Summary
The γ-tocopherol methyltransferase (γ-TMT) is an important enzyme regulating synthesis of four tocopherols (α, γ, β and δ). In this report, we investigated the role of γ-TMT in regulating abiotic stress within chloroplasts. The At γ-tmt overexpressed via the tobacco chloroplast genome accumulated up to 7.7% of the total leaf protein, resulting in massive proliferation of the inner envelope membrane (IEM, up to 8 layers). Such high level expression of γ-TMT converted most of γ-tocopherol to α-tocopherol in transplastomic seeds (~10 fold higher) in the absence of abiotic stress. When grown in 400 mM NaCl, α-tocopherol content in transplastomic TMT leaves increased up to 8.2-fold and 2.4-fold higher than wild-type leaves. Likewise, under heavy metal stress α-tocopherol content in the TMT leaves increased up to 7.5-fold, twice higher than in the wild-type. Under extreme salt stress, the wild-type accumulated higher starch and total soluble sugars but TMT plants were able to regulate sugar transport. Hydrogen peroxide and superoxide content in wild-type increased up to 3-fold within 48 hours of NaCl stress when compared to TMT plants. The ion leakage from TMT leaves was significantly less than wild-type plants under abiotic stress and with less malondialdehyde, indicating lower lipid peroxidation. Taken together, these studies show that α-tocopherol plays a crucial role in the alleviation of salt and heavy metal stresses by decreasing ROS, lipid peroxidation and ion leakage, in addition to enhancing vitamin E conversion. Increased proliferation of the IEM should facilitate studies on retrograde signaling from chloroplast to the nucleus.
Keywords: chloroplast transformation, abiotic stress tolerance, γ-tocopherol methyltransferase
Introduction
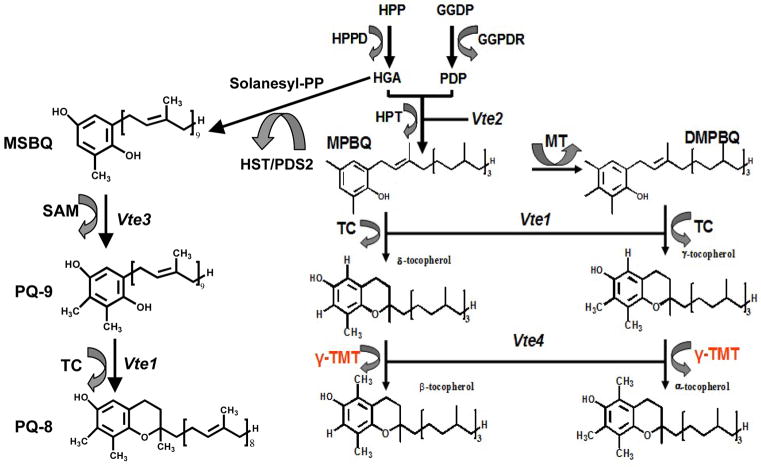
Vitamin E is the collective term for eight structurally similar tocochromanol compounds including four tocopherols (α, γ, β and δ) and four tocotrienols (α, γ, β, and δ), which differ from one another by the number and position of methyl groups on the chromanol ring and are synthesized exclusively in photosynthetic organisms, including plants, algae and some cyanobacteria (DellaPenna, 2005; DellaPenna and Pogson, 2006). Recently, a ninth form of vitamin E, plastochromanol-3 derived from plastochinol-9 was reported by two groups (Zbierzak et al., 2010, Mene-Saffrane et al., 2010, Figure 1). Tocopherols are lipid-soluble antioxidants and play an important role in the plastid antioxidant network by eliminating ROS. It is also known that ROS is generated as a byproduct of photosynthesis, which participates in lipid peroxidation of chloroplast membranes (Asensi-Fabado and Munné-Bosch, 2010). Among the four tocopherols, α-tocopherol shows the highest vitamin E activity and is the most active lipid-soluble antioxidant (Schneider, 2005). Alpha-tocopherol rapidly reacts with the peroxyl radical and blocks further reactions.
Figure 1.
The biosynthetic pathway of tocopherols and PC-8 in plants. HPP-hydroxyphenylpyruvate; HPPD-HPP dioxygenase; HGA-homogentisic acid; GGDP-geranylgeranyl diphosphate; GGDPR-GGDP reductase; PDP-phytyl diphosphate; HPT-HGA phytyltransferase; MPBQ-2-methyl-6-phytyl-1,4-benzoquinol; DMPBQ-2,3-dimethyl-6-phytyl-1,4-benzoquinol; MSBQ-2-methyl-6-solanesyl-1,4-benzoquinol; PQ-9 -plastoquinol-9 ; PC-8-plastochromanol-8; MT- MPBQ methyltransferase; TC-tocopherol cyclase; γ –TMT- γ-tocopherol methyltransferase. Vte1, vte2, vte3 and vte4 are mutants of TC, HPT, SAM and γ–TMT in Arabidopsis, respectively.
The γ-tocopherol is biosynthetic precursor of α-tocopherol suggesting that the final step of the α-tocopherol biosynthetic pathway catalyzed by γ-tocopherol methyl transferase (γ-TMT) is the rate limiting step (Shintani and Dellapenna, 1998), as explained in Figure 1. The last steps are catalyzed by tocopherol cyclase (TC) and γ-tocopherol methyltransferase (γ-TMT), yielding four kinds of tocopherols. Detailed description of the PQ-9 and PC-8 synthesis pathways is available in recent reports (Mene-Saffrane and DellaPenna, 2010; Zbierzak et al., 2010). In plants, α-tocopherol plays an important role in maintaining the redox status, thylakoid structure and chloroplast function during plant development, and in plants exposed to abiotic stress (Munné-Bosch and Alegre, 2002; Sattler et al., 2004). The vitamin E2 (vte2) mutant isolated form Arabidopsis thaliana was defective in homogentisate phytyltransferase (HPT) and lacked all tocopherols and pathway intermediates (Figure 1). Previous research showed that vte2 mutants were severely impaired in seed longevity, early seedling development (Sattler et al., 2004), and were less cold tolerant when compared to wild-type plants (Maeda et al., 2006). The vte1 mutant was defective in tocopherol cyclase (TC) and also deficient in all tocopherols. However, it accumulates the redox-active biosynthetic intermediate 2, 3-dimethyl-6-phytyl- 1, 4-benzoquinol (DMPBQ) (Figure 1) (Sattler et al., 2003). The vte1 plants were virtually identical to wild-type plants at all developmental stages when grown at 100 to 120 μmol• m−2 •s−1 high light stress (Porfirova et al., 2002; Sattler et al., 2004). They did not exhibit the lipid peroxidation phenotype observed in germinating vte2 seedlings, suggesting that DMPBQ may compensate for the lack tocopherols as a lipid-soluble antioxidant during seed germination (Sattler et al., 2004). The vte1 plants showed a more rapid induction of lipid peroxidation than the wild-type under extreme conditions (Havaux et al., 2005). The vte4 mutant was defective in γ-tocopherol methyltransferase, resulting in the deficiency of α-tocopherol but increased γ-tocopherol accumulation (Bergmüller et al., 2003; Cela et al., 2011). The transcript levels of ethylene signaling pathway genes were severely impaired in the vet4 mutant mature leaves when exposed to salt stress (Cela et al., 2011). Further studies showed additional functions of tocopherols including the inhibition of enzymatic activity, regulation of gene transcription and signaling (Russin et al., 1996, Hofius et al., 2004, Sakuragi et al., 2006; Cela et al., 2011).
