Abstract
Semaphorin3A (sema3A) was recently identified as an early diagnostic biomarker of acute kidney injury. However, its role as a biomarker and/or mediator of chronic kidney disease (CKD) related to diabetic nephropathy is unknown. We examined the expression of sema3A in diabetic animal models and in humans and tested whether sema3A plays a pathogenic role in the development of diabetic nephropathy. The expression of sema3A was localized to podocytes and epithelial cells in distal tubules and collecting ducts in control animals, and its expression was increased following induction of diabetes. Quantification of sema3A urinary excretion in three different diabetic mouse models showed that excretion was increased as early as 2 weeks after induction of diabetes and increased over time, in conjunction with the development of nephropathy. Consistent with the mouse data, increased sema3A urinary excretion was detected in diabetic patients with albuminuria, particularly in those with macroalbuminuria. Genetic ablation of sema3A, or pharmacological inhibition with a novel sema3A inhibitory peptide, protected against diabetes-induced albuminuria, kidney fibrosis, inflammation, oxidative stress and renal dysfunction. We conclude that sema3A is both a biomarker and a mediator of diabetic kidney disease and could be a promising therapeutic target in diabetic nephropathy.
Keywords: biomarker, sema3A, diabetic nephropathy, albuminuria
Introduction
In the United States, approximately 20 million people (7% of the population) are estimated to have diabetes, and the incidence of diabetes is increasing. Diabetes has become the primary cause of end-stage renal disease (ESRD); approximately 44% of new patients entering dialysis in the U.S. are diabetics [1,2]. Progress in the development of new diagnostic tests and treatments for diabetic nephropathy has been impeded by an incomplete understanding of disease pathogenesis. Diabetic nephropathy is a complex disease involving many cell types leading to structural and functional abnormalities which include hyperfiltration with glomerular hypertension, renal hypertrophy, increased glomerular basement membrane thickness, tubular atrophy and interstitial fibrosis. These events lead to the development of proteinuria, worsening systemic hypertension and ultimately progressive loss of kidney function. Current therapeutics for diabetic nephropathy focus on improved glycemic and blood pressure control, which provide little protection against renal disease progression [3]. Unfortunately, there is no test available to diagnose the early development of diabetic nephropathy and no effective treatment to prevent or reverse its course.
Semaphorins are secreted or membrane-associated glycoproteins that have been grouped into eight classes based upon their structural elements and amino acid sequences. Proteins in semaphorin classes 1, 4, 5, 6 and 7 are membrane-associated, whereas those in classes 2 and 3, and the viral semaphorins, are secreted. They are characterized structurally by a conserved ~400 amino acid sema domain [4]. Semaphorins are classically described as collapsing factors and mediators of axon repulsion, although they may also act as context-dependent chemoattractants. They have also been shown to have roles in cardiovascular development and in the regulation of immune cell antigen presentation. In addition, semaphorins are known to regulate cell motility and attachment in axon guidance, vascular growth, immune cell regulation and tumor progression [4–7]. Semaphorin3A (sema3A) is a chemorepellent with multiple guidance functions, including axon pathfinding, cardiac and peripheral vascular patterning and branching morphogenesis. Semaphorin3A gene deletion results in perinatal lethality, suggesting an indispensable role in developmental biology [8]. Sema3A signaling is mediated by a complex of the binding receptor neuropilin 1 and the signaling receptors plexinA1 or A3 [9,10]. Both sema3A and its receptor neuropilin 1 are expressed in the developing glomerulus, and sema3A inhibits ureteric bud branching by downregulation of glial cell-line-derived neurotrophic factor [7]. Semaphorin3A expression persists in adult podocytes and collecting tubules [11,12] and is highly induced after acute tubular injury, leading to increased excretion of sema3A in urine in both mice and humans [13–15]. However, whether sema3A expression is increased in diabetic kidney disease (DKD), and its utility as a biomarker for early diagnosis of DKD, are unknown. Moreover, the role of endogenously-produced sema3A in diabetic kidney disease is unknown. The purpose of the present study was to determine whether sema3A excretion in urine is increased early in the course of diabetes in an experimental animal model, and its functional role in nephropathy.
Materials and Methods
Animals
Experiments utilized male C57BL/6J, 20-week old C57BL/KsJ (wild-type or db/db; JAX#000642, BKS.Cg-Dock7m+/+Leprdb/J, homozygous for Lprdb) and 8 or 30-week old DBA/2J mice (wild-type or with the Akita mutation (JAX# 007562, D2.B6-Ins2Akita/MatbJ), C3H/HeJ (Jax #000659) and sema3A mutant mice (C3;B6-Sema3am808Ddg/J) (Jax# 014646). Sema3A mutant mice were created by chemical-induced point mutation, which inactivates sema3A receptor binding. Sema3A mutant mice were transferred into the C3H background by backcrossing at Jackson Laboratories. All protocols were approved by the Institutional Animal Care and Use Committee of the Georgia Health Sciences University (approval number: BR10-10-369) and followed the American Physiological Society Guidelines for the Care and Use of Laboratory Animals. Mice were housed under conditions of constant temperature and humidity and exposed to a 12:12-hour light-dark cycle. Animals were allowed free access to drinking water and normal chow.
STZ-Induced diabetes
The diabetes induction protocol followed for mice was described by the Animal Models of Diabetic Complication Consortium (AMDCC) (http://www.diacomp.org/shared/protocols.aspx) [16–18]. Eight-week-old C3H, sema3A mutant mice, and DBA/2J mice (Jackson Laboratories) were given streptozotocin (STZ) (50 mg/kg BW in citrate buffer) in multiple doses (every 24hr, total of five doses). Blood glucose and urine albumin were measured at 1 week after STZ injection and every three weeks thereafter. 6–10 animals/group were used in all experiments.
