Abstract
We report on 348 patients ≥ 70 years (median age 78 years) with acute myeloid leukemia (> 50% with secondary AML) randomized to receive either 600 mg or 300 mg of tipifarnib orally twice daily on days 1–21 or days 1–7 and 15–21, repeated every 28 days (4 treatment regimens). Responses were seen in all regimens, with overall response rate (CR + CRi + PR) highest (20%) among patients receiving tipifarnib 300 mg twice daily on days 1–21. Toxicities were acceptable. Unless predictors of response to tipifarnib are identified, further study as a single agent in this population is unwarranted.
Keywords: tipifarnib, farnesyltransferase inhibitor, acute myeloid leukemia, older, untreated
Introduction
The prognosis of older adults with acute myeloid leukemia (AML) has not improved over the last three decades and remains grim [1]. In a recent analysis of 2,657 Medicare beneficiaries with AML, the mortality was 86% and 94% at 1 and 2 years after initial diagnosis, respectively [2]. Of note, only 30% of patients received any form of intravenous chemotherapy in the two years following diagnosis (44% of those aged 65–74 years, 24% of those aged 75–84 years, and 6% of those aged 85 years and above) [2], suggesting a hesitation of physicians to subject many of these patients to systemic chemotherapy. In fact, Older AML patients often have difficulty tolerating intensive therapy due to poor performance status, impaired organ function, and co-morbid illnesses. On the other hand, as these patients more likely have unfavorable risk karyotypes, antecedent hematologic disorders such as myelodysplastic syndromes (MDS), and expression of the multi-drug resistance protein, they are less likely than younger adults to respond to conventional chemotherapy [3]. Indeed, two randomized trials comparing intensive induction therapy with palliative therapy for older patients with de novo AML produced conflicting results [4,5]. Although both studies reported a higher remission rate with intensive cytotoxic therapy, only one of these studies demonstrated a survival advantage following induction chemotherapy. Thus, effective therapies that can be tolerated by older AML patients are needed.
The RAS signaling pathway has been proposed as a rational pharmacologic target for treatment of the hematologic malignancies [6]. Activating mutations of the RAS proto-oncogene have been described in AML blasts [7]. Since RAS activity depends on post-translational farnesylation, a number of inhibitors of farnesyltransferase have been developed in an effort to perturb RAS signaling [8]. Tipifarnib is an oral farnesyltransferase inhibitor with activity in the treatment of MDS and high-risk AML patients [9,10,11,12,13]. For example, in a phase II trial, previously-untreated AML patients (N=158) received tipifarnib 600 mg twice daily for 21 consecutive days every 28 days [13]. The patient population had a median age of 74 years, prior MDS in 75%, and unfavorable blast cytogenetics in 47%. Fourteen percent achieved a complete remission with a median duration of remission of 7.3 months. However, there was no correlation of response with RAS mutation status, inhibition of protein farnesylation or activation of other signal transduction molecules in this study or other investigations. Although survival was better in patients who achieved a response, but there are no data available regarding the further therapy of these patients with chemotherapy; thus, the relative value of tipifarnib for the treatment of AML remains unclear.
Neurotoxicity was identified as a dose limiting toxicity associated with tipifarnib in prior phase I studies [9,10]. However, Kirschbaum and colleagues explored an interrupted schedule of tipifarnib in patients with predominantly relapsed and refractory AML [14]. Dose-limiting neurotoxicity was not observed, when tipifarnib was administered twice daily for seven consecutive days every 14 days. Responses were also observed with this every other week schedule. Whether neurotoxicity can be reduced without sacrificing efficacy in AML by lowering the dose or altering the schedule of the drug is unknown. Given these uncertainties, SWOG S0432 (ClinicalTrials.gov Identifier:NCT00093418) was a randomized phase II study which included the regimen previously described by Lancet et al. [13] and three alternative regimens with either lower dose or a more fractionated schedule. The primary objective of S0432 was to test whether any of the four different regimens of tipifarnib was sufficiently effective and tolerable for patients age 70 or over with previously untreated AML to warrant phase III study.
