Abstract
Objective
Polycystic Ovary Syndrome (PCOS) is associated with reduced adipose tissue lipolysis that can be rescued by aerobic exercise. We aimed to identify differences in gene expression of perilipins and associated targets in adipose tissue in women with PCOS before and after exercise.
Design and Methods
We conducted a cross-sectional study in 8 women with PCOS and 8 women matched for BMI and age with normal cycles. Women with PCOS also completed a 16-week prospective aerobic exercise-training study. Abdominal subcutaneous adipose tissue biopsies were collected, and primary adipose-derived stromal/stem cell cultures were established from women with PCOS before 16 weeks of aerobic exercise training (n=5) and controls (n=5). Gene expression was measured using real time PCR, in vitro lipolysis was measured using radiolabeled oleate, and PLIN3 protein content was measured by western blotting.
Results
The expression of PLIN1, PLIN3, and PLIN5, along with coatomers ARF1, ARFRP1, and βCOP were ~80% lower in women with PCOS (all p<0.05). Following exercise training, PLIN3 was the only perilipin to increase significantly (p<0.05), along with coatomers ARF1, ARFRP1, βCOP, and Sec23a (all p<0.05). Furthermore, PLIN3 protein expression was undetectable in the cell cultures from women with PCOS vs. controls. Following exercise training, in vitro adipose oleate oxidation, glycerol secretion, and PLIN3 protein expression were increased, along with reductions in triglyceride content and absence of large lipid droplet morphology.
Conclusions
These findings suggest that PLIN3 and coatomer GTPases are important regulators of lipolysis and triglyceride storage in the adipose tissue of women with PCOS.
Keywords: Lipolysis, Insulin, Obesity, Fatty acid oxidation, Hyperandrogenemia, PAT protein
Introduction
Polycystic Ovary Syndrome (PCOS) is a complex endocrine and reproductive disorder affecting approximately 4–7% of women of reproductive age 1, 2. As a principle cause of infertility in reproductive aged women, PCOS is characterized by the presence of menstrual disterbances, hyperandrogenemia, and ovarian cysts 3. Similarly, approximately 70% of women with PCOS have increased adiposity 4, and between 20–43% have insulin resistance and reduced glucose control 5, 6. One possible culprit speculated to contribute to this irregular metabolic phenomena is defects within the adipose tissue 7.
Adipose tissue functions as a storage reservoir for excess lipid, a reserve that must be readily lipolyzed upon increased energy demand. Inefficient ability of the adipose tissue to mobilize and secrete free fatty acids during conditions of energy demand have been linked to conditions of impaired glucose tolerance and type 2 diabetes 8–10. It has been previously documented that women with PCOS have dysfunctions in lipase activity and catecholamine-mediated lipolysis 11–13. Furthermore it has been reported that testosterone can reduce: 1) catecholamine-stimulated adipose tissue lipolysis 14, 15; 2) expression of beta-adrenergic receptors in adipose tissue 14, 15, and 3) adrenergic stimulated lipolysis in the brown adipose tissue 16. Possible molecular targets, such as the expression of lipases, have been implicated as factors regulated by testosterone 15, 17, 18, thus offering a possible relationship between impaired lipolysis and the hyperadrogenemic states seen in women with PCOS.
Exercise training has been shown to improve several metabolic impairments typical of PCOS 19–21, and has been shown to enhance adipose tissue lipolysis 22, 23. Previous studies from our group revealed an increase in basal and pharmacologically stimulated lipolysis with isoproterenol from the adipose tissue following exercise training 24. The molecular markers that regulate lipolysis in the adipose tissue of women with PCOS however have not been thoroughly studied.
Prior investigations have shown that a single nucleotide polymorphism within the perilipin gene (PLIN1) that exists in women with PCOS is associated with impaired glucose tolerance and increased LDL 25, prehaps implicating a potential role for the perilipin family of proteins, lipases, and related factors effecting adipose tissue lipolysis. Recent investigations have also identified several coatomer GTPase proteins (ARF1, Sec23a, βCOP, GBF1, ARFRP1), which are usually involved in ER-to-Golgi transport, and that are involved in the delivery of adipose tissue triglyceride lipase (ATGL) to lipid droplets, particularly those lipid droplets coated by perilipin 3 (PLIN3) 26. We have recently shown that PLIN3 and coatomer GTPases in skeletal muscle tissue and in primary human skeletal muscle cultures were associated with increased fat oxidation following exercise and lipolytic stimulation 27. We, therefore, hypothesized that these novel mediators of lipolysis might also be differentially expressed in the adipose tissue of women with PCOS, where lipolysis is impaired, and upregulated following aerobic exercise training. We investigated the expression of lipases, perilipins, and coatomer GTPases from adipose tissue in a cross-sectional cohort of 8 women with PCOS compared to women with normal menses matched for age, BMI, and percent body fat. Furthermore, in the women with PCOS, we investigated the effects of a 16-week aerobic exercise training program on lipolysis both in adipose tissue and in stromal-derived adipose cultures. Our results indicate previously unexplored potential roles for perilipin 3 (PLIN3) and coatomers, (ADP-ribosylation factor 1 (ARF1), ARF related peptide 1 (ARFRP1), coatomer complex 1 subunit beta (β-COP1), and coatomer complex 2 subunit 23a (Sec23a)) in the regulation of lipolysis in the adipose tissue.
