when the subject was alone in the home cage. If they did not drink, subsequent habituation sessions occurred in the presence of their attachment figure or entire family group. At the end of the habituation sessions the volume of Tang consumed was measured. Since some test sessions had more than one monkey in the cage, it was necessary to use the time spent drinking instead of the volume consumed. Habituation sessions were therefore scored for time spent drinking, which was correlated with the volume consumed. Subjects were tested when they had drunk from the bottles at least once. If subjects did not drink during the habituation sessions, the bottles were put on the cages for the entire day with bottles being placed on their home cage in the morning and taken down before lights off. If an animal did not drink within two weeks of habituation, he was replaced with another male. This was necessary for one Lone male and one Natal male.
There are several reasons for the difficulty in habituating subjects to drink. First, titi monkeys have been shown to be extremely neophobic and often survey novel objects through visual investigation instead of physical exploration [Fragaszy and Mason 1978]. The use of a novel water bottle most likely inhibited approach and drinking behavior. Additionally, titi monkeys are a very habitual species and have difficulty in incorporating a new task or food into their daily routine [Fragaszy 1980]. Past colony experience has shown that Tang is a substance that titi monkeys prefer, however they had not been recently exposed to it due to colony concerns about sugar intake. Introducing a new task, drinking from a water bottle, and reintroducing a substance that the monkeys had not recently been exposed to recently likely contributed to the difficulty in habituation.
Test Sessions
Subjects underwent eight 30-minute test sessions. Food bowls from the previous day were removed prior to their morning meal and animals were not fed until after testing. However, there were some instances where subjects ate small amounts of biscuits that were still on the cage floor. Subjects were not water deprived.
Lone males underwent all eight sessions alone. Paired and Natal males experienced four sessions with the attachment figure (females for Paired males, and fathers for Natal males) and four sessions without the attachment figure. When males were tested without attachment figures, the pair-mate and/or family members were removed from the home cage and moved to a location outside of visual but not auditory contact. Even though family members and pair-mates were in auditory contact, there were only a few incidences of removed animals vocalizing. When Natal males were being tested with their father, the mother and all siblings over the age of five months were captured in transfer boxes and moved to a location out of visual contact. One Natal male had a sibling under the age of five months. To control for the disturbance of entering the home cage with a transfer box, an experimenter would enter the home cage of Lone males and Paired males being tested with their pair-mate with a transfer box prior to testing sessions. The male whose female gave birth in the middle of the experiment underwent one session with his pair-mate and the new infant, but the infant was on the mother for the entire session.
After all appropriate monkeys were removed from the cage, a bottle of the 2.5% Tang solution and a bottle of water were placed on the front door of the home cage. The water was colored orange with McCormick© food coloring to match the color of the Tang. The location of Tang and water was counterbalanced over the eight test sessions. The 30-minute sessions were filmed with the subject as the focal object. The order of sessions with and without the attachment figure was counterbalanced.
Two testing sessions occurred each day, one at 0830 (Early session) and the other at 0930 (Late session). An individual subject was tested once per day. For each test session, a subject from each Social Status (one Paired, one Natal, and one Lone) was tested simultaneously. The same cohort of three animals was tested together throughout the eight testing sessions. Session time (Early/Late), Social Context (With attachment figure/Without attachment figure), and bottle location were all approximately counterbalanced so all groups experienced the same combination of session time, Social Context (except Lone males), and bottle location. At the end of each session, any animals removed from the home cage during testing were returned and all individuals were given their morning meal.
Filming sessions were scored for affiliative behaviors, arousal behaviors, time spent drinking Tang and water, and preference for Tang. Time spent drinking was measured in seconds and preference for Tang was expressed as a percent of total time spent drinking. See Table 1 for ethogram. The behavioral ethogram was similar to the one used by Fernandez-Duque and colleagues [1997]. Behaviors included those involved in affiliation (i.e. tail-twining), territoriality (i.e. chest-rubbing), arousal (i.e. piloerection), and vocalizations (i.e. peeps, long calls). Anogenital rubbing and chest-rubs have been considered to be reflective of behavioral arousal and possibly territorial scent marking [Mason 1966; Moynihan 1966]. Titi monkeys are a territorial species and scent marking behaviors could aid in a pair maintaining their territory. Peeps mostly occur when an individual is isolated and long calls can be heard during territorial duetting, intraspecific disputes, and long distance communication [Mason 1966; Moynihan 1966] Drinking was scored when an animal placed its mouth on the end of the water bottle spout. Balks were also measured and were defined as when a subject did not drink for the entire session.
Table 1.
Ethogram of Non-Drinking and Drinking Behaviors
| Behavior* | Description |
|---|---|
| Non-Drinking Behavior | |
| Aggression | Vigorous grasping, pulling, slapping, or biting |
| Anogenital Rubbing | The anogenital region is in contact with a surface and the rear part of the body moves back and forth |
| Back-Arch | The back is arched (as in a frightened cat), the subject may also have its arms and trunk lifted off the perch |
| Chest-Rub | The chest is moved with pressure and friction against a perch or surface; it may also be pressed in a downward motion with the hands or arms |
| Contact (sec) | Passive physical contact between subjects that does not involve tail-twining |
| Grooming (sec) | Combing through the fur of the attachment figure |
| Isolation Peeps | Short, high pitched vocalizations which usually occur in rapid succession |
| Lip-Smack | Repeated and rapid opening and closing of the mouth |
| Locomotion (sec) | Movement of at least one body length |
| Long-Calls (sec) | Loud, sustained vocalizations |
| Mount/Thrust | Common usage |
| Piloerection | Hair erect, most noticeably in the tail |
| Proximity (sec) | Subjects are within arm’s reach of attachment figure |
| Receive Grooming (sec) | The focal animal is being groomed by the attachment figure |
| Tail-Lash | Repetitive swinging of the whole tail from side to side (arcs greater than 45 degrees) |
| Tail-Twine (sec) | Sitting side-by-side with tails wrapped around each other for at least one rotation |
| Drinking Behavior | |
| Tang Preference % | (Tang/Total Drinking) x 100 |
| Tang (sec) | Places mouth on the spout from the bottle containing Tang |
| Water (sec) | Places mouth on the spout from the bottle containing Water |
| Total Drinking (sec) | Total time with mouth spent on the bottle spout |
Behaviors followed by (sec) are durations measured in seconds. All other behaviors are frequencies.
All procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee. Research methods adhered to the American Society of Primatologists Principles for the Ethical Treatment of Non Human Primates and the legal requirements for nonhuman primate research in the United States.
Statistics
Data were analyzed using a generalized linear mixed model (GLMM). Two models were run. The first model included all three groups and included Social Status and Test Session with ID as a random variable. The second model only included Paired and Natal males and included Social Status, Social Context, Social Status x Social Context interaction, Test Session, and ID as a random variable. If data were not normally distributed, square-root or quad-root transformations were performed to normalize the data. There were some instances where transformations did not completely normalize the data; however, the GLMM was still performed. This was done because although the ANOVA F-test is strongest for normally distributed data, when comparing means of non-normally distributed data, the ANOVA F-test is still recommended [Feir-Walsh and Toothaker 1974]. Post hoc comparisons were done using t-tests. The P-value was set at 0.05, and all tests were two-tailed.
RESULTS
Fifty-five habituation sessions were used to calculate the correlation between volumes drunk and time spent drinking. There was a significant positive correlation between volume drunk and time spent drinking, Pearson’s (r=0.85, P<0.0001).
Balks
A balk was defined as when a subject did not drink anything for the entire 30-minute session. Balks were compared between each group. Mean (± SEM) of balks for Paired, Lone, and Natal males were 2.17 (±1.33), 3.67 (±1.15), and 3.5 (±1.34), respectively. There was no significant difference in balks between groups (F=0.42, df=2,15, P=0.6660).
Drinking Data
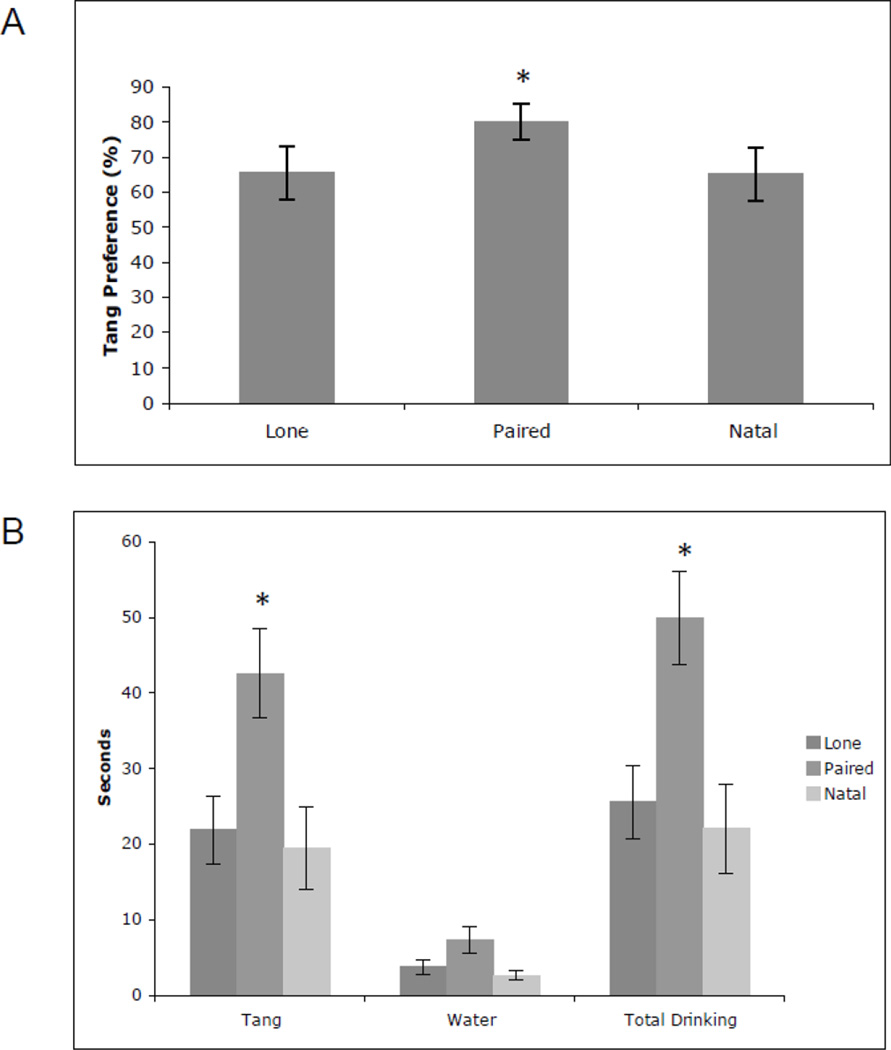
Data were analyzed excluding balked sessions. Since one Paired male and one Natal male balked all eight sessions, sample sizes for analyses on drinking behavior were N=6 (Lone males), N=5 (Paired males), and N=5 (Natal males). Tang Preference is expressed as a percent of total time drinking and all other variables are in seconds. There was a significant effect of Social Status on Tang Preference (F=4.83, df=2,64, P=0.0111) (Fig. 1A). Post-hoc tests revealed that Paired males had a greater preference for Tang compared to Lone males (t=2.81, df=9, P=0.0064) and Natal males (t=2.20, df=8, P=0.0313). There was also a significant effect of Social Status on time spent drinking Tang (F=8.94, df=2,64, P=0.0004) and total time spent drinking (F=10.26, df=2,64, P=0.0001) (Fig. 1B). Paired males spent significantly more time drinking Tang compared to Lone (t=3.15, df=9, P=0.0025) and Natal males (t=3.74, df=8, P=0.0004). They also spent more time drinking compared to Lone (t=3.23, df=9, P=0.0019) and Natal males (t=4.09, df=8, P=0.0001). Social Status had no significant effect on time drinking water (F=1.09, df=2,64, P=0.3416) (Fig. 1B).
