Abstract
Objective
The maternal-fetal interface must modulate immune function to allow tolerance of fetal cells while still reacting to pathogens to suppress infection. Human leukocyte antigen-G (HLA-G) is a class Ib major histocompatibility complex protein involved in maternal-fetal tolerance. We posited that alterations in placental HLA-G expression predispose women to preterm birth. The aim of this study was to compare HLA-G expression in the maternal-fetal interface of term versus preterm human placentas.
Methods
We performed a cross-sectional study of specimens from the basal plate of the human placenta from women enrolled in a tissue specimen and clinical data consortium. Immunohistochemistry with digital microscopic analysis was used to quantify HLA-G protein expression in the basal plate from preterm and term placentas.
Results
Preterm birth <37 weeks occurred in 29.5% of 149 singleton pregnancies. HLA-G-positive cells occupied one-third of the basal plates, and the HLA-G-positive area was increased by 14% in placentas from preterm births than in those from term births (32.1% in term placentas versus 36.6% in preterm placentas).
Conclusion
Although HLA-G is required for maternal tolerance of the semi-allogeneic fetus, higher levels of HLA-G expression at the maternal fetal interface is associated with preterm birth.
Keywords: Maternal-fetal tolerance, placenta, extravillous trophoblast, infection
Introduction
Infection is one of the most consistently identified risk factors for preterm birth (PTB); intrauterine infection contributes to at least 25% of PTBs, and 11% to 80% of cases of PTB are associated with intraamniotic colonization [1,2]. Thus, the maternal-fetal interface must simultaneously modulate immune function to allow tolerance of semi-allogeneic fetal cells while reacting appropriately to pathogens to suppress infection.
One mechanism used to identify cells as “self” or “non-self” to the host immune system is expression of major histocompatibility complex (MHC) proteins, including human leukocyte antigens (HLAs). Dysregulation of antigen recognition by the HLA system occurs in autoimmune disease, transplant organ rejection, and tumor growth[3]. Human leukocyte antigen-G (HLA-G) is a class Ib (nonclassic) MHC protein involved in maternal tolerance of the fetus[4–9]. HLA-G is expressed as a membrane-bound protein on the surface of invasive extravillous trophoblast cells, in the cytoplasm of these cells, and in the plasma as soluble antigens[9]. HLA-G modulates uterine natural killer cells, T lymphocytes, and the balance of T-helper cells during implantation and throughout pregnancy[4,5,10–19]. HLA-G expression by trophoblastic cells is advantageous for maternal acceptance of the antigenically distinct fetus and may be required for normal pregnancy maintenance[20]. However, higher expression of HLA-G may shift the balance from immunotolerance toward infection suceptibility.
We recently reported the presence of intracellular bacteria at the maternal-fetal interface within fetal extravillous trophoblasts in the human placental basal plate of both complicated and uncomplicated pregnancies[21]. We demonstrated that these HLA-G+ cells are not only a preferred location for intracellular bacteria, but permit their survival and growth over time [22]. Here, we tested the hypothesis that modulation of placental HLA-G expression is one mechanism underlying predisposition to PTB. The primary aim of this study was to compare the expression of HLA-G protein in the maternal-fetal interface of term and preterm placentas. The secondary aim was to determine whether or not expression of HLA-G was associated with bacterial colonization at the maternal-fetal interface.
Methods
Study Design
This is a cross-sectional study of patients enrolled into the Women and Infants’ Health Specimen Consortium, an ongoing bio-specimen and clinical data collection core at Washington University in Saint Louis. The Washington University School of Medicine human studies review board approved this investigation. Patients were enrolled during their prenatal course or at the time of admission to labor and delivery and followed prospectively for gestational age at delivery and placental specimen collection. Trained research personnel used close-ended data collection forms to abstract obstetric diagnoses. All maternal demographic characteristics, medical history, obstetric history, and neonatal outcomes were collected from review of prenatal records, medical charts, and patient interviews.
All obstetric diagnoses were made by the treating physician team. Bacterial vaginosis (BV) was classified as positive if the diagnosis of BV (based on Amsel’s criteria[23]) had been made at any time during the pregnancy. “Any vaginal infection” was defined as a composite exposure of BV, chlamydia, gonorrhea, or trichomonas during the pregnancy. Group B streptococcus was considered positive if a recto-vaginal culture was positive within five weeks of delivery or urine culture was positive at any time during the pregnancy. Positive chorioamnionitis was classified on the basis of either histopathological examination of the chorioamnion or umbilical cord or clinical symptoms (maternal fever or maternal/fetal tachycardia) requiring treatment with broad-spectrum antibiotics during labor.