Materials and Methods
Participants and study design
Eight obese women with PCOS and eight age, BMI, and percent fat matched healthy women without clinical signs of abnormal menses or hyperandrogenemia were recruited in this study (anthropometric characteristics are provided in Table 1). The results reported for this investigation were an ancillary project to a clinical study, which was designed and powered to determine the effect of an aerobic exercise program on body composition and whole body insulin resistance in obese women diagnosed with PCOS. The original study was powered using the mean and standard deviation of glucose disposal rate (GDR) to estimate sample sizes required to measure a minimum 20% change in GDR from baseline. With a prospective study design (paired) with a target power of 80% and a significance level set to α=0.05, we can conclude that only 6 subjects would be needed to detect a 20% change in GDR from baseline. The main outcomes of this study were previously reported in Moro et al. 24 and in Redman et al. 28 The diagnosis of PCOS was assessed by the Rotterdam criteria. 29, 30 Women with PCOS had to possess two of the following criteria: confirmation by medical history of menstrual irregulatiry (oligo- or amenorrhea), presence of more than 10 ovarian follicles 2–9mm in diameter as assessed by MRI, or either clinical (hirsutism score) or serum measures of androgen excess (elevated free androgen index, FAI). Other causes of oligomenorrhea (hyperprolactinemia, congenital adrenal hyperplasia, Cushing’s syndrome, hyperthyroidism) were excluded by medical history. Women in the control group were excluded from participation in our study for exercise training, use of contraceptive medications, and menstrual cycle irregularity together with androgen excess. All women in our control group had FAI values below 3.6 as defined as a cut-off value for FAI in the assessment of PCOS according to Hahn et al. 31 Additionally, all women in our control group reported regular menstruation, thus confirming that they did not have PCOS based on the Rotterdam guidelines. Potential subjects were excluded from participating in either group if they smoked, were taking any medications, had current or past history of cardiovascular disease, hypertension (>140/90 mmHg), diabetes (type 1 or type 2), kidney, liver or heart disease, alcoholism or substance abuse, were pregnant or trying to become pregnant and were unable to comply with exercise training program. Adipose tissue measures are reported in all participants included in this study. Fat cell size was determined using osmium fixation and counted using a multisizer 3 Coulter counter (Beckman Coulter, Brea, CA) as previously described 32. In vitro measures of stromal-derived adipocytes are reported from five women with PCOS before and after exercise due to availability of adipose tissue material for the isolation and culturing of stromal-vascular cells. For immunocytochemistry analysis of lipid droplet comparisons and protein immunoblotting, controls were taken from 5 women with normal menstrual cycling unrelated to the original study that were matched for age (24.2 ± 2.3 yrs, p = 0.37 compared to women with PCOS) and BMI (26.1 ± 2.5 kg/m2, p = 0.83 compared to women with PCOS)—this was necessary because the collection of stromal-derived adipose cultures was not part of the initial study protocol for the control subjects. The study design and protocol was approved by the institutional review board of Pennington Biomedical Research Center, and all volunteers gave written informed consent. This study was registered at clinicaltrials.gov (NCT01150539).
Table 1.
Anthropometic, metabolic, and serum markers for both the cross-sectional study between controls and women with PCOS, and the exercise training intervention in the women with PCOS only.
| Women with PCOS | Control vs PCOS | Pre- vs Post- exercise | |||
|---|---|---|---|---|---|
| Control | Pre-Exercise | Post-Exercise | P value | P value | |
| Age (yr) | 29.7 ± 12.4 | 27.0 ± 2.9 | -- | 0.65 | -- |
| Weight (kg) | 78.9 ± 15.7 | 82.2 ± 18.2 | 81.4 ± 21.6 | 0.74 | 0.69 |
| BMI (kg/m2) | 26.5 ± 5.4 | 30.8 ± 4.2 | 30.4 ± 5.8 | 0.17 | 0.66 |
| Total body fat (%) | 29.9 ± 9.8 | 36.8 ± 5.0 | 34.8 ± 6.1 | 0.19 | 0.21 |
| FM (kg) | 23.3 ± 9.3 | 30.8 ± 11.1 | 29.3 ± 13 | 0.23 | 0.39 |
| FFM (kg) | 55.6 ± 15.9 | 51.4 ± 7.9 | 52.1 ± 9.0 | 0.60 | 0.24 |
| Visceral AT (kg) | 1.4 ± 0.8 | 1.2 ± 0.5 | 1.1 ± 0.7 | 0.75 | 0.64 |
| Subcutaneous AT (kg) | 11.4 ± 1.8 | 10.9 ± 4.8 | 10.5 ± 5.7 | 0.86 | 0.47 |
| IHL (AU) | 0.013 ± 0.017 | 0.13 ± 0.17 | 0.03 ± 0.05 | 0.16 | 0.20 |
| IMCL (soleus, AU) | 0.006 ± 0.003 | 0.006 ± 0.004 | 0.007 ± 0.005 | 0.89 | 0.27 |
| Glucose, fasting (mg/dL) | 103.5 ± 28.6 | 82.2 ± 6.3 | 89.6 ± 5.5 | 0.14 | 0.08 |
| Insulin, fasting (mg/dL) | 11.0 ± 10.2 | 9.8 ± 3.9 | 12.4 ± 5.6 | 0.81 | 0.51 |
| HOMA-IR (AU) | 3.4 ± 4.2 | 2.0 ± 0.8 | 2.7 ± 1.2 | 0.50 | 0.42 |
| GDR/EMBS (mg/kgFFM +17.7) | 6.8 ± 4.3 | 6.3 ± 1.2 | 7.6 ± 1.6 | 0.79 | 0.02 |
| Resting Metabolic Rate/FFM (kcal/day/kg) | 30.3 ± 4.8 | 30.7 ± 5.0 | 30.0 ± 2.7 | 0.91 | 0.62 |
| Resting RQ | 0.80 ± 0.03 | 0.81 ± 0.02 | 0.80 ± 0.01 | 0.37 | 0.21 |
| Clamp RQ | 0.93 ± 0.07 | 0.90 ± 0.04 | 0.90 ± 0.05 | 0.50 | 0.83 |
| ΔRQ (Clamp vs Resting) | 0.13 ± 0.07 | 0.09 ± 0.02 | 0.10 ± 0.03 | 0.26 | 0.72 |
| VO2max (mL/min/kg) | 27.7 ± 10.0 | 29.5 ± 3.0 | 33.6 ± 4.3 | 0.71 | 0.04 |
| Fat Cell Size (nL) | 0.69 ± 0.31 | 0.68 ± 0.13 | 0.76 ± 0.25 | 0.92 | 0.40 |
| DHEA-S (ug/mL) | 135.3 ± 49.6 | 159.0 ± 73.6 | 192.0 ± 79.4 | 0.47 | 0.02 |
| Testosterone (ng/dL) | 35.4 ± 11.7 | 92.4 ± 41.2 | 64.8 ± 25.3 | 0.002 | 0.61 |
| SHBG (nmol/L) | 24.1 ± 4.6 | 30.9 ± 31.7 | 34.2 ± 19.3 | 0.56 | 0.75 |
| FAI (AU) | 1.2 ± 0.6 | 18.8 ± 14.2 | 13.9 ± 17.5 | 0.02 | 0.67 |