Administration of sema3A inhibitory peptide to diabetic mice
DBA mice were made diabetic as described above. 4 weeks after confirmation of diabetes, animals received sema3A inhibitory peptide (10μg/animal, sequence: N-Ac-HAVEHGFMQTLLKVTLE-NH2)[19] or scrambled peptide in vehicle (0.1% BSA) every 24hr (intraperitoneally) for 6 weeks. 24hr urine was collected and then mice were sacrificed. Kidney tissues were processed for RNA and histological analysis. Urine was used to quantify albumin excretion rate.
Immunohistochemical analysis
Kidneys were fixed in 10% buffered formalin overnight at room temperature, transferred to 70% ethanol for 24 h, and paraffin embedded. The kidneys were sectioned at a thickness of 4 μm onto Superfrost plus slides and processed. Slides were incubated in the absence or presence of primary antibodies to sema3A (Cat# ab23393, Abcam) (1:500 dilution), macrophages (Cat# ab56297) (Abcam) (1:100 dilution) or α-smooth muscle actin (Cat# ab5694) (1:250 dilution) in humidified chambers overnight at 4°C, followed by incubation with biotin-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) or goat-anti-rat FITC (Vector Labs) for 1 hr at room temperature. Color was developed after incubation with ABC reagent (Vector Lab). The stained sections were photographed with an Olympus BX40 microscope (Olympus America, Melville, NY) on bright-field setting fitted with a digital camera (Olympus DP12; Olympus America) (magnification x660). For assessment of injury, 5 μM sections were stained with periodic acid-Schiff (PAS) followed by hematoxylin. Fibrosis was determined with Mason’s trichrome staining. Tubular injury was assessed in PAS-stained sections using a semiquantitative scale [20] in which the percentage of cortical tubules showing dilation, epithelial cell necrosis, brush-border loss, cast formation and apoptotic bodies in the cortex was assigned a score: 0 = normal; 1 = <10%; 2 = 10–25%; 3 = 26–75%; 4 = >75%. 10 fields of 40X magnification were examined and averaged. The individual scoring the slides was blinded to the genotype of the animal.
Quantification of mouse sema3A in serum and urine
Mice were placed in metabolic cages (Nalgene) for a 24 h acclimation period, followed by a 24 h period where urine was collected. Mouse sema3A was quantified using an ELISA kit (Cat no: CSB-EL020980MO, Cedarlane Laboratories USA). Human sema3A was quantified in diabetic human urine samples using an ELISA kit (Catalog # MBS732622, My Biosource, Inc., San Diego, CA).
Measurement of urine albumin excretion rate (AER)
Excretion of urinary albumin was determined using albumin-to-creatinine ratio (ACR) in 24 hr urine collections. Twenty-four-hour urine was collected using Nalgene® Metabolic Cage System (Rochester, NY). Nalgene metabolic cages are designed to allow efficient separation of urine and feces from a single mouse. The concentration of albumin in urine was quantified by using ELISA assay (Bethyl Laboratories, Inc., Montgomery, TX), and urine creatinine was determined using creatinine assay kit (Diazyme Laboratories, Poway, CA).
Assessing kidney functions
Kidney function was assessed by measuring blood urea nitrogen (Bioassay systems).
Quantification of podocyte injury marker nephrin-1 and oxidative stress in urine
Nephrin-1 and thiobarbituric acid reactive substance (TBARS) excretion in urine was quantified using kits (Cat#1019 and cat#1020 respectively, Exocell Inc., Philadelphia, PA). Nephrin-1 was expressed as microgram/24hr urine and microgram/mg of creatinine. TBARS was expressed as micromole/24hr urine and micromole/mg of creatinine.
Cytokine and chemokine measurement
Cytokines and chemokines in urine were measured using ELISA array kit from eBioscience.
Quantification of mRNA by real-time RT-PCR
RNA was isolated using TRIZOL reagent (Life Technologies, Grand Island, NY). Real-time RT-PCR was performed using an Applied Biosystems Inc. 7700 Sequence Detection System (Foster City, California, USA). 3 μg total RNA was reverse transcribed in a reaction volume of 40 μl using Omniscript RT kit and random primers. The product was diluted to a volume of 150 μl, and 5 μl aliquots were used as templates for amplification using the SYBR Green PCR amplification reagent (Qiagen) and gene specific primers. The primer sets used were: mouse TNFα (forward: GCATGATCCGCGACGTGGAA; reverse: AGATCCATGCCGTTG GCCAG), MCP-1 (forward: ATGCAGGTCCCTGTCATG; reverse: GCTTGAGGTGGTTGTGGA), ICAM-1 (forward: AGATCACATTCACGGTGCTG; reverse: CTTCAGAGGCAGGAAACAGG), COX-1 (forward: GAATGCCACCTTCATCCGAGAAG; reverse: GCTCACATTGGAGAAGGACTCC), COX-2 (forward: GCGACATACTCAAGCAGGAGCA; reverse: AGTGGTAACCGCTCAGGTGTTG) and IL-6 (forward : GATGCTACCAAACTGGATATAATC; reverse : GGTCCTTAGCCACTCCTTCTGTG). The amount of DNA was normalized to the β-actin signal amplified in a separate reaction (forward primer: AGAGGGAAATCGTGCGTGAC; reverse: CAATAGTGATGACCTGGCCGT).
Morphometry
Glomerular size was calculated as described before [21]. Briefly, Glomerular size was determined using ImageJ analysis software (US National Institutes of Health, Bethesda, MD, USA http://imagej.nih.gov/ij). The largest area on sections was measured in three to four different mice per group with at least ten glomeruli of each mouse.
For quantification of mesangial extracellular matrix, 3-μm sections from paraformaldehyde-fixed, paraffin-embedded kidney slices were stained using Periodic Acid-Schiff’s reagent (PAS). Mesangial area was expressed quantitatively by calculating the percentage of the total glomerular area that was PAS positive and nucleus free area [22]. Fifteen glomerular tufts per animal were chosen randomly for analysis.
