Abstract
To better understand the roles that hormones and experience play in infant survival and maternal behavior in a biparental primate species, we analyzed urinary estrone (E1C) and pregnanediol glucuronide (PdG) from 24 socially housed titi monkey (Callicebus cupreus) females over 54 pregnancies (N = 1430 samples). Pregnancies were categorized according to whether the infant survived (N = 35) or not (N = 19), and by maternal parity (primiparous: N = 9; multiparous: N = 45). Mothers of infants that survived had a significantly greater drop in PdG from the third trimester to the first week postpartum than mothers of infants that did not survive. Multiparous mothers had a greater increase in PdG from the first to the third trimester as well as greater increases in the E1C:PdG ratio from the first trimester to the third trimester and from the third trimester to the first week postpartum. There were positive relationships between third trimester PdG and maternal carrying and nursing during the first week postpartum, and between maternal age and carrying during the infant’s first month of life. There was a negative correlation between maternal age and PdG during the third trimester. These results suggest that elevated progesterone during late pregnancy followed by progesterone withdrawal immediately following parturition is associated with greater probability of infant survivorship and maternal behavior in this species, and that older females engage in more postpartum maternal care.
Keywords: steroid hormones, maternal behavior, maternal parity, infant survival
INTRODUCTION
Steroid hormones, including estrogen (E) and progesterone (P), during late pregnancy originate from multiple organs and serve multiple functions. The primary sources of these hormones include the mother, the fetus, and the placenta, and the main purpose of the hormones is to facilitate communication between these three sources so as to orchestrate a successful gestation [Chambers and Hearn, 1985; Godfrey, 2002; Haig, 1996; Rutherford, 2009]. An additional function of these hormones that has evolved in mammalian lineages is to prepare females for labor, birth, and maternal behavior following birth. Of particular interest to this study is whether these hormones appear to influence maternal care and, ultimately, infant survival, and whether female parity predicts their concentrations in a monogamous nonhuman primate.
E concentrations, which peak before the onset of labor [Moltz et al., 1970; Terkel and Rosenblatt, 1972; Yoshinaga et al., 1969], have been associated with the initiation of maternal behaviors in many mammalian species. Critically, E is most effective at initiating maternal behaviors when acting synergistically with other ovarian hormones. Moltz and colleagues [Moltz et al., 1970] experimentally induced maternal behaviors in ovariectomized nulliparous rat dams by administering estradiol (E2), P, and prolactin at specific time points during gestation. The simultaneous withdrawal of P and stimulation by E are critical to initiating maternal behaviors [Hauser and Gandelman, 1985].
P primarily functions in creating a hospitable environment for the developing embryo. In most mammals, a drop in P immediately precedes the onset of labor [Brown et al., 2006], however, the drop is less dramatic in primates than in other species [Power and Schulkin, 2006]. Because of the critical role P plays during gestation and the events immediately leading up to parturition, several studies have investigated the role that this hormone may play in the initiation of maternal behaviors. In rats, treatment with estrogen induces maternal behaviors in virgin females [Siegel and Rosenblatt, 1975a], while simultaneous P treatment inhibits this behavioral change [Siegel and Rosenblatt, 1975b]. In ewes, vaginal stimulation is necessary to inhibit aggression towards offspring but is only effective following hormonal priming with E and P [Kendrick and Keverne, 1991]. In common marmosets, a more dramatic prepartum drop in P is associated with greater maternal motivation postpartum [Pryce, 1993]. However, in baboons, those mothers that had the greatest drop from prepartum pregnanediol glucuronide (PdG; a metabolite of P), to postpartum PdG maintained less contact with their infants—the exact opposite pattern observed in marmosets [Bardi et al., 2004]. In a study comparing rhesus and Japanese macaques, no differences in maternal style were associated with PdG [Bardi et al., 2003]. One possible explanation for the contrasting findings between marmosets, baboons, and macaques is that a critical factor in determining the role that PdG plays in initiating maternal behavior is the social system of the species. That is, PdG signaling may have a different meaning in a species that regularly engages in biparental care of offspring and a species in which care of offspring is nearly entirely achieved by females. Further, other hormonal profiles (e.g. oxytocin, estrogen, prolactin) of females in different social structures are very likely different, which could influence the expression, and subsequent urinary concentrations of PdG [Ramirez et al., 2004; Shively et al., 2007; Wilson et al., 2005].
Maternal parity, or the amount of maternal experience a female has, is also known to influence maternal care and, ultimately, infant survival in mammals. Generally, more maternal experience is associated with better maternal care [Epple, 1978; Hrdy, 1999; Maestripieri et al., 1997; Tardif et al., 1984]. This may be due to the following: increased physical fitness of the older mother leading to greater reproductive success [Cameron et al., 2000]; increased knowledge of how to successfully raise offspring leading to greater reproductive success (e.g. [Fairbanks, 1996; Green, 1990]); and the initial gestation having “primed” the female to behave maternally resulting in multiparous females behaving appropriately, while primiparous females may not [Caraty and Skinner, 1999; Kendrick and Keverne, 1991; Keverne, 1996; Rosenblatt and Siegel, 1981].