This data reflects the 5 females with PCOS for whom we were able to obtain primary stromal-derived adipose cultures both before and after exercise intervention. EMBS: Estimated Mean Body Size, FM: Fat Mass, FFM: Fat Free Mass, GDR: Glucose Disposal Rate, RQ: Respiratory Quotient, DHEA-S: Dehydroepiandrosterone Sulfate, SHGB: Sex Hormone Binding Globulin, FAI: Free Androgen Index, AT: Adipose Tissue, IHL: Intrahepatic lipid, IMCL: Intramyocellular lipid
All women were examined at baseline, and the women with PCOS were reexamined after 16 weeks of aerobic exercise training. At each time-point, study testing occurred over 3 days. In order to reduce confounding factors associated with dietary differences and metabolic testing, we provided participants a standard diet of 50% carbohydrate, 35% fat, and 15% protein 2 days prior to testing and throughout the duration of testing. Following an overnight fast, blood and subcutaneous abdominal adipose tissue samples were collected, body composition (percent body fat surmised from all regions of the body) was assessed by dual energy x-ray absorptiometry (DXA, QDR 4500A; Hologics, Bedford, MA), and insulin sensitivity was determined by a hyperinsulinemic-euglycemic clamp (120-minutes at 80mU/min/m2) as previously described 28. Aerobic capacity (VO2max) was measured during a graded treadmill test (TrueMax 2400; ParvoMedics, Salt Lake City, UT). Serum testosterone and sex hormone binding globulin were determined by an automated chemiluminescent immunoassay on the Immulite 2000 (Siemens Healthcare Diagnostics, Deerfield, IL). Serum glucose and serum insulin were measured by an enzymatic assay on a Beckman Coulter DXC 600 (Beckman Coulter, Brea, CA). Ovarian morphology, abdominal subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) as well as intrahepatic lipid and intramyocellular lipid (IMCL) of the soleus was examined before and after 16 weeks of aerobic exercise training using a 3T MRI/MRS (GE 3.0T Signa EXCITE MRI, GE Healthcare, Pittsburgh, PA; results provided in Table 1).
Magnetic Resonance Imaging and Spectroscopy
Abdominal fat: An 8 channel torso-array coil was placed over the chest/abdomen area and 3.4 mm slices (1.7 mm intersection gap) were acquired from the highest point of the liver to the inferior pole of the right kidney. A total of approximately 220 images were acquired on each participant. Total (TAT), visceral (VAT) and subcutaneous (SAT) abdominal adipose tissue mass were calculated using the Analyze™ software package (CNSoftware, Rochester, MN). Muscle and hepatic lipid content: For the muscle intramyocellular lipid measures, the right leg was positioned inside a 1H knee coil with the knee in extension and the ankle in a neutral position. Separate water suppressed PRESS boxes (10 × 7.5 × 7.5 mm voxels) were collected from the tibialis anterior (n=1), soleus (n=3) and peanut oil phantom (n=1). For the liver lipid content, with the participant lying prone, a 1H body coil was placed over the torso and a single PRESS box (30 × 30 × 30 mm) was collected in an area of the liver free from heavy vascularization. Data were analyzed using the jMRUi software package. Ovarian morphology: An 8 channel torso array coil was used to acquire coronal (T2 weighted fast spin echo (FSE), short T1 inversion recovery (STIR), and T1 weighted localizer), sagittal (T1 weighted localizer only) and axial (T2 weighted FSE and STIR) sequences. Images were acquired from the highest point of the uterus or ovaries through the bottom of the ovaries with 4mm thick slices and 1 mm intersection gap for a total of approximately 40 images. Images were analyzed using Analyze™ 8.1 (CNSoftware, Rochester, MN) by a trained analyst. Ovaries, as well as ovarian follicles, were located and quantified on each coronal FSE image. Follicles were located on an image and followed down through the remaining consecutive images to ensure that each individual follicle was identified as such. The total number of follicles in each ovary was calculated using this technique. The volume of each follicle was calculated based on the number of pixels measured in each image and the number of consecutive images in which the follicle was located.
Subcutaneous Adipose Tissue Biopsy
Subcutaneous adipose tissue was obtained from the left upper quadrant of the abdomen with a 5-mm Bergstrom needle using the Bergstrom technique. The skin was cleansed with povidone-iodine solution, a sterile drape was placed over the incision site, and local anesthesia (5 mL 1:1 mixture of 0.5% bupivicaine and 2% lidocaine) was administered. An approximate 1cm incision was made and adipose tissue was collected.
Exercise training program
Aerobic exercise was performed under supervision at the Pennington Health and Fitness Center, five times per week. Aerobic exercise was prescribed on an individual basis with the objective to achieve specified exercise energy expenditure (ExEE) in each session. During the first four weeks, the target ExEE was 4% of the participants’ estimated energy requirement for weight maintenance, and was incremented to 6% for weeks 5–8, to 8% for weeks 9–12 and to 10% for weeks 13–16. All exercises were performed on a treadmill at 55% VO2max, a moderate intensity. The necessary speed and gradient to achieve the ExEE was estimated from a linear regression of O2 uptake and workload during the VO2max test and the ExEE was confirmed once during each 4-week interval by indirect calorimetry. Heart rate was monitored during all sessions to verify ExEE. The exercise time necessary to complete the energy expenditure target was 23±1 minutes per session during weeks 1–4, 35±1 minutes per session during weeks 5–8, 47±2 minutes per session during weeks 9–12 and 58±2 minutes per session during weeks 13–16. In order to prevent variation in results due to dietary influences during the exercise intervention, we asked participants to maintain their dietary habits throughout the 16-weeks of intervention as they had prior to study enrollment. Additionally, we requested updates on food intake during site visits for exercise sessions.