Patient recruitment and sample collection
This is a post-hoc study in which 87 out of 92 previously collected diabetic urine samples were assayed [23]. Type 1 and type 2 diabetic patients who were visiting a diabetes specialty clinic were recruited between April 2009 and September 2009 at University Medical Center Groningen, Groningen, The Netherlands. The study protocol was approved by the local ethics committee at University Medical Center Groningen and the Human Assurance Committee at the Georgia Health Sciences University. Patients were stratified by the amount of albuminuria based on a first morning urine void. We included 87 diabetic patients, of whom 40 had normoalbuminuria, 38 had microalbuminuria and 9 had macroalbuminuria (Supplementary table 1).
Statistical analyses
Data are presented as the mean ± SEM. p< 0.05 was considered significant. Statistical analyses were performed using Graphpad Instat 3 (GraphPad Software, San Diego, CA, USA). All assays were performed in duplicate or triplicate. Statistical significance was assessed by an unpaired, two-tailed Student t-test for single comparison or ANOVA for multiple comparisons.
Results
Sema3A expression in kidney is highly induced in diabetes
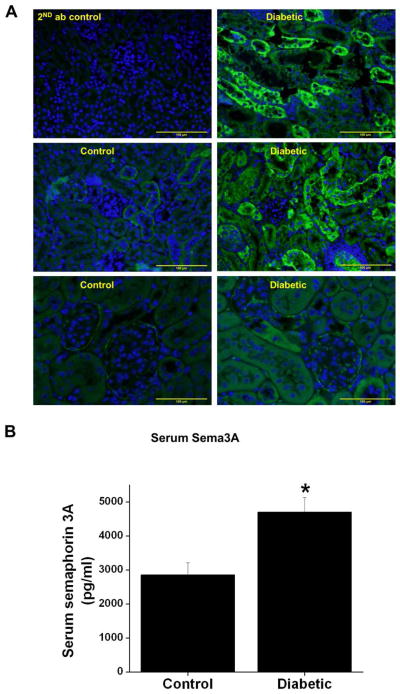
Our previous studies had demonstrated that sema3A is a tubular injury marker that predicts the development of acute kidney injury accurately much earlier than serum creatinine in experimental animals and humans [15]. However, its usefulness as a marker of chronic kidney disease is unknown. Moreover, the expression pattern in chronic kidney disease has not determined. Sema3A is mainly expressed in the podocytes and distal and collecting duct epithelial cells in normal kidney, and its expression is highly induced in podocytes and distal tubules after induction of diabetes (Figure 1A). Quantification of sema3A in the circulation showed a significant increase in diabetic animals over control animals (Figure 1B).
Figure 1.
A. Immunofluorescence localization of sema3A in the control and diabetic mouse kidney. Sema3A staining (green) was seen in podocytes and distal and collecting tubules of control kidneys, and intensity is increased in diabetic mice. Scale bar: 100 μM. B. Quantification of serum sema3A in control and diabetic mouse. Diabetes increased serum concentration of sema3A significantly as compared to control. *, p<0.001 vs. control. n=6.
Sema3A excretion in urine is elevated early in diabetes and correlates with renal injury in different mouse models of diabetes
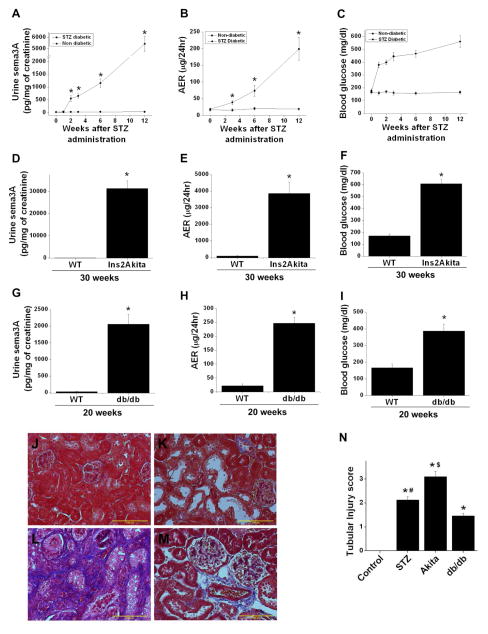
To determine whether sema3A excretion is increased early in diabetes, urine sema3A was quantified as described in Methods. Increased excretion of sema3A was detected 2 weeks after induction of diabetes by STZ and rose in time-dependent fashion, reaching over 200 fold by 12 weeks of diabetes (Figure 2A). Albumin excretion likewise increased after induction of diabetes, displaying a similar kinetic pattern as sema3A (Figure 2B). Blood glucose rose early, as expected, and remained elevated throughout the period of the study (Figure 2C). Since STZ-induced diabetes is known to promote tubular toxicity, we used two additional genetic models of type 1 and type 2 diabetes, namely Ins2Akita and db/db mice. As shown in Figure 2D, urine sema3A was increased over 1000 fold in 30 week old Ins2Akita mice as compared to their wild-type counterparts. The increase in sema3A was associated with severe albuminuria and elevated blood glucose (Figure 2E&F). Also, sema3A excretion was increased over 100 fold in 20 week old db/db mice as compared to wild-type mice (Figure 2G). Albuminuria was moderately increased in db/db mice, consistent with a more moderate diabetic phenotype in these mice (Figure 2H) as compared to the other diabetic models (Figure 2A &D). Blood glucose is significantly elevated in db/db mice as compared to WT mice (Figure 2I).
Figure 2.
Sema3A excretion in urine, albumin excretion ratio (AER) and blood glucose levels in diabetic mouse models. A–C: Data from STZ-induced diabetes in C57BL/6J mice. D–F: Data from 30 week old WT and ins2Akita diabetic mice. G–I: Data from 20 week old db/db diabetic mice. Sema 3A excretion and proteinuria were increased significantly in all three models. *, p<0.001 vs. WT control. n=6–8 for each time point. J–M: Kidney interstitial fibrosis assessed by Masson’s trichrome staining. Extensive fibrosis was seen in STZ diabetic kidney (K), Ins2Akita diabetic mouse kidney (L) and db/db diabetic mouse kidney (M) as compared to non-diabetic kidney (J). N. Tubular injury score was significantly increased in all three diabetic groups as compared to control kidney, with the highest tubular injury seen in ins2akita mice kidney. Scale bar: 100 μM. *, p<0.0001 vs. control. #, p<0.05 vs. db/db and Akita. $, p<0.05 vs. all other groups.
