Abstract
Background
Impairment in instrumental activities of daily living (IADL) starts as individuals with amnestic mild cognitive impairment (MCI) transition to Alzheimer’s disease (AD) dementia. However, most IADL scales have not shown IADL alterations in clinically normal (CN) elderly. The objective of this study was to determine which of the IADL-related Everyday Cognition (ECog) scale items are most sensitive for detection of early functional changes.
Methods
We assessed 290 CN and 495 MCI participants from the Alzheimer’s Disease Neuroimaging Initiative. We performed logistic regression analyses predicting the probability of CN vs. MCI diagnosis using only the 17 participant-based and 17 informant-based ECog items related to IADL. We then performed Cox regression analyses to predict progression from CN to MCI. All analyses were adjusted for demographic characteristics.
Results
We found that worse performance on “remembering a few shopping items” (participant and informant-based p<0.0001), “remembering appointments” (participant and informant-based p<0.0001), “developing a schedule in advance of anticipated events” (participant-based p=0.007), “balancing checkbook” (participant-based p=0.02), and “keeping mail and papers organized” (informant-based p=0.002) best discriminated MCI from CN. We found that worse performance on “keeping mail and papers organized” (participant-based Hazard Ratio (HR)=2.27, p=0.07) marginally predicted greater hazard of progressing from CN to MCI.
Conclusions
Our results indicate that a few simple questions targeting early functional changes, addressed either to the individual or informant, can effectively distinguish between CN elderly and individuals with MCI. Additionally, one of the above questions related to organization suggested which CN individuals are likely to progress to MCI.
Keywords: Activities of daily living, Alzheimer’s disease, clinical assessment, daily functioning, clinically normal elderly, mild cognitive impairment
Introduction
Instrumental activities of daily living (IADL) consist of activities such as managing the finances, organizing papers and documents, planning and scheduling activities for the day, driving or using public transportation, shopping for food or clothes, preparing meals, and performing household chores. Patients with Alzheimer’s disease (AD) dementia and their caregivers view impairment in IADL as the burdensome, disabling, and practical everyday extensions of cognitive dysfunction. Impairment in IADL starts as individuals with amnestic mild cognitive impairment (MCI) transition to AD dementia. Multiple scales have been used to demonstrate the presence of mild IADL impairment in MCI [1–7]. However, most of these scales have not been sensitive to IADL alterations in clinically normal (CN) elderly.
In recent years the concept of preclinical AD has gained strength and materialized in the form of research criteria, defining preclinical AD as the presence of amyloid with or without neurodegeneration in asymptomatic or minimally symptomatic older individuals [8]. These minimal symptoms are often thought of as early cognitive or behavioral alterations reported subjectively by individuals or measured objectively by sensitive neuropsychological tests. Accordingly, early alterations in high level IADL may be present at the stage of preclinical AD and need to be captured by more sensitive assessments [9]. The Everyday Cognition (ECog) scale combines subjective report of both cognitive and IADL difficulties obtained from individuals, as well as informants who know them well, which makes it especially appealing for assessing individuals with preclinical AD and those with MCI, also referred to as prodromal AD [10].
The objective of this study was to determine which of the IADL-related ECog items are most sensitive for detection of early functional changes. The identified items can then be used to inform future research studies, the development of more sensitive IADL scales targeting preclinical AD extending to prodromal AD, assessment of early IADL changes in secondary prevention trials in preclinical AD, and ultimately screening of asymptomatic to mildly symptomatic elderly individuals at risk for AD in the primary care setting. To accomplish this goal, we performed a cross-sectional analysis in which we determined which ECog items best discriminated between CN elderly and individuals with MCI in hopes of finding IADL changes that are clearly associated with MCI (prodromal AD) and would be important to focus on when targeting early AD symptoms. We then performed a longitudinal analysis in which we determined which ECog items best predicted progression from CN to MCI in hopes of determining which early IADL changes already present in CN elderly are of greater importance and may signal impending decline and development of the clinical stages of AD. Since some of these IADL alternations might be confounded by or even directly attributed to demographic characteristics, we adjusted for age, sex, level of education, and an estimate of premorbid intelligence. Both education and premorbid intelligence were high in our sample, but these variables do not necessarily represent the same thing; therefore, we adjusted for both.