The majority of research in this field has been conducted in either rodents or sheep. While these models have made many contributions to our current understanding of the role of neuroendocrine mechanisms in maternal behavior, behavioral and physiological differences exist between these taxa and those more closely related to humans. Perhaps the most relevant difference to this study is the fact that the endocrine patterns and the sources of certain gestational hormones differ between primates and other mammals [Power and Schulkin, 2006]. In most mammalian species P is converted to E2 by the placenta. This means that elevations in P are followed by elevations in E2 and reduction in P particularly in late term gestation when the placenta is most efficient [Smith et al., 2005]. In primates, the placenta is incapable of converting P to E2 because it lacks the critical enzyme 17α-hydoxylase-17,20-lyase [Kallen, 2004]. The source of E2 accounting for the late gestation rise is conversion from Dehydroepiandrosterone sulfate (DHEAS) from the fetal adrenal gland. Thus, in primates, but not in rodents, late term elevations in E2 do not result in obligatory decreases in P and both hormones can be simultaneously elevated [Rainey et al., 2004].
While nonhuman primates generally are more similar to humans than rodents, the majority of primate species are distinct from humans in their parental behaviors. That is, in most primate species, parental behavior comes exclusively from the mother, whereas human families have exhibited a wide range of parental styles throughout history, among them biparental care, which is the norm in modern Western culture [Feldman et al., 2010]. Titi monkeys (Callicebus cupreus) are particularly appropriate for modeling human parental behavior and its underlying physiology because they, like humans, exhibit social monogamy [Fuentes, 1998] and biparental care [Fragaszy et al., 1982; Mendoza and Mason, 1986]. Titi monkeys also exhibit dramatic variability in their expression of parental care. In most cases, the father is the primary carrier of infants, and the mother carries only for short periods of time to allow the infant to nurse [Mendoza and Mason, 1986]. As a result, the infant will only show an adrenocortical response to separation from its father, not from its mother [Hoffman et al., 1995]. In certain individuals, however, the mother is the primary, or even exclusive, carrier of the infant. The mechanisms that are responsible for these individual differences are not known, but if the behavioral similarities between titi monkeys and humans are driven by similarities in physiology, then titis would represent an ideal model to study the physiology underlying human parental care and could yield information about individual differences in parental care present in humans.
This study was designed to investigate the association between maternal hormones (estrogens and progestins), maternal experience, maternal behavior, and infant survivorship in a monogamous New World primate, the coppery titi monkey. We predicted that a greater peak in estrogens prior to parturition and a greater drop in progestins at parturition would result in a stronger expression of maternal behaviors and result in a greater likelihood of offspring survival. In addition, we predicted that mothers with previous experience rearing offspring would have greater infant survival, a greater prepartum estrogen peak, and a more dramatic drop in progestins than inexperienced mothers.
METHODS
All procedures used in this study were approved by the University of California, Davis Institutional Animal Care and Use Committee, and adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non Human Primates and the legal requirements of the United States.
Subjects
Subjects were 24 coppery titi monkey (Callicebus cupreus) females housed at the California National Primate Research Center (CNPRC). All females had been raised, and at the time of the study were living, in family groups consisting of an adult male and female and their offspring, a social living condition that approximates the species typical pattern displayed in free-ranging titi monkeys. All females were sexually mature (mean age = 4.18; range: 2.8–6.4) and had been paired with a sexually mature male for at least one year. Aside from infrequent removal for health or experimental purposes all animals lived continuously with their mates and offspring during the study and were housed in cages (2.13 m × 1.27 m × 1.27 m), identical to the housing described previously [Mendoza, 1999]. Twice daily (at 0800 and 1300 hrs) titi monkeys were fed a diet consisting of monkey chow, cottage cheese, marmoset jelly, apples, raisins, baby carrots, and vitamins.
Sample collection and analysis
Urine samples were collected during the first morning void at 0600 hr once per week. Within 30 min of collection samples were transferred to polypropylene tubes and stored at −20°C until assay. Samples from the entire gestation (gestation length based on [Valeggia et al., 1999]) and the first three months post-partum were assayed for urinary concentrations of estrogen (E1C) and progesterone (PdG) metabolites (1430 urine samples from 54 pregnancies). Most females were represented multiple times in the data set (mean number of pregnancies = 2.45; range: 1–6).