To determine whether increased urinary sema3A is associated with the severity of histological injury, tissue sections were stained with Masson’s trichrome. As shown in Figure 2, STZ induced diabetes (K) and db/db mice kidney tissue (M) show moderate levels of fibrosis (less than 5% interstitium is fibrotic), whereas Ins2Akita (L) showed extensive interstitial fibrosis (over 30% of interstitium is fibrotic). Moreover, PAS stained section were analyzed for tubular injury. As shown in Figure 2N, db/db mice, which exhibited the smallest relative increase in urinary sema3A excretion amongst the three diabetic models (Figure 2A, D & G), showed the lowest level of tubular injury. In contrast, Ins2Akita mice, which exhibited the greatest relative increase in urinary sema3A excretion, had the most tubular injury, while db/db mice exhibited an intermediate phenotype.
Sema3A is significantly elevated in urine from diabetic patients with nephropathy
To determine whether sema3A is increased in diabetic human patient urine and correlates with the severity of albuminuria, sema3A was quantified as described in Methods. As shown in supplementary Figure 1, a non-significant increase in sema3A urinary excretion was seen in diabetes without microalbuminuria. However, the level of sema3A was significantly increased in diabetics with microalbuminuria and more so in those with macroalbuminuria.
Sema3A plays a pathogenic role in the development diabetic nephropathy
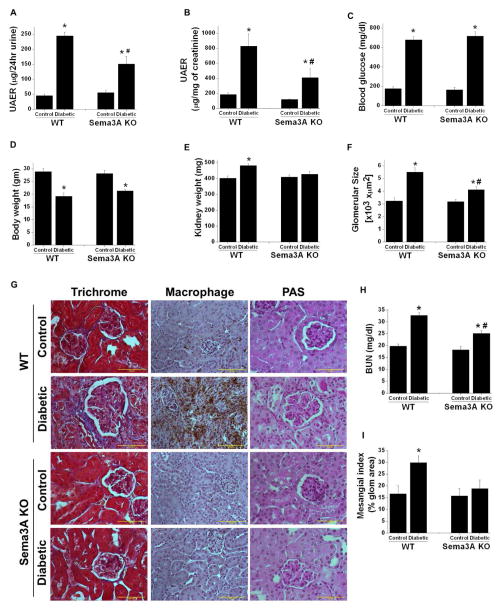
Previous studies had demonstrated that exogenous administration of recombinant sema3A induced proteinuria by disrupting podocyte foot processes [24]. However, the role of endogenous sema3A in diabetic nephropathy is unknown. Sema3A mutant mice and wild-type controls were made diabetic as described in Methods. Non-diabetic control mice gained weight as expected (Figure 3D) and did show increase in plasma glucose (Figure 3C). Administration of STZ induced hyperglycemia in both WT and sema3A knockout animals (Figure 3C). However, diabetes induced albuminuria (Figure 3A & B), kidney hypertrophy (Figure 3D) and increase in glomerular area (Figure 3F) in WT mice. These changes were minimal in diabetic sema3A mutant mice (Figure 3A–F).
Figure 3.
Sema3A mutant (KO) mice are resistant to diabetes induced albuminuria. Sema3A mutant mice are in C3H background. A and B: Albumin excretion rate expressed as μg/24hr urine and μg/mg of creatinine, respectively. Diabetes induced a large increase in albuminuria in WT mice which was reduced significantly in sema3A mutant mice. *, p<0.001 vs. control. #, p<0.001 vs. WT diabetic mice. C. Blood glucose in WT and sema3A mutant control and diabetic mice. *, p<0.0001 vs. control. D. Diabetes significantly reduced body weight in both WT and sema3A mutant mice. *, p<0.05 vs. control. n=8–10. E. Diabetes induced kidney hypertrophy is significantly reduced in sema3A mutant mice. *, p<0.001 vs. control. #, p<0.001 vs. WT diabetic mice. n=8–10. F. Diabetes induced a significant increase in glomerular area in WT mice which was reduced in sema3A mutant mice with diabetes. *, p<0.05 vs. control. #, p<0.05 vs. WT diabetic mice. G. Diabetes induced glomerularsclerosis and macrophage infiltration in WT and sema3A knockout mice. Trichrome staining shows fibrosis of glomeruli and the interstitium of WT diabetic mice kidney which was drastically reduced in sema3A knockout diabetic mice kidney. Similarly, extensive macrophage infiltration was seen in WT diabetic kidney as compared to control kidney. Macrophage infiltration is largely absent in sema3A knockout diabetic kidney. PAS stained section showing expansion of glomerular area, increase cellularity and deposition of proteinaceous materials in WT diabetic glomeruli which are reduced in sema3A mutant kidney section. Scale bar: 100 μM. H. Diabetes induced a significant increase in BUN in WT mice as compared to control, which was significantly reduced in sema3A KO diabetic mice. *, p<0.01 vs. control. #, p<0.05 vs. WT diabetic mice. n=6–8. I. Diabetes induced a significant increase in mesangial matrix expansion in WT mice as compared sema3A mutant mice. *, p<0.05 vs. other groups. n=8.