Tissue Harvest
Placental samples of enrolled patients were collected, processed, and analyzed for this investigation from May 2011 through May 2012 as previously described[21]. Briefly, placentas were collected within four hours of delivery and refrigerated immediately. Sterile technique was used to excise three specimens (5–8 mm in diameter) from the inner two-thirds of the basal plate surface of the placenta. Specimens were fixed for 48 hours at room temperature in 10% neutral buffered formalin prior to embedding in paraffin.
Immunohistochemistry
Five-micron sections of embedded tissues were deparaffinized and rehydrated with xylene and ethanol. Slides were then blocked with blocking buffer (1% bovine serum albumin, 0.3% Triton X-100, in phosphate buffered saline (PBS)) for a minimum of 30 minutes. Monoclonal mouse anti-human HLA-G antibody 4H84 (Santa Cruz Biotechnology, Inc. Dallas, TX[24]) was applied at 4 °C for 8–12 hours. This antibody binds to both membrane-bound and soluble intracellular isoforms of HLA-G [25]. Slides were washed with PBS and then incubated with biotinylated rabbit-anti-mouse antibody (Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature. Signal was detected with VECTASTAIN R.T.U Elite ABC Reagent kit (Vector Laboratories) according to the manufacturer’s instructions. Slides were counter stained with the Brown-Hopps modification of the Gram stain (Fisher Scientific, St. Louis, MO) as previously described[21].
Digital Microscopy and Quantification of HLA-G
Slides were scanned with a Nanozoomer 2.0-HT (Bridgewater, NJ) digital brightfield microscopy system. Visiomorph DP software (Visiopharm, Broomfield, CO) was used to detect and quantify HLA-G-expressing cells in the digitized images. After pre-processing for detection of pixels, the entire tissue specimen on the slide was analyzed according to an individualized protocol optimized for the current study. Image analysis protocols were designed to define three main slide components: 1) basal plate tissue positive for HLA-G; 2) basal plate tissue negative for HLA-G; and 3) glass, to account for small tissue irregularities where glass slide showed between tissue fragments. Each slide was individually examined to guard against false analysis of debris or smudge. An investigator blinded to the gestational age and clinical characteristics of the patients from which the specimens were derived oversaw the digitalized protocol analysis. To control for variation in size and shape of basal plate tissue specimens, both the HLA-G-positive and -negative areas were measured, and the percentage of the total basal plate surface area positive for HLA-G was calculated.
Histopathological analysis for intracellular bacteria
The presence or absence of intracellular bacteria was diagnosed by two independent observers, including IUM, described in detail elsewhere[21]. Briefly, 5-micron sections were deparaffinized and stained with the Brown-Hopps modification of the Gram stain to maximize bacterial detection[26]. Ten random fields of each specimen per 100 μm2 were examined for the presence or absence of bacteria.
Statistical Analysis
The exposure for this investigation was HLA-G protein in the human placental basal plate. Percent basal plate surface area positive for HLA-G protein was examined in preterm and term placentas. Preterm birth was defined as delivery at < 37 weeks’ gestation, which was based on the best obstetric estimate determined by last menstrual period and ultrasound dating. The association between expression of HLA-G and the presence or absence of intracellular bacteria was examined as a secondary outcome.
Maternal demographic characteristics were compared by using chi-square or Fischer exact tests. The percent of surface area positive for HLA-G was normally distributed (as determined by the SK test) so parametric means and standard deviations were used for comparisons. The correlation between percent surface area positive for HLA-G and gestational age was examined using Spearman’s rho. Percent surface area positive for HLA-G by gestational age strata was explored using descriptive statistics. The student’s t-test was used to compare HLA-G protein expression (percent basal plate surface area positive for HLA-G as a continuous variable) in term versus preterm placentas. To examine HLA-G protein expression as a dichotomous variable, “high” was arbitrarily defined as ≥75th percentile of HLA-G-positive cell surface area, and “normal” was defined as < 75th percentile. The chi square test was used to calculate the relative risk and 95% confidence intervals of an association between high HLA-G and PTB. Backward stepwise logistic regression was used to control for pertinent confounding variables, and the likelihood ratio test was used to assess the impact of removing covariates from the model. Stratified analysis was performed to compare HLA-G positivity in the subgroups of spontaneous and indicated labor.