All urine samples were assayed via enzyme immunoassays (EIAs) for pregnanediol-3-glucuronide (PdG; [Monro et al., 1991]) and estrone-1-conjugates (E1C; [Shideler et al., 1990]) that had been previously validated for titi monkeys [Valeggia, 1996]. All samples were assayed for creatinine (Cr) concentrations in order to correct hormone concentrations for diluteness of urine [Jaffe, 1886] and concentrations are reported as pg of hormone per mg Cr. Inter-assay coefficients of variance were 1.55% and 1.72% and intra-assay coefficients of variance were 0.89% and 2.39% for PdG and E1C, respectively.
Behavioral analysis
One-hour video recordings were conducted for each female three times per week following the birth of her infant. Observations were one hour in length with the infant as the focal individual. Recordings were scored for maternal behaviors of interest included nursing, carrying (measured as duration), anogenital licking, investigation, and face licking (measured as frequency; see Table I for description of behaviors). Video recordings were scored using Behavior Tracker v. 1.5 (www.behaviortracker.com) by individuals blind to the reproductive status of the females.
Table I.
Description of a number maternal behaviors typically expressed by Callicebus cupreus
| Maternal behavior | Description |
|---|---|
| Carrying | Mother carries infant |
| Nursing | Infant observed in nursing position on mother |
| Anogenital licking | Mother licks anogenital region of infant |
| Investigation | Mother inspects infant, either while carrying or not |
| Face licking | Mother licks the face of the infant |
Infant weighing
Infants were weighed at six days of age. Following the initial weighing, weights were recorded every 14 days until the offspring reached nine months of age.
Statistical analyses
Infants were categorized as “surviving” if they survived to at least two weeks of age (survive = 35 infants; not survive = 19 infants). This survival rate (65.8%) is similar to that found in other New World monkey species [Tardif et al., 1984]. Infant mortality in this study was due to either developmental problems resulting in complications in gestation or insufficient communication between the infant and parents postpartum. Generally, early gestational complications resulted in either spontaneous abortion of the fetus or a stillborn infant, and late gestational complications resulted in insufficient communication between the infant and parents, and ultimately to parental rejection. In either case, the exact cause of infant death was often unknown.
Mothers were categorized as being multiparous if at least one previous offspring was confirmed alive at birth (N = 45 pregnancies). That is, she had at least one previous opportunity to behave maternally. This categorization excludes cases where the infant was found dead and we were unable to determine whether the infant was born alive or dead. Those females were considered primiparous (N = 9 pregnancies). Hormone concentration means were calculated for each female for each of her pregnancies if samples were obtained from multiple pregnancies.
Samples were categorized according to the time relative to parturition that they were obtained: first, second, and third trimester, and the 1st week postpartum. We used generalized linear mixed models (GLIMMIX macro in SAS 9.1) that controlled for maternal age and infant weight at first weighing, with maternal identity as a random factor, to investigate whether E1C, PdG, or E1C:PdG ratio were significant predictors of infant survival. In addition, we investigated maternal parity as a predictor of maternal E1C, PdG, and E1C:PdG ratio (Table II).
Table II.
Variables used in mixed models.
| Dependent variable | Independent variables |
|---|---|
| Infant survival | Maternal hormones, maternal behaviors, maternal parity (controlling for maternal age, infant weight at one week). |
| Maternal hormones, maternal behaviors | Maternal parity, maternal age |
| Infant weight at one week*, infant weight at one month*, infant growth rate | Maternal parity, maternal hormones, maternal behaviors, maternal age |
| Nursing, week 1 Carrying, week 1 |
Maternal hormones |
Infant weight was only collected from infants that survived.
Correlations between hormone concentrations and maternal behaviors occurring in the same time period were calculated, as well as correlations between prepartum hormone concentrations and postpartum behaviors. Correlational analysis was used to determine if maternal hormones or behaviors were predictive of infant growth rate. Correlational analyses were used to determine if maternal age was associated with differences in maternal hormones and behaviors. In all correlation analyses the Hochberg-Benjamini false discovery rate (FDR) correction was used to adjust p-values for multiple tests [Benjamini and Hochberg, 1995]. Chi-square analysis was used to analyze proportions of surviving offspring between multiparous and primiparous mothers. T tests were used to compare infant growth rates across maternal parity.