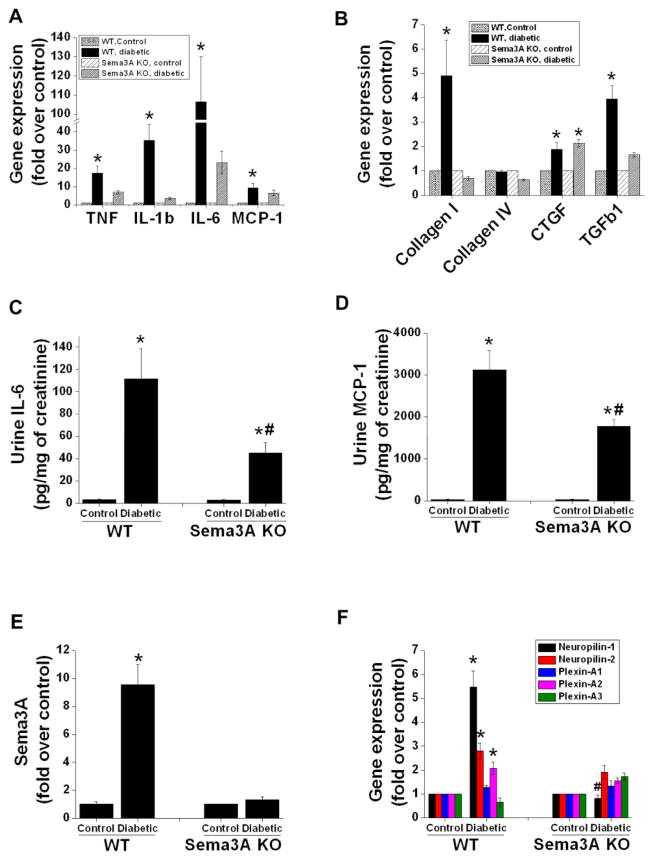
Glomerulosclerosis and interstitial fibrosis are a hallmark of diabetic nephropathy. As shown in Figure 3G, WT mice with diabetes developed glomerulosclerosis and interstitial fibrosis, which was strongly suppressed in sema3A mutant mice. Moreover, extensive macrophage infiltration was observed in the kidneys of WT diabetic mice, which was drastically reduced in sema3A mutant mice. Consistent with glomerular changes and increased albuminuria, diabetes induced kidney dysfunction as measured by BUN which was significantly increased in WT mice as compared to control and sema3A mutant diabetic mice showed much less increase in BUN as compared to diabetic WT mice (Figure 3H). Consistent with glomerular changes and kidney dysfunction, mesangial matrix expansion was significantly increased in WT which was suppressed in sema3A mutant mice (Figure 3I). In addition, the expression of inflammatory cytokines (Figure 4A) and fibrotic genes (Figure 4B) was significantly upregulated in the kidneys of WT mice with diabetes. These changes were minimal in Sema3A mutant mice kidney. Consistent with these findings, excretion of IL-6 (Figure 4C) and MCP-1 (Figure 4D) in urine were significantly increased in diabetic WT mice and suppressed by inactivation of sema3A.
Figure 4.
Diabetes-induced pro-inflammatory and pro-fibrotic gene expression in the kidney, and urinary excretion of cytokines, is diminished in sema3A KO mice. A. Inflammatory cytokine and chemokine expression in kidney was significantly increased in WT diabetic mice and blunted by sema3A gene deletion. *, p<0.001 vs. other groups. B. Expression of profibrotic genes collagen I, CTGF and TGFβ1 is significantly induced in kidneys of WT diabetic mice as compared to control mice; sema3A gene deletion reduced collagen I and TGFβ1 but not CTGF expression. *, p<0.05 vs. other groups. IL- 6 excretion (C) and MCP-1 (D) excretion in urine was significantly increased by diabetes in WT mice, which was reduced in sema3A KO mice. *, p<0.001 vs. control. #, p<0.05 vs. WT diabetic animals. Expression of sema3A (E) and sema3A receptors (F) in kidneys of control and diabetic mice. Both sema3A and several of its receptors were induced by diabetes in WT mice, which was blunted in sema3A KO mice. *, p<0.001 vs. other groups. #, p<0.05 vs. control. n=6–8.
As expected, diabetes induced a large increase in sema3A mRNA expression in WT mice but not in sema3A mutant mice (Figure 4E). Interestingly, diabetes also increased the expression of sema3A receptors neuropilin-1 and -2, as well as plexin-A1, in WT mice, which was blunted in sema3A mutant mice (Figure 4F).
Sema3A inhibitory peptide suppresses the development of diabetic nephropathy
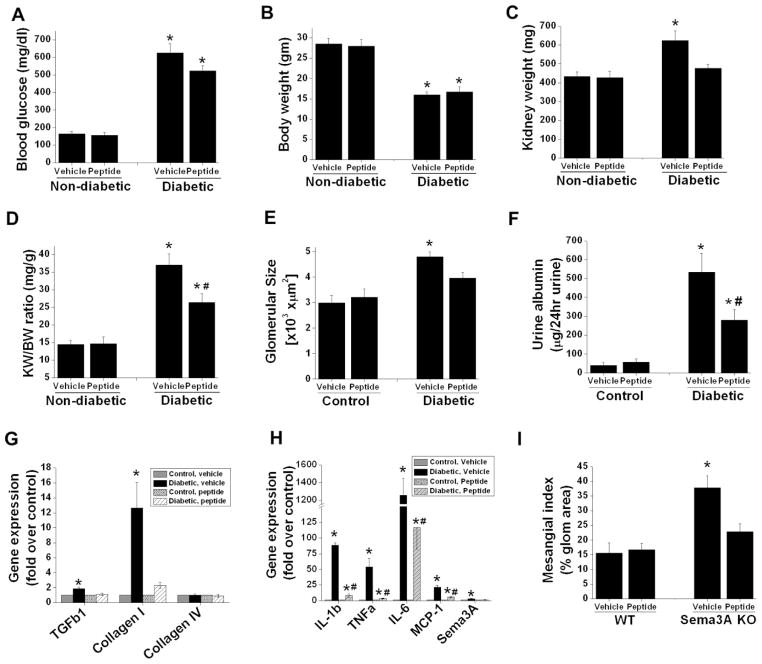
In order to translate our findings in sema3A mutant animals to a model suitable for therapeutic intervention, we administered a small inhibitory peptide against sema3A, or a scrambled inactive peptide control, to diabetic or control DBA mice as described in Methods. As shown in Figure 6, the peptide did not affect blood glucose levels (Figure 5A) or body weight (Figure 5B) after induction of diabetes. However, administration of sema3A inhibitory peptide reduced kidney hypertrophy as seen by decreased kidney weight (Figure 5C) and body weight/kidney weight ratios (Figure 5D). Consistent with increase in kidney hypertrophy, diabetes increased glomerular area (Figure 5E) and mesangial matrix expansion (Figure 5I) in vehicle treated mice kidney but not in inhibitory peptide treated mice kidney. In addition, vehicle treated DBA diabetic animals developed severe albuminuria which was drastically reduced in sema3A inhibitory peptide treated diabetic animals (Figure 5F& G). Moreover, neither the inhibitory peptide nor the scrambled peptide affected protein excretion (Figure 5E& F) or renal function in non-diabetic animals (Figure 6B). It is interesting to note that when the treatment was initiated, all diabetic animals had developed proteinuria, suggesting that sema3A inhibition may be useful for reversing the nephropathy. Moreover, the sema3A inhibitory peptide markedly attenuated the expression of pro-fibrotic and pro-inflammatory genes in diabetic animals (Figure 5G and H).