Stromal-derived adipocyte cultures, immunofluorencenc staining, lipolysis, triglyceride determination, and oleate oxidation
Adipose tissue samples from subcutaneous abdominal depot were collected under aseptic conditions and isolated stromovascular (SV) cells were cultured as previously described 33 and differentiated as previously described 34 with modifications: DMEM-F12 (1:1) medium plus 10 mg/ml transferin, 33 μM biotin, 17 μM calcium pantothenate, 0.5 μM insulin, 0.1 μM dexamethasone, 0.2 nM triiodothyronin, as well as 0.5 μM Roziglitazone, and 540 μM IBMX during the last 48 hours of culture. All experiments were performed on cells following 9 days of differentiation. Cultures were stained for lipids (BODIPY, 10 μg/ml) and DNA (DAPI, 300 nM) (Invitrogen, Carlsbad, CA). Images were obtained using the Leica TCS SP5 AOBS resonant scanning confocal microscope (Leica AG, Wetzlar, Germany). The lipolysis assay was performed over 3 hr by adding 0.2 ml of HBSS + BSA 2%. At the end of incubation, medium was collected for measurement of glycerol, performed in duplicate using the free glycerol reagent (Sigma-Aldrich, St Louis, MO) and adjusted for the triglyceride content. Triglyceride levels were then measured using a glycerol phosphate oxidase triglyceride determination kit (Sigma-Aldrich, St Louis, MO) and normalized to the protein content. Cultures were preincubated for 3 hr with [1-14C] oleic acid (1 μCi/ml; PerkinElmer, Boston, MA) and non-labeled (cold) oleic acid (100 μM). Oleic acid was coupled to fatty acid-free BSA in a molar ratio of 5:1. Following incubation, 14CO2 was measured as previously described 35 The cells were then lysed in 0.2 ml of SDS 0.1% for determination of cell-associated label uptake and protein content for normalization. All assays were performed in duplicate.
Real-time qRT-PCR and Western Blotting
Total RNA was extracted from approximately 100 mg of adipose tissue using miRNEasy Kits (Qiagen, Valencia, CA) according to the manufacturer’s specifications. RNA extracts were converted into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and stored at −20°C until Real Time-PCR was performed. Gene expression was carried out using Real Time-PCR with TaqMan gene expression assays-on-demand on the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Gene expression assays were performed for the following genes: PLIN1 (Hs00160173_m1), PLIN2 (Hs00765634_m1), PLIN3 (Hs00998421_m1), PLIN4 (Hs00287411_m1), PLIN5 (Hs00965990_m1), GBF1 (Hs00188327_m1), ARF1 (Hs00796826_s1), ARFRP1 (Hs00182389_m1), βCOP (Hs00200674_m1), Sec23a (Hs00197232_m1), ATGL (Hs00386101_m1), CGI-58 (Hs01104373_m1), MGL (Hs00200752_m1), DGAT2 (Hs01045913_m1), mtGPAT (Hs00326039_m1), PPIA (Hs99999904_m1). Relative gene expression was assessed using a standard curve of known concentrations of mRNA and normalized to Cyclophilin A (PPIA) gene expression. Protein extracts from adipose culture were immunoblotted and probed with an antibody against PLIN3 (Novus Biologicals, Littleton, CO) and normalized against the loading control GAPDH (AbCam, Cambridge, MA).
Statistical analyses
All analyses were performed using GraphPad Prism Software, version 5.0 (GraphPad Software, La Jolla, CA). The Mann-Whitney test was used for cross-sectional comparisons between PCOS and Obese Control women when data was not normally distributed, and an Independent Samples t-test was used when the data was normally distributed. Paired t-tests were used to compare variables from baseline to the end of the exercise training intervention; except when the data was not normally distributed, Wilcoxon Signed Ranked Paired tests were used. P < 0.05 was considered statistically significant. All graphical data is presented as mean ± SEM.
Results
Comparison of controls vs. women with PCOS
Compared to the control participants, women with PCOS had elevated serum testosterone and free androgen index, as expected, but they did not differ in terms of sex hormone binding globulin (SHGB) (Table 1). Otherwise, control participants were effectively matched to women with PCOS for body composition, insulin sensitivity (fasting glucose, fasting insulin, glucose disposal rate, HOMA-IR), and energy metabolism (resting metabolic rate, fasting respiratory quotient (RQ), metabolic flexibility (i.e. changes between the fasting RQ and RQ during the steady state of the hyperinsulinemic euglycemic clamp) and VO2Max (Table 1). Importantly, women with PCOS did not differ from control participants with regard to mean subcutaneous fat cell size (Table 1).
Women with PCOS did however differ significantly from controls in terms of subcutaneous adipose tissue gene expression for candidates responsible for lipolysis, lipid droplet perilipin proteins, and coatomer proteins. As shown in Figure 1A, perilipin 1, 3, and 5 (PLIN1, PLIN3, and PLIN5, respectively) were ~80–90% lower in women with PCOS when compared to controls, while perilipin 2 (PLIN2) and perilipin 4 (PLIN4) were approximately 3-fold higher. Expression mRNA levels of coatomer GTPases ARF1, ARFRP1, and βCOP (Figure 1B) were ~80% lower in women with PCOS compared to controls, with GBF1 and Sec23a being expressed ~9- and 7-fold higher, respectively. Finally, lipase mRNA expression of ATGL and monoglycerol lipase (MGL) were reduced by 90% and 65% respectively, with no difference in the expression of CGI-58 (Figure 1C).
Figure 1.