Materials and Methods
In this North American Intergroup study between SWOG, CALGB, and ECOG, eligible patients with newly diagnosed AML other than acute promyelocytic leukemia were treated. Eligible patients had reached their seventieth birthday and could not be considered candidates for, or must have declined, conventional AML induction chemotherapy. They could not have received prior therapy for AML other than hydroxyurea. Patients had to have adequate renal and hepatic function, and the white blood cell (WBC) count had to be less than 30,000/μL at the time of registration. Eligible patients could have a history of MDS, but could not have received intensive chemotherapy or stem cell transplantation. All patients provided written informed consent in accordance with local policies, federal regulations, and the declaration of Helsinki. Patients were randomized to receive one of four different regimens: arm 1, 600 mg twice daily on days 1–21; arm 2, 600 mg twice daily on days 1–7 and 15–21; arm 3, 300 mg twice daily on days 1–21 days; arm 4, 300 mg twice daily on days 1–7 and 15–21. Cycles were repeated every 28 days until disease progression or unacceptable toxicity. Bone marrow biopsies and aspirates were scheduled after every even number of cycles of tipifarnib, beginning with cycle 2. Patients achieving complete remission (CR) or CR with incomplete hematologic recovery (CRi) were to receive three additional cycles and then discontinue therapy. Complete response (CR), CR with incomplete hematologic recovery (CRi), and partial response (PR) were defined according to the International Working Group Guidelines [15]. Patients achieving partial remission (PR) or having stable disease could continue treatment until progression of AML.
Each phase II regimen was evaluated separately. The sample size of each phase II study was based on the following considerations. If a regimen’s true response rate was ≤10%, then further evaluation of that regimen would be unwarranted. However, if the response rate was ≥30%, then further investigation of that regimen would be considered reasonable. A two-stage accrual design was used for each regimen. If a single CR, CRi or PR was not observed in the first 15 patients, then the arm would be closed to further accrual. Otherwise, accrual would continue until an additional 59 patients were entered. If 15 or greater of the 74 patients responded, then further investigation would be warranted. All four treatment arms continued to full accrual. Additional patients were registered in order to account for potentially ineligible patients. Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 3. The data analysis was performed on August 3, 2012.
Results
Three hundred forty-eight patients were registered in 18 months between September 15, 2004 and February 15, 2006. Eighteen patients were excluded from analysis due to diagnosis other than AML, WBC above 30,000/μL, no protocol therapy, and incorrect regimen administered. The patient demographics are shown in Table 1. The median age in each arm was 78 years, with some patients being older than the age of 90 years. The majority of patients were men. At least 98% of patients in each arm had a performance status of 0–2. Between 48% and 52% of patients in each phase II study had either an antecedent hematologic disorder or treatment-related AML. Cytogenetic analysis was only available for patients entered by SWOG. Of the 167 eligible and evaluable SWOG patients included in this analysis, 124 patients (74%) had pretreatment specimens submitted and were evaluable by conventional cytogenetic analysis (Table 2). Normal diploid karyotypes were only seen in 22–37% of patients in each study arm. An unfavorable risk karyotype was observed in 41–59% of patients in each study. Karyotypes with ≥3 numeric and/or structural abnormalities were seen in 30–41% of patients. The most frequently encountered karyotypic abnormalities were monosomy 5 or del(5q) (32% of karyotypes) and monosomy 7 or del(7q) (23% of karyotypes). The core binding factor-related translocations were observed in only 3 patients.
Table 1.
Patient characteristics according to treatment arm
| 600 mg bid day 1–21 (n=80) | 600 mg bid d 1–7, 15–21 (n=85) | 300 mg bid day 1–21 (n=82) | 300 mg bid d 1–7, 15–21 (n=84) | ||
|---|---|---|---|---|---|
| Age (years) | |||||
| Median | 78 | 78 | 78 | 78 | |
| Range | 70–91 | 70–94 | 70–92 | 70–91 | |
| Gender | |||||
| Male | 68% | 58% | 72% | 64% | |
| AML onset | |||||
| De novo | 50% | 49% | 48% | 52% | |
| MDS | 50% | 44% | 46% | 43% | |
| Therapy | 0% | 7% | 6% | 5% | |
| Zubrod PS | |||||
| 0 | 25% | 27% | 28% | 23% | |
| 1 | 58% | 45% | 47% | 50% | |
| WBC, median | 2.9 | 2.9 | 2.4 | 4.7 | |
| HGB, median | 9.3 | 9.5 | 9.5 | 9.5 | |
| PLT, median | 66 | 44 | 61 | 45 | |
| Marrow blasts (%)1 | 44 | 40 | 45 | 40 | |
| Peripheral blasts (%) | 11 | 9 | 4 | 17 |
Table 2.