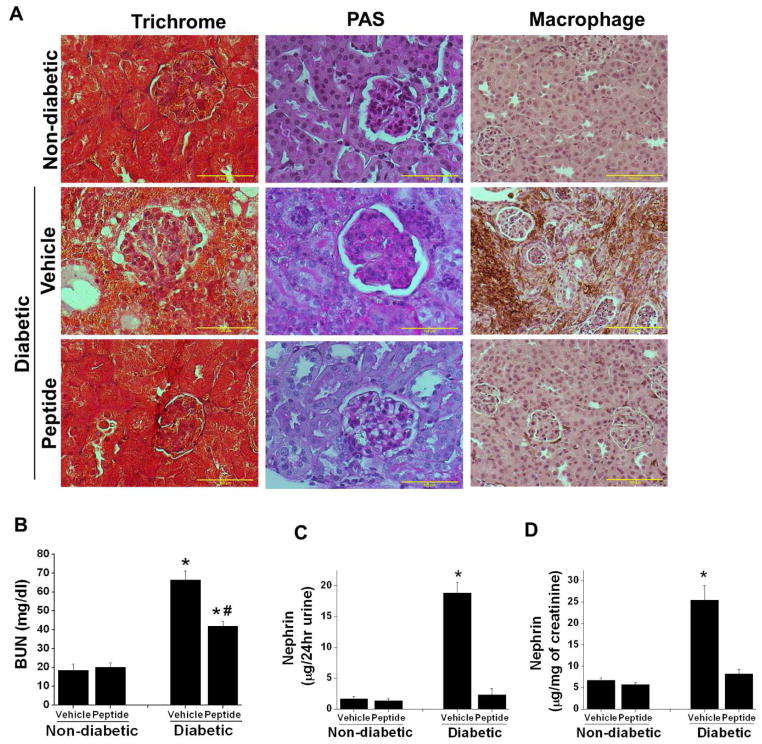
Figure 6.
Sema3A inhibitory peptide administration suppressed diabetes induced gloermulosclerosis, interstitial fibrosis, macrophage infiltration, kidney dysfunction. and nephrin-1 excretion in urine. DBA/2J mice made diabetic by administering low dose of STZ and non-diabetic animals received citrate buffer as described in Methods. 4 weeks after confirmation of diabetes animals received either 10μg/animal/day of scrambled peptide (vehicle) or sema3A complimentary inhibitory peptide (peptide) for another 6 weeks. Kidney tissue was processed for histopathology. A. Fibrosis and glomerular sclerosis was assessed by Masson’s trichrome staining and PAS staining. Diabetes induced extensive damage in parenchyma and glomerulosclerosis in vehicle treated mice which was suppressed in sema3A peptide treated diabetic animal kidney. In addition, diabetes induced significant increase in macrophage infiltration in vehicle treated mice kidney which was reduced in sema3A antagonist peptide treated diabetic mice kidney. Scale bar: 100 μM. B. Kidney function was measured by quantifying blood urea nitrogen (BUN). Administration of sema3A inhibitory peptide significantly reduced diabetes induced kidney dysfunction. *, p<0.001 vs. non-diabetic animals. #, p<0.05 vs. Vehicle treated diabetic animals. n=6–8. C & D. Podocyte injury marker nephrin-1 was quantified as described in Methods. Diabetes induced a large increase in nephrin-1 excretion in vehicle treated diabetic mice as compared to controls which was largely suppressed in peptide treated diabetic mice. *, p<0.001 vs. all other groups. n=6.
Figure 5.
Sema3A complimentary peptide administration suppressed diabetes induced albuminuria and inflammation. DBA/2J mice made diabetic by administering low dose of STZ as described in Methods. Non-diabetic animals received citrate buffer. 4 weeks after confirmation of diabetes animals received either 10μg/animal/day of scrambled peptide (vehicle) or sema3A complimentary inhibitory peptide (peptide) for another 6 weeks. Animal tissue and urine was processed for histopathology and albumin quantification. A. Blood glucose significantly elevated both in vehicle and peptide treated diabetic animals. *,p<0.001 vs. non-diabetic animals. B. Body weight significantly reduced in both vehicle treated and peptide treated diabetic mice. *, p<0.001 vs. non-diabetic mice. Administration of inhibitor peptide to sema3A significantly reduced diabetes induced kidney weight (C) and kidney weight/bodyweight ratio (D) suggesting that reduction of kidney hypertrophy with peptide treatment. *, p<0.05 vs. non-diabetic group. #, p<0.05 vs. vehicle treated diabetic group. E. Diabetes induced a significant increase in glomerular area in vehicle treated diabetic mice which was reduced with sema3A antagonist peptide treated diabetic mice. *, p<0.05 vs. all other groups. n=6–8. F. Albumin excretion rate expressed as μg/24hr urine. *, p<0.001 vs. other groups. G. Profibrotic gene expression is significantly increased in diabetic animals treated with vehicle as compared to control animals. Administration of peptide completely suppressed profibrotic gene expression. *, p<0.01 vs. other groups. H. Diabetes induced a large increase in inflammatory cytokine and chemokine expression as compared control animals which was largely suppressed in peptide administered diabetic animal kidney. *, p<0.001 vs. other groups. #, p<0.05 vs. control. n=6–8. I. Diabetes induced a significant increase in mesangial matrix expansion in vehicle treated group as compared inhibitory peptide treated group. *, p<0.05 vs. other groups. n=6.
