Abstract
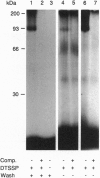
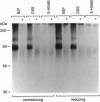
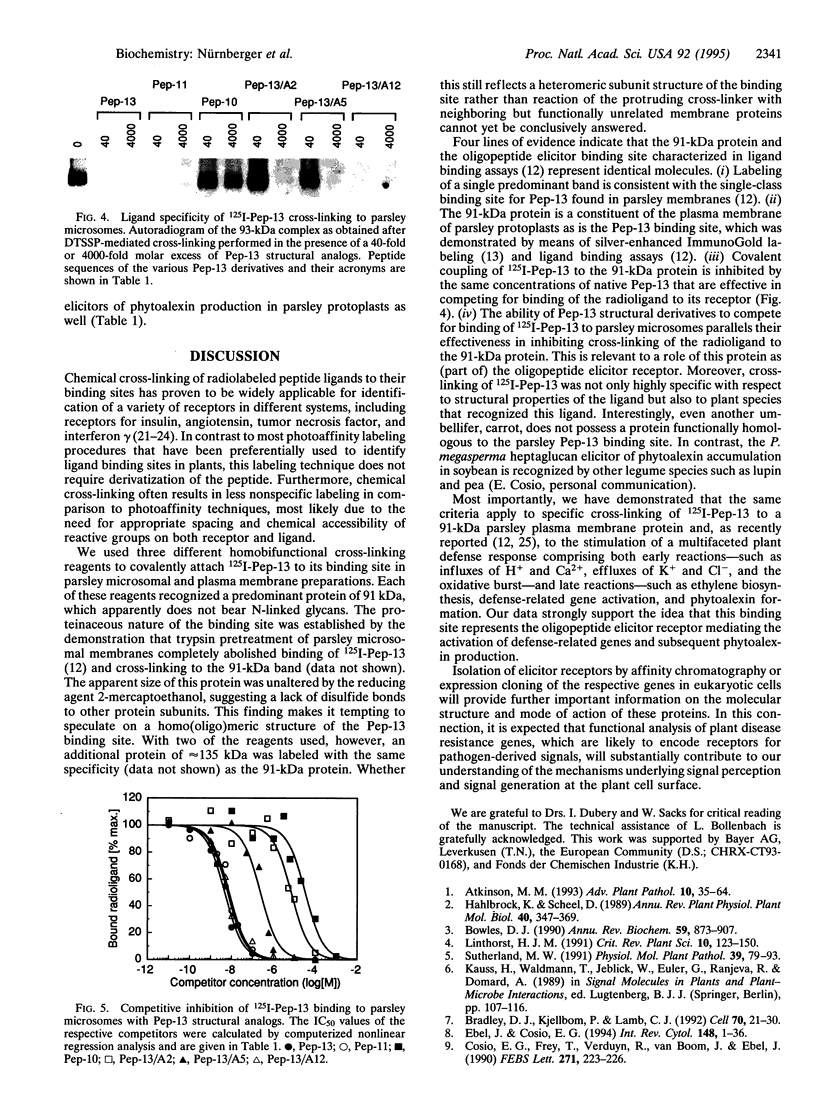
An oligopeptide elicitor from Phytophthora megasperma f.sp. glycinea (Pep-13) that induces phytoalexin accumulation in cultured parsley cells was radioiodinated and chemically cross-linked to its binding site in microsomal and plasma membrane preparations with each of three homobifunctional reagents. Analysis by SDS/PAGE and autoradiography of solubilized membrane proteins demonstrated labeling of a 91-kDa protein, regardless of which reagent was used. Cross-linking of this protein was prevented by addition of excess unlabeled Pep-13. The competitor concentration found to half-maximally reduce the intensity of the cross-linked band was 6 nM, which is in good agreement with the IC50 value of 4.7 nM, obtained from ligand binding assays. No crosslinking of 125I-labeled Pep-13 was observed by using microsomal membranes from three other plant species, indicating species-specific occurrence of the binding site. Coupling of 125I-Pep-13 to the parsley 91-kDa protein required the same structural elements within the ligand as was recently reported for binding of 125I-Pep-13 to parsley microsomes, elicitor-induced stimulation of ion fluxes across the plasma membrane, the oxidative burst, the expression of defense-related genes, and phytoalexin production. These findings suggest that the 91-kDa protein identified in parsley membranes is the oligopeptide elicitor receptor mediating activation of a multicomponent defense response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basse C. W., Fath A., Boller T. High affinity binding of a glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993 Jul 15;268(20):14724–14731. [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kjellbom P., Lamb C. J. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992 Jul 10;70(1):21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Cheong J. J., Hahn M. G. A specific, high-affinity binding site for the hepta-beta-glucoside elicitor exists in soybean membranes. Plant Cell. 1991 Feb;3(2):137–147. doi: 10.1105/tpc.3.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio E. G., Frey T., Ebel J. Identification of a high-affinity binding protein for a hepta-beta-glucoside phytoalexin elicitor in soybean. Eur J Biochem. 1992 Mar 15;204(3):1115–1123. doi: 10.1111/j.1432-1033.1992.tb16736.x. [DOI] [PubMed] [Google Scholar]
- Cosio E. G., Frey T., Verduyn R., van Boom J., Ebel J. High-affinity binding of a synthetic heptaglucoside and fungal glucan phytoalexin elicitors to soybean membranes. FEBS Lett. 1990 Oct 1;271(1-2):223–226. doi: 10.1016/0014-5793(90)80411-b. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D., Orchansky P., Revel M., Rubinstein M. The human interferon-gamma receptor. Purification, characterization, and preparation of antibodies. J Biol Chem. 1987 Jun 25;262(18):8483–8487. [PubMed] [Google Scholar]
- Nürnberger T., Nennstiel D., Jabs T., Sacks W. R., Hahlbrock K., Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994 Aug 12;78(3):449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Sen I., Bull H. G., Soffer R. L. Isolation of an angiotensin II-binding protein from liver. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1679–1683. doi: 10.1073/pnas.81.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]