Cytogenetic analysis according to treatment arm
| 600 mg bid day 1–21 (n=80) | 600 mg bid d 1–7, 15–21 (n=85) | 300 mg bid day 1–21 (n=82) | 300 mg bid d 1–7, 15–21 (n=84) | ||
|---|---|---|---|---|---|
| Evaluable1 | 71% | 83% | 68% | 74% | |
| Normal karyotype | 35% | 29% | 23% | 28% | |
| Risk group2 | |||||
| Intermediate | 54% | 37% | 35% | 38% | |
| Unfavorable3 | 42% | 54% | 58% | 47% | |
| Karyotype complexity | |||||
| ≥ 3 | 31% | 37% | 38% | 34% | |
| ≥ 5 | 15% | 31% | 31% | 25% |
Data from 167 SWOG patients; 124 with evaluable cytogenetic analysis
Only 3 patients had CBF translocations; not (15;17)
−5/5q- in 34%, −7/7q- in 23%
Treatment results are presented in Table 3. The CR rates ranged from 1–11% in the four arms, with the overall response rates ranging from 6% to 20%. None of the treatment arms met the pre-defined response criterion of ≥ 15 responses among the first 74 allocated patients. Of the four arms, the overall response rate was highest following treatment with tipifarnib 300 mg twice daily on days 1–21 every 28 days (p = 0.0068). The response rates in the other three arms were not significantly different from each other. The median time to CR and CRi was 37 days in arm 1, 68 days in arm 2, 78 days in arm 3, and 64.5 days arm 4. The median number of treatment cycles delivered in patients achieving CR, CRi and PR were 4 in arm 1, 5 in arm 2, 6 in arm 3 and 6.5 in arm 4. Of the patients not achieving a CR, CRi, or PR, 148 received at least two cycles of tipifarnib, and 70 of these patients (47%) received at least three cycles.
Table 3.
Patient outcomes in the four phase II tipifarnib studies of S0432
| 600 mg bid day 1–21 (n=80) | 600 mg bid d 1–7, 15–21 (n=85) | 300 mg bid day 1–21 (n=82) | 300 mg bid d 1–7, 15–21 (n=84) | ||
|---|---|---|---|---|---|
| CR, CRi, PR | 16% | 12% | 20% | 5% | |
| 95% CI | 9–26% | 6–20% | 12–30% | 1–12% | |
| CR | 8% | 5% | 11% | 1% | |
| CRi | 8% | 6% | 4% | 4% | |
| PR | 1% | 1% | 5% | 0% | |
| Fatal toxicity | 8% | 4% | 2% | 0% | |
| Median survival | 104 days | 116 days | 111 days | 106 days | |
| 12 month overall survival | 13% | 25% | 28% | 15% | |
| 12 month relapse-free survival | 11% | 20% | 17% | 0% |
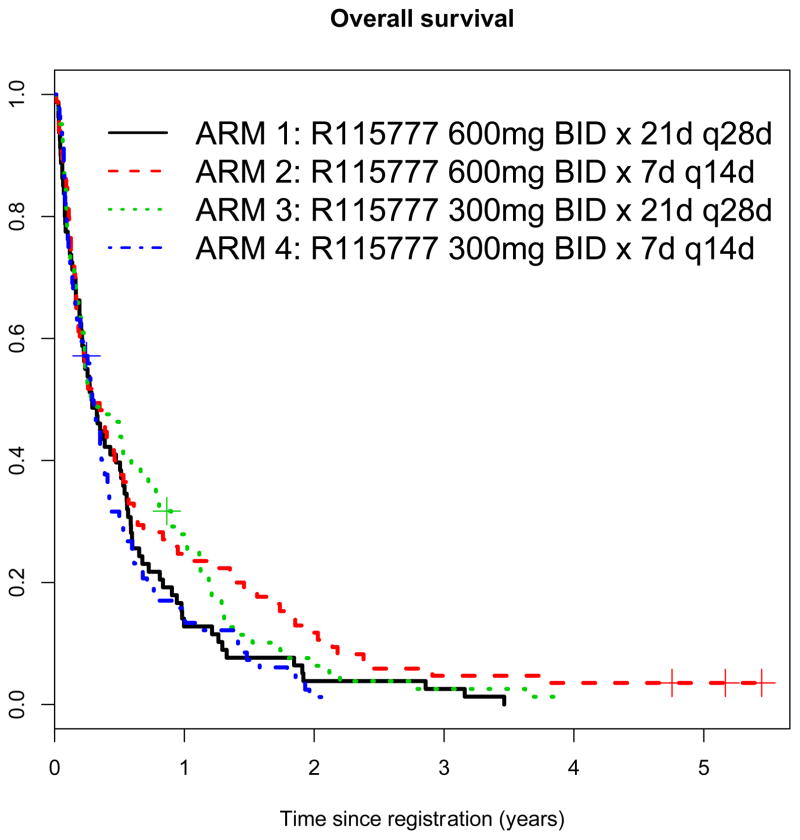
A WBC count at presentation below the median was associated with a higher probability of response in univariate analysis; there was no association of response with prior MDS or cytogenetic risk category (Table 4). However, the studies were not designed to be sufficiently powerful to detect such associations. There was also no difference in relapse free survival and overall survival between the four treatment arms (Table 3, Figure 1).
Table 4.
Association of patient characteristics with response
| No Response (N, %) | Response (N, %) | P value | |
|---|---|---|---|
| WBC < 2900 | 136 (81) | 32(19) | < 0.001 |
| WBC > 2900 | 152 (93) | 11 (7) | |
| AML, de novo | 145 (88) | 20 (12) | 0.74 |