Materials and Methods
Participants
Data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study (adni.loni.usc.edu, PI Michael W. Weiner), a large multi-center, observational study previously described in detail [3,11]. The primary goals of ADNI are to develop improved methods for obtaining imaging, clinical, cognitive and biological data in AD research, to establish a large database describing the progression of CN, MCI, and mild AD dementia participants, and to accelerate the development of biomarkers as surrogate outcome measures in AD clinical trials. ADNI is the result of a partnership among multiple public and private entities, from over 50 sites across North America [11].
Seven hundred and eighty five participants (290 CN and 495 MCI at baseline) were evaluated every 6 to 12 months. Participants at baseline were ages 55 to 94 (inclusive), were generally in good health or with stable medical conditions, did not have significant cerebrovascular disease and had a Modified Hachinski Ischemic Score [12] ≤ 4, did not have active psychiatric disorders and had a Geriatric Depression Scale, short form [13] ≤ 5, and had a study partner able to provide collateral information about the participant’s daily functioning, cognition, and behavior.
Participants were assigned to diagnostic groups (CN or amnestic MCI) by site investigators at baseline and at subsequent visits as previously described [3,11]. At baseline, CN participants had a Clinical Dementia Rating (CDR) [14] global score of 0, Mini-Mental State Examination (MMSE) [15] score of 24–30 (inclusive), and no significant memory impairment (performed within 1.5 standard deviations of education adjusted cut-off scores on the delayed recall portion of one Logical Memory story (LM-IIa) of the Wechsler Memory Scale-Revised (WMS-R) [16]). MCI participants were amnestic, single or multiple domain, had a CDR global score of 0.5 and memory box score ≥ 0.5, MMSE score of 24–30 (inclusive), a memory complaint, objective memory loss on the WMS-R LM-IIa, essentially preserved IADL (the site investigator had access to the CDR, which includes IADL items, but no cut-off on a specific test was used to determine this; it was based on qualitative clinical judgment by the site investigator), and were not demented. Of note, the ECog was not used in the assignment of diagnostic groups at baseline. For follow-up diagnoses, the ECog was available to the site investigator determining the diagnosis of the participant. However, it was used infrequently and more emphasis was placed on neuropsychological testing, the CDR, and the Functional Activities Questionnaire [17].
The Institutional Review Board (IRB) of each participating ADNI site approved the study. Written informed consent was obtained from all participants and study partners prior to initiation of any study procedures in accordance with local IRB guidelines.
Clinical Assessments
The Everyday Cognition (ECog) is a subjective scale that assesses cognitive function and IADL related to 6 domains [5,10]. The ECog consists of 39 items, 17 of which are specific to IADL (this was determined by an experienced clinician, GAM). It is administered separately to the participant and to the informant. The score range for each item is 1–4 (higher scores indicate greater impairment; 1 = better or no change compared to 10 years ago; 2 = questionable/occasionally worse; 3 = consistently a little worse; and 4 = consistently much worse). The total score on the ECog has been previously shown to successfully discriminate between CN elderly and individuals with MCI [5].
Statistical Analyses
Statistical and graphical analyses were performed using SAS Version 9.3 and JMP Pro Version 10.
Preliminary Protected Multiple Test Correction
As a preliminary multivariate approach to protect from chance findings given the large number of ECog items (17 participant-based and 17 informant-based) for tests of MCI versus CN, we first ran 34 t-tests of diagnostic group mean differences on the respective ECog items, using a non-parametric resampling stepdown permutation test (the SAS Multtest Procedure) to adjust the p values for the multiple tests (all 34 tests were pooled as a single collective for this purpose). This method provides more powerful tests than a Bonferroni or Sidak correction would because these latter techniques assume tests are independent whereas ECog item inter-correlations clearly indicate otherwise (see Results). The resampling method adjusts on the basis of observed correlations. However, for comparison, we also adjusted p values with Bonferroni, stepdown Sidak and false discovery rate (FDR) methods. The FDR is more powerful than the Bonferroni or Sidak, but at the price of tolerating a specified expected small percentage of false positives. We also ran the Bonferroni, stepdown Sidak and FDR corrections on p values for the diagnostic group effect in 34 separate analyses of covariance (ANCOVAs) corresponding to the 34 different ECog items as dependent variables respectively, co-varying for age.