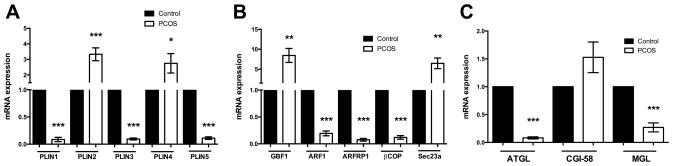
Gene expression from abdominal subcutaneous adipose tissue from women with PCOS verses age, BMI, and percent fat matched controls (cross-sectional study). A) Perilipin family of protein gene expression of all five perilipin proteins shows drastic differences in expression. Perilipin 1 (PLIN1), perilipin 3 (PLIN3), and perilipin 5 (PLIN5) are all expressed approximately 90% lower in females with PCOS compared to controls. Perilipin 2 (PLIN2) and perilipin 4 (PLIN4) are expressed about 3 times higher when compared to controls. B) Coatomer proteins involved in ER-to-Golgi transport were also differentially regulated on the gene expression level. ADP-ribosylation Factor 1 (ARF1), ARF related protein 1 (ARFRP1), and Beta-coatomer (βCOP, part of the COPI complex) were drastically reduced in women with PCOS. GDP-exchange Brefeldin A resistant Factor 1 (GBF1, which operated in conjunction with ARF1) and Sec23a (part of the COPII complex) were both expressed approximately 8 fold higher in the women with PCOS. C) Expression of Adipose Triglyceride Lipase (ATGL) and Monoglyceride Lipase (MGL) were both reduced in women with PCOS on the gene expression level, while there was no statistical difference in the expression of the ATGL co-activator CGI-58. *p < 0.05; **p < 0.01; ***p < 0.001
Effect of Sixteen-Weeks of Aerobic Exercise Training in Women with PCOS
Aerobic exercise resulted in a ~10% improvement in maximal aerobic capacity (VO2Max, p=0.04) and a ~20% improvement in glucose disposal rate (p=0.02) (Table 1). No other changes were noted in terms of body composition, abdominal adipose depot size (visceral or subcutaneous depots), ectopic lipid accumulation (in the liver or muscle), or in fat cell size (Table 1). However, alterations were seen in the gene expression in the adipose tissue following exercise. Of all the measured perilipins, only expression of PLIN3 increased significantly (p<0.05; Figure 2A), whereas PLIN1, PLIN2, PLIN4 and PLIN5 did not change. On average, ARF1, ARFRP1, βCOP, and Sec23a expressions were increased by approximately 5-, 8-, 7-, and 4- fold, respectively (p<0.05), with no changes in GBF1 (Figure 2B). Additionally, the lipases ATGL, MGL and the ATGL co-activator CGI-58, increased significantly after sixteen weeks of exercise training (p<0.05; Figure 2C).
Figure 2.
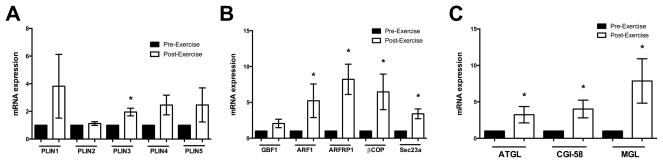
Gene expression targets in adipose tissue known to be involved in lipid droplet regulation and lipolysis were altered at 16 weeks of exercise training in the women with PCOS (Exercise training intervention, women with PCOS only). A) Gene expression of the perilipin family of proteins from before to after exercise demonstrates that only PLIN3 is significantly elevated following exercise training. B) Coatomer gene expression reveals that ARF1, ARFRP1, βCOP, which were grossly decreased when compared to controls before exercise training, revealed an increase in their expression. Sec23a was also increased following exercise training. C) Gene expression of ATGL and MGL, which was blunted when compared to controls at baseline, increased with exercise training. Additionally, CGI-58 increased following exercise training. *p < 0.05
Primary Adipose Culture Lipid Droplet Morphology and Lipolysis
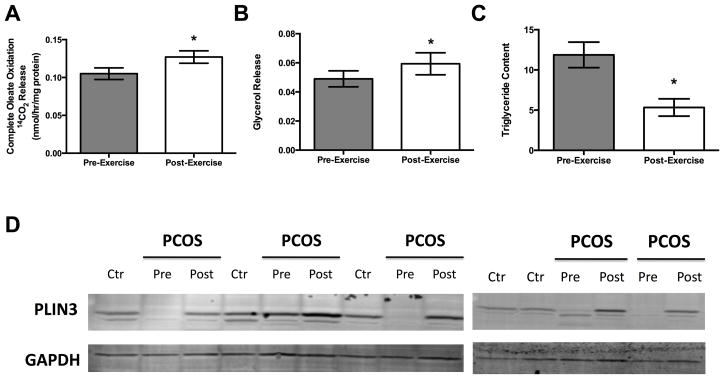
As shown in Figure 3, primary adipocyte cultures revealed larger lipid droplet morphology in women with PCOS compared to control, which decreased in size after exercise training, and thus more closely resembled the morphology of control females (Figure 3). In vitro measures of fat oxidation and lipolysis in primary cultured adipocytes showed that oleate oxidation and glycerol release were increased following exercise training (p<0.05, Figure 4A and 4B). Likewise, total triglyceride content was reduced after exercise (p<0.05, Figure 4C). Since PLIN3 was the only perilipin to significantly increase in adipose tissue on the mRNA level, we measured the protein level of PLIN3 in primary adipose culture. We found virtually nonexistent levels of PLIN3 in 4 out of 5 women with PCOS, while the expression of PLIN3 was present in controls (Figure 4D). Exercise induced protein expression of PLIN3 in those 4 women with PCOS, who had no protein expression of PLIN3 before exercise, and was increased compared to pre-exercise expression in the one woman with PCOS, who had PLIN3 protein expression present before exercise training (Figure 4D).
Figure 3.
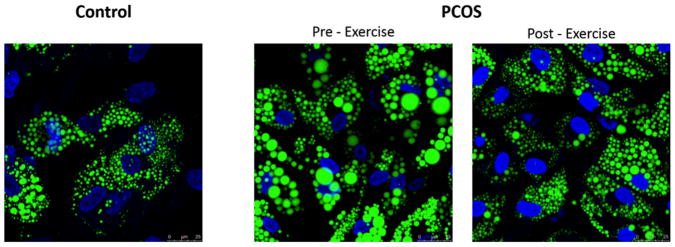
Representative images of stromal derived pre-adipocytes cultured and differentiated into adipocytes. Women with PCOS possessed large lipid droplets when compared to control donors matched by age, BMI, and % fat. Lipid droplet morphology appeared to resemble that of controls in our cross-sectional study following 16 weeks of aerobic exercise training in women with PCOS. Bodipy 494 (Green) was used to identify lipid and DAPI (Blue) to identify nuclei.
Figure 4.