RESULTS
Infant survival
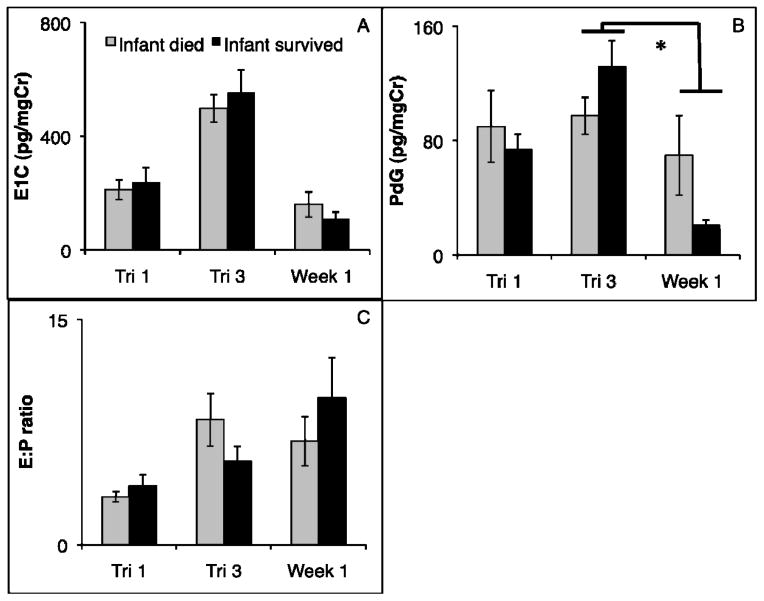
Neither mean E1C levels, nor mean PdG levels at any time point predicted infant survival (all Ps > 0.25; Fig. 1A). However, the drop in mean PdG from the third trimester to the first week postpartum was significantly greater in mothers of surviving infants than mothers of infants that did not survive (Fig. 1B; F1,22 = 4.87, P = 0.04). There were no significant differences in the E1C:PdG ratio at any time point, or across time points.
Figure 1.
E1C (A), PdG (B) and E1C:PdG ratio (C) averages at relevant time points (first trimester, last trimester, and first week postpartum) for mothers of infants that survived (dark) and mothers of infants that died (light). Mothers of infants that survived showed a greater drop in PdG from the third trimester to the first week postpartum (* = p < 0.05).
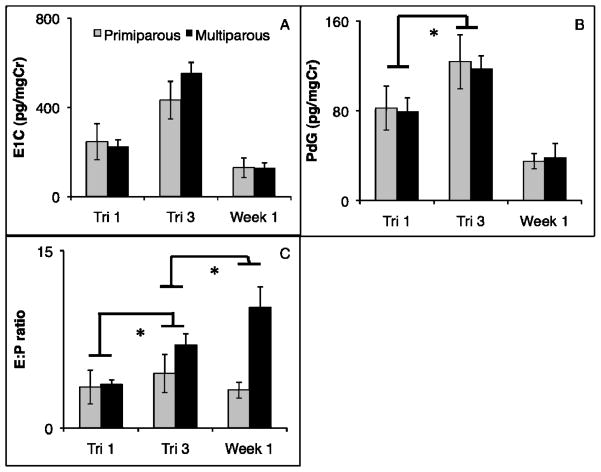
Maternal parity and age
Infants of multiparous mothers were more likely to survive than infants of primiparous mothers (χ2 = 7.29, df = 1, P < 0.01). Maternal parity was not predictive of E1C at any time point, or of the change in E1C across time points (all Ps > 0.20; Fig. 2A). The change in PdG from the first to the third trimester was significantly greater among multiparous mothers (Fig. 2B; F1,24 = 12.17, P < 0.01). The increase in the E1C:PdG ratio from the first trimester to the third trimester was significantly greater in multiparous mothers (Fig. 2C; F1,21 = 30.33, P < 0.01). Similarly, a significantly greater increase in the E1C:PdG ratio was seen in multiparous mothers from the third trimester to the first week postpartum (Fig. 2C; F1,28 = 83.36, P < 0.01).
Figure 2.
E1C (A), PdG (B) and E1C:PdG ratio (C) averages at relevant time points (first trimester, last trimester, and first week postpartum) for multiparous (dark) and primiparous (light) mothers. The increase in PdG from first trimester to third trimester was greater in multiparous mothers. E:P showed a greater increase in multiparous mothers, both in the change from first to third trimester, and third trimester to first week postpartum (* = p < 0.05 for change between time points between groups).
There were no significant differences in maternal behavior between primiparous and multiparous mothers. There were no differences between primiparous and multiparous mothers in infant growth rate (P = 0.37), infant weight at one week (P = 0.44), or infant weight at one month (P = 0.30). No significant correlations between infant growth rate and any maternal behaviors or maternal hormone concentrations were found (all Ps > 0.18).
Maternal age was negatively associated with PdG during the third trimester (r52 = −0.445, p < 0.01). Maternal age among mothers of infants that survived was positively associated with carrying during the first week postpartum (r21 = 0.464, p < 0.05) and the first month postpartum (r21 = 0.466, p < 0.05). Maternal age did not differ significantly between mothers of infants who survived and mothers of infants that did not survive (t52 = 0.123, p = 0.90).
Maternal hormone X behavior correlations
No significant correlations were found between either E1C or PdG and maternal behaviors measured within the same time period as the hormones were collected (all Ps > 0.21). Significant positive correlations were found between third trimester PdG and maternal carrying during the first week (Fig. 3A; r53 = 0.374, P = 0.03) and maternal nursing during the first week (Fig. 3B; r53 = 0.475, P < 0.01).