| AML, secondary | 143 (86) | 23 (14) | |
| Karyotype, favorable/intermediate | 46 (90) | 5 (10) | 0.24 |
| Karyotype, unfavorable | 58 (97) | 2 (3) |
Figure 1.
Overall survival by arm
At least one toxicity of grade 3 or higher was seen in more than 50% of patients in each arm, with the most common grade 3 and 4 toxicities (> 20% in each arm) being febrile neutropenia and fatigue. The most common grade 1 and 2 toxicities (> 20% in each arm) were gastrointestinal (anorexia, nausea, diarrhea), rash and pruritis, creatinine elevation, and hepatic enzyme and bilirubin elevations. Confusion was seen in more than 10% of patients only in arm 1. The number of patients withdrawing from protocol therapy due to toxicity were 19 (24%) in arm 1, 8 (9%) in arm 2, 10 (12%) in arm 3, and 4 (5%) in arm 4. Fatal toxicities were also more commonly observed in patients assigned to receive 600 mg twice daily for 21 consecutive days (arm 1, see table 3).
Discussion
There was rapid accrual to SWOG S0432 with almost 350 previously untreated older AML patients registered in just eighteen months, indicating the interest in exploring lower intensity treatment strategies in this difficult-to-treat patient subset. Unfortunately, none of the 4 arms of this phase II study of tipifarnib in this patient population achieved response rates worthy of further investigation according to the initial design of S0432. However, tipifarnib 300 mg bid on days 1–21 did result in a 20% overall response rate (CR, CRi and PR) and an 11% CR rate. The observed response rates were similar to those reported by Lancet and colleagues (14% CR) [13] and Harousseau and colleagues (8% CR, 3% PR) [16]. Responses were seen in all treatment arms with acceptable toxicity, but there was a higher rate of neurologic toxicity and treatment-related mortality with 600 mg twice daily for 21 consecutive days. Thus, based on efficacy and toxicity, the results from S0432 suggest that a tipifarnib regimen of 300 mg twice daily for 21 out of 28 days is optimal for older AML patients.
Low response rates have been seen in older patients with AML with other commercially available agents, including low dose cytarabine (15–20%) [17], gemtuzumab ozogamicin (18–21%) [18,19], azacitidine (18%) [20], and decitabine (25%) [21]. Nonetheless, in phase III studies, cytarabine [17], azacitidine [20] and decitabine [22] have each been associated with improvement in survival of older AML patients compared with hydroxyurea or supportive care alone. In contrast, there was no difference in overall survival of AML patients over age 70 years treated with tipifarnib 600 mg twice daily for 21 days every 28 days compared with best supportive care including hydroxyurea [16]. However, our findings from SWOG S0432 suggest that the optimal dose of tipifarnib in terms of both efficacy and tolerability may not have been used in this prior phase III study.