Salt stress impairs plant growth by different mechanisms, including inducting osmotic imbalance, ion toxicity (Kumar et al., 2004), and oxidative stress by increasing ROS production (Krasensky and Jonak, 2012). However, very little is known about the role of tocopherols in protecting plants from salt stress. It has been shown that HPT: RNAi transgenic tobacco plants with decreased total tocopherol contents increased the sensitivity to salt stress. However, the TMT: RNAi plants showed increased tolerance to osmotic stress and methyl viologen but decreased tolerance to salt stress, in which transgenic plants accumulated higher γ-tocopherol than α-tocopherol, suggesting that α-tocopherol may play specific role in salt stress (Abbasi et al., 2007)
Metal toxicity in plants is also mediated through several mechanisms (Ruiz and Daniell, 2009, Ruiz et al., 2011). For example, the transition redox-active metals like Fe and Cu could interfere with cellular oxygen in plant tissues. Their autoxidation function results in the formation of ROS by Fenton-type reactions and also leads to pro-oxidant conditions within cells (Halliwell and Gutteridge, 1986). The other common mechanism of metal toxicity is related to their strong ability to bind to nitrogen, oxygen, and sulphur atoms (Nieboer and Richardson, 1980). Also most heavy metals can bind low-molecular-weight compounds like glutathione peptides and cysteine residues. Consequently, severe changes in the cell redox status occur when they are exposed to metals, due to glutathione depletion. Finally, the replacement of one metal in the metallic cofactors of enzymes by another metal results in a strong inhibition of their activity. Living plants exhibit severe oxidative injury generated either directly by redox-active metals or indirectly by metal-induced metabolic perturbations, when exposed to toxic metals (Valko et al., 2005). However, only a few reports are available investigating the role of tocopherols in protecting plants from metal-induced stress. Collin et al., (2008) observed that transcripts encoding enzymes of the vitamin E biosynthetic pathway increased response to metal exposure and the vitamin E-deficient mutant exhibited an enhanced sensitivity towards Cu and Cd metals, suggesting that vitamin E could play a crucial role in the oxidative stress induced by heavy metals.
In this report, we expressed the At γ-TMT in tobacco chloroplasts to evaluate the feasibility of high level expression of a heterologous membrane protein in chloroplasts and its consequence on abiotic stress tolerance.
Results
Construction of pLD-G10-γ-TMT for chloroplast transformation
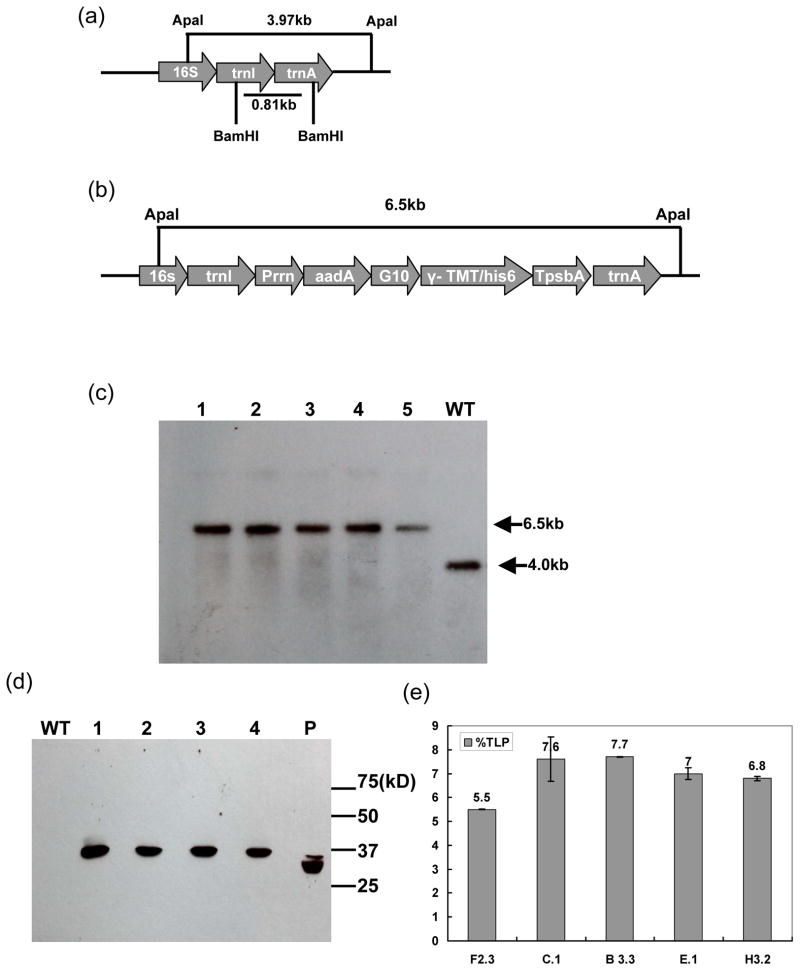
The pLD vector developed in our lab has been used for chloroplast transformation in several studies (Kota et al., 1999; Jin et al., 2011; Jin et al., 2012; Kohli et al., 2013). The trnA and trnI genes in the pLD vector were used for homologous recombination between the transformation vector and the chloroplast genome. The γ-tmt gene was inserted into the pLD-G10-γ-TMT vector, downstream of the aadA gene and 5′ translation control region of bacteriophage T7 gene 10. The aadA gene conferred resistance to spectinomycin and was used as the selection marker. The 5′ translation control region of bacteriophage T7 gene 10 contained strong ribosome binding sites (Dhingra et al., 2004; Ruhlman et al., 2010) and was placed upstream of the of γ-tmt gene coding sequence. A 6x His-tag was introduced to the 3′ end of the γ-tmt gene to facilitate detection and purification of γ-TMT expressed in chloroplasts. Expression of the aadA and γ-tmt genes was regulated by the 16s rRNA promoter (Prrn) and transcripts were stabilized by the 3′ untranslated region (UTR) of psbA (TpsbA) (Figure 2b).
Figure 2.
Creation and characterization of TMT transplastomic lines by Southern, western blot analysis and ELISA. (a) The native chloroplast genome showing both homologous recombination sites (trnI and trnA) and the restriction enzyme sites used for Southern blot analysis. (b) The pLD-G10-γ-TMT chloroplast vector map (c) Southern blot hybridized with the flanking sequence probe showing homoplasmy. Lanes 1 to 5: T0 transplastomic lines; WT, wild-type. (d) Western blot analysis of wild-type (WT), transplastomic TMT and his-tag standard protein. Protein extracts were resolved by SDS-PAGE and transferred to nitrocellulose membrane. WT, wild-type tobacco ; SD: his-tag protein standard (~30kD GenScript Inc); lanes 1–4, his-TMT transplastomic lines- F2.3, C.1, B 3.3 and E.1. (e) ELISA quantification of his-TMT in five independent transplastomic lines-F2.3, C.1, B3.3, E.1 and H3.2.
Evaluation of transgene integration into the chloroplast genome
Southern blot was used to determine homoplasmy and confirm site-specific integration of the pLD-G10-γ-TMT cassette into the chloroplast genome (Figure 2a–b). In wild-type, there was a 4.0-kb hybridizing fragment, while there was a 6.5-kb fragment in all the transplastomic lines (Figure 2c). The larger size fragments in the DNA from TMT plants confirmed site-specific integration of the transgenes into the spacer region between the trnI and trnA genes (Figure 2b–c). Furthermore, the absence of a 4.0-kb fragment (observed in the wild-type) in the transgenic lines confirmed that homoplasmy had been achieved.
Higher expression level of TMT in transplastomic lines but without obvious phenotype change
Western blot analysis showed the estimated 38 kDa protein as predicted by tmt cDNA sequence in 4 transplastomic lines but not in the wild-type, when probed with the anti-his-tag antibody (Figure 2d). ELISA was used to quantify expression levels of the γ-TMT in five transplastomic lines (F2.3, C.1, B3.3, E.1 and H3.2) following our previously published protocols (Jin et al., 2012). His-tag TMT accumulated up to 5.5–7.7 % of the total leaf protein (TLP) (Figure 2e). The transplastomic TMT lines F 2.3 and B 3.3 showed lowest (5.5% TLP) and highest (7.7 % TLP) expression level, respectively. This variation of expression levels in different transplastomic lines could be due to time of harvest, leaf age, etc.