Figure 3.
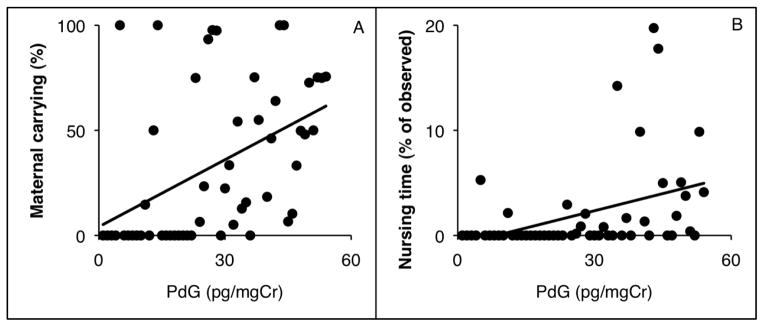
Relationship between maternal PdG and maternal carrying (A) and nursing (B). PdG during the third trimester was associated with the percent of time the infant was observed being carried by its mother (r53 = 0.374, adjusted P = 0.03), and the amount of time the infant was observed nursing (r53 = 0.475, adjusted P < 0.01) as a percent of the total time observed during the first week postpartum.
DISCUSSION
The main objectives of this study were to investigate the association between maternal estrogens and progestins, maternal experience, maternal behaviors, and infant survivorship. E1C concentrations were not associated with any maternal behaviors, nor did E1C differ between primiparous and multiparous mothers or between mothers of infants that survived and those that did not. Changes in PdG differed between pregnancies that resulted in surviving vs. non-surviving infants, and between multiparous and primiparous mothers. Although PdG concentrations at no single time point predicted infant survival, the drop in PdG from the third trimester to the first week postpartum was greater in mothers whose infants survived.
In female sheep central administration of P increases responsiveness towards infant signals and likelihood to perform maternal behaviors [Kendrick and Keverne, 1991; Wang et al., 1995]. We did not find differences in maternal behavior in the immediate postpartum period, but multiparous mothers showed a greater increase in PdG from the first to the third trimester, and multiparous females had greater rates of infant survival.
One mechanism by which P could influence maternal behaviors is through its impact on other neurotransmitter systems. P withdrawal following sustained elevations in P has been shown to increase oxytocin (OT) mRNA in the paraventricular nucleus of female rats [Amico et al., 1997; Blyth et al., 1997]. Central OT has long been implicated in the onset of maternal behaviors [reviewed in Insel and Young, 2001; Kendrick et al., 1987; Keverne, 1996], and steroid treatment enhances OT expression in both the magnocellular and parvocellular cells of the paraventricular nucleus (PVN) [Thomas et al., 1999]. A greater drop in P might result in increased OT expression, which is likely to have peripheral consequences in milk letdown [Saltzman and Maestripieri, 2011] as well as behavioral consequences [Champagne, 2008; Dwyer and Smith, 2008; Meddle et al., 2007]. The ability to provide milk to offspring is certainly critical to offspring survival in any mammalian species. Elevated P during late pregnancy followed by a dramatic decrease in P at parturition may also result in optimal central OT expression in the immediate postpartum and may be causally associated with maternal behavior and infant survival. However, this study was correlational in design, so we can not determine if hormones influenced survival and maternal behavior. It is equally possible that signals from the offspring, either in utero or postpartum, affected maternal hormone production. Further, maternal age or experience could influence her hormone profiles.
The hormones excreted by the mother may not necessarily originate from her tissues. These hormones are equally likely to have come from either the developing fetus or the placenta and may represent a mechanism through which the fetus communicates with the placenta [Albrecht and Pepe, 1999]. Higher pre-partum P affects not only the mother, but also the developing fetus, making the infant fully developed at parturition [Kleemann et al., 1994]. Therefore, if P can serve as a signal from the fetus and, in turn, can affect development of the fetus, differences in urinary PdG during gestation may represent differences in fetoplacental health [Kallen, 2004]. Although differences in urinary PdG during gestation were present, they do not appear to explain any variability in infant birth weights. A multitude of variables contribute to infant birth weight, and while PdG is a marker of developmental signalling, it seems in this case that other signals outweighed the effects of PdG [Chiesa et al., 2008; Goedhart et al., 2010].