Ex vivo oleate oxidation studies revealed that stromal derived adipose cultures from women with PCOS had increased completed oleate oxidation as measured by 14C labeled CO2 (A) after 16 weeks of exercise training. Furthermore, glycerol release into the culture media was increased (B) and total triglyceride content was reduced (C) in the adipose cultures of women with PCOS after exercise training. D) Both cross-sectional and exercise intervention expression of PLIN3, the only perilipin protein to increase in adipose tissue in the women with PCOS following exercise training on the gene expression level, were found to be virtually nonexistent in 4 out of the 5 females with PCOS donors when compared to controls in their primary adipocyte cultures. Likewise, expression levels increased in all 5 females with PCOS after exercise training in their primary adipose cultures. *p < 0.05
Discussion
Our data highlights a previously unrecognized, potential role of perilipin 3 in adipose tissue lipolysis in women with PCOS. We show for the first time that the expressions of PLIN3, along with PLIN1 and PLIN5, is greatly reduced in adipose tissue of women with PCOS when compared to age- and body composition- and metabolically-matched females. PLIN3 mRNA expression is increased—the only perilipin protein to significantly increase—following 16 weeks of aerobic exercise training in women with PCOS. Additionally, stromal-derived primary adipose cultures from PCOS women revealed that virtually no PLIN3 protein is expressed before exercise training, but becomes expressed following exercise training coupled with increases in oleate oxidation. We previously reported that exercise training in this cohort increased adipose tissue lipolysis under adrenergic stimulation 24. Our data suggest that PLIN3 may in part contribute to enhanced adipose tissue lipolytic stimulation.
The exercise benefits associated with improving the symptomatology of PCOS has been thoroughly investigated. Studies have shown improvements for women with PCOS in insulin resistance19, 20, 36, 37, serum lipids36, 38, and cardiovascular disease risk19, 20, 36, 39. Additionally, menstrual cycle improvements have been shown following exercise intervention in women with PCOS19, 36, 40. Though some studies show reductions in body weight with exercise19, 20, 36, several studies have shown that benefits occur without weight loss28, 37, 41 and suggest that exercise for PCOS would be recommended even if weight loss were not achieved. Our study showed improvements in insulin resistance as measured by euglycemic-hyperinsulinemic clamps (Table 1) and improvements in menstrual function28. However, despite all these benefits of exercise, few studies have investigated molecular targets in the adipose tissue from women with PCOS. This seems important given the reported defects in adipose tissue function previously shown in women with PCOS (reviewed in 7). We previously reported that 16 weeks of aerobic exercise can improve basal and catacholemine stimulated lipolysis of adipose tissue24. A prior investigation showed a single nucleotide polymorphism found in women with PCOS for the perilipin gene25, indicating a potential for defects in the perilipin family of proteins, which are involved in lipolysis. Here, we have investigated targets that regulate adipose tissue lipolysis, with an emphasis on PLIN3 given the upregulation of gene expression seen in the adipose tissue with exercise.
Studies centered on the perilipin family of proteins have focused heavily on the involvement of PLIN1 in the regulation of lipolysis in adipose tissue (reviewed in 42) via a coupling of PLIN1 phosphorylation and ATGL activation 43, 44. However, the possible role of PLIN3 regulation in adipose lipolysis has been for the most part overlooked. Studies have shown PLIN3 to be highly expressed in adipocytes and described to have a preference for PLIN3 to coat smaller lipid droplets 45. Additionally, studies in HeLa cells have revealed a colocalization of ATGL to PLIN3 coated lipid droplets during lipolytic stimulus 26, 46. Furthermore, a handful of studies investigating the role of PLIN3 in skeletal muscle lipolysis demonstrated that PLIN3 colocalizes to lipid droplets in rats during epinephrine and muscle contractile stimulation 47, 48. Work from our group has recently shown that PLIN3 expression is upregulated following aerobic exercise in skeletal muscle, upregulated in response to lipolytic stimulation in human primary skeletal myotube cultures, and is positively associated with both ex vivo skeletal muscle and in vivo whole body fat oxidation 27. Our data here, using both in vivo and in vitro systems, shows that the association between PLIN3 and lipolysis is evident in the adipose tissue of women with PCOS, indicating PLIN3 as a potential novel mediator of lipolysis.
Our data also highlights previously uninvestigated coatomer GTPases that might potentially be involved in mediating lipolysis. We show a lower expression of ARF1, ARFRP1, and βCOP1 in women with PCOS compared to controls; the expression of these targets increases greatly following 16 weeks of aerobic exercise training in the women with PCOS. Soni et al. showed that ATGL was delivered to PLIN3 coated lipid droplets: a phenomenon that was inhibited when either treated with brefeldin A, a compound known to inhibit ARF1, or when several of the coatomer GTPases, such as ARF1, Sec23a, βCOP, and GBF1 were knocked down 26. Studies from our group have shown that ARF1, Sec23a, and ARFRP1 increase their expression with aerobic exercise in skeletal muscle and with lipolytic stimulation in primary skeletal muscle cultures 27. Finally, Guo et al. found differential expression of coatomer GTPases with regards to differences in lipid droplet morphology 49. Likewise, we show that independent of differences in fat cell size, women with PCOS possess larger lipid droplet morphology when compared to their age and body composition matched controls. Lipid droplet morphology more closely resembled control females following 16 weeks of aerobic exercise in the women with PCOS. Alterations in these coatomer GTPases may perhaps be involved in regulating lipolysis not only as a mediator of lipase delivery to lipid droplets, but also as a regulator of lipid droplet morphology. Further functional investigations are warranted to determine these mechanisms.
We also observed reduced mRNA expression of lipases (both ATGL and MGL) in the adipose tissue of women with PCOS, compared to controls. The expression of lipases increased with exercise, which would be expected given the increase in both in vivo and in vitro lipolysis. One aspect of lipase expression that remains elusive is that prior investigations have shown that testosterone mediates the expression of lipases 17, 18. Our data shows that lipase expression is increased following exercise training with no decreases in circulating concentrations of total testosterone or free androgen index, perhaps indicating that aerobic exercise can increase adipose tissue lipolysis despite altering testosterone expression. Furthermore, we speculate that the increases in lipase expression we observed are not solely responsible for the increased lipolysis following exercise training. In fact, previous reports show that lipases do not act alone on lipid droplets to facilitate lipolysis, but require a chaperone such as the perilipin proteins (reviewed in 42) or certain coatomer GTPases 26.