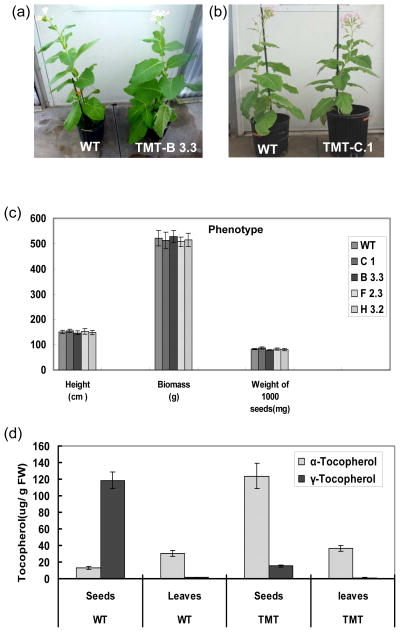
Although the transplastomic lines B3.3 and C.1 showed higher expression level of TMT, the phenotype of these lines including plant height, seeds weight and biomass did not show obvious changes when compared to wild-type control (Figure 3a–c).
Figure 3.
Phenotype, tocopherol content and composition in wild-type and transplastomic TMT leaves. (a–b) Transplastomic lines (TMT C.1 and B3.3) showing normal biomass and flowering when compared to wild-type plants. (c) Quantitative data of phenotype from five transplastomic lines and wild-type plants. The plant height and biomass were recorded at the end of their vegetative phase (four-month-old plants) and data presented are means and SD of five individual lines of one representative population. The biomass represents the weight of whole plant excluding the root biomass. (d) tocopherol content and composition in TMT transplastomic and wild-type (WT) tobacco tissues. Tocopherols were extracted in 100% methanol from the mature tobacco leaves and seeds and were quantified using HPLC as described in “Materials and Methods.” The data shown are means and SD from three independent samples.
Expression of TMT in chloroplast results in conversion of most γ-tocopherol to α-tocopherol
To investigate the impact of overexpression of γ-tmt in chloroplasts, the composition of tocopherol pools in leaves and seeds was analyzed by HPLC. In the mature leaf of TMT and wild-type tobacco, the tocopherol pool was dominated by α-tocopherol. In the TMT plant leaves, α-tocopherol accounted up to 97% of total tocopherol with negligible amounts of γ-tocopherol. Similarly, in wild-type plant leaves, 95% of tocopherol is α-tocopherol (Figure 3d). In the seeds of wild-type, the tocopherol pool consisted more than 90% of γ-tocopherol and less than 10% of α-tocopherol. In contrast, there was only 7.6% of γ-tocopherol in the tocopherol pool of TMT seeds and α-tocopherol accounted for 92 % of total tocopherol. The content of α-tocopherol in the TMT seeds is 9.6 times higher than in the wild-type seeds, while there was no significant change of total tocopherol content between TMT and wild-type seeds (Figure 3d).
In these experiments, the β-and δ-tocopherols were hardly detectable. In most plant species including tobacco, α- and γ-tocopherol are the predominant tocopherol forms and β- and δ-tocopherols accounted for less than 5% of the total tocopherol content (Grusak and Dellapenna, 1999). So, it is very common to detect only α- and γ-tocopherol as reported in previous studies (Falk et al., 2003 ; Li et al., 2011). Taken together, α- tocopherol is dominated in both TMT and wild-type plant leaves. However, seeds of wild-type are enriched in γ-tocopherol, while α-tocopherol accumulated to high levels in TMT seeds because of the expression of γ-TMT converting most of γ-tocopherol to α-tocopherol.
Structure of the chloroplast inner envelope membrane in wild-type and transplastomic TMT plants
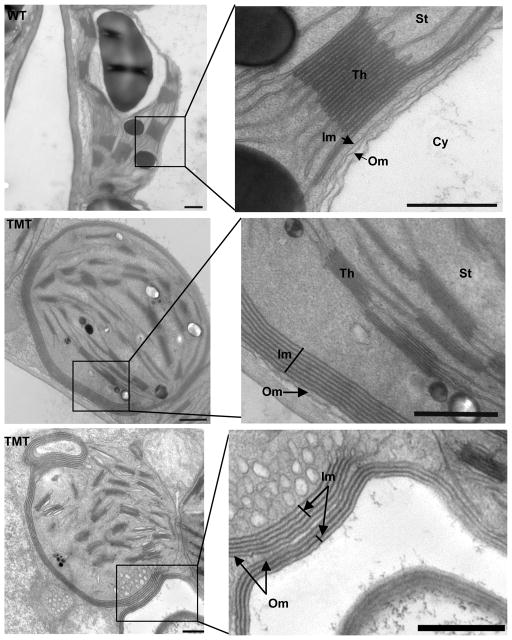
To evaluate the effect of overexpression of inner membrane localized enzyme –TMT on the chloroplast structure, the ultrastructure of chloroplast was investigated by transmission electron microscopy (TEM). While control chloroplasts showed a typical kidney shape, TMT chloroplasts were spherical as often observed previously in transplastomic chloroplasts (Sigh et al., 2008; De Cosa et al., 2000; Fernandez San-Millan, 2003; Verma et al., 2013). Wild-type chloroplasts showed two layers of membranes - outer and inner membranes of chloroplast envelope (Figure 4, wild-type). Interestingly, chloroplasts from TMT leaves exhibited multiple layers of inner membranes (Figure 4, TMT). When compared to the uneven surface of inner membrane of wild-type chloroplast, the inner membranes of TMT showed uniform distance between each layer and appeared well defined. To evaluate the effect of expression of γ-TMT membrane protein in chloroplasts, transplastomic plants were grown under identical conditions in the greenhouse. Phenotype of TMT showed no significant difference when compared to wild-type plants despite massive proliferation of the inner envelope membrane (Figure 3a–c).
Figure 4.
Ultrastructure of the chloroplast envelope membranes in wild-type and transplastomic tobacco plants. Nine small pieces (3–4 mm) were dissected from three mature leaves of transplastomic TMT lines (C.1, B 3.3) and wild-type plants for TEM studies. The left panels show chloroplasts of leaf tissue from wild-type and TMT plants (bars = 0.5 μm). The right panels show higher magnifications of the boxed regions (bars = 0.5um). The positions of the thylakoids (Th), the stroma (St), the outer and inner envelope membrane (Om, Im), the cytoplasm (Cy), are labeled.
Expression of TMT in chloroplast decreases the susceptibility to salt and heavy metal stress by increasing the α-tocopherol content
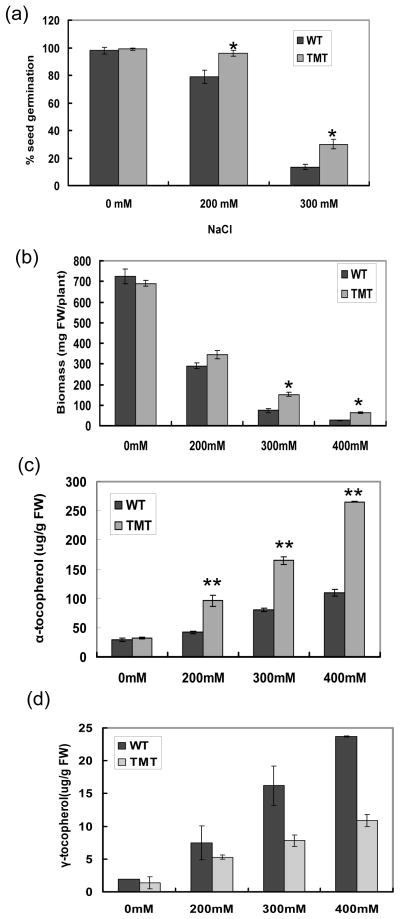
We evaluated the effects of NaCl stress on the seed germination, growth and heavy metal toxicity in both wild-type and TMT transplastomic lines. The percentage of seed germination of TMT was significantly higher (P<0.05) than the germination of wild-type seeds on the MS medium supplemented with 200 or 300 mM NaCl (Figure 5a). The biomass of wild-type plants significantly decreased with the increasing NaCl concentration. The average weight of thousand seeds of wild-type plants was 26 mg on 400 mM NaCl, which diminished 96% when compared to the plants grown on the medium without NaCl (Figure 5b). The vegetative growth of TMT transplastomic plants showed slightly higher tolerance to the salinity stress. The average biomass was 1.2, 2.1 and 2.4 times higher than corresponding wild-type plants on the medium supplemented with 200, 300 and 400 mM NaCl, respectively (Figure 5b).