Given the biologic complexity of AML, it would be naïve to expect high response rates with a small molecule inhibitor in unselected AML patients. Of note, the therapeutic targets of the farnesyltransferase inhibitors in the myeloid malignancies remain elusive. Specifically, response to tipifarnib did not correlate with RAS mutation status, farnesyltransferase inhibition, blast karyotype, or other clinical features in phase I and II studies of this agent in patients with myeloid malignancies [9,13]. On the other hand, the RASGRP1:APTX gene expression ratio has been shown to predict response to tipifarnib in both previously-untreated and relapsed AML patients [23], suggesting that selection of subsets of patients most suitable for treatment with tipifarnib may be feasible. Clearly, reliable identification of predictors of response to tipifarnib, to define the population of AML patients with the greatest expected benefit from this agent, would be a prerequisite to consider further clinical testing of tipifarnib.
Acknowledgments
Funding: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA46368, CA27057, CA20319, CA14028, CA46441, CA13238, CA63848, CA46113, CA76448, CA35178, CA11083, CA22433, CA35090, CA35431, CA67575, CA12644, CA58416, CA74647, CA46282, CA13612, CA37981, CA45808, CA76447, CA35176, CA76429, CA45807, CA45450, CA42777, CA35119, CA04919, CA63850, CA41287, CA21115, CA17145 and in part by Janssen Research & Development. Study sponsors had no involvement in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Contributors: HE, FA, KK, MS,RL, MT conceived and designed the study; HE, MK, MT, RL, FA provided study materials or patients; HG, MO, KK collected and assembled the data; HE, RW, MO, KK analyzed and interpreted the data; HE, RW, MO, KK participated in drafting the article; all authors read and approved the final manuscript.
Conflict of Interest Statement: The authors have no competing financial interests to declare in relation to the work described, with competing interests defined as those of a financial nature that, through their potential influence on behavior or content, or from perception of such potential influences, could undermine the objectivity, integrity or perceived value of the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 2.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Archives of Internal Medicine. 2002;162:1597–1603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Löwenberg B, Zittoun R, Kerkhofs H, Jehn U, Abels J, Debusscher L, Cauchie C, Peetermans M, Solbu G, Suciu S, Stryckmans P. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization of Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 5.Tilly H, Castaigne S, Bordessoule D, Casassus P, Le Prise P-Y, Tetian G, Desablens B, Henry-Amar M, Degos L. Low-Dose Cytarabine versus Intensive Chemotherapy in the Treatment of Acute Nonlymphocytic Leukemia in the Elderly. J Clin Oncol. 1990;8:272–279. doi: 10.1200/JCO.1990.8.2.272. [DOI] [PubMed] [Google Scholar]
- 6.CW, Reuter M, Morgan MA, Bergmann L. Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies. Blood. 2000;96:1655–1669. [PubMed] [Google Scholar]
- 7.Radich JP, Kopecky KJ, Appelbaum F, Willman CL, Collins SJ. N-ras mutations in acute myelogenous leukemia: A review of the current literature and an update of the Southwest Oncology Group experience. Leukemia and Lymphoma. 1992;6:325–334. [Google Scholar]
- 8.Rowinsky EK, Windle JJ, Von Hoff DD. Ras Protein Farnesyltransferase: A Strategic Target for Anticancer Therapeutic Development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 9.Karp JE, Lancet JE, Kaufmann SH, End DW, Wright JJ, Bol K, Horak I, Tidwell ML, Liesveld J, Kottke TJ, Ange D, Buddharaju L, Gojo I, Highsmith WE, Belly RT, Hohl RJ, Rybak ME, Thibault A, Rosenblatt J. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. 2001;97:3361–3369. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 10.Kurzrock R, Kantarjian HM, Cortes JE, Singhania N, Thomas DA, Wilson EF, Wright JJ, Freireich EJ, Talpaz M, Sebti SM. Farnesyltransferase inhibitor R115777 in myelodysplastic syndrome: clinical and biologic activities in the phase I setting. Blood. 2003;102:4527–4534. doi: 10.1182/blood-2002-11-3359. [DOI] [PubMed] [Google Scholar]