Fig. 1.
A) Mean (± SEM) Tang preference in percentage for Lone, Paired, and Natal males. Paired males had a significantly greater preference for Tang compared to Lone and Natal males. B) Mean (± SEM) time Lone, Paired, and Natal males spent drinking Tang, water, and total time drinking. Paired males spent significantly more time drinking Tang and total time spent drinking compared to Lone and Natal males. There were no significant differences in time spent drinking water. *Significantly different compared to Lone and Natal males P<0.05.
There was a significant effect of Test Session on time spent drinking Tang (F=2.25, df=7,64, P=0.0410) and the effect of Test Session on total time spent drinking approached significance (F=2.14, df=7,64, P=0.0516). In general, drinking was less between sessions two and seven compared to the first two sessions. Drinking then increased during session eight.
There was no significant effect of Social Context or interaction between Social Status and Social Context on any drinking variable.
Non-drinking Data
A summary of all dyadic behavioral data can be found in Table 2. Paired males spent significantly more time in Contact (t=4.28, df=10 P=0.0002) and Tail-Twining (t=5.19, df=10 P<0.0001) during the test sessions compared to Natal males. Paired males also spent more time in Proximity compared to Natal males, but this effect only approached significance (t=1.89, df=10, P=0.0567). Paired males spent more time Receiving Grooming compared to Natal males (t=4.91, df=10, P<0.0001); however, Natal and Paired males spent an equal amount of time Grooming (t=0.65, df=10, P=0.5216). Despite the fact that Paired males engaged in more affiliative behavior than Natal males, they also engaged in significantly more bouts of Aggression compared to Natal males (t=2.44, df=10, P=0.0209).
Table 2.
Dyadic behaviors comparing Paired (N=6) and Natal (N=6) males
| Social Status |
||||
|---|---|---|---|---|
| Behavior | Paired | Natal | t-statistic | P-Value |
| Proximity | 188.63 ± 26.99 | 138.04 ± 22.23 | 1.98 | 0.0567 |
| Contact | 358.13 ± 51.14 | 182.04 ± 72.94 | 4.28 | 0.0002 |
| Tail-Twine | 214.79 ± 58.10 | 9.88 ± 9.88 | 5.19 | <0.0001 |
| Grooming | 18.96 ± 6.73 | 16.13 ± 7.39 | 0.65 | 0.5216 |
| Receive Grooming | 14.13 ± 4.03 | 1.96 ± 1.79 | 4.91 | <0.0001 |
| Aggression | 1.08 ± 0.35 | 0.38 ± 0.16 | 2.44 | 0.0209 |
| Mount/Thrust | 0.04 ± 0.04 | 0 ± 0 | 0.92 | 0.3466 |
Bold Significant difference between Paired and Natal males P<0.05
A summary of non-drinking behavior by Social Status, between different Social Contexts, and comparing Paired and Natal males in different Social Contexts can be found in tables 3, 4, and 5, respectively. There was a significant effect of Social Status on Anogenital Rubbing (F=6.24, df=2,119, P=0.0026) with Paired males engaging in more Anogenital Rubbing compared to Lone (t=2.98, df=10, P=0.0035) and Natal males (t=3.13, df=10, P=0.0022). There was no effect of Social Context (t=0.77, df=11, P=0.4429) but there was a significant interaction between Social Status and Social Context (F=5.43, df=1,75, P=0.0224) in that Paired males engaged in more Anogenital Rubbing when tested alone compared to when they were with their pair-mate (t=2.27, df=4, P=0.0283).
Table 3.
Non-drinking behaviors comparing Paired (N=6), Natal (N=6), and Lone (N=6) males
| Social Status |
|||||
|---|---|---|---|---|---|
| Behavior | Paired | Natal | Lone | F-Statistic | P-value |
| Anogenital Rubbing | 1.52 ± 0.42a,c | 0.35 ± 0.09 | 0.48 ± 0.14 | 6.24 | 0.0026 |
| Back-Arch | 1.19 ± 0.37 | 0.65 ± 0.29b,c | 1.52 ± 0.32 | 5.17 | 0.0070 |
| Chest-Rub | 0.81 ± 0.27c | 3.44 ± 0.93b,c | 0.10 ± 0.09 | 15.02 | <0.0001 |
| Lip-Smack | 3.85 ± 0.98 | 2.04 ± 0.61b,c | 5.52 ± 1.09 | 8.69 | 0.0003 |
| Piloerection | 0.88 ± 0.29 | 0.48 ± 0.18 | 1.40 ± 0.28a,c | 5.26 | 0.0064 |
| Locomotion | 111.90 ± 13.73 | 126.46 ± 12.18 | 177.04 ± 18.01a,c | 5.23 | 0.0066 |
| Isolation Peeps | 123.58 ± 28.59c | 208.00 ± 67.89c | 32.90 ± 9.10 | 6.40 | <0.0023 |
| Long-Calls | 36.60 ± 7.60 | 33.92 ± 8.68 | 70.15 ± 13.55a,c | 3.37 | 0.0376 |
| Tail-Lash | 1.38 ± 0.45 | 0.73 ± 0.27 | 2.04 ± 0.53a | 3.15 | 0.0465 |
Bold Significant difference between Paired, Natal and Lone males P<0.05
=significantly different compared to Natal males, P<0.05
=significantly different compared to Lone males, P<0.05
=significantly different compared to Paired males, P<0.05
Table 4.