Figure 5.
Seed germination efficiency, biomass and tocopherol content of wild-type and TMT transplastomic lines under salt stress. Seeds from wild-type and TMT (line B3.3) plants were germinated in MS basal medium or with additional 200, 300 mM NaCl. The percent seed germination was calculated based on three repeated experiments (90–100 seeds taken for each replicate). (a) Percentage of seed germination on NaCl. The values are means from three independent experiments. (b) Biomass of wild-type and TMT plants (four-week old) grown on medium supplemented with different concentrations of NaCl. The data are the means and SDs of five independent measurements. (c, d) Alpha- and Gamma- tocopherol content in leaves of the wild-type and transgenic tobacco plants. The results are from three replicate samples ±SD. *, P<0.05, **,P<0.01
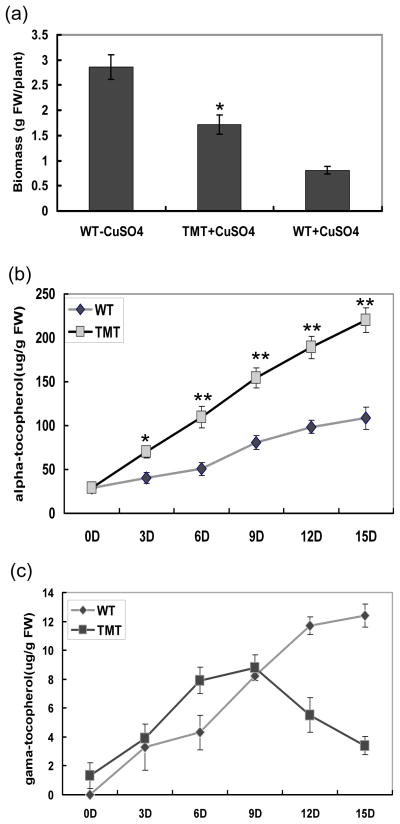
Wild-type plants (six-week old) also showed sensitivity toward heavy metal stress. After 15 days treatment with 25 mM Cu, the biomass of wild-type plants decreased 72% when compared to plants grown in unchallenged condition. In contrast, the average weight of TMT plants was 2.1 times higher than the weight of wild-type plants (Figure 6a).
Figure 6.
Evaluation of biomass and tocopherol content under heavy metal stress. (a) Biomass of plants (six-week old) exposed to 25 mM Cu for 2 weeks. For control, no Cu was added to the nutrient solution. The data are the means and SDs of five independent measurements. (b) and (c) Alpha- and Gamma- tocopherol content in leaves of the wild-type and transplastomic lines-B 3.3. Measurements were done every three days. The results are from three replicate samples ±SD. *, P<0.05, **, P< 0.01
Also, we evaluated tocopherol accumulation in TMT and wild-type leaves under stress conditions. For wild-type plants, the total content was ~31.2 ug/g FW under unchallenged condition, which increased up to 133 ug/g FW during the salt stress (400 mM) and α-tocopherol increased from 29.3 to 109.3 ug/g FW (Figure 5c). In contrast, the α-tocopherol content in TMT plant leaves exposed to 400 mM NaCl was 265.5 ug/g FW, which was 8.2 times higher than TMT plants under unchallenged condition (32.2 ug/g FW) and 2.4 times higher than wild-type plants under salt stress (Figure 5c). Gama- tocopherol content also increased both in the wild-type and TMT leaves but wild-type always had higher content of γ-tocopherol than TMT leaves under salt stress (Figure 5d).
Under Cu stress, α-tocopherol content in wild-type leaves gradually increased from 29 ug/g FW to 108.6 ug/g FW within 15 days. A striking 7.5 times increase in the α-tocopherol concentration was measured in TMT plant leaves. At all time points examined, α-tocopherol content in TMT leaves was almost twice higher than in wild-type leaves (Figure 6b). The change of γ-tocopherol content in wild-type leaves showed similar pattern to α-tocopherol content, which gradually increased with the time exposed to Cu stress (Figure 6c). However, the γ-tocopherol content in TMT plant leaves increased up to 9 days of Cu treatment and then dropped to level on the second day (Figure 6c). Therefore, the level of tocopherol were found to be up-regulated in tobacco in response to salt and metal stress and higher concentration of α-tocopherol accumulated in TMT plants than in the wild-type plants under these stress conditions.
Role of anti-oxidative compounds under salt and metal stress
Accumulation of anthocyanins, as an indicator of senescence, stress and damage was investigated. After four weeks of salt stress, anthocyanin content increased in wild-type under low salt stress (200 mM). By increasing NaCl concentration to 400 mM, the anthocyanin content soared up to 85 μg/g FW, which is 5.2 times higher than wild type grown on 400mM NaCl. The anthocyanins in the TMT plants showed the same trend as the wild-type in response to salt stress (Figure S1 a). Unlike salt stress, accumulation of anthocyanins in the wild-type and TMT showed different trends under Cu stress. On the 12th day of Cu stress, the anthocyanins content reached the maximum and then decreased. There was no significant difference in the accumulation of anthocyanins between the wild-type and TMT plants under Cu stress (Figure S1 a-1).
The ascorbate pool size in TMT plant leaves was similar to wild-type in control and high salinity stress (Figure S1 b and c), which was evaluated with increase of NaCl concentration. Cu stress resulted in a more pronounced increase of the ascorbate pool than salt stress (1218.7 nmol/g FW on 25 mM Cu when compared to 948.7 nmol/g FW 400 mM NaCl). After prolonged metal stress, the ascorbate pool size gradually increased. However, there was no significant difference in the accumulation of ascorbate between wild-type and TMT plants under Cu stress (Figure S1 b-1 and c-1). In general, it appears that tocopherol accumulation did not significantly influence the antioxidant compounds in chloroplasts under abiotic stress.
ROS accumulation significantly decreased in TMT plants under salt stress
In this project, we evaluated the ROS levels in wild-type and TMT plants when exposed to 300 mM NaCl for 12, 24 and 48 hrs, respectively. Hydrogen peroxide was evaluated with 3, 3′-diaminobenzidine tetrahydrochlorde hydrate (DAB) staining. Wild-type plantlets showed deeper color than the TMT plants at all the time points (Figure 7). The content of hydrogen peroxide in wild-type increased up to 3.2 fold within 48 hrs under NaCl stress when compared to TMT plants (Figure 7).
Figure 7.
Visualization and quantification of hydrogen peroxide by DAB staining. Three-week-old MS-grown wild-type and TMT transplastomic plantlets were subjected to 300 mM NaCl for 12, 24 or 48 hours and then were visualized by DAB staining. For each group, 15 plantlets are shown. Quantification of generated H2O2 after NaCl treatment were repeated 3 times (15 plantlets were used in each experiment). The data shown are means and SD from three independent experiments. *, P< 0.05.
The superoxide radical increased steadily under NaCl stress, showing deeper blue color after nitroblue tetrazolium (NBT) staining (Figure 8). In contrast, there was no significant color change in TMT plantlets within 48 hrs under NaCl stress (Figure 8). The content of NBT in wild-type was 2.0, 2.7 and 3.1 times higher than in TMT plantlets when subjected to NaCl stress for 12, 24 and 48 hrs, respectively (Figure 8). These data indicate that the generation of ROS was decreased or eliminated in TMT transplastomic plantlets under NaCl stress.