Primary Cross-sectional Analyses
In order to determine which of the ECog items best differentiated participants with baseline diagnoses of CN versus MCI, we conducted a backward elimination (cutoff <0.05) logistic regression analysis using all 17 ECog items as the initial pool of predictors. Separate analyses were performed for the participant-based ECog items and the informant-based ECog items. Covariates associated with diagnosis (see Table 1) were included in the model—baseline age, sex, years of education, and the American National Adult Reading Test intelligence quotient (AMNART IQ) [18] (an estimate of premorbid intelligence, serving as a proxy of cognitive reserve).
Table 1.
Baseline demographics and characteristics of participants.
| All Participants | CN | MCI | |
|---|---|---|---|
| n | 785 | 290 | 495 |
| Age* | 74.1 ± 7.6 | 76.1 ± 6.7 | 72.9 ± 7.9 |
| Sex (% male)** | 54.1% | 49.0% | 57.2% |
| Education | 16.2 ± 2.7 | 16.4 ± 2.7 | 16.1 ± 2.8 |
| AMNART IQ* | 119.4 ± 10.2 | 121.2 ± 9.5 | 118.3 ± 10.5 |
| MMSE* | 28.4 ± 1.6 | 29.1 ± 1.2 | 28.0 ± 1.7 |
| CDR Sum of Boxes* | 0.9 ± 1.0 | 0 ± 0.1 | 1.5 ± 0.9 |
AMNART IQ (American National Adult Reading Test intelligence quotient), CDR (Clinical Dementia Rating), CN (clinically normal), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination). All values (except n and sex) represent mean ± standard deviation.
p<0.0001 for CN vs. MCI
p<0.05 for CN vs. MCI
Primary Longitudinal Analyses
We employed Cox proportional hazards regression models to assess time to change in diagnosis from a baseline diagnosis of CN to an endpoint diagnosis of MCI. Only participants diagnosed as CN at baseline were included in these analyses and those who remained stable at CN were treated as “censored” observations providing partial information on time to change in diagnosis (i.e., we know they had not transitioned to a diagnosis of MCI at the time of their last study visit). Predictors were tested in a backward elimination algorithm (cutoff p<0.05). The initial pool of predictors in the Cox regressions was the same as that used in the cross-sectional analyses. After backward elimination produced an optimal subset of predictors, the validity of the proportional hazard model assumption was tested for each of the retained predictors using a Kolmogorov test comparing residuals with simulations under a null hypothesis.
Results
Table 1 provides baseline demographics and characteristics of all participants and for each diagnostic group (CN, MCI). There were significant differences between diagnostic groups for AMNART IQ, MMSE, and CDR Sum of Boxes in expected directions. There was a significantly higher proportion of males for the MCI than CN group, and the MCI group was significantly younger than the CN group. These differences were among those adjusted for in our analyses.
Table 2 shows descriptive statistics for the baseline participant-based and informant-based individual ECog item scores and the total ECog score. The mean for the MCI group was significantly higher than that for the CN group on all 34 IADL-related ECog items (17 participants-based and 17 informant-based), according to the unadjusted t-test p values and using any of the p value adjustment methods (permutation, stepdown Sidak, Bonferroni, or FDR). This was also true for the adjusted means for MCI versus CN in the ANCOVAs for all correction methods. Therefore, in a univariate descriptive sense, there appears to be a real difference between the MCI and CN groups with the MCI group having a higher mean (worse IADL) on every one of the 34 ECog items. As such, any significant ECog differences between MCI and CN groups reported below are not likely to be chance effects related to multiple testing. Table 2 also provides a measure of effect size—the percent of the variance of each ECog item accounted for by the diagnostic group difference.