Variables comparing Paired and Natal males (N=12) in different social contexts
| Social Context |
||||
|---|---|---|---|---|
| Behavior | With | Without | t-Statistic | P-Value |
| Anogenital Rubbing | 0.65 ± 0.15 | 1.23 ± 0.41 | −0.77 | 0.4429 |
| Back-Arch | 0.71 ± 0.24 | 1.13 ± 0.40 | −1.33 | 0.1871 |
| Chest-Rub | 0.65 ± 0.30 | 3.60 ± 0.91 | −3.92 | 0.0002 |
| Lip-Smack | 4.08 ± 1.01 | 1.81 ± 0.54 | 3.31 | 0.0015 |
| Locomotion | 101.54 ± 10.79 | 136.81 ± 14.47 | −1.95 | 0.0547 |
| Piloerection | 0.73 ± 0.28 | 0.63 ± 0.19 | 1.26 | 0.2099 |
| Isolation Peeps | 14.29 ± 4.97 | 317.29 ± 67.09 | −11.14 | <0.0001 |
| Long-Calls | 34.73 ± 8.37 | 35.79 ± 7.94 | −0.30 | 0.8723 |
| Tail-Lash | 1.17 ± 0.37 | 0.94 ± 0.38 | 0.02 | 0.9825 |
Bold Significant difference between test sessions with an attachment figure and without an attachment figure
Table 5.
Non-dyadic behaviors in Paired (N=6) and Natal (N=6) males in different social conditions
| Social Context |
|||||
|---|---|---|---|---|---|
| Behavior | Social Status |
With | Without | F-Statistic* | P-value |
| Anogenital Rubbing |
Paired | 0.79 ± 0.26 | 2.25 ± 0.77 | 5.43 | 0.0224 |
| Natal | 0.50 ± 0.15 | 0.21 ± 0.08 | |||
| Back-Arch | Paired | 0.96 ± 0.39 | 1.42 ± 0.63 | 0.00 | 0.9599 |
| Natal | 0.46 ± 0.27 | 0.83 ± 0.51 | |||
| Chest-Rub | Paired | 0.13 ± 0.07 | 1.50 ± 0.51 | 1.82 | 0.181 |
| Natal | 1.17 ± 0.58 | 5.71 ± 1.66 | |||
| Lip-Smack | Paired | 6.63 ± 1.72 | 1.08 ± 0.54 | 28.69 | <0.0001 |
| Natal | 1.54 ± 0.82 | 2.54 ± 0.92 | |||
| Piloerection | Paired | 1.25 ± 0.54 | 0.50 ± 0.20 | 3.36 | 0.0707 |
| Natal | 0.21 ± 0.10 | 0.75 ± 0.33 | |||
| Locomotion | Paired | 82.17 ± 13.28 | 141.63 ± 22.75 | 2.17 | 0.1451 |
| Natal | 120.92 ± 16.34 | 132.00 ± 18.34 | |||
| Isolation Peeps | Paired | 4.67 ± 2.90 | 242.50 ± 45.85 | 3.41 | 0.0689 |
| Natal | 23.92 ± 9.20 | 392.08 ± 125.73 | |||
| Long-Calls | Paired | 35.13 ± 10.32 | 38.08 ± 11.38 | 0.12 | 0.737 |
| Natal | 34.33 ± 13.42 | 33.50 ± 11.31 | |||
| Tail-Lash | Paired | 1.79 ± 0.68 | 0.96 ± 0.61 | 0.98 | 0.3245 |
| Natal | 0.54 ± 0.28 | 0.92 ± 0.46 |
F-Statisitic is for interaction between Social Status and Social Context
Bold Significant interaction between Social Status and Social Context
There was a significant effect of Social Status on Chest-Rubs (F=15.02, df=2,119, P<0.0001). Paired (t=2.61, df=10, P=0.0102) and Natal males (t=5.48, df=10, P<0.0001) Chest-Rubbed significantly more than Lone males. Natal males Chest-Rubbed significantly more than Paired males (t=−2.87, df=10, P=0.0049). Social Context also had a significant effect on Chest-Rubs: males tested without their attachment figure Chest-Rubbed more than when tested with their attachment figure (t=−3.92, df=11, P=0.0002).
Social Status had a significant effect on Back-Arches (F=5.17, df=2,119, P=0.007) where Paired (t=2.01, df=10, P=0.047) and Lone males (t=−3.18, df=10, P=0.002) engaged in more Back-Arches than Natal males. Social Status also had a significant effect on Tail-Lashing (F=3.77, df=2,119, P=0.0259). Lone males Tail-Lashed more than Natal males (t=−2.74, df=10, P=0.0071). Piloerection was significantly affected by Pair-Status (F=5.26, df=2,119, P=0.0064). Lone males exhibited Piloerection more than Paired (t=−2.32, df=10, P=0.0219) and Natal males (t=−3.12, df=10, P=0.0022).
There was a significant effect of Social Status on Locomotion (F=5.23, df=2,119, P=0.0066). Lone males spent more time in Locomotion than Paired (t=−3.20, df=10, P=0.0018) and Natal males (t=−2.02, df=10, P=0.0457). For Paired and Natal males the effect of Social Context on Locomotion approached significance. Males tested without an attachment figure engaged in more Locomotion (t=1.95, df=11, P=0.0547).
Social Status had a significant effect on Lip-Smacks (F=8.69, df=2,119, P=0.0003). Paired (t=2.28, df=10, P=0.0245) and Lone males (t=−4.16, df=10, P<0.0001) exhibited significantly more Lip-Smacks than Natal males. For Natal and Paired males there was a significant effect of Social Context (t=3.31, df=11, P=0.0015) with males tested with their attachment figure, Lip-Smacking significantly more with their attachment figure compared to when tested alone. There was a significant interaction between Social Status and Social Context (F=28.69, df=1,75, P<0.0001). Paired males tested with their attachment figure Lip-Smacked significantly more than when tested without their attachment figure (t=6.60, df=10, P<0.0001) but this effect was not found in Natal males (t=−1.37, df=10, P=0.1780).