We recognize that our results and conclusions are based on observational data and associations between increases in lipolysis, and expression of PLIN3 and coatomer GTPases following exercise, and does not establish causative results. However, given the preponderance of our findings and data presented in prior investigations from our group and others, we believe that these associations are novel and relevant to highlight potentially new, unrecognized targets that may in part mediate adipose tissue lipolysis. Additionally, it is noted that we only conducted the exercise intervention in the women with PCOS, since our original design was a prospective exercise intervention study for the women with PCOS. Although we did not conduct an exercise intervention in our control group since the cross-section component was added later, the goal of the study involving the control group was to perform the same set of state-of-the-art assessments (clamp, MRI, fat cell size, DXA) and to obtain a control group matched on the basis of metabolic phenotype. The cross-sectional comparison demonstrates novel and robust differences between the women with PCOS and controls and is able to further demonstrate that exercise in women with PCOS can rescue defects in lipolysis to levels observed in control subjects. Due to our rigorous study design, supervised and carefully monitored exercise interventions, and extensive phenotyping analyses of the women with PCOS before and after their exercise intervention, we are confident that we were adequately able to identify the upregulation of PLIN3 gene expression as well as the coatomer GTPases, thus demonstrating their potential role in the improvements in adipose tissue lipolysis following exercise training. Further investigations would be necessary to understand specific mechanistic roles of PLIN3 in adipose tissue lipolysis.
In conclusion, based on prior evidence from single nucleotide polymorphisms in the perilipin gene 25, we investigated the expression of the perilipin family of proteins in the adipose tissue from women with PCOS, and have shown that several perilipin proteins are differentially expressed in women with PCOS verses age- and body composition-matched control females. We have also shown that coatomer GTPases (ARF1, Sec23a, βCOP, GBF1, ARFRP1) are differentially expressed in women with PCOS. Sixteen weeks of aerobic exercise training significantly increased PLIN3 expression as well as coatomer GTPases (ARF1, Sec23a, βCOP, GBF1, ARFRP1). Stromal-derived primary adipose cultures showed increases in in vitro lipolysis, oleate oxidation, and reductions in triglyceride content following exercise training. Additionally, adipose cultures revealed virtually no PLIN3 protein expression before exercise, which was then increased/became expressed following exercise training. Finally, primary adipose cultures demonstrated a large lipid droplet morphology, which was altered by exercise training, despite the fact that there were no differences in fat cell size from adipose tissue cross-sectionally. These data highlights previously unrecognized and novel potential targets that might be responsible for improving exercise mediated lipolysis in the adipose tissue of women with PCOS.
Acknowledgments
Funding
This investigation was supported in part by a grant from the Health and Performance Enhancement Division of Pennington Biomedical Research Center (L.M.R.) and a Nutrition Obesity Research Center (NORC) grant NIH 1P30 DK072476 (E.R.). This work utilized the Genomics Core Facility and Cell Biology and Bioimaging Core at Pennington Biomedical Research Center, which are supported in part by COBRE (NIH P20 GM103528) and NORC (NIH 1P30-DK072476). L.M.R. is supported by NIH grants R00HD060762 and U01DK094418.
The authors would like to thank the Nursing Staff of Pennington Biomedical Research Center. We also wish to thank Jamie LaGrange and Blaine Masinter for their assistance with Adipose Tissue RNA isolation, and we wish to thank Richard Carmouche and Susan Newman of the Genomics Core Facility of Pennington Biomedical Research Center. We also wish to thank the Cell Biology and Cell Imaging Core Facility of Pennington Biomedical Research Center. Finally, we wish to sincerely thank all the research volunteers for their participation in this study.
Footnotes
Declaration of interest
The authors have nothing to declare.
Author’s Contribution
J.C. conceived the experiment, performed experiments, analyzed data, and wrote the manuscript; S.B. contributed to discussion and reviewed/edited the manuscript; C.M. performed experiments and reviewed/edited the manuscript; Y.T. performed experiments and reviewed/edited the manuscript; P.E. performed experiments and reviewed/edited the manuscript; D.B. performed experiments and reviewed/edited the manuscript; E.R. contributed to discussion, conceived of the experiment, and reviewed/edited the manuscript; L.R. provided helpful discussion, conceived the experiment, and reviewed/edited the manuscript. All authors gave final approval of the manuscript prior to submission. J.C. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 3.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:162–168. doi: 10.1210/jc.2007-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrabian F, Khani B, Kelishadi R, Kermani N. The prevalence of metabolic syndrome and insulin resistance according to the phenotypic subgroups of polycystic ovary syndrome in a representative sample of Iranian females. J Res Med Sci. 2011;16:763–769. [PMC free article] [PubMed] [Google Scholar]
- 6.Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–1217. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 7.Villa J, Pratley RE. Adipose tissue dysfunction in polycystic ovary syndrome. Curr Diab Rep. 2011;11:179–184. doi: 10.1007/s11892-011-0189-8. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom L, Langin D, Reynisdottir S, Dauzats M, Arner P. Adipocyte lipolysis in normal weight subjects with obesity among first-degree relatives. Diabetologia. 1996;39:921–928. doi: 10.1007/BF00403911. [DOI] [PubMed] [Google Scholar]
- 9.Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest. 1994;93:2590–2599. doi: 10.1172/JCI117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynisdottir S, Wahrenberg H, Carlstrom K, Rossner S, Arner P. Catecholamine resistance in fat cells of women with upper-body obesity due to decreased expression of beta 2-adrenoceptors. Diabetologia. 1994;37:428–435. doi: 10.1007/BF00408482. [DOI] [PubMed] [Google Scholar]
- 11.Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, Hellstrom M, Lonn L, Olivecrona G, Stener-Victorin E, Lonn M. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 12.Faulds G, Ryden M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003;88:2269–2273. doi: 10.1210/jc.2002-021573. [DOI] [PubMed] [Google Scholar]