Table 2.
Descriptive statistics for baseline participant and informant-based ECog individual item and total scores.
| Participant | Informant | |||||
|---|---|---|---|---|---|---|
| ECog Items | CN | MCI | % Variance | CN | MCI | % Variance |
| “Remembering a few shopping items without a list”* | 1.6 ± 0.7 | 2.4 ± 1.0 | 15.9 | 1.2 ± 0.5 | 2.2 ± 1.0 | 24.9 |
| “Remembering appointments, meetings, or engagements”* | 1.4 ± 0.6 | 2.1 ± 1.0 | 15.0 | 1.2 ± 0.5 | 2.1 ± 1.0 | 20.9 |
| “Following a map to find new location” | 1.1 ± 0.4 | 1.5 ± 0.8 | 6.2 | 1.1 ± 0.4 | 1.6 ± 0.9 | 9.6 |
| “Reading a map and helping with directions when someone else is driving” | 1.2 ± 0.4 | 1.5 ± 0.8 | 4.8 | 1.1 ± 0.4 | 1.7 ± 0.9 | 10.5 |
| “Finding one’s car in a parking lot” | 1.3 ± 0.5 | 1.7 ± 0.8 | 6.1 | 1.1 ± 0.4 | 1.6 ± 0.8 | 9.4 |
| “Finding the way back to a meeting spot in the mall or other location” | 1.2 ± 0.5 | 1.6 ± 0.8 | 6.8 | 1.1 ± 0.3 | 1.6 ± 0.8 | 10.5 |
| “Finding one’s way around a familiar neighborhood” | 1.1 ± 0.3 | 1.3 ± 0.6 | 5.0 | 1.0 ± 0.2 | 1.3 ± 0.5 | 5.7 |
| “Finding one’s way around a familiar store” | 1.1 ± 0.3 | 1.3 ± 0.6 | 4.6 | 1.0 ± 0.2 | 1.2 ± 0.6 | 4.7 |
| “Finding one’s way around a house visited many times” | 1.0 ± 0.2 | 1.2 ± 0.5 | 3.5 | 1.0 ± 0.1 | 1.1 ± 0.3 | 1.7 |
| “Planning the sequence of stops on a shopping trip” | 1.2 ± 0.4 | 1.5 ± 0.8 | 7.8 | 1.1 ± 0.3 | 1.6 ± 0.8 | 11.4 |
| “Anticipating weather changes and planning accordingly” | 1.1 ± 0.3 | 1.3 ± 0.6 | 3.9 | 1.1 ± 0.3 | 1.2 ± 0.5 | 3.7 |
| “Developing a schedule in advance of anticipated events”* | 1.1 ± 0.3 | 1.4 ± 0.7 | 8.0 | 1.1 ± 0.4 | 1.6 ± 0.8 | 9.7 |
| “Keeping living and work space organized” | 1.5 ± 0.6 | 1.8 ± 0.9 | 3.1 | 1.3 ± 0.6 | 1.7 ± 1.0 | 5.8 |
| “Balancing the checkbook without error”* | 1.2 ± 0.4 | 1.5 ± 0.8 | 5.7 | 1.1 ± 0.3 | 1.5 ± 0.9 | 8.2 |
| “Keeping financial records organized” | 1.2 ± 0.5 | 1.6 ± 0.8 | 7.0 | 1.1 ± 0.4 | 1.7 ± 0.9 | 10.2 |
| “Keeping mail and papers organized”*/** | 1.4 ± 0.6 | 1.8 ± 0.9 | 4.9 | 1.2 ± 0.6 | 1.8 ± 1.0 | 10.7 |
| “Using an organized strategy to manage medication schedule involving multiple medications” | 1.1 ± 0.4 | 1.3 ± 0.6 | 3.8 | 1.1 ± 0.3 | 1.4 ± 0.7 | 5.4 |
| Total ECog score (39 items) | 51.2 ± 11.0 | 68.9 ± 20.2 | 44.2 ± 9.4 | 64.8 ± 22.2 | ||
CN (clinically normal), ECog (Everyday Cognition), MCI (mild cognitive impairment). ECog item score range: 1–4 (higher scores indicate greater impairment). Values in CN and MCI columns represent mean ± standard deviation. Values in % Variance columns represent the percent of the variance of each ECog item accounted for by the diagnostic group difference.