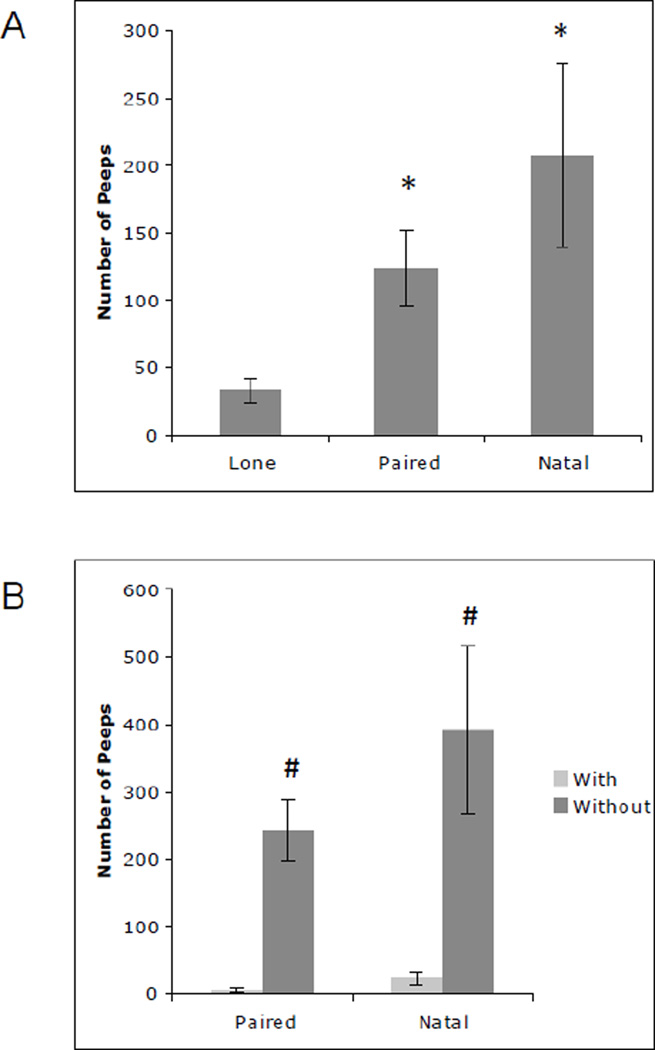
Social Status had a significant effect on Long-Calls (F=3.37, df=2,119, P=0.0376). Lone males Long-Called more compared to Paired (t=−2.14, df=10, P=0.0343) and Natal males (t=− 2.34, df=10, P=0.0208). Social Context significantly affected Isolation Peeps with males tested without their attachment figure emitting more Isolation Peeps compared to when they were tested with their attachment figure (t=−11.14, df=11, P<0.0001). Social Status also had a significant effect on Isolation Peeps (F=6.40, df=2,119, P=0.0023) where Paired (t=−2.42, df=10, P=0.0173) and Natal males (t=−3.49, df=10, P=0.0007) emitted significantly more Isolation Peeps compared to Lone males (Fig. 2A). This effect, however, was driven by the significantly greater number of Isolation Peeps that Paired and Natal males emitted when tested alone (t=− 10.02, df=10, P<0.0001; t=−7.08, df=10, P<0.0001, respectively) (Fig. 2B). There was also a significant effect of Test Session on Isolation Peeps F=2.55, df=7,8, P=0.0175).
Fig. 2.
A) Mean (± SEM) number of isolation peeps emitted by Lone, Paired, and Natal males. Paired and Natal males peeped significantly more compared to Lone males. B) Mean (± SEM) number of isolation peeps emitted by Paired and Natal males when tested with their attachment figure and without their attachment figure. Paired and Natal males tested without their attachment figure peeped significantly more compared to when tested with their attachment figure. *Significantly different compared to Lone males P<0.05. #Significantly different compared to test sessions with an attachment figure P<0.0001
DISCUSSION
The goal of this study was to examine qualitative differences in titi monkey bonds and whether these differences influence affiliation, arousal, and response to a sweet rewarding stimulus, specifically Tang. As expected based on previous studies, we found that when paired males and adult offspring are separated from their attachment figure, they undergo a behavioral separation response reflected primarily through a significant increase in isolation peeps and locomotor behavior, although the latter only approached significance (P=0.0547). This response to separation has also been seen in six-month-old titi infants separated from their father [Hoffman et al. 1995], but this is the first study to show that this behavioral reaction in response to an acute separation from the father continues into adulthood. This complements previous research that found that extended separation of adult offspring from its father results in long lasting increases in cortisol [Mendoza et al. 2000; Valeggia 1996], which may be indicative of an attachment lasting into sexual maturity.
In addition to supporting the idea that an offspring’s attachment to its father continues into adulthood, we provided evidence that the bidirectional bond between male and female and the unidirectional bond from offspring to father can be measured through affiliative behaviors and not solely through separation paradigms. A traditional method of measuring social attachment is through the use of a separation paradigm and measuring the behavioral and/or physiological stress response upon separation [Ainsworth and Bell 1970; Mason and Mendoza 1998; Mendoza et al. 1980]. These measures often include vocalizations and hypothalamic- pituitary-adrenal (HPA) activity [Erickson et al. 2005; Hoffman et al. 1995; Kalin et al. 1988; Mendoza et al. 1980; Mendoza and Mason 1986a; Panksepp et al. 1980]. The present study found that Paired males spend significantly more time in contact and tail-twining with their pair-mate compared to sons with their fathers. Paired males also received significantly more grooming from their pair-mate than Natal males received from their fathers. Studies quantifying affiliative behaviors, such as passive contact, have been shown to be a valid measure of social bonds [Aragona and Wang 2004; Carter et al. 1995; Williams et al. 1992].