Results
Study Population Characteristics
The study population consisted of 149 women with singleton pregnancies. Demographic characteristics are summarized in Table 1. Of the 44 women who had the primary outcome of PTB < 37 weeks, 31 had spontaneous PTB and 13 had indicated PTB.
Table 1.
Baseline demographic characteristics of the study population.
| Characteristic | Incidence (n=149) |
|---|---|
| Maternal age, years (mean ± standard deviation) | 28.7 ± 6.0 |
| Maternal age >35 years old (%) | 33.5 |
| Body mass index (mean ± standard deviation) | 33.4 ± 8.8 |
| Black race (%) | 51.2 |
| Preeclampsia or gestational hypertension (%) | 26.7 |
| Prior preterm delivery (%) | 19.7 |
| Preterm delivery <37 weeks (%) | 29.5 |
| Preterm delivery <34 weeks (%) | 16.1 |
| Preterm delivery <28 weeks (%) | 6.0 |
| Preterm premature rupture of membranes(%) | 14.9 |
| Vaginal delivery (%) | 55.0 |
HLA-G Protein Expression is Higher in Placentas from Preterm than Term Births
A representative example of a digital microscopy image of a tissue section immunostained with HLA-G antibody and counterstained with the Brown-Hopps modification of the Gram stain is shown on the left in Figure 1. The right side shows the same image after digital image analysis.
Figure 1.
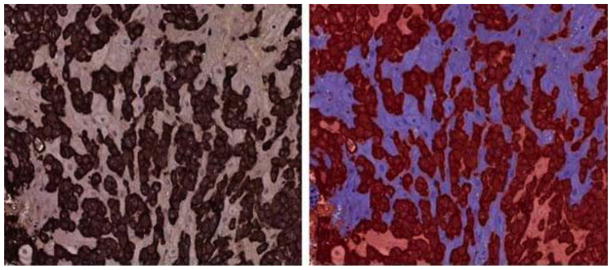
Digital microscopy of basal plate with HLA-G positive cells labeled dark brown (left) and Visiomorph image analysis (right) after classification of HLA-G positive cells (red) and HLA-G negative cells (blue).
We first explored the mean basal plate surface area positive for HLA-G by gestational age strata shown in table 2. We then compared the correlation between HLA-G surface area and gestational age at delivery as two continuous variables using Spearman’s rho. There was a nonsignificant negative correlation between surface area positive for HLA-G and increasing gestational age (r = −0.12, p=0.11). Percent surface area positive for HLA-G did not differ by mode of delivery (vaginal, 32.8% ± 11.5% vs. cesarean, 35.2% ± 12.1%, P=0.30). However, placentas from preterm birth had a significantly larger percentage basal plate surface area positive for HLA-G compared to placentas from term birth (Table 3). Furthermore, spontaneous preterm labor was associated with higher HLA-G positivity than spontaneous labor at term. In contrast, HLA-G expression in placentas from preterm indicated labor was not significantly different from HLA-G expression in term- indicated placentas (Table 3).
Table 2.
Mean Surface Area Positive for HLA-G by Gestational Age Category
| Gestational Age Category (Weeks) | Percent Surface Area Positive for HLA-G |
|---|---|
| 23–27 (n=13) | 37.9 ± 15.4% |
| 28–32 (n=16) | 32.5 ± 13.6% |
| 33–36 (n=28) | 36.2 ± 13.1% |
| 37–39 (n=63) | 32.3 ± 11.7% |
| 40–42 (n=29) | 31.2 ± 10.8% |
Table 3.