Figure 8.
Visualization and quantification of superoxide radical by NBT staining. Three-week-old MS-grown wild-type and TMT transplastomic plantlets were subjected to 300 mM NaCl for 12, 24 or 48 hours and then were visualized by NBT staining. For each group, 15 plantlets are shown. Quantification of generated superoxide radical after NaCl treatment were repeated 3 times (15 plantlets were used in each experiment). The data shown are means and SD from three independent experiments. *, P <0.05.
Lipid peroxidation and membrane damage decreased by expression of TMT in chloroplasts under salt and metal stress
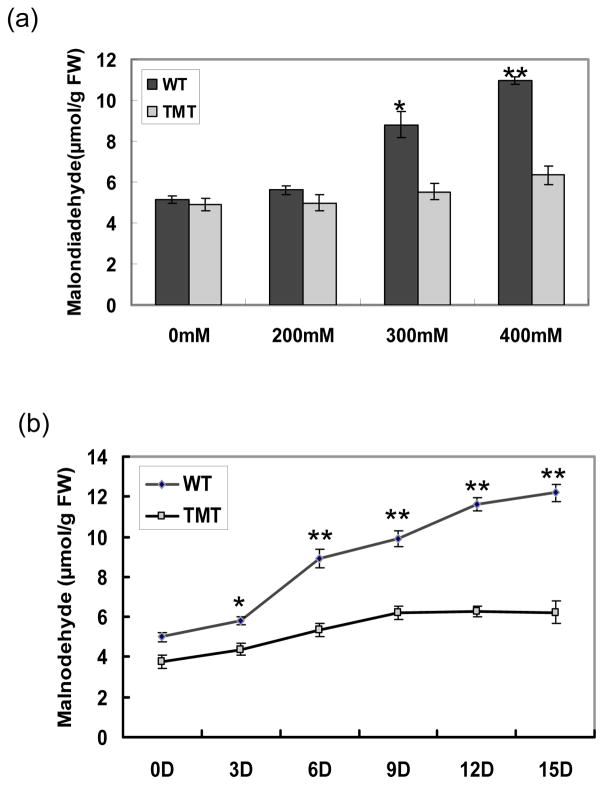
Under low stress condition (200 mM NaCl), lipid peroxidation in wild-type and TMT plants showed a slight increase (Figure 9a), which is in good agreement with the slightly higher ion leakage observed in wild-type and TMT plants (Figure S2 a). With the increase of salt stress, the content of MDA in wild-type plants increased dramatically. The MDA concentration in wild-type plants grown on 400 mM NaCl was 10.98 μm/g FW, which is twice higher than under unchallenged condition. Compared to wild-type, the TMT plants accumulated less (p< 0.05) MDA when exposed to salt stress (Figure 9a). The ion leakage measurement from TMT coincides with lipid peroxidation assay, which showed significantly less (p< 0.05) ion leakage than wild-type plants (Figure S2 a).
Figure 9.
Lipid peroxidation under salt and heavy metal stress (Cu): (a) Both TMT transplastomic and wild-type tobacco plants were grown on the MS medium supplemented with 0, 200, 300, 400 mM of NaCl. Lipid peroxidation was assayed in leaf samples after 4 weeks of stress by determining the amount of malondialdehyde (MDA). (b) TMT and wild-type tobacco plants were irrigated with standard nutrient solution containing 25 mM CuSO4. Control plants were grown similarly, but without CuSO4. Lipid peroxidation was assayed from leaf samples every 3 days by determining the amount of MDA. Data shown here is the mean and SD from five independent measurements. *, P<0.05, **,P <0.01
Under Cu stress, wild-type leaves accumulated much higher MDA than TMT leaves at all time points (Figure 9b). Fifteen days after the onset of metal stress, the MDA content increased to 12.2 μm/g FW in wild-type leaves, which is almost twice higher than in TMT leaves. Unlike the salt stress, ion leakage in wild-type leaves reached the maximum level on day 9 and then stabilized. In the TMT leaves, ion leakage showed the similar tendency to wild-type but there was less ion leakage than in the wild-type plants at all the time points (Figure S2 b). Taken together, the data shown here indicate that upregulation of α-tocopherol in transgenic chloroplasts increased membrane integrity under salt and metal stress.
Expression of γ-TMT altered sugar accumulation but not amino acid metabolism under stress conditions
Total amino acid contents in both wild-type and TMT plant leaves were elevated with the increase of NaCl concentration. Total amino acid accumulated 4.7 and 4.6-fold in wild-type and TMT plants respectively on 400 mM NaCl when compared to un-supplemented controls. Notably, proline increased 7.1 and 7.3-fold in wild-type and TMT respectively when grown on 200 mM NaCl. Proline accounted up to 36.3% of the total amino acid pool (increased around 18-fold) in both wild-type and TMT plants under 400 mM NaCl stress. Under salt stress, the amino acid accumulation in both TMT and wild-type plants did not show any significant difference (Figure S3 a and b). Under Cu stress, the trend of total amino acid and proline was similar to the NaCl stress as shown in the Figure S3 a-1 and b-1. There was a 3.4 and 3.0-fold increase of total amino acid content in wild-type and TMT plants at the end of Cu stress, when compared to the onset of stress.
The changes of carbohydrate were similar to the amino acid pool. There was no significant change of starch content in both wild-type and TMT plant when 200 mM NaCl was supplied. However, under extreme stress (400 mM), the starch accumulation in wild-type was significantly higher (P<0.05) than in TMT plants. Total soluble sugars in both wild-type and TMT were found to correlate directly with increasing salt stress, which were increased about 3.2 and 2.4-fold in wild-type and TMT grown on 400 mM NaCl, when compared to respective controls. Significant higher total soluble sugar accumulation was observed in wild-type plants than in the TMT plants when more than 300 mM NaCl was supplied (Figure S3 c and d).
Cu stress induced clearly different response in starch metabolism in wild-type and TMT. The starch content increased about 3.0 and 2.9-fold and reached to maximum value 9 days after the stress onset and then decreased gradually (Figure S3 c-1). However, the accumulation of the total soluble sugars exhibited a steady increase at all time points (Figure S3 d-1). We did not observe significant difference between wild-type and TMT plants in carbohydrate accumulation under metal stress.
Taken together, the TMT lines did not show significant difference in carbohydrate and free amino acid contents than the wild-type under Cu stress. Under extreme salt stress, the wild-type accumulated significantly higher starch and total soluble sugars but not free amino acid than TMT plants.
Discussion
The total tocopherols content and composition vary enormously in plant tissues. Generally, α-tocopherol is dominant in green leafy tissue; however such tissues have relatively low content of total tocopherols e.g. 10–50 μg/g FW when compared to seeds (300–1200 μg/g FW) (Grusak and Dellapenna, 1999). The abundant α-tocopherol in photosynthetic apparatus presumably indicates a critical functional role. When compared to the leaf tissue, γ-tocopherol is more abundant in seeds and α-tocopherol is a minor component because of the γ-TMT activity is likely the rate limiting step in seeds.
In this project, we expressed the γ-TMT in tobacco chloroplasts to investigate its role in abiotic stress tolerance. The γ-TMT converted γ-tocopherol to α-tocopherol in seeds and α-tocopherol content was elevated 10-fold in the absence of any abiotic stress. Under abiotic stress, α-tocopherol content increased up to 7–8 fold in leaves. This is the first report of a foreign membrane protein expressed in chloroplasts as there is only a single report of expressing an endogenous membrane protein (Singh et al., 2008). The γ-tocopherol methyltransferase (γ-TMT) is an IM enzyme which plays important role in the lipid synthesis in chloroplasts (Soll et al., 1985; Zbierzak et al., 2010). In the present study, overexpression of γ-TMT in tobacco chloroplasts accumulated up to 7.7 % of total leaf protein and induced significant membrane proliferation. Increase in α-tocopherol content under abiotic stress in leaves and proliferation of the inner envelope membrane provides an ideal system to investigate retrograde signaling between chloroplast and nucleus, as illustrated in a recent study (Kwon et al., 2013).