ECog items (bolded) that best discriminated between CN and MCI participants.
ECog items (bolded) that best predicted progression from CN to MCI.
The ECog items correlated positively (pooled across diagnostic groups). They tended to correlate moderately (typically with an r value of about 0.5) across different items within the participant-based items and within the informant-based items, but corresponding items across the participant/informant distinction correlated less well (typically r=0.3), suggesting a strong method variance with regards to the source of information, as well as a conceptual component to the scores.
Primary Cross-sectional Analyses
Separate models were used for the participant-based and informant-based ECog items. The 17 IADL-related ECog items were entered as simultaneous predictors along with the covariates of baseline age, sex, years of education, and AMNART IQ in a backward elimination logistic regression predicting the probability of being assigned a diagnosis of MCI versus CN at baseline.
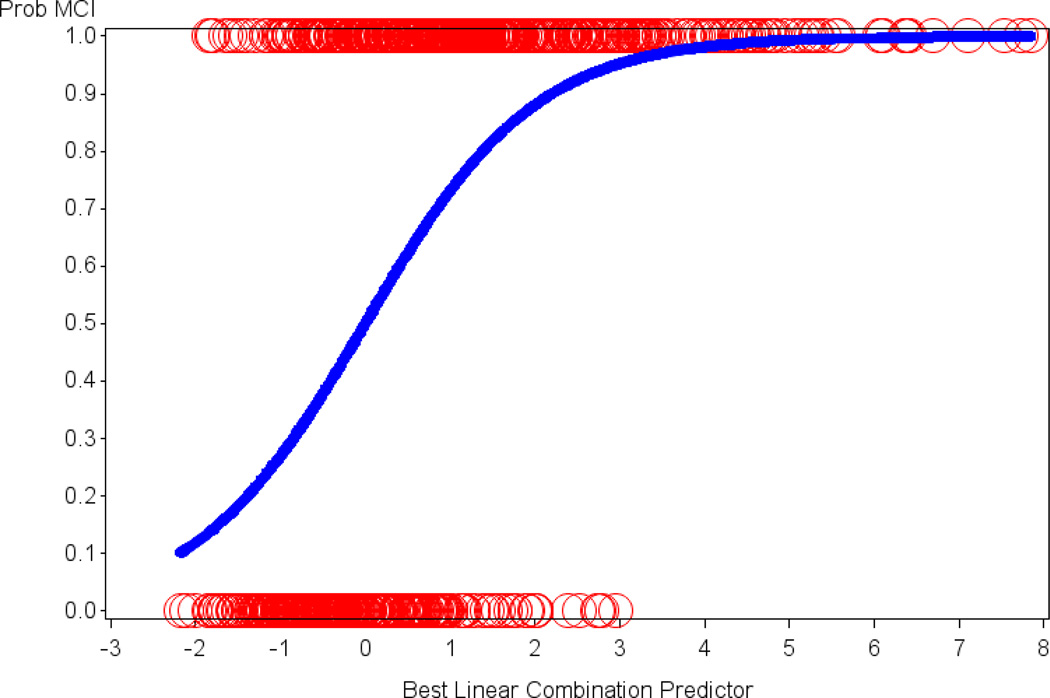
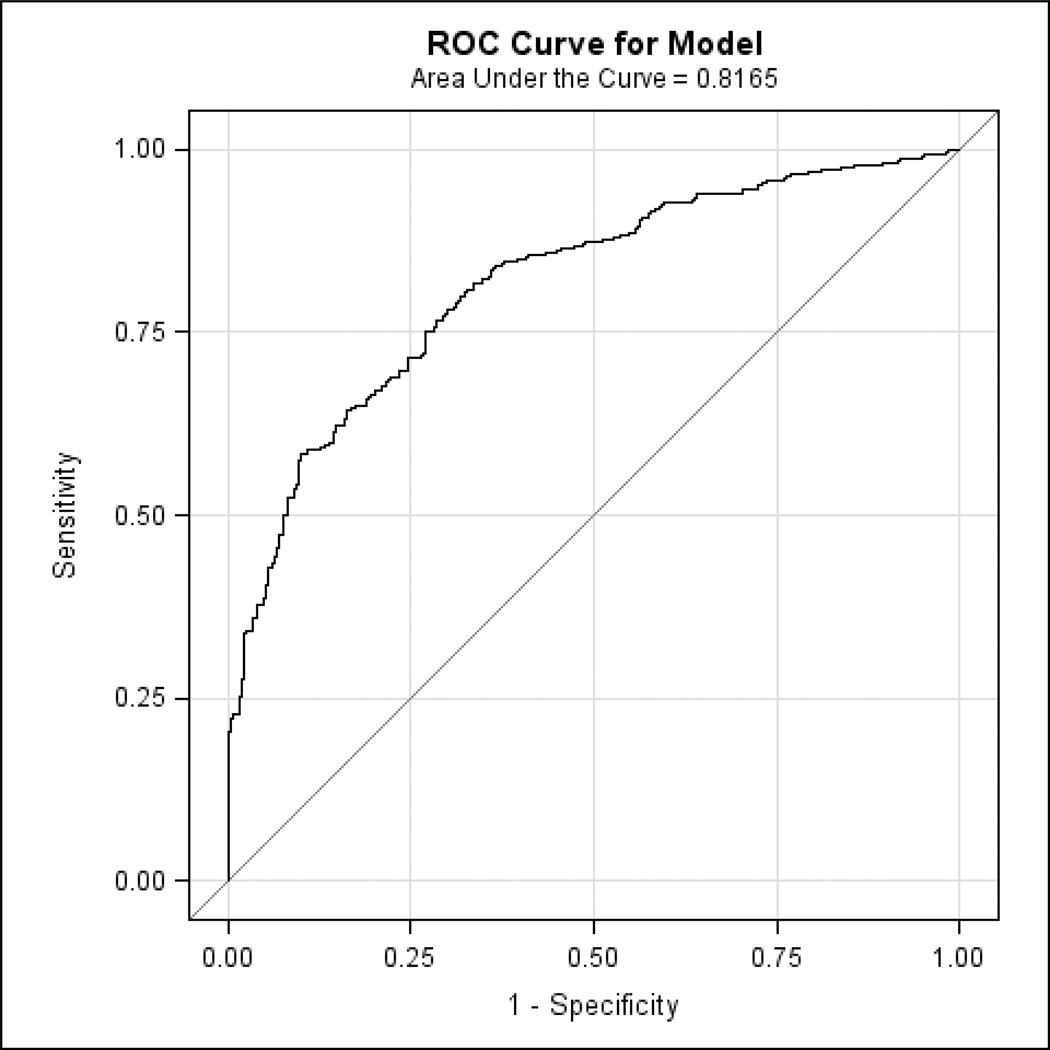