Percent surface area of basal plate positive for HLA-G positive cells in term compared to preterm placentas.
| Term | Preterm | p-value | |
|---|---|---|---|
| Percent Surface Area HLAG+ Entire Population n=149 |
32.1 ± 11.9 | 36.6 ± 12.7 | 0.03 |
| Percent Surface Area HLAG+ Spontaneous Labor n=80 |
30.8 ± 13.4 | 37.1± 13.0 | 0.04 |
| Percent Surface Area HLAG+ Indicated Labor n=44 |
32.6 ± 11.4 | 36.5 ± 14.1 | 0.53 |
To facilitate comparison, we dichotomized the percentage of the surface area that was positive for HLA-G into “high”, defined as ≥75th percentile, and “normal”, defined as <75th percentile. The 75th percentile corresponded to 41% positivity, significantly above the average positivity noted even within preterm placentas. No significant differences were found between baseline demographic characteristics or important confounding variables in women with high HLA-G tissue expression and those with normal HLA-G tissue expression (Table 4). Specifically, black race, smoking status, vaginal infection, Group B streptococcus positivity, and administration of corticosteroids for fetal lung maturity did not differ in women with high versus normal HLA-G expression in the basal plate. There was also no association between high HLA-G tissue expression and need for antibiotics during labor or the diagnosis of chorioamnionitis. High HLA-G tissue expression was associated with a 2.8-fold increased risk for PTB <37 weeks even after controlling for prior PTB. Stratification by spontaneous and indicated labor revealed that high HLA-G expression was associated with an increased risk for spontaneous preterm delivery but not indicated preterm delivery (Table 5).
Table 4.
Baseline demographic characteristics of women with high or normal expression of HLA-G in the basal plate.
| Characteristic | High* HLA-G in basal plate tissue (n=38) | Normal* HLA-G in basal plate tissue (n=111) | P-value |
|---|---|---|---|
| Maternal Age >35 years (%) | 23.7 | 36.9 | 0.14 |
| Body Mass Index (mean ± sd) | 32.6 ± 9.2 | 33.7 ± 8.6 | 0.53 |
| Black Race (%) | 48.6 | 52.2 | 0.72 |
| Tobacco Use (%) | 13.8 | 18.3 | 0.58 |
| Prior PTB (%) | 17.1 | 20.7 | 0.66 |
| Gestational Hypertensive Disease (%) | 28.6 | 27.1 | 0.87 |
| Gestational Diabetes (%) | 11.4 | 8.7 | 0.65 |
| Bacterial Vaginosis During Pregnancy (%) | 17.6 | 7.7 | 0.10 |
| Any Vaginal Infection During Pregnancy (%) | 26.3 | 16.2 | 0.17 |
| Urinary Tract Infection During Pregnancy (%) | 28.6 | 31.5 | 0.75 |
| Chorioamnionitis (%) | 28.6 | 16.3 | 0.12 |
| Steroids for Fetal Lung Maturity (%) | 23.7 | 27.0 | 0.69 |
| Antibiotics During Labor (%) | 54.3 | 41.3 | 0.18 |
| Group B Streptococcus Positive (%) | 25.7 | 32.9 | 0.44 |
| Vaginal Delivery (%) | 51.4 | 56.5 | 0.60 |
| Cesarean Section Without Labor (%) | 13.2 | 18.0 | 0.50 |
High defined as ≥75th percentile. Normal defined as < 75th percentile.
Table 5.
PTB association with high HLA-G basal plate expression
| Outcome | High** HLAG | Normal** HLAG | Relative Risk (95% CI) | Adjusted Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| PTB <37 weeks | 44.7 | 24.3 | 2.5 (1.1–5.5) | 2.8* (1.2–6.5) | 0.02 |
| Spontaneous PTB <37 weeks | 53.8 | 32.9 | 2.4 (1.0–5.9) | 3.0* (1.1–8.3) | 0.03 |
| Indicated PTB <37 weeks | 23.5 | 9.6 | 2.4 (0.7–8.1) | 2.5* (0.5–11.5) | 0.24 |
After controlling for prior preterm birth
High defined as ≥75th percentile. Normal defined as < 75th percentile.
Given our previous report of the presence of intracellular bacteria in the basal plate, as a secondary aim we investigated whether HLA-G protein expression level was associated with the presence of intracellular bacteria. Placentas with and without intracellular bacteria did not differ with respect to basal plate HLA-G positivity (33.4% vs. 33.2%, P=0.9).
Discussion
The data show that extravillous trophoblasts positive for HLA-G occupy about one-third of the surface area of the basal plate of human placentas, and that placentas from PTBs have significantly more HLA-G-positive cell-surface area than those from placentas at term. One interpretation of the finding of increased HLA-G positive cell surface area in preterm placentas is that we are capturing a normal physiologic decrease in placental HLA-G expression as gestation progresses. An alternative explanation is that increased placental HLA-G is associated with pathological processes underlying preterm birth.