Several reports showed that tocopherols play an important role in sugar export and starch metabolism under stress. The tocopherol-deficient potato and maize mutants exhibited callose plugs at the plasmodesmata, which disturbed sugar transport and metabolism (Provencher et al., 2001; Hofius et al., 2004). Maeda et al. (2006) reported that vte 2 mutant in Arabidopsis showed rapid decrease in the photoassimilate export upon chilling by callose deposition in the phloem parenchyma. In this project, we observed that high salinity stress (400 mM NaCl) leads to a significantly higher accumulation of total soluble sugars and starch in leaves of wild-type but not in γ-TMT transplastomic plant leaves. Therefore, the α-tocopherol content in wild-type leaves was sufficient to regulate sugar export under normal conditions but not under high salinity stress. However, transplastomic TMT plants accumulated higher levels of α-tocopherol and were able to regulate sugar transport under high salinity conditions. Because γ-tocopherol increased under salinity stress and didn’t help in regulation of sugar transport, α-tocopherol and not any other tocopherol should play an important role in the sugar export and carbohydrate metabolism.
Environmental stress leads to excess levels of reactive oxygen species (ROS) causing oxidative damage (Kwon et al., 2013). There are four common forms of ROS generated during photosynthesis, which are hydrogen peroxide (H2O2), the superoxide radical (O2·-), the hydroxyl radical (HO-·), and singlet oxygen (1O2) (De Carvalho, 2008). Recent reports showed that α-tocopherol confers protection to membranes to reduce the extent of lipid peroxidation in seeds and leaves by quenching singlet oxygen and reacting with lipid peroxy radicals (Sattler et al., 2004; Havaux et al., 2005). In the present study, the TMT transplastomic plantlets that accumulated higher content α-tocopherol under salt stress showed significantly less accumulation of ROS, suggesting that α-tocopherol plays a protective role under abiotic stress conditions. Lipid peroxidation is one of the byproducts and indicators of ROS, which results in leaky membranes. Quantification of MDA showed that there was less lipid peroxidation in the TMT plants under salt or heavy metal stress and improved integrity of membrane significantly decreased ion leakage.
Alpha-tocopherol protects membrane lipid by reacting with fatty acid peroxyl radicals, which are the primary products of lipid peroxidation and then intercepts the chain reaction (Burton and Ingold, 1981). Previous reports have tried to understand the role of tocopherol under a variety of abiotic stress conditions. However, the observed results led to more questions. In the present research, the γ-TMT transplastomic plants subjected to salt and metal stress showed increase of α-tocopherol content. TMT plants with higher α-tocopherol levels showed higher tolerance to stress by improving membrane integrity. Under unchallenged conditions, α-tocopherol content in wild-type and transplastomic plants did not differ but significant difference was observed under stress conditions, suggesting that γ-TMT activity in leaves is up-regulated by abiotic stress. Expression of TMT in chloroplasts increased the α-tocopherol content in seeds ~10-fold when compared to wild-type seeds, under normal conditions without any stress. Alpha-tocopherol works with other cellular components including ascorbate, glutathione, carotenoids and anthocyanins to maintain the antioxidant status. When there is α-tocopherol deficiency, other antioxidants could partly compensate this. In contrast, the data from present study show that TMT plants accumulating the same level of ascorbate, anthocyanin and carotenoids content as the wild-type are more tolerant to salt and metal stress. This could be explained by higher α-tocopherol contents resulting from higher conversion of γ to α-tocopherol catalyzed by higher level expression of γ-TMT. These data also suggests that the γ-TMT activity is not only limited in the seeds but is also limited in leaves under stress conditions and therefore higher γ-TMT activity is essential for enhanced abiotic stress tolerance. Further studies could involve expression of the entire pathway of vitamin E biosynthesis, as multigene engineering is feasible in chloroplasts (Decosa et al., 2001; Kumar et al., 2013).
Experimental protocol
Construction of vector pLD-G10-γ-TMT for tobacco chloroplast transformation
The full length cDNA of γ-tocopherol methyltransferase (γ-tmt) gene (U13225) was ordered from the Arabidopsis Biological Resource Center at the Ohio State University. A Pair of primers, Toco-Forward and Toco-Reverse was designed to generate two restriction enzyme sites. The Toco-Forward primer: GAATTCCATATGAAAGCAACTCTAGCAGCACC contained the NdeI restriction site and the Toco-Reverse primer: TTGCTCTAGATTAGTGATGAT GATGATGATGGAGTGGCTTCTGGCAAGTGATG had the 6x-His tag as well as a XbaI restriction site. After γ-tmt cDNA was cloned by PCR and it was ligated into the pCR-Blunt II-TOPO vector (Invitrogen Company, CA, USA). After ligation, enzyme digestion with NotI and XbaI confirmed that the γ-tmt gene was inserted in the right orientation. Finally, the γ-tmt gene was inserted into the pLD -G10-XylDV chloroplast transformation vector by digestion with NdeI and NotI enzyme. Since the γ-tmt cDNA also has the NdeI restriction sites, partial digestion was performed to avoid cutting off the γ-tmt sequence. The pLD-G10 vector has been used for chloroplast transformation in our lab for several years (Guda et al., 2000; Dhingra et al., 2004; Ruhlman et al., 2010). The expression of aadA, as well as γ-tmt gene, was regulated by the Prrn (16s rRNA promoter) and 3′ UTR (3′-untranslated region) of psbA (TpsbA). The TpsbA was placed downstream of the γ-tmt and aadA genes to provide transcript stability. In the final vector -pLD-G10-γ-TMT, γ-tmt gene is regulated by the 5′ translation control region of bacteriophage T7 gene 10. The final vector pLD-G10-γ-TMT was sequenced to verify coding regions and regulatory elements.
Chloroplast transformation and molecular characterization of transplastomic plants
Young leaves of wild-type tobacco (Nicotiana tabacum var Petit Havana) were bombarded using the biolistic device PDS1000/He and transplastomic lines were obtained as described previously (Jin et al., 2012; Kwon et al., 2013). To confirm homoplasmy of transplastomic plants, Southern blot analysis was performed according to our previous reports (Verma et al., 2008; Lee et al., 2011). Quantification of TMT protein/enzyme in five transplastomic lines (F2.3, C.1, B3.3, E.1 and H3.2 by ELISA was performed following our previous published protocols (Jin et al., 2012).
Transmission electron microscopy (TEM)
Nine small pieces were dissected (3–4 mm) from three mature leaves (at the end of vegetable growth) of transplastomic TMT lines-C.1, B 3.3 and wild-type plants were observed by TEM followed the protocol described by Singh et al., (2008).
Seed germination, plant growth under salt, heavy metal stress
Seeds from the wild-type and TMT (line B3.3) plants were germinated in Murashige and Skoog (MS) basal medium, or with additional 200, 300 mM NaCl. The percent seed germination was calculated based on three repeated experiments (90–100 seeds taken for each replicate). For NaCl stress, seeds of transplastomic TMT lines-B3.3 and wild-type were germinated on Murashige and Skoog (MS) medium for 4 weeks, and then transferred to MS medium containing 0, 200, 300, and 400 mM NaCl as indicated in the figure legends for four weeks or transferred to MS medium containing 300 mM NaCl for 12, 24 and 48 hrs for hydrogen peroxide and superoxide radical staining in a 16-h/8-h light/dark cycle at 25 °C. For the heavy metal stress, 4 week-old seedlings grown on MS medium were transferred on Jiffy pellet for 2 more weeks of acclimation in the incubation room at 25 °C in a 16-h/8-h light/dark cycle. After that, plants were irrigated with nutrient solution supplemented with CuSO4 at a final concentration of 25 mM for 15 days.