However, there could be more than one reason for the differences in contact and tail-twining between Paired and Natal males. As stated before, one possibility is that the relationship between adult offspring and father is different than that between a male and his pair-mate. It has been shown that fathers lack a bond with their infants demonstrated by an absence of a behavioral and physiological response upon separation from their infant [Hoffman et al. 1995; Mendoza and Mason 1986b]. If this lack of a bond persists when the offspring is an adult then the father may be less motivated to maintain physical contact with his son. Another reason for differences seen in these affiliative behaviors could actually be reflective of the attachment between the father and his pair-mate. When the Natal male was tested with his father, the father was separated from his pair-mate. This separation could have resulted in a shift of motivation in the father from grooming and engaging in physical contact with his offspring to regaining proximity with his pair-mate. This shift in priorities would result in a decrease in grooming offspring. This could also potentially contribute to the effect seen in contact and tail-twining. Future research measuring these behaviors when the entire family group is present could clarify the reason for differences seen in affiliative behaviors.
Interestingly, even though Paired males engaged in more affiliative behavior with their pair-mate compared to Natal males, we observed more aggression between Paired males and their females compared to Natal males and their fathers. This finding was unexpected since aggression is rare in titi monkeys, especially between males and their pair-mate [Mason 1966]. Most of the aggression seen between the Paired males and their pair-mate was over access to the bottle of Tang, which could provide an explanation for this effect. Paired males also lip-smacked significantly more than Natal males. Lip-smacking appears to serve multiple functions and be reflective of different affective states. Lip-smacking in titi monkeys can function as an affiliative behavior, as well as be an indicator of arousal [Fernandez-Duque et al. 1997; Fernandez-Duque et al. 2000; Moynihan 1966]. In the present study, lip-smacking often occurred after bouts of aggression. In other primate species lip-smacking has been seen to function as a method for reconciliation after aggression, but is also associated with affiliation and mating [Maestripieri 1997].
We saw differences in scent marking behavior. Paired males emitted significantly more anogenital rubbing compared to Natal and Lone males. Differences between Paired and Natal males may reflect both age and Social Status within the family group. In golden lion tamarins (Leontopithecus rosalia rosalia) it has been found that parents emit more anogenital scent marking than their offspring [Ruiz-Miranda and Kleiman 2002]. Differences between Paired and Lone males may be due to living conditions. It has been observed that socially isolated golden lion tamarins emit very low levels of scent marking [Mack and Kleiman 1978; Ruiz-Miranda and Kleiman 2002]. Paired males emitted more anogenital rubbing when they were tested alone. This may be reflective of levels of arousal after being separated from his pair-mate.
We found that Paired and Natal males chest-rubbed significantly more than Lone males and that more chest-rubs were emitted when tested alone. The low levels of scent marking that isolated housed males exhibited likely resulted in this difference. Again, this effect may be similar to that found in isolated golden lion tamarins [Mack and Kleiman 1978; Ruiz-Miranda and Kleiman 2002]. Males tested without their attachment figure exhibited significantly more chest-rubs than when they were tested with their pair-mate. This may be indicative of higher levels of behavioral arousal. This behavior has been seen to increase in other situations reflecting behavioral arousal in titi monkeys such as being exposed to a conspecific stranger [Fernandez-Duque et al. 2000] or interactions with neighboring groups [Mason 1966]. Natal males also emitted more chest-rubs compared to Paired males. This difference between Natal and Paired males as well as being tested with and without an attachment figure may partially be driven by the large numbers of chest-rubs emitted by Natal males when tested alone. This may signify developmental changes in males. In golden lion tamarins, sternal scent marking begins when offspring are sub-adult and entering into young adulthood [Hoage 1982]. Lone males appear to be more easily aroused than males in other social conditions. Lone males locomoted, long-called, and piloerected significantly more than Paired and Natal males. All of these behaviors are indicators of emotional arousal [Cubicciotti and Mason 1975; Fernandez-Duque et al. 1997; Mason 1966; Moynihan 1966]. In addition to differences in Social Status, there could also be a developmental component to these differences in arousal behavior.
One of the major goals of the study was to discover whether having an attachment figure, and/or the presence of that individual, was associated with differences in response to the rewarding aspects of a sweet stimulus. We hypothesized that males who were living with their attachment figure would have a decreased preference for Tang and spend less time drinking Tang. However, this is not what happened. Paired males had a greater preference for Tang and drank more Tang compared to Lone and Natal males. We also found that Paired males spent more time drinking compared to Lone and Natal males, which was most likely driven by the large amount of time spent drinking Tang. Whether a male was tested with or without his attachment figure had no effect on his drinking behavior.
Even though Lone males displayed a preference for Tang, their preference for Tang and the time they spent drinking Tang was significantly less compared to Paired males. One possibility for this effect could be due to length of time Lone males were living by themselves. Social isolation has been utilized to act as a model of neuropsychiatric illnesses and is viewed as a chronic stressor. Social isolation results in negative affect, decreased body weight, and chronically high levels of glucocorticoids [Grippo et al. 2011; Grippo et al. 2007a; Grippo et al. 2007b; Grippo et al. 2007c; Grippo et al. 2008; Kim and Kirkpatrick 1996; Panksepp 2003; Wallace et al. 2009]. In prairie voles and rats, social isolation results in anhedonia reflected by a decrease in consumption of a sucrose solution and have been shown to be more behaviorally reactive to stressors such as resident-intruder tests and the elevated plus maze [Grippo et al. 2007a; Grippo et al. 2007b; Grippo et al. 2007c; Grippo et al. 2008; Wallace et al. 2009]. Although Lone males did drink less Tang compared to Paired males, differences in behavior may not be indicative of chronic stress. Increased levels of locomotion and long-calls observed in Lone males could be reflective of a natural history state where males are searching for a pair-mate after leaving the natal group. Physiologically, the chronic stressor of long term isolation in prairie voles results in an increase in glucocorticoids [Kim and Kirkpatrick 1996]. A similar effect is seen in socially isolated titi monkeys [Mendoza et al. 2000]. Additionally, the metabolic profile observed in socially isolated titi monkeys is similar to individuals with certain psychopathologies related to chronic stress [Laugero et al. 2011] Even though Lone males appear to have a physiological phenotype of chronic stress, it may not be the only factor contributing to a decrease in Tang drinking. Both Lone and Natal males drank an equivalent amount of Tang, and Natal males were not under a situation of chronic stress. In the wild, titi monkey adult offspring live in the natal group [Mason 1966] and removal from the natal group results in an increase in cortisol [Mendoza et al. 2000]. It is therefore possible that changes in neurobiology upon pairing and not chronic stress are contributing to differences in drinking behavior, although it may not be DA driven.