Spontaneous PTB secondary due to either preterm rupture of membranes or spontaneous onset of preterm labor is often distinguished from indicated PTB because of medical complications such as preeclampsia or fetal growth restriction. Spontaneous PTB has been linked more strongly to infectious etiologies than indicated PTB[27–31]. In a subgroup analysis of our data, spontaneous PTB was associated with increased basal plate HLA-G, whereas indicated PTB was not. Our data are consistent with the findings of Kusanovic et al., who demonstrated that levels of soluble HLA-G in the amniotic fluid were elevated in patients with spontaneous preterm labor[32].
Other studies have found increased maternal serum HLA class I (classic) antibodies to be associated with spontaneous PTB and fetal inflammatory responses, suggesting a component of maternal-anti-fetal rejection in the pathogenesis of PTB[33,34]. Thus, our finding that non-classic (immuno-tolerant) HLA protein is higher on fetally derived cells from PTB than from term birth suggests that maternal-fetal interactions may jointly contribute to pregnancy tolerance or rejection[33,34]. Our findings of increased surface area positive for HLA-G in preterm placentas may represent a vulnerability to infection mediated preterm birth, or alternatively, HLA-G basal plate expression may normally decrease as pregnancy progresses toward normally timed parturition. In contrast, Zhou et al. examined the basal plates in 32 cases of inflammatory preterm labor and observed that inflammation was associated with decreased HLA-G expression[24]. However, the assay used by these authors, grading HLA-G expression level as “normal”, “weak”, or “none”, was different from our assay. Instead of measuring relative staining intensity, we measured the relative proportion of total basal plate architecture occupied by HLA-G-positive cells. An increase could be either due to an increased number of cells or larger cells.
Our previous findings of intracellular bacteria in the basal plates of both term and preterm placentas and the tendency for intracellular bacteria to preferentially localize to HLA-G positive extravillous trophoblast [21,22] raised the question of whether increased HLA-G expression may predispose the placenta to bacterial invasion. Although work from our group documented invasion of HLA-G positive extravillous trophoblasts by E. coli and L. monocytogenes in placental explant model, in this analysis we found no association between high HLA-G and clinical outcomes such as chorioamnionitis or the need for antibiotics during labor. Interestingly, pathogenic brucellae species including, Brucella abortus, which cause complications during pregnancy such as abortion, also invade and form inclusions in HLAG+ extravillous trophoblasts in culture. The pathogens exclusively localize to HLAG+ cells irrespective of how few or how many cells were present, suggesting the HLAG+ levels may correlate with infectivity [35]. However, we only sampled small biopsies of the maternal basal plate and found no association between the presence of intracellular bacteria and HLA-G expression level. It is possible that intracellular bacteria were present in other areas of the placenta. Future next-generation sequencing-based analyses of whole basal plate tissues to determine bacterial colonization would definitively address this issue.
Our study is not without limitations. First, immunohistochemistry is associated with unavoidable variations due to tissue characteristics, tissue amount, antigen presence, and reagent variability. However, the use of digital microscopy and digital analysis of the slides avoided the subjectivity of human assessments. A digitalized protocol defining tissue positivity versus negativity was applied uniformly to all specimens by an investigator blinded to the clinical conditions of the study patient. Additionally, a major benefit of digital image analysis is that, rather than sampling random areas, the entire tissue specimen can be analyzed in an unbiased manner. Furthermore, we chose an HLA-G antibody that binds to both soluble and membrane-bound forms of HLA-G. Further investigations will be necessary to elucidate which isoform is critical for the maternal-fetal immune tolerance cascade. A second limitation is that, because perinatal epidemiologists have questioned the distinction between spontaneous and indicated PTB and have suggested significant etiologic overlap between the two[36–38], we specified a primary outcome of all PTB regardless of subtype. Although we conducted a subgroup analysis and found increased expression of HLA-G in placentas from spontaneous PTB but not inicated PTB, the indicated PTB subgroup was small. Thus, we cannot rule out the possibility of type II error.
In conclusion, we report a positive association between increased placental expression of HLA-G and preterm birth. Although placental HLA-G expression can neither be manipulated nor directly measured to aid in clinical management, our findings contribute toward understanding the complex maternal-fetal interactions in term and preterm birth and can shed light on one of the major unsolved obstetric problems of our time.