Determination of tocopherol content
Four-week old wild-type and TMT transplastomic planlets were exposed to salt for four weeks or heavy metal stress for two weeks and then leaves of similar age/size were harvested and ground in liquid nitrogen and then extracted by 200 μl 100% methanol for 1 h at 30 °C. For seed extraction, 50 mg mature seeds from wild-type and TMT plants were used in each experiment. After incubation, samples were centrifuged at 13,000 ×g for 10 min and supernatant was saved. The remaining pellet was re-extracted twice with 200 μl 100% methanol under similar conditions. All pooled supernatants were stored at −20 °C until further use. The tocopherol recovered from tobacco tissues was measured by HPLC system (Perkin Elmer Series 225 LC). The extracts were injected on a Brownlee SPP HILIC column (Perkin Elmer) with hexane/isopropanal (99.5:0.5, V/V) mobile phase. The identification and quantification of tocopherols was based on the retention time tocopherol standards.
Staining and quantification of superoxide radical and hydrogen peroxide
Visualization and quantification of hydrogen peroxide and superoxide were performed according to the method described by Ramel et al.(2009) with suitable modifications. Hydrogen peroxide was visualized by 3, 3′-diaminobenzidine (DAB) staining.
Superoxide radical was detected with nitroblue tetrazolium (NBT) staining method. These experiments were repeated three times (15 plantlets for each time).
Lipid peroxidation measurement
Lipid peroxidation in tobacco leaf tissues was measured by quantification of MDA in the samples according to the methods described by Rao and Sresty (2000).
Statistical analyses
Differences between transplastomic TMT plants and wild-type on different treatments were determined by one-way analysis of variance (ANOVA).
Supplementary Material
Figure S1 Total anthocyanin, ascorbate and dedydroascorbate under salt and heavy metal stress. For the salinity stress, plants were grown in 0 to 400 mM salt added to the MS culture medium and measurements were done 4 weeks after growth. For the Cu stress, TMT and wild-type tobacco plants were irrigated with standard nutrient solution containing CuSO4 at a final concentration of 25 mM and measurements were done every three days (a) and (a-1), total anthocyanin; (b) and (b-1), total ascorbate content; (c) and (C-1) total dehydroascorbate content. Anthocyanin, ascorbate, dehydroascorbate content were determined spectrophotometrically as described in Experimental protocol. The data shown are means and SD from five replicate samples.
Figure S2 Ion leakage under salt and heavy metal stress (a) Both TMT transplastomic and wild-type tobacco plants were grown on the half MS medium supplied 0, 200, 300, 400 mM of NaCl. Membrane damage was assayed in stressed plants by determining ion leakage from leaf discs (six 9-mm leaf discs for each time point) (b) TMT and wild-type tobacco plants were irrigated with standard nutrient solution containing 25 mM CuSO4. Control plants were grown similarly, but without CuSO4. Membrane damage was assayed from leaf samples every 3 days of stressed plants by determining ion leakage from leaf discs. Data shown here is the mean and SD from three independent measurements. *, P <0.05.
Figure S3 Amino acid and carbohydrate contents in leaves from TMT and wild-type tobacco plants. For the salinity stress (Left column), plants were grown on 0 to 400 mM salt in the MS medium and measurements were made after 4 weeks. For Cu stress, TMT and wild-type tobacco plants were irrigated with standard nutrient solution containing 25 mM CuSO4 and measurements were made every three days. (a) and (a-1): the total amino acid; (b) and (b-1): proline; (c) and (c-1): starch; (d) and (d-1): total soluble sugars. The data shown are mean and SD of five independent measurements from one representative experiment. *, P<0.05
Acknowledgments
Authors thank Lee Barker for technical assistance. This project was supported in part by NIH grants R01 GM 63879, R01 HL 109442, R01 HL 107904 to Henry Daniell.
References
- Abrahám E, Hourton-Cabassa C, Erdei L, Szabados L. Methods for determination of proline in plants. Methods in Molecular Biology. 2010;639:317–331. doi: 10.1007/978-1-60761-702-0_20. [DOI] [PubMed] [Google Scholar]
- Asensi-Fabado MA, Munné-Bosch S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010;15:582–592. doi: 10.1016/j.tplants.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Burton GW, Ingold KU. Autoxidation of biological molecules. 1.The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vivo. J Am Chem Soc. 1981;103:6472–6477. [Google Scholar]
- Cela J, Chang C, Munne-Bosch S. Accumulation of γ-Rather than α-Tocopherol Alters Ethylene Signaling Gene Expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol. 2011;52:1389–1400. doi: 10.1093/pcp/pcr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho MHC. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Sig and Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCosa B, Moar W, Lee SB, Miller M, Daniell H. Hyper-expression of the Bt Cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nature Biotechnology. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Portis AR, Daniell H. Enhanced translation of a chloroplast expressed RbcS gene restores SSU levels and photosynthesis in nuclear antisense RbcS plants. Proc Natl Acad Sci USA. 2004;101:6315–6320. doi: 10.1073/pnas.0400981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D. Progress in the dissection and manipulation of vitamin E synthesis. Trends Plant Sci. 2005;10:574–579. doi: 10.1016/j.tplants.2005.10.007. [DOI] [PubMed] [Google Scholar]
- DellaPenna D, Pogson BJ. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu Rev Plant Biol. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- Falk J, Andersen G, Kernebeck B, Krupinska K. Constitutive over-expression of barley 4-hydroxyphenylpyruvate dioxygenase in tobacco results in elevation of the vitamin E content in seeds but not in leaves. FEBS Lett. 2003;540:35–40. doi: 10.1016/s0014-5793(03)00166-2. [DOI] [PubMed] [Google Scholar]
- Fournier E. Colorimetric quantification of carbohydrates. In: Wrolstad RE, editor. Current Protocols in Food Analytical Chemistry. Hoboken, NJ, USA: John Wiley and Sons; 2001. pp. E1.1.1–E1.1.8. [Google Scholar]
- Fernandez-San Millan, Mingeo-Castel A, Miller M, Daniell H. A chloroplast transgenic approach to hyper-express and purify Human Serum Albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol J. 2003;1:71–79. doi: 10.1046/j.1467-7652.2003.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak M_A, Dellapenna D. Improving the nutrient composition of plants to enhance human nutrition and health. Ann Rev Plant Physiol Plant Mol Biol. 1999;50:133–161. doi: 10.1146/annurev.arplant.50.1.133. [DOI] [PubMed] [Google Scholar]
- Guda C, Lee SB, Daniell H. Stable expression of biodegradable protein based polymer in tobacco chloroplasts. Plant Cell Rep. 2000;19:257–262. doi: 10.1007/s002990050008. [DOI] [PubMed] [Google Scholar]
- Guisti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE, editor. Current Protocols in Food Analytical Chemistry. Hoboken, NJ, USA: John Wiley and Sons; 2001. pp. F1.2.1–F1.2.13. [Google Scholar]
- Halliwell B, Gutteridge JMC. Iron and free radical reactions: two aspects of antioxidant protection. Trends in Biochemical Science. 1986;11:375. [Google Scholar]