Mothers with previous experience showed a greater increase in PdG from the first to third trimesters. While maternal parity was not predictive of E1C, the increase in the E1C:PdG ratio was greater in multiparous mothers, both from the first to the third trimesters, and from the third trimester to the first week postpartum. High E1C:PdG ratio during late pregnancy has been associated with initiation of maternal behavior and responsiveness in macaques [Bardi et al., 2001; Maestripieri, 1999]. Increased responsiveness towards infants may account for the increased survival rate of infants born to multiparous mothers as compared to primiparous mothers. Alternatively, we found a significant negative association between maternal age and PdG during the third trimester, as well as a negative association between maternal age and the drop in PdG from the third trimester to the first week postpartum. We also found a positive association between maternal age and carrying during the first week postpartum. Together these findings suggest that while maternal hormones may indeed influence behavior, maternal age may influence behavior as well – either directly through behavioral changes associated with aging not measured in this study, or indirectly through changes in hormonal profiles. Of particular interest here is that the negative relationship between maternal age and two measures of PdG concentration suggest that younger females have a “better” hormone profile, given that higher PdG during the third trimester and greater drops in PdG from the third trimester to the first week postpartum were observed in mothers of infants that survived. However, when the infant survives, older mothers engage in more maternal carrying of the infant.
The data presented here suggest that the decrease in maternal PdG from the third trimester to the first week postpartum is associated with increased infant survival. Additionally, this study provides evidence that differences in maternal parity and hormones are associated with differences in maternal behaviors and infant survival. While no causal relationship can be determined from this study alone, at least some of the mechanisms involved with the onset of maternal behavior and infant survival are similar to those that have been more thoroughly studied in rodents. As was previously mentioned, this species of monkey is fairly unique in that it is socially monogamous and engages in biparental care. Differences between this work and previous studies on this topic may provide insight into differences between monogamous and nonmonogamous primates in maternal behavior and the underlying physiology that drives that behavior. In addition, the patterns observed here may provide some understanding of the individual differences in parental care styles present in humans.
Table III.
Summary of general linear mixed model significant statistics
| Model | n | F-value | P-value |
|---|---|---|---|
| Infant survival | |||
| Drop in maternal PdG from 3rd trimester to 1st week | 23 | 4.87 | 0.038 |
| Maternal parity | |||
| Rise in PdG from 1st to 3rd trimester | 24 | 12.17 | 0.002 |
| Rise in E1C:PdG ratio from 1st to 3rd trimester | 21 | 30.33 | <0.001 |
| Rise in E1C:PdG ratio from 3rd trimester to 1st week | 28 | 83.36 | <0.001 |
Acknowledgments
The authors thank L. Laughlin and T. Yim for their contributions to the sample analysis. This work was supported by funding from the Good Nature Institute (to KLB), the National Institutes of Health (HD053555 to KLB, RR00169 to CNPRC) and the Schwall Fellowship in Biomedical Research (to MRJ).
References
- Albrecht ED, Pepe GJ. Central integrative role of oestrogen in modulating the communication between the placenta and fetus that results in primate fecal-placental development. Placenta. 1999;20:129–39. doi: 10.1053/plac.1998.0359. [DOI] [PubMed] [Google Scholar]
- Amico JA, Thomas A, Hollingshead DJ. The duration of estradiol and progesterone exposure prior to progesterone withdrawal regulates oxytocin mRNA levels in the paraventricular nucleus of the rat. Endocrine Research. 1997;23:141–56. doi: 10.3109/07435809709031849. [DOI] [PubMed] [Google Scholar]
- Bardi M, French JA, Ramirez SM, Brent L. The role of the endocrine system in baboon maternal behavior. Biological Psychiatry. 2004;55:724–32. doi: 10.1016/j.biopsych.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum sex steroid changes and maternal style in rhesus and japanese macaques. General and Comparative Endocrinology. 2003;133:323–31. doi: 10.1016/s0016-6480(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Fujita S, Borgognini-Tarli SM, Huffman MA. Hormonal correlates of maternal style in captive macaques (macaca fuscata, m. Mulattaci) International Journal of Primatology. 2001;22:647–662. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:12. [Google Scholar]
- Blyth BJ, Hollingshead DJ, Amico JA. Time course of induction of oxytocin messenger ribonucleic acid levels in the hypothalamic paraventricular nucleus of ovariectomized rats following gonadal steroid administration. Life Sciences. 1997;60:2427–33. doi: 10.1016/s0024-3205(97)00327-5. [DOI] [PubMed] [Google Scholar]