We found 4 significant participant-based ECog items, for each of which a higher score (worse IADL) predicted greater probability of being assigned a diagnosis of MCI as compared to CN: “remembering a few shopping items without a list” (p<0.0001; Odds Ratio (OR) per unit=1.79, 95% Confidence Interval (CI)=1.41, 2.29), “remembering appointments, meetings, or engagements” (p<0.0001; OR=2.14, 95% CI=1.60, 2.90), “developing a schedule in advance of anticipated events” (p=0.007; OR=2.10; 95% CI=1.24, 3.68), and “balancing the checkbook without error” (p=0.02; OR=1.56; 95% CI=1.08, 2.32). Lower age (p<0.0001; OR for decade of age=0.48, 95% CI=0.37, 0.62) and lower estimated premorbid intelligence (AMNART IQ) (p=0.002; OR for 10 points=0.75, 95% CI=0.62, 0.90) also predicted higher probability of MCI for this sample and were controlled for. The model as a whole was highly significant (p<0.0001). Figure 1 illustrates the relation of the optimal linear combination of logistic regression predictors (the 4 participant-based ECog items above, age, and AMNART IQ) to the probability of being assigned a diagnosis of MCI versus CN. Additionally, the specificity and sensitivity of this linear combination was high, with the area under the receiver operating characteristic (ROC) curve equal to 0.82 (see Figure 2). When repeating the above analyses with only the 4 participant-based ECog items excluding the covariates (age and AMNART IQ), the area under the ROC curve was minimally reduced to 0.78.