We found that paired males consumed more of an external reward compared to unpaired subjects, which is in opposition to the findings in paired prairie voles and rat dams where alterations in the DAergic system and reward circuitry result in a decrease in the response to an external non-social rewarding stimulus [Ferris et al. 2005; Liu et al. 2011; Mattson et al. 2001]. These contrasting results could be due to differences in the rewarding stimulus. Psychostimulants were administered in studies using rat dams and prairie voles while the present study used a sweet reward. Another possibility is that DA is not involved in the present study but rather another system such as the opioid system, which is also involved in social attachment and sweet rewards [Machin and Dunbar 2011; Olszewski and Levine 2007; Panksepp et al. 1980; Shapiro et al. 1989; Yamamoto et al. 2000].
One caveat of this study is the significant age difference between Natal males and the other two groups (Lone and Paired males). The age difference could play a role in the difference in drinking behavior between Paired and Natal males. However, Natal males’ drinking behavior was similar to that of Lone males, who were also significantly older than Natal males. Age could play a role in the differences found in behaviors such lip-smacking and back-arching since both of these behaviors were different between Natal males and Lone and Paired males.
In conclusion, we observed that Lone males have higher levels of behavioral arousal reflected through higher rates of locomotion, piloerection, and long-calls compared to Paired and Natal males. In addition, adult offspring undergo a behavioral distress response upon separation from their father indicated by an increase in isolation peeps. Finally, the Social Status of a titi monkey male affects its response to a sweet stimulus with Paired males having a greater preference and drinking more of a sweet solution compared to an adult male living alone or an adult male remaining in its natal group. This difference could reflect neurobiological changes in response to isolation or to the formation of a pair-bond.
ACKNOWLEDGEMENTS
We would like to acknowledge our funding for this research provided by NIH (#HD053555 to KLB and #RR00169 to the California National Primate Research Center) and the Good Nature Institute (to KLB), as well Allison Perkeybile and Michael Jarcho for their help in data collection. We also want to acknowledge the University of California, Davis Institutional Animal Care and Use Committee and the helpful comments of two anonymous reviewers.
REFERENCES
- Ainsworth MD, Bell SM. Attachment, exploration, and separation: Illustrated by behavior of one-year-olds in a strange situation Child Development. 1970;41(1):49–67. [PubMed] [Google Scholar]
- Aragona BJ, Detwiler JM, Wang Z. Amphetamine reward in the monogamous prairie vole. Neuroscience Letters. 2007;418(2):190–194. doi: 10.1016/j.neulet.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. Journal of Neuroscience. 2003;23(8):3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9(1):133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Opposing regulation of pair bond formation by cAMP signaling within the nucleus accumbens shell. Journal of Neuroscience. 2007;27(48):13352–13356. doi: 10.1523/JNEUROSCI.3216-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang ZX. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. Ilar Journal. 2004;45(1):35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139(3):813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. Journal of Neuroscience. 2000;20(10):3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Devries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model Neuroscience and Biobehavioral Reviews. 1995;19(2):303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cubicciotti DDI, Mason WA. Comparative Studies of Social Behavior in Callicebus and Saimiri: Male-Female Emotional Attachments. Behavioral Biology. 1975;16:185–197. doi: 10.1016/s0091-6773(76)91296-7. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Research. 2006;1126(1):76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceeds of the National Academy of Sciences of the United States of America. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Erickson K, Gabry KE, Schulkin J, Gold P, Lindell S, Higley JD, Champoux M, Suomi SJ. Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Developmental Psychobiology. 2005;46(4):331–339. doi: 10.1002/dev.20061. [DOI] [PubMed] [Google Scholar]
- Feir-Walsh BJ, Toothaker LE. An empirical comparison of the ANOVA F-test, normal scores test, and Kruskal-Wallis test under violation of assumptions. Educational and Psychological Measurement. 1974;34:789–799. [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch) American Journal of Primatology. 1997;43(3):225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mason WA. Effects of Pair-Bond and Social Context on Male-Female Interactions in Captive Titi Monkeys (Callicebus moloch, Primates: Cebidae) Ethology. 2000;106:1067–1082. [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. Journal of Neuroscience. 2005;25(1):149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragaszy DM. Comparative studies of squirrel monkeys (Saimiri) and titi monkeys (Callicebus) in travel tasks. Zeitschrift für Tierpsychologie. 1980;54(1):1–36. doi: 10.1111/j.1439-0310.1980.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Mason WA. Response to Novelty in Saimiri and Callicebus: Influence of Social Context. Primates. 1978;19(2):311–331. [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2000;114(1):173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, LaRocca MA, Bates SL, Porges SW. 24Hour Autonomic Dysfunction and Depressive Behaviors in an Animal Model of Social Isolation: Implications for the Study of Depression and Cardiovascular Disease. Psychosomatic Medicine. 2011;73(1):59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007a;69(2):149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32(8–10):966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biological Psychiatry. 2007c;62(10):1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and Anxiety. 2008;25(6):E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Research. 2001;904(1):76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2004;286(1):R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: A microdialysis study. Pharmacology Biochemistry and Behavior. 1993;45(3):673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hoage R. Social and physical maturation in captive lion tamarins, Leontopithecus rosalia rosalia. Smithsonian Contributions to Zoology. 1982;354:1–56. [Google Scholar]