- Brown AG, Leite RS, Strauss JF., 3rd Mechanisms underlying “functional” progesterone withdrawal at parturition. Annals of the New York Academy of Sciences. 2006;1034:36–49. doi: 10.1196/annals.1335.004. [DOI] [PubMed] [Google Scholar]
- Cameron EZ, Linklater WL, Stafford KJ, Minot EO. Aging and improving reproductive success in horses: Declining residual reproductive value or just older and wiser? Behavioral and Ecological Sociobiology. 2000;47:243–249. [Google Scholar]
- Caraty A, Skinner DC. Progesterone priming is essential for the full expression of the positive feedback effect of estradiol in inducing preovulatory gonadotropin-releasing hormone surge in the ewe. Endocrinology. 1999;140:165–170. doi: 10.1210/endo.140.1.6444. [DOI] [PubMed] [Google Scholar]
- Chambers PL, Hearn JP. Embryonic, foetal, placental development in the common marmoset (callithrix jacchus) Journal of Zoology. 1985;207:545–561. [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in Neuroendocrinology. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa C, Osborn JF, Haass C, Natale F, Spinelli M, Scapillati E, Spinelli A, Pacifico L. Ghrelin, leptin, igf-1, igfbp-3, and insulin concentrations at birth: Is there a relationship with fetal growth and neonatal anthropometry? Clinical Chemistry. 2008;54:550–8. doi: 10.1373/clinchem.2007.095299. [DOI] [PubMed] [Google Scholar]
- Dwyer CM, Smith LA. Parity effects on maternal behaviour are not related to circulating oestradiol concentrations in two breeds of sheep. Physiology & Behavior. 2008;93:148–54. doi: 10.1016/j.physbeh.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Epple G. Reproductive and social behavior of marmosets with special reference to captive breeding. Primates in Medicine. 1978;10:50–62. [PubMed] [Google Scholar]
- Fairbanks LA. Individual differences in maternal style: Causes and consequences for mothers and offspring. Advances in the Study of Behavior. 1996;25:579–611. [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Schwarz S, Shimosaka D. Longitudinal observations of care and development of infant titi monkeys (callicebus moloch) American Journal of primatology. 1982;2:191–200. doi: 10.1002/ajp.1350020207. [DOI] [PubMed] [Google Scholar]
- Fuentes A. Re-evaluating primate monogamy. American Anthropologist. 1998;100:890–907. [Google Scholar]
- Godfrey K. The role of the placenta in fetal programming-- a review. Placenta. 2002;23:S20–S27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- Goedhart G, Vrijkotte TG, Roseboom TJ, van der Wal MF, Cuijpers P, Bonsel GJ. Maternal cortisol and offspring birthweight: Results from a large prospective cohort study. Psychoneuroendocrinology. 2010;35:644–52. doi: 10.1016/j.psyneuen.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Green WCH. Reproductive effort and associated costs in bison (bison bison): Do older mothers try harder? Behavioral Ecology. 1990;1:148–160. [Google Scholar]
- Haig D. Placental hormones, genomic imprinting, and maternal-fetal communication. Journal of Evolutionary Biology. 1996;9:357–380. [Google Scholar]
- Hauser H, Gandelman R. Lever pressing for pups: Evidence for hormonal influence upon maternal behavior of mice. Hormones and Behavior. 1985;19:454–68. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Hoffman KA, Mendoza SP, Hennessy MB, Mason WA. Responses of infant titi monkeys, callicebus moloch, to removal of one or both parents: Evidence for paternal attachment. Developmental Psychobiology. 1995;28:399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. Mother nature. New York: Pantheon; 1999. [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Neuroscience Reviews. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Jaffe M. Measurement of creatinine using picric acid. Zeitschrift fur Physiologische Chemie. 1886;10:391–400. [Google Scholar]
- Kallen CB. Steroid hormone synthesis in pregnancy. Obstetrics and Gynecology Clinics of North America. 2004;31:795–816. doi: 10.1016/j.ogc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB. Importance of progesterone and estrogen priming for the induction of maternal behavior by vaginocervical stimulation in sheep: Effects of maternal experience. Physiology & Behavior. 1991;49:745–50. doi: 10.1016/0031-9384(91)90313-d. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behavior in sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Psychopharmacology of maternal behavior. Journal of Psychopharmacology. 1996;10:16–22. doi: 10.1177/026988119601000104. [DOI] [PubMed] [Google Scholar]
- Kleemann DO, Walker SK, Seamark RF. Enhanced fetal growth in sheep administered progesterone during the first three days of pregnancy. Journal of Reproduction and Fertility. 1994;102:411–417. doi: 10.1530/jrf.0.1020411. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Changes in social behavior and their hormonal correlates during pregnancy in pig-tail macaques. International Journal of Primatology. 1999;20:707–718. [Google Scholar]
- Maestripieri D, Wallen K, Carroll KA. Infant abuse runs in families of group-living pigtail macaques. Child Abuse Neglect. 1997;21:465–571. doi: 10.1016/s0145-2134(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Mendoza SP. Squirrel monkeys. In: Poole T, editor. The ufaw handbook on the care and management of laboratory animals. 7. Oxford: Blackwell Science Ltd; 1999. pp. 591–600. [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (callicebus moloch) Animal Behaviour. 1986;34:1336–1347. [Google Scholar]