Consistent with reduction in albuminuria, renal fibrosis, glomerulosclerosis and macrophage infiltration (Figure 6A), kidney dysfunction (Figure 6B) was also reduced in diabetic mice treated with the sema3A inhibitory peptide as compared to control. Consistent with improved glomerular morphology and kidney function, podocyte injury marker nephrin-1 excretion in urine was also completely suppressed in sema3A inhibitory peptide treated group (Figure 6C & D) as compared to vehicle treated group. In addition, either genetic ablation of sema3A or treatment with the sema3A inhibitory peptide markedly attenuated diabetes-induced oxidative stress in the kidney, as evidenced by reduce urinary excretion of TBARS (supplementary Figure 2). These results suggest that sema3A inhibitory peptide can potentially be used as a therapeutic drug for diabetic nephropathy.
Discussion
The rising prevalence of diabetes worldwide is associated with increased morbidity and mortality [25]. Nephropathy is a major complication of diabetes and is the commonest cause of end-stage renal disease. Here, we report that the secreted glycoprotein sema3A is highly induced early in the time course of diabetic nephropathy and correlates with albuminuria in both mice and diabetic patients. Our studies with genetic ablation and inhibitory peptide models demonstrate that sema3A plays a pathogenic role in the development of diabetic nephropathy, contributing to albuminuria, kidney fibrosis and inflammation, and renal dysfunction.
Consistent with previous studies, we observed that in the absence of diabetes, renal sema3A expression is primarily localized to podocytes and epithelial cells in distal tubules and collecting ducts. Diabetes induced a large increase in sema3A expression in the aforementioned cells, with some staining also appearing in proximal tubular epithelial cells. Co-localization of sema3A with megalin was inconsistent, however, suggesting that the protein’s presence in proximal tubules may be due to uptake of filtered sema3A by proximal tubular epithelial cells rather than in situ production. Increases in both circulating and urinary sema3A was observed in experimentally-induced diabetes. However, the level of increase in urine was several fold higher than in plasma, suggesting that the bulk of urinary sema3A originated from tubular epithelial cells. Similarly, the sema3A receptor neuropilin-1 was observed to be highly induced in proximal tubular epithelial cells in diabetes, suggesting that sema3A signaling in proximal tubular epithelial cells promotes nephropathy and interstitial fibrosis. Our prior studies suggested that sema3A is proteolytically degraded and appears in urine as multiple fragments [26]. However, whether proteolysis is required for its pathogenic activity in diabetes is unknown. In addition, in the absence of diabetes, its urinary excretion is limited, suggesting that sema3A biological activity may be controlled at the level of secretion or proteolysis.
Human diabetic patients also exhibited a progressive increase in sema3A excretion in urine that correlated with the severity of albuminuria, corroborating our findings in diabetic mice. The signal for the induction of sema3A in diabetic nephropathy is unknown. It could be inferred from findings of sema3A expression in acute models of kidney injury that the induction in diabetes might be due to epithelial cell injury, but further studies are required to substantiate this hypothesis. Previous in vivo studies demonstrated that the administration of recombinant sema3A induced transient massive proteinuria by disrupting the podocyte foot process [24]. However, the mechanisms whereby sema3A disrupts podocytes are not clear. Sema3A receptors, in the absence of ligands, can promote integrin-matrix interactions, thereby maintaining cellular attachment and polarization [27]. In the presence of ligands, the sema3A receptor plexinA1 mediates inactivation of RAS by GTP hydrolysis, thus inhibiting integrin-matrix interactions and causing cell retraction and disruption of polarity [27]. As endogenously expressed sema3A in healthy kidneys does not disrupt podocytes, however, it appears that sema3A levels are insufficient to perturb integrin-matrix interactions or cellular integrity in physiologic states.
Sema3A mutant mice were observed to be resistant to diabetes-induced proteinuria, interstitial fibrosis, and renal dysfunction. These findings are consistent with earlier studies employing infusion of recombinant sema3A protein that showed nephrotoxic effects of the protein [24]. The primary cell type responsible for sema3A’s pathological effects in the kidney is unknown. Since sema3A is expressed in podocytes and tubular epithelial cells, and is secreted into the circulation, it may act in multiple cellular locations to mediate nephropathy. Future studies using tissue-specific sema3A receptor mutant mice will be required to address this question.
Our studies in sema3A mutant mice were supported by the use of a sema3A inhibitory peptide which had previously been identified to inhibit sema3A-induced growth cone collapse [19]. However, use of this peptide for treating disease states associated with elevated sema3A expression had not been reported. We synthesized and used this peptide in our diabetic model. Consistent with the data in sema3A mutant mice, administration of the sema3A inhibitory peptide drastically reduced proteinuria in our diabetic model. The peptide also favorably modulated pro-inflammatory and pro-fibrotic gene expression, resulting in a beneficial impact on renal pathology and function. The fact that the peptide was effective when administered after the onset of proteinuria raises the intriguing possibility that sema3A could be an attractive target for therapeutic intervention in diabetic nephropathy.
Our studies also demonstrated that renal oxidative stress is dramatically reduced by genetic ablation of sema3A or by administration of the sema3A inhibitory peptide in our diabetic model (supplementary Figure 2). The mechanism underlying these effects is not clear, as sema3A has not been reported to directly regulate oxidant generating pathways such as NADPH oxidases. However, sema3A was reported to augment TLR4 signaling [28]. Therefore, it is possible that blocking sema3A may favorably modulate other inflammatory and oxidative stress pathways such as TLR4 to suppress diabetes-induced kidney injury.
In summary, we report that sema3A is highly induced in mouse models of diabetes and in human diabetic patients. The level of sema3A in urine correlates with severity of nephropathy. Genetic ablation or pharmacological inhibition of sema3A protects against diabetes-induced albuminuria, fibrosis, inflammation, and renal dysfunction. Sema3A appears to be an attractive target for pharmacological intervention to treat diabetic nephropathy.