- 13.Ek I, Arner P, Bergqvist A, Carlstrom K, Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997;82:1147–1153. doi: 10.1210/jcem.82.4.3899. [DOI] [PubMed] [Google Scholar]
- 14.Dicker A, Ryden M, Naslund E, Muehlen IE, Wiren M, Lafontan M, Arner P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47:420–428. doi: 10.1007/s00125-003-1324-0. [DOI] [PubMed] [Google Scholar]
- 15.Arner P. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie. 2005;87:39–43. doi: 10.1016/j.biochi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Monjo M, Rodriguez AM, Palou A, Roca P. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144:4923–4930. doi: 10.1210/en.2003-0537. [DOI] [PubMed] [Google Scholar]
- 17.Anderson LA, McTernan PG, Harte AL, Barnett AH, Kumar S. The regulation of HSL and LPL expression by DHT and flutamide in human subcutaneous adipose tissue. Diabetes Obes Metab. 2002;4:209–213. doi: 10.1046/j.1463-1326.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 18.Zang H, Ryden M, Wahlen K, Dahlman-Wright K, Arner P, Linden Hirschberg A. Effects of testosterone and estrogen treatment on lipolysis signaling pathways in subcutaneous adipose tissue of postmenopausal women. Fertil Steril. 2007;88:100–106. doi: 10.1016/j.fertnstert.2006.11.088. [DOI] [PubMed] [Google Scholar]
- 19.Vigorito C, Giallauria F, Palomba S, Cascella T, Manguso F, Lucci R, De Lorenzo A, Tafuri D, Lombardi G, Colao A, Orio F. Beneficial effects of a three-month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:1379–1384. doi: 10.1210/jc.2006-2794. [DOI] [PubMed] [Google Scholar]
- 20.Giallauria F, Palomba S, Maresca L, Vuolo L, Tafuri D, Lombardi G, Colao A, Vigorito C, Francesco O. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2008;69:792–798. doi: 10.1111/j.1365-2265.2008.03305.x. [DOI] [PubMed] [Google Scholar]
- 21.Palomba S, Falbo A, Giallauria F, Russo T, Rocca M, Tolino A, Zullo F, Orio F. Six weeks of structured exercise training and hypocaloric diet increases the probability of ovulation after clomiphene citrate in overweight and obese patients with polycystic ovary syndrome: a randomized controlled trial. Hum Reprod. 2010;25:2783–2791. doi: 10.1093/humrep/deq254. [DOI] [PubMed] [Google Scholar]
- 22.Bukowiecki L, Lupien J, Follea N, Paradis A, Richard D, LeBlanc J. Mechanism of enhanced lipolysis in adipose tissue of exercise-trained rats. Am J Physiol. 1980;239:E422–429. doi: 10.1152/ajpendo.1980.239.6.E422. [DOI] [PubMed] [Google Scholar]
- 23.Stich V, de Glisezinski I, Galitzky J, Hejnova J, Crampes F, Riviere D, Berlan M. Endurance training increases the beta-adrenergic lipolytic response in subcutaneous adipose tissue in obese subjects. Int J Obes Relat Metab Disord. 1999;23:374–381. doi: 10.1038/sj.ijo.0800829. [DOI] [PubMed] [Google Scholar]
- 24.Moro C, Pasarica M, Elkind-Hirsch K, Redman LM. Aerobic exercise training improves atrial natriuretic peptide and catecholamine-mediated lipolysis in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:2579–2586. doi: 10.1210/jc.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Ng MC, Hayes MG, Yoshiuchi I, Tsuchiya T, Robertson H, Cox NJ, Polonsky KS, Bell GI, Ehrmann DA. Variation in the perilipin gene (PLIN) affects glucose and lipid metabolism in non-Hispanic white women with and without polycystic ovary syndrome. Diabetes Res Clin Pract. 2009;86:186–192. doi: 10.1016/j.diabres.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122:1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Covington JD, Galgani JE, Moro C, LaGrange JM, Zhang Z, Rustan AC, Ravussin E, Bajpeyi S. Skeletal Muscle Perilipin 3 and Coatomer Proteins are Increased Following Exercise and are Associated with Fat Oxidation. PLoS One. 2014 doi: 10.1371/journal.pone.0091675. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redman LM, Elkind-Hirsch K, Ravussin E. Aerobic exercise in women with polycystic ovary syndrome improves ovarian morphology independent of changes in body composition. Fertil Steril. 2011;95:2696–2699. doi: 10.1016/j.fertnstert.2011.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 31.Hahn SWK, Tan S, Mann K, Janssen OE. Prognostic value of free testosterone and free androgen index in detecting the polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006:114. [Google Scholar]
- 32.Pasarica M, Xie H, Hymel D, Bray G, Greenway F, Ravussin E, Smith SR. Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes Care. 2009;32:900–902. doi: 10.2337/dc08-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, Jensen MD, Kirkland JL. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 34.Hauner H, Petruschke T, Russ M, Rohrig K, Eckel J. Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia. 1995;38:764–771. doi: 10.1007/s001250050350. [DOI] [PubMed] [Google Scholar]
- 35.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:3373–3380. doi: 10.1210/jc.2008-0751. [DOI] [PubMed] [Google Scholar]
- 37.Bruner B, Chad K, Chizen D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl Physiol Nutr Metab. 2006;31:384–391. doi: 10.1139/h06-007. [DOI] [PubMed] [Google Scholar]
- 38.Brown AJ, Setji TL, Sanders LL, Lowry KP, Otvos JD, Kraus WE, Svetkey PL. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med Sci Sports Exerc. 2009;41:497–504. doi: 10.1249/MSS.0b013e31818c6c0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. Heart rate recovery improves after weight loss in overweight and obese women with polycystic ovary syndrome. Fertil Steril. 2010;93:1173–1178. doi: 10.1016/j.fertnstert.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, Colao A, Vigorito C, Zullo F, Orio F. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23:642–650. doi: 10.1093/humrep/dem391. [DOI] [PubMed] [Google Scholar]
- 41.Randeva HS, Lewandowski KC, Drzewoski J, Brooke-Wavell K, O’Callaghan C, Czupryniak L, Hillhouse EW, Prelevic GM. Exercise decreases plasma total homocysteine in overweight young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:4496–4501. doi: 10.1210/jc.2001-012056. [DOI] [PubMed] [Google Scholar]
- 42.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Hu L, Dalen K, Dorward H, Marcinkiewicz A, Russell D, Gong D, Londos C, Yamaguchi T, Holm C, Rizzo MA, Brasaemle D, Sztalryd C. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J Biol Chem. 2009;284:32116–32125. doi: 10.1074/jbc.M109.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–19155. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 46.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7:106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prats C, Donsmark M, Qvortrup K, Londos C, Sztalryd C, Holm C, Galbo H, Ploug T. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J Lipid Res. 2006;47:2392–2399. doi: 10.1194/jlr.M600247-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ. Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am J Physiol Regul Integr Comp Physiol. 2013;304:R644–650. doi: 10.1152/ajpregu.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]