- Hoffman KA, Mendoza SP, Hennessy MB, Mason WA. Responses of infant titi monkeys, Callicebus moloch, to removal of one or both parents: evidence for paternal attachment. Developmental Psychobiology. 1995;28(7):399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain and behavior. 2011;10(3):375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Research. 1988;440(2):285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biological Psychiatry. 1996;40(9):918–922. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Quartely Review of Biology. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Smilowitz JT, German JB, Jarcho MR, Mendoza SP, Bales KL. Plasma omega 3 polyunsaturated fatty acid status and monounsaturated fatty acids are altered by chronic social stress and predict endocrine responses to acute stress in titi monkeys. Prostaglandins Leukotrienes and Essential Fatty Acids. 2011;84(3–4):71–78. doi: 10.1016/j.plefa.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang ZX. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(3):1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. The Journal of Neuroscience. 2011;31(22):7960–7966. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin AJ, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148(9–10):985–1025. [Google Scholar]
- Mack DS, Kleiman DG. Distribution of scent marks in different contexts in captive lion tamarins, Leontopithecus rosalia (Primates) In: Rothe H, Wolters HJ, Hearns JP, editors. Biology and Behaviour of Marmosets. Göttingen, West Germany: Eigenverlag Rothe; 1978. pp. 181–190. [Google Scholar]
- Maestripieri D. Gestural communication in macaques: Usage and meaning of nonvocal signals. Evolution of Communication. 1997;1:193–222. [Google Scholar]
- Martinez-Hernandez J, Lanuza E, Martinez-Garcia F. Selective dopaminergic lesions of the ventral tegmental area impair preference for sucrose but not for male sexual pheromones in female mice. European Journal of Neuroscience. 2006;24(3):885–893. doi: 10.1111/j.1460-9568.2006.04944.x. [DOI] [PubMed] [Google Scholar]
- Mason WA. Social organization of the south american monkey, Callicebus Moloch: A preliminary report. Tulane Studies in Zoology. 1966;13:23–28. [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23(8):765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JJ. Comparison of two positive reinforcing stimuli: Pups and cocaine throughout the postpartum period. Behavioral Neuroscience. 2001;115(3):683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mendoza SP. Squirrel monkeys. In: Poole T, editor. The UFAW Handbook on the Care and Management of Laboratory Animals. Seventh ed. Oxford: Blackwell Science Ltd; 1999. pp. 591–600. [Google Scholar]
- Mendoza SP, Capitanio JP, Mason WA. Chronic social stress: Studies in non-human primates. In: Moberg GP, Mench JA, editors. Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. New York, New York: CABI Publishing; 2000. pp. 227–247. [Google Scholar]
- Mendoza SP, Coe CL, Smotherman WP, Kaplan J, Levine S. Functional consequences of attachment: A comparison of two species. In: Bell RW, Smotherman WP, editors. Maternal Influences and Early behavior. Jamaica, N.Y: Spectrum Publications; 1980. [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology and behavior. 1986a;38(6):795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch) Animal Behaviour. 1986b;35(5):1336–1347. [Google Scholar]
- Moynihan M. Communication in the titi monkey, Callicebus. Journal of Zoology. 1966;150:77–127. [Google Scholar]
- Muscat R, Willner P. Effects of dopamine receptor antagonists on sucrose consumption and preference. Psychopharmacology (Berl) 1989;99(1):98–102. doi: 10.1007/BF00634461. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiology and behavior. 2007;91(5):506–512. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Feeling the pain of social loss. Science. 2003;302(5643):237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neuroscience and Biobehavioral Reviews. 1980;4(4):473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Progress in Neurobiology. 2004;73(3):179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Research. 1995;693(1–2):21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Ruiz-Miranda CR, Kleiman DG. Conspicuousness and complexity: Themes in lion tamarin communication. In: Kleiman DG, Rylands AB, editors. Lion tamarins: Biology and conservation. Washington: Smithsonian Institution Press; 2002. pp. 233–254. [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. Journal of Pharmacology and Experimental Therapeutics. 2003;305(1):1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE. Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): A radiolabeled clearance study and endogenous excretion under varying social conditions. Hormones and behavior. 2007;51(3):436–442. doi: 10.1016/j.yhbeh.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Meyer ME, Dewsbury DA. Affiliative behavior in voles: effects of morphine, naloxone, and cross-fostering. Physiology and behavior. 1989;46(4):719–723. doi: 10.1016/0031-9384(89)90357-0. [DOI] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and behavior. 2010a;57(2):255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic hotspots: Generating sensory pleasure in the brain. In: Kringelbach ML, Berridge KC, editors. Pleasures Brain of the Brain. Oxford: Oxford University Press; 2010b. pp. 27–49. [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Hormones and behavior. 2010;58(4):614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towell A, Muscat R, Willner P. Effects of pimozide on sucrose consumption and preference. Psychopharmacology (Berl) 1987;92(2):262–264. doi: 10.1007/BF00177926. [DOI] [PubMed] [Google Scholar]
- Valeggia CR. Social influences on the development of sexual physiology and behavior in titi monkey females (Callicebus moloch) [PhD] Davis: University of California, Davis; 1996. [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nature Neuroscience. 2009;12(2):200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female priarie voles (Microtus ochrogaster): The role of social and sexual experience. Hormones and behavior. 1992;26(3):339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of Neuroendocrinology. 1994;6(3):247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Maeda S. Effects of taste stimulation on beta-endorphin levels in rat cerebrospinal fluid and plasma. Physiology and behavior. 2000;69(3):345–350. doi: 10.1016/s0031-9384(99)00252-8. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Wang ZX. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neuroscience and Biobehavioral Reviews. 2011a;35(3):498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Dietz DM, Wang H, Kabbaj M, Wang ZX. Amphetamine alters behavior and mesocorticolimbic dopamine receptor expression in the monogamous female prairie vole. Brain Research. 2011b;1367:213–222. doi: 10.1016/j.brainres.2010.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]