- 11.Fenaux P, Raza A, Mufti GJ, Aul C, Germing U, Kantarjian H, Cripe L, Kerstens R, De Porre P, Kurzrock R. A muticenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood. 2007;109:4158–4163. doi: 10.1182/blood-2006-07-035725. [DOI] [PubMed] [Google Scholar]
- 12.Harousseau J-L, Lancet JE, Reiffers J, Löwenberg B, Thomas X, Huguet F, Fenaux P, Zhang S, Rackoff W, De Porre P, Stone R. A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory and relapsed acute myeloid leukemia. Blood. 2007;109:5151–5156. doi: 10.1182/blood-2006-09-046144. [DOI] [PubMed] [Google Scholar]
- 13.Lancet JE, Gofo I, Gotlib J, Feldman EJ, Greer J, Liesveld JL, Bruzek LM, Morris L, Park Y, Adjei AA, Kaufmann SH, Garrett-Mayer E, Greenberg PL, Wright JJ, Karp JE. A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood. 2007;109:1387–1394. doi: 10.1182/blood-2006-04-014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschbaum MH, Synold T, Stein AS, et al. A phase I trial dose-escalation study of tipifarnib on a week-on, week-off schedule in relapsed, refractory, or high risk myeloid leukemia. Leukemia. 2011;25:1543–1547. doi: 10.1038/leu.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standard for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Harousseau J-L, Martinelli G, Jedrzejczak WW, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114:1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 17.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive chemotherapy. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 18.Amadori S, Suciu S, Stasi R, Willemze R, Mandelli F, Selleslag D, Denzlinger C, Muus P, Stauder R, Berneman Z, Pruijt J, Nobile F, Cassibba V, Marie J-P, Beeldens F, Baila L, Vignetti M, de Witte T. Gemtuzumab ozogamicin (Mylotarg) as single-agent treatment for frail patients 61 years of age and older with acute myeloid leukemia: final results of AML-15B, a phase 2 study of the European Organisation for Research and Treatment of Cancer and Gruppo Italiano Malattie Ematologiche dell’Adulto Leukemia Groups. Leukemia. 2005;19:1768–1773. doi: 10.1038/sj.leu.2403901. [DOI] [PubMed] [Google Scholar]
- 19.Estey EH, Thall PF, Giles FJ, Wang X-M, Cortes JE, Beran M, Pierce SA, Thomas DA, Kantarjian HM. Gemtuzumab ozogamicin with or without interleukin 11 in patients 65 years of age or older with untreated acute myeloid leukemia and high-risk myelodysplastic syndrome: comparison with idarubicin plus continuous-infusion, high dose cytosine arabinoside. Blood. 2002;99:4343–4349. doi: 10.1182/blood.v99.12.4343. [DOI] [PubMed] [Google Scholar]
- 20.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine Prolongs Overall Survival Compared with Conventional Care Regimens in Elderly Patients with Low Bone Marrow Blast Count Acute Myeloid Leukemia. J Clin Oncol. 2012;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 21.Cashen AF, Shiller GJ, O’Donnell MR, DePersio JF. Multicenter, Phase II Study of Decitabine for the First-Line Treatment of Older Patients with Acute Myeloid Leukemia. J Clin Oncol. 2009;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Decitabine versus Patient Choice, with Physician Advice, of Either Supportive Care or Low-Dose Cytarabine for the Treatment of Older Patients with Acute Myeloid Leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raponi M, Lancet JE, Fan H, Dossey L, Lee G, Gojo I, Feldman EJ, Gotlib J, Morris LE, Greenberg PL, Wright JJ, Harousseau J-L, Löwenberg B, Stone RM, De Porre P, Wang Y, Karp JE. A two-gene classifier for predicting response to the farnesyltransferase inhibitor tipifarnib in acute myeloid leukemia. Blood. 2008;111:2589–2596. doi: 10.1182/blood-2007-09-112730. [DOI] [PubMed] [Google Scholar]