Supplementary Material
Key Messages.
Diabetes induced sema3A excretion in urine.
Increased semaphorin3A was associated with severity of albuminuria.
Seme3A mediated diabetes induced glomerulosclerosis.
Peptide based inhibition of semaphorin3A suppressed diabetic nephropathy.
Acknowledgments
Funding:
This work was supported by a R01grant (1R01DK083379 - 01A3) to Ganesan Ramesh from NIH-NIDDK. PR and RM are recipients of American Heart Association Postdoctoral fellowship.
Footnotes
Author Contributions: RM and PR. researched data, Wrote manuscript. CJ. Researched data/reviewed/edited manuscript. FN. Researched data, reviewed/edited manuscript. RTN. Reviewed/edited manuscript. MB, NLW. Reviewed and edited manuscript. GR. Wrote manuscript/Edited the manuscript.
Conflict of interest statement: The results presented in this paper have not been published previously in whole or part. All authors declared that they have no conflict of interest related to this study.
Reference List
- 1.Rossing P, de Zeeuw D. Need for better diabetes treatment for improved renal outcome. Kidney Int Suppl. 2011:S28–S32. doi: 10.1038/ki.2010.513. [DOI] [PubMed] [Google Scholar]
- 2.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS. Risk for ESRD in Type 1 Diabetes Remains High Despite Renoprotection. Journal of the American Society of Nephrology. 2011;22:545–553. doi: 10.1681/ASN.2010040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hostetter TH. Prevention of end-stage renal disease due to type 2 diabetes. N Engl J Med. 2001;345:910–912. doi: 10.1056/NEJM200109203451209. [DOI] [PubMed] [Google Scholar]
- 4.Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumanogoh A, Kikutani H. Immune semaphorins: a new area of semaphorin research. J Cell Sci. 2003;116:3463–3470. doi: 10.1242/jcs.00674. [DOI] [PubMed] [Google Scholar]
- 6.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–431. doi: 10.1007/s10555-007-9097-4. [DOI] [PubMed] [Google Scholar]
- 7.Tufro A, Teichman J, Woda C, Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech Dev. 2008;125:558–568. doi: 10.1016/j.mod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 9.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 10.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 11.Villegas G, Tufro A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech Dev. 2002;119(Suppl 1):S149–S153. doi: 10.1016/s0925-4773(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 12.Robert B, Zhao X, Abrahamson DR. Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am J Physiol Renal Physiol. 2000;279:F275–F282. doi: 10.1152/ajprenal.2000.279.2.F275. [DOI] [PubMed] [Google Scholar]
- 13.Reeves WB, Kwon O, Ramesh G. Netrin-1 and kidney injury. II. Netrin-1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F731–F738. doi: 10.1152/ajprenal.00507.2007. [DOI] [PubMed] [Google Scholar]
- 14.Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395–401. doi: 10.2215/CJN.05140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayakumar C, Ranganathan P, Devarajan P, Krawczeski CD, Looney S, Ramesh G. Semaphorin 3A Is a New Early Diagnostic Biomarker of Experimental and Pediatric Acute Kidney Injury. PLoS ONE. 2013;8:e58446. doi: 10.1371/journal.pone.0058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris SM, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 Mediates Diabetic Renal Injury. Diabetes. 2011;60:3015–3022. doi: 10.2337/db11-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breyer MD, Böttinger E, Brosius FC, Coffman TM, Harris RC, Heilig CW, Sharma K for the AMDCC. Mouse Models of Diabetic Nephropathy. Journal of the American Society of Nephrology. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 18.Brosius FC, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T for the Animal Models of Diabetic Complications Consortium. Mouse Models of Diabetic Nephropathy. Journal of the American Society of Nephrology. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams G, Eickholt BJ, Maison P, Prinjha R, Walsh FS, Doherty P. A complementary peptide approach applied to the design of novel semaphorin/neuropilin antagonists. Journal of Neurochemistry. 2005;92:1180–1190. doi: 10.1111/j.1471-4159.2004.02950.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289:F166–F174. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 21.Tak E, Ridyard D, Badulak A, Giebler A, Shabeka U, Werner T, Clambey E, Moldovan R, Zimmerman M, Eltzschig H, Grenz A. Protective role for netrin-1 during diabetic nephropathy. J Mol Med. 2013;91:1071–1080. doi: 10.1007/s00109-013-1041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Schin M, Saha J, Burke K, Holzman LB, Filipiak W, Saunders T, Xiang M, Heilig CW, Brosius FC., III Podocyte-specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. Am J Physiol Renal Physiol. 2010;299:F91–F98. doi: 10.1152/ajprenal.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauta FL, Boertien WE, Bakker SJL, van Goor H, van Oeveren W, de Jong PE, Bilo H, Gansevoort RT. Glomerular and Tubular Damage Markers Are Elevated in Patients With Diabetes. Diabetes Care. 2011;34:975–981. doi: 10.2337/dc10-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tapia R, Guan F, Gershin I, Teichman J, Villegas G, Tufro A. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2007;73:733–740. doi: 10.1038/sj.ki.5002726. [DOI] [PubMed] [Google Scholar]
- 25.Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Jayakumar C, Ranganathan P, Devarajan P, Krawczeski CD, Looney S, Ramesh G. Semaphorin 3A Is a New Early Diagnostic Biomarker of Experimental and Pediatric Acute Kidney Injury. PLoS ONE. 2013;8:e58446. doi: 10.1371/journal.pone.0058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yebra M, Montgomery AMP, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, Tessier-Lavigne M, Cirulli V. Recognition of the Neural Chemoattractant Netrin-1 by Integrins [alpha]6[beta]4 and [alpha]3[beta]1 Regulates Epithelial Cell Adhesion and Migration. Developmental Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 28.Wen H, Lei Y, Eun SY, Ting Y. Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor 4 and sepsis-induced cytokine storm. The Journal of Experimental Medicine. 2010;207:2943–2957. doi: 10.1084/jem.20101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.