- Moltz H, Lubin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiology & Behavior. 1970;5:1373–7. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Monro CJ, Stabenfeldt GH, Cragun JR, Addiego LA, Overstreet JW, Lasley BL. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clinical Chemistry. 1991;37:838–844. [PubMed] [Google Scholar]
- Power ML, Schulkin J. Functions of corticotropin-releasing hormone in anthropoid primates: From brain to placenta. American Journal of Human Biology. 2006;18:431–47. doi: 10.1002/ajhb.20521. [DOI] [PubMed] [Google Scholar]
- Pryce CR. The regulation of maternal behavior in marmosets and tamarins. Behavioural Processes. 1993;30:201–224. doi: 10.1016/0376-6357(93)90133-C. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Rehman KS, Carr BR. The human fetal adrenal: Making adrenal androgens for placental estrogens. Seminal Reproductive Medicine. 2004;22:327–336. doi: 10.1055/s-2004-861549. [DOI] [PubMed] [Google Scholar]
- Ramirez SM, Bardi M, French JA, Brent L. Hormonal correlates of changes in interest in unrelated infants across the peripartum period in female baboons (papio hamadryas anubis sp.) Hormones and Behavior. 2004;46:520–8. doi: 10.1016/j.yhbeh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Siegel HI. Factors governing the onset and maintenance of maternal behavior among nonprimate mammals: The role of hormonal and non-hormonal factors. In: Gubernick DJ, Klopfer PH, editors. Parental care in mammals. New York: Plenum; 1981. pp. 13–76. [Google Scholar]
- Rutherford JN. Fetal signaling through placental structure and endocrine function: Illustrations and implications from a nonhuman primate model. American Journal of Human Biology. 2009;21:745–753. doi: 10.1002/ajhb.20923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. The neuroendocrinology of primate maternal behavior. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2011;35:1192–1204. doi: 10.1016/j.pnpbp.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shideler SE, Monro CJ, Tell L, Owiti G, Laughlin L, Chatterton RT, Jr, Lasley BL. The relationship of serum estradiol and progesterone concentrations to the enzyme immunoassay measurements of urinary estrone conjugates and immunoreactive pregnanediol-3-glucuronide in macaca mulatta. American Journal of Primatology. 1990;22:113–122. doi: 10.1002/ajp.1350220205. [DOI] [PubMed] [Google Scholar]
- Shively CA, Wood CE, Register TC, Willard SL, Lees CJ, Chen H, Sitruk-Ware RL, Tsong YY, Cline JM. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32:981–90. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Estrogen induced maternal behavior in hysterectomized-ovariectomized virgin rats. Physiology & Behavior. 1975a;14:465–471. doi: 10.1016/0031-9384(75)90012-8. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Progesterone inhibition of estrogen-induced maternal behavior in hysterectomized-ovariectomized virgin rats. Hormones and Behavior. 1975b;6:223–230. doi: 10.1016/0018-506x(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, Nicholson R, Clifton V, Zakar T. The regulation of human parturition. In: Power ML, Schulkin J, editors. Birth, distress and disease: Placenta-brain interactions. Cambridge: Cambridge University Press; 2005. pp. 74–87. [Google Scholar]
- Tardif SD, Richter CB, Carson RL. Effects of sibling-rearing experience on future reproductive success in two species of callitrichidae. American Journal of Primatology. 1984;6:377–380. doi: 10.1002/ajp.1350060408. [DOI] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Humoral factors underlying maternal behavior at parturition: Cross transfusion between freely moving rats. Journal of comparative and physiological psychology. 1972;3:365–371. doi: 10.1037/h0032965. [DOI] [PubMed] [Google Scholar]
- Thomas A, Shughrue PJ, Merchenthaler I, Amico JA. The effects of progesterone on oxytocin mrna levels in the paraventricular nucleus of the female rat can be altered by the administration of diazepam or ru486. Journal of neuroendocrinology. 1999;11:137–144. doi: 10.1046/j.1365-2826.1999.00294.x. [DOI] [PubMed] [Google Scholar]
- Valeggia CR. Dissertation. Davis: University of California; 1996. Social influences on the development of sexual physiology and behavior in titi monkey females (callicebus cupreus) [Google Scholar]
- Valeggia CR, Mendoza SP, Fernandez-Duque E, Mason WA, Lasley B. Reproductive biology of female titi monkeys (callicebus moloch) in captivity. American journal of primatology. 1999;47:183–95. doi: 10.1002/(SICI)1098-2345(1999)47:3<183::AID-AJP1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wang MW, Crombie DL, Hayes JS, Heap RB. Aberrant maternal behaviour in mice treated with a progesterone receptor antagonist during pregnancy. The Journal of endocrinology. 1995;145:371–7. doi: 10.1677/joe.0.1450371. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic- pituitary-adrenal (lhpa) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Hawkins RA, Stocker JF. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969;85:103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]