Acknowledgments
Financial Support:
Dr. Stout is supported by Washington University CTSA grant (UL1 TR000448) and NICHD T32 (5 T32 HD055172-02). Digital microscopy and image analysis were supported by the Alafi Neuroimaging Laboratory at Washington University in Saint Louis (National Institute of Health Neuroscience Blueprint Interdisciplinary Center Core Grant P30 NS057105).
Dr. Mysorekar is supported by a Preventing Prematurity Initiative grant from the Burroughs Wellcome Fund and a Prematurity Research Initiative Investigator award from the March of Dimes
The Women and Infants’ Health Specimen Consortium is supported by grants from the Washington University ICTS (NIH UL1 RR024992).
We thank Dr. D. Michael Nelson for comments, Dr. Krzysztof Hyrc and Mr. Gary London in the Alafi Neuroimaging lab for their expertise in digital microscopy and image analysis. We thank Carolyn Bower, Ms. Rebecca Gunkel, and Ms. Qiuhong Zhao for their assistance with this study, and Dr. Deborah Frank for editorial assistance.
Footnotes
Disclosure: The authors report no conflict of interest
Human Subjects Approval: Washington University Institutional Review Board approved this investigation (#09-1807)
Declarations of Interests: The authors report no declarations of interest.
References
- 1.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 2.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiina T, Inoko H, Kulski JK. An update of the HLA genomic region, locus information and disease associations: 2004. Tissue Antigens. 2004;64(6):631–49. doi: 10.1111/j.1399-0039.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 4.Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A. 1997;94(21):11520–5. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanai T, Fujii T, Kozuma S, Miki A, Yamashita T, Hyodo H, Unno N, Yoshida S, Taketani Y. A subclass of soluble HLA-G1 modulates the release of cytokines from mononuclear cells present in the decidua additively to membrane-bound HLA-G1. J Reprod Immunol. 2003;60(2):85–96. doi: 10.1016/s0165-0378(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 6.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19(7):681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 7.Hunt JS, Petroff MG, Morales P, Sedlmayr P, Geraghty DE, Ober C. HLA-G in reproduction: studies on the maternal-fetal interface. Hum Immunol. 2000;61(11):1113–7. doi: 10.1016/s0198-8859(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 8.Shankarkumar U, Shankarkumar A, Chedda Z, Ghosh K. Role of 14-bp deletion/insertion polymorphism in exon 8 of the HLA-G gene in recurrent spontaneous abortion patients. J Hum Reprod Sci. 2011;4(3):143–6. doi: 10.4103/0974-1208.92289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hviid TV. HLA-G in human reproduction: aspects of genetics, function and pregnancy complications. Hum Reprod Update. 2006;12(3):209–32. doi: 10.1093/humupd/dmi048. [DOI] [PubMed] [Google Scholar]
- 10.Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S, Puppo F. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol. 2003;33(1):125–34. doi: 10.1002/immu.200390015. [DOI] [PubMed] [Google Scholar]
- 11.Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, Bensussan A, Le Bouteiller P. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164(12):6100–4. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- 12.Kanai T, Fujii T, Kozuma S, Yamashita T, Miki A, Kikuchi A, Taketani Y. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod. 2001;7(2):195–200. doi: 10.1093/molehr/7.2.195. [DOI] [PubMed] [Google Scholar]
- 13.Le Gal FA, Riteau B, Sedlik C, Khalil-Daher I, Menier C, Dausset J, Guillet JG, Carosella ED, Rouas-Freiss N. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol. 1999;11(8):1351–6. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 14.LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci U S A. 2004;101(18):7064–9. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouas-Freiss N, Naji A, Durrbach A, Carosella ED. Tolerogenic functions of human leukocyte antigen G: from pregnancy to organ and cell transplantation. Transplantation. 2007;84(1 Suppl):S21–5. doi: 10.1097/01.tp.0000269117.32179.1c. [DOI] [PubMed] [Google Scholar]