Figure 1.
The relation of the optimal linear combination of logistic regression predictors to the probability of being assigned a diagnosis of MCI versus CN is illustrated. Age, AMNART IQ and 4 of the participant-based ECog items were retained in the final model. The y-axis signifies the probability of MCI with 1 = MCI and 0 = CN. The circles represent actual subjects whereas the sigmoid curve is the predicted probability of being assigned a diagnosis of MCI as opposed to CN. AMNART IQ (American National Adult Reading Test intelligence quotient), CN (clinically normal), ECog (Everyday Cognition), MCI (mild cognitive impairment).
Figure 2.
The ROC curve corresponding to the logistic regression model indicates the specificity and sensitivity of the optimal linear combination of 4 participant-based ECog items (and the covariates of age and AMNART IQ) retained in the final model discriminating MCI from CN. AMNART IQ (American National Adult Reading Test intelligence quotient), CN (clinically normal), ECog (Everyday Cognition), MCI (mild cognitive impairment), ROC (receiver operating characteristic).
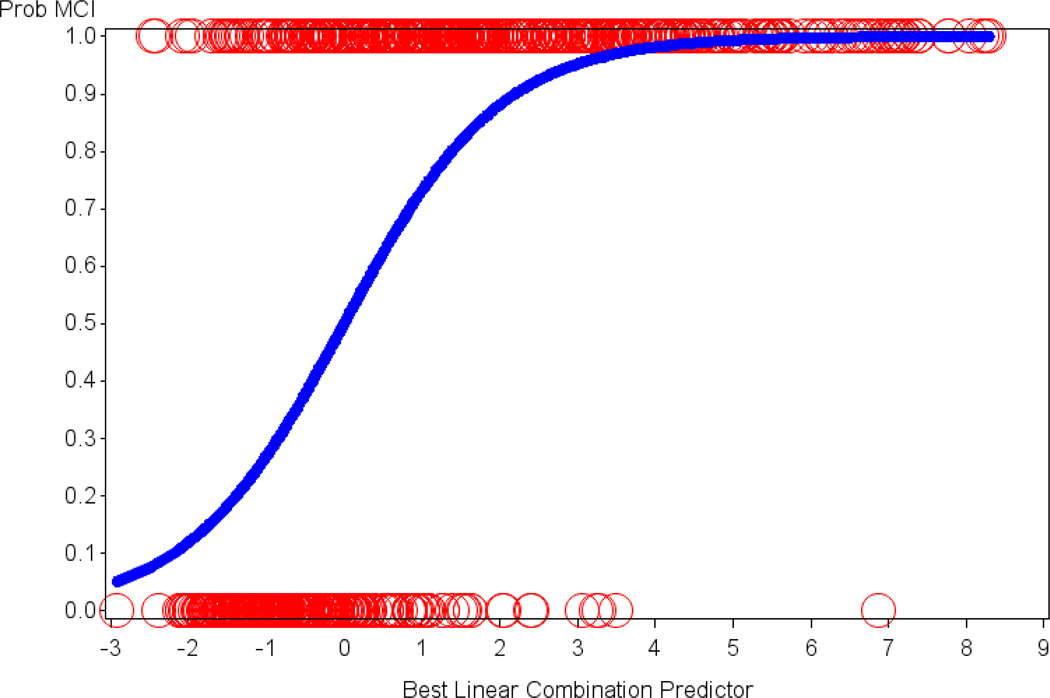