- Hofius D, Hajirezaei M, Geiger M, Tschiersch H, Melzer M, Sonnewald U. RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol. 2004;135:1256–1268. doi: 10.1104/pp.104.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofius D, Hajirezaei MR, Geiger M, Tschiersch H, Melzer M, Sonnewald U. RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol. 2004;135:1256–1268. doi: 10.1104/pp.104.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Kanagaraj A, Verma D, Lange T, Daniell H. Release of hormones from conjugates: chloroplast expression of β-glucosidase results in elevated phytohormone levels associated with significant increase in biomass and protection from aphids or whiteflies conferred by sucrose esters. Plant Physiol. 2011;155:222–235. doi: 10.1104/pp.110.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhang XL, Daniell H. Pinellia ternata agglutinin expression in chloroplasts confers broad spectrum resistance against aphid, whitefly, lepidopteran insects, bacterial and viral pathogens. Plant Biotech J. 2012;10:313–327. doi: 10.1111/j.1467-7652.2011.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli N, Westerveld D, Ayache AC, Verma A, Shil P, Prasad T, Zhu P, Chan SC, Li Q, Daniell H. Oral delivery of bioencapsulated proteins across blood–brain and blood–retinal barriers. Molecular Therapy. 2014;22:535–546. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota M, Daniell H, Varma S, Garczynski SF, Gould F, Moar WJ. Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc Natl Acad Sci USA. 1999;96:1840–1845. doi: 10.1073/pnas.96.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany. 2012;63:1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Dhingra A, Daniell H. Plastid expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–2854. doi: 10.1104/pp.104.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Hahn FM, Baidoo E, Kahlon TS, Wood DF, McMahan CM, Cornish K, Keasling JD, Daniell H, Whalen MC. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mavalonate pathway in chloroplasts. Metabolic Engineering. 2012;14:19–28. doi: 10.1016/j.ymben.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Verma D, Jin S, Singh ND, Daniell H. Release of proteins from chloroplasts induced by reactive oxygen species during biotic and abiotic stress. PLoS One. 2013;8:e67106. doi: 10.1371/journal.pone.0067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotech J. 2013;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. Biochem J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Li BC, Jin SX, Daniell H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegin-1 in chloroplast to control viral and bacterial infections. Plant Biotech J. 2011;9:100–115. doi: 10.1111/j.1467-7652.2010.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MR, Li Y, Li HQ, Wu GJ. Improvement of paper mulberry tolerance to abiotic stresses by ectopic expression of tall fescue FaDREB1. Tree Physiol. 2012;32:104–113. doi: 10.1093/treephys/tpr124. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang G, Hou R, Zhou Y, Gong R, Sun X, Tang K. Engineering tocopherol biosynthetic pathway in lettuce. Biologia Plantarum. 2011;55:453–460. [Google Scholar]
- Mene-Saffrane L, DellaPenna D. Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol Biochem. 2010;48:301–309. doi: 10.1016/j.plaphy.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Mene-Saffrane L, Jones AD, Dellapenna D. Plastochromanol-8 and tocopherols are essential lipid-soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc Natl Acad Sci USA. 2010;107:17815–17820. doi: 10.1073/pnas.1006971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci. 2002;21:31–57. [Google Scholar]
- Nieboer E, Richardson DHS. The replacement of the nondescript term ‘heavy metal’ by a biologically significant and chemically significant classification of metal ions. Environmental Pollution, (Serial B) 1980;1:3–26. [Google Scholar]
- Provencher LM, Miao L, Sinha N, Lucas WJ. Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell. 2001;13:1127–1141. doi: 10.1105/tpc.13.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Bogard M, Couee I, Gouesbet G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biology. 2009;9:28. doi: 10.1186/1471-2229-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KVM, Sresty TVS. Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan) in response to Zn and Ni stress. Plant Sci. 2000;157:113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Rosen H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957;67:10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Ruhlman T, Verma D, Samson N, Daniell H. Role of heterologous elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz ON, Alvarez D, Torres C, Roman L, Daniell H. Metallothionein expression in chloroplasts enhances mercury accumulation and phytoremediation capability. Plant Biotechnol J. 2011;9:609–617. doi: 10.1111/j.1467-7652.2011.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz ON, Daniell H. Genetic engineering to enhance mercury phytoremediation. Curr Opin Biotechnol. 2009;20:213–219. doi: 10.1016/j.copbio.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russin WA, Evert RE, Vanderveer PJ, Sharkey TD, Briggs SP. Modification of a specific class of plasmodesmata and loss of sucrose export ability in a sucrose export defective1 maize mutant. Plant Cell. 1996;8:645–658. doi: 10.1105/tpc.8.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragi Y, Maeda H, Dellapenna D, Bryant DA. a-Tocopherol plays a role in photosynthesis and macronutrient homeostasis of the cyanobacterium Synechocystis sp. PCC 6803 that is independent of its antioxidant function. Plant Physiol. 2006;141:508–521. doi: 10.1104/pp.105.074765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D. Characterization of tocopherol cyclases from higher plants and cyanobacteria: evolutionary implications for tocopherol synthesis and function. Plant Physiol. 2003;132:2184–2195. doi: 10.1104/pp.103.024257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- Shintani D, DellaPenna D. Elevating the vitamin E content of plants through metabolic engineering. Science. 1998;282:2098–2100. doi: 10.1126/science.282.5396.2098. [DOI] [PubMed] [Google Scholar]
- Singh ND, Li M, Lee SB, Schnell D, Daniell H. Arabidopsis Tic40 expression in tobacco chloroplasts results in massive proliferation of the inner envelope membrane and upregulation of associated proteins. Plant Cell. 2008;20:3405–3417. doi: 10.1105/tpc.108.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J, Schultz G, Joyard J, Douce R, Block M. Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch Biochem Biophys. 1985;238:290–299. doi: 10.1016/0003-9861(85)90167-5. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Verma D, Samson NP, Koya V, Daniell H. A protocol for expression of foreign genes in chloroplasts. Nat Protoc. 2008;3:739–758. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- Verma D, Jin S, Kanagaraj A, Singh ND, Jaiyanth D, Kolattukudy PE, Miller M, Daniell H. Expression of fungal cutinase and swollenin in tobacco chloroplasts reveals novel enzyme functions and/or substrates. PLoS One. 2013;8:e57187. doi: 10.1371/journal.pone.0057187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbierzak AM, Kanwischer M, Wille C, Vidi PA, Giavalisco P, Lohmann A, Briesen I, Porfirova S, Brehelin C, Kessler F, Dormann P. Intersection of the tocopherol and plastoquinol metabolic pathways at the plastoglobule. Biochem J. 2010;425:389–399. doi: 10.1042/BJ20090704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Total anthocyanin, ascorbate and dedydroascorbate under salt and heavy metal stress. For the salinity stress, plants were grown in 0 to 400 mM salt added to the MS culture medium and measurements were done 4 weeks after growth. For the Cu stress, TMT and wild-type tobacco plants were irrigated with standard nutrient solution containing CuSO4 at a final concentration of 25 mM and measurements were done every three days (a) and (a-1), total anthocyanin; (b) and (b-1), total ascorbate content; (c) and (C-1) total dehydroascorbate content. Anthocyanin, ascorbate, dehydroascorbate content were determined spectrophotometrically as described in Experimental protocol. The data shown are means and SD from five replicate samples.
Figure S2 Ion leakage under salt and heavy metal stress (a) Both TMT transplastomic and wild-type tobacco plants were grown on the half MS medium supplied 0, 200, 300, 400 mM of NaCl. Membrane damage was assayed in stressed plants by determining ion leakage from leaf discs (six 9-mm leaf discs for each time point) (b) TMT and wild-type tobacco plants were irrigated with standard nutrient solution containing 25 mM CuSO4. Control plants were grown similarly, but without CuSO4. Membrane damage was assayed from leaf samples every 3 days of stressed plants by determining ion leakage from leaf discs. Data shown here is the mean and SD from three independent measurements. *, P <0.05.
Figure S3 Amino acid and carbohydrate contents in leaves from TMT and wild-type tobacco plants. For the salinity stress (Left column), plants were grown on 0 to 400 mM salt in the MS medium and measurements were made after 4 weeks. For Cu stress, TMT and wild-type tobacco plants were irrigated with standard nutrient solution containing 25 mM CuSO4 and measurements were made every three days. (a) and (a-1): the total amino acid; (b) and (b-1): proline; (c) and (c-1): starch; (d) and (d-1): total soluble sugars. The data shown are mean and SD of five independent measurements from one representative experiment. *, P<0.05