- 16.Sanders SK, Giblin PA, Kavathas P. Cell-cell adhesion mediated by CD8 and human histocompatibility leukocyte antigen G, a nonclassical major histocompatibility complex class 1 molecule on cytotrophoblasts. J Exp Med. 1991;174(3):737–40. doi: 10.1084/jem.174.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A. 2003;100(15):8856–61. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solier C, Aguerre-Girr M, Lenfant F, Campan A, Berrebi A, Rebmann V, Grosse-Wilde H, Le Bouteiller P. Secretion of pro-apoptotic intron 4-retaining soluble HLA-G1 by human villous trophoblast. Eur J Immunol. 2002;32(12):3576–86. doi: 10.1002/1521-4141(200212)32:12<3576::AID-IMMU3576>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer A, Lukassen HG, van Cranenbroek B, Weiss EH, Braat DD, van Lierop MJ, Joosten I. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod. 2007;13(2):123–33. doi: 10.1093/molehr/gal100. [DOI] [PubMed] [Google Scholar]
- 20.Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, Bencini E, Menicucci A, Baricordi OR. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol. 2002;32(2):311–5. doi: 10.1002/1521-4141(200202)32:2<311::AID-IMMU311>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, Roehl KA, Nelson DM, Macones GA, Mysorekar IU. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208(3):226, e1–7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mysorekar IU, Cao B. Microbiome in Parturition and Preterm Birth Seminars in Reproductive. Medicine. 2014;32:50–55. doi: 10.1055/s-0033-1361830. [DOI] [PubMed] [Google Scholar]
- 23.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Bianco K, Huang L, Nien JK, McMaster M, Romero R, Fisher SJ. Comparative analysis of maternal-fetal interface in preeclampsia and preterm labor. Cell Tissue Res. 2007;329(3):559–69. doi: 10.1007/s00441-007-0428-0. [DOI] [PubMed] [Google Scholar]
- 25.Paul P, Rouas-Freiss N, Moreau P, Cabestre FA, Menier C, Khalil-Daher I, Pangault C, Onno M, Fauchet R, Martinez-Laso J, et al. HLA-G, -E, -F preworkshop: tools and protocols for analysis of non-classical class I genes transcription and protein expression. Hum Immunol. 2000;61(11):1177–95. doi: 10.1016/s0198-8859(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz DA, Munger R, Lazzaro B, Lumb G. A modified Brown and Hopps stain for identification of gram-positive and gram-negative microorganisms in glycol methacrylate-embedded tissues. Arch Pathol Lab Med. 1989;113(2):181–3. [PubMed] [Google Scholar]
- 27.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, Hauth JC. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(1):43, e1–5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg RL, Mercer BM, Meis PJ, Copper RL, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network Obstet Gynecol. 1996;87(5 Pt 1):643–8. doi: 10.1016/0029-7844(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg RL, Thom E, Moawad AH, Johnson F, Roberts J, Caritis SN. The preterm prediction study: fetal fibronectin, bacterial vaginosis, and peripartum infection. NICHD Maternal Fetal Medicine Units Network Obstet Gynecol. 1996;87(5 Pt 1):656–60. doi: 10.1016/0029-7844(96)00034-8. [DOI] [PubMed] [Google Scholar]
- 31.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–35. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 32.Kusanovic JP, Romero R, Jodicke C, Mazaki-Tovi S, Vaisbuch E, Erez O, Mittal P, Gotsch F, Chaiworapongsa T, Edwin SS, et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2009;22(12):1151–66. doi: 10.3109/14767050903019684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, Chiang PJ, Kusanovic JP, Hassan SS, Yeo L, et al. Characterization of the Fetal Blood Transcriptome and Proteome in Maternal Anti-Fetal Rejection: Evidence of a Distinct and Novel Type of Human Fetal Systemic Inflammatory Response. Am J Reprod Immunol. 2013 doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Romero R, Xu Y, Miranda J, Yoo W, Chaemsaithong P, Kusanovic JP, Chaiworapongsa T, Tarca AL, Korzeniewski SJ, et al. Detection of Anti-HLA Antibodies in Maternal Blood in the Second Trimester to Identify Patients at Risk of Antibody-Mediated Maternal Anti-Fetal Rejection and Spontaneous Preterm Delivery. Am J Reprod Immunol. 2013;70(2):162–75. doi: 10.1111/aji.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salcedo SP, Chevrier N, Lacerda TL, Ben Amara A, Gerart S, Gorvel VA, de Chastellier C, Blasco JM, Mege JL, Gorvel JP. Pathogenic brucellae replicate in human trophoblasts. J Infect Dis. 2013;207(7):1075–83. doi: 10.1093/infdis/jit007. [DOI] [PubMed] [Google Scholar]
- 36.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195(3):643–50. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189(4):1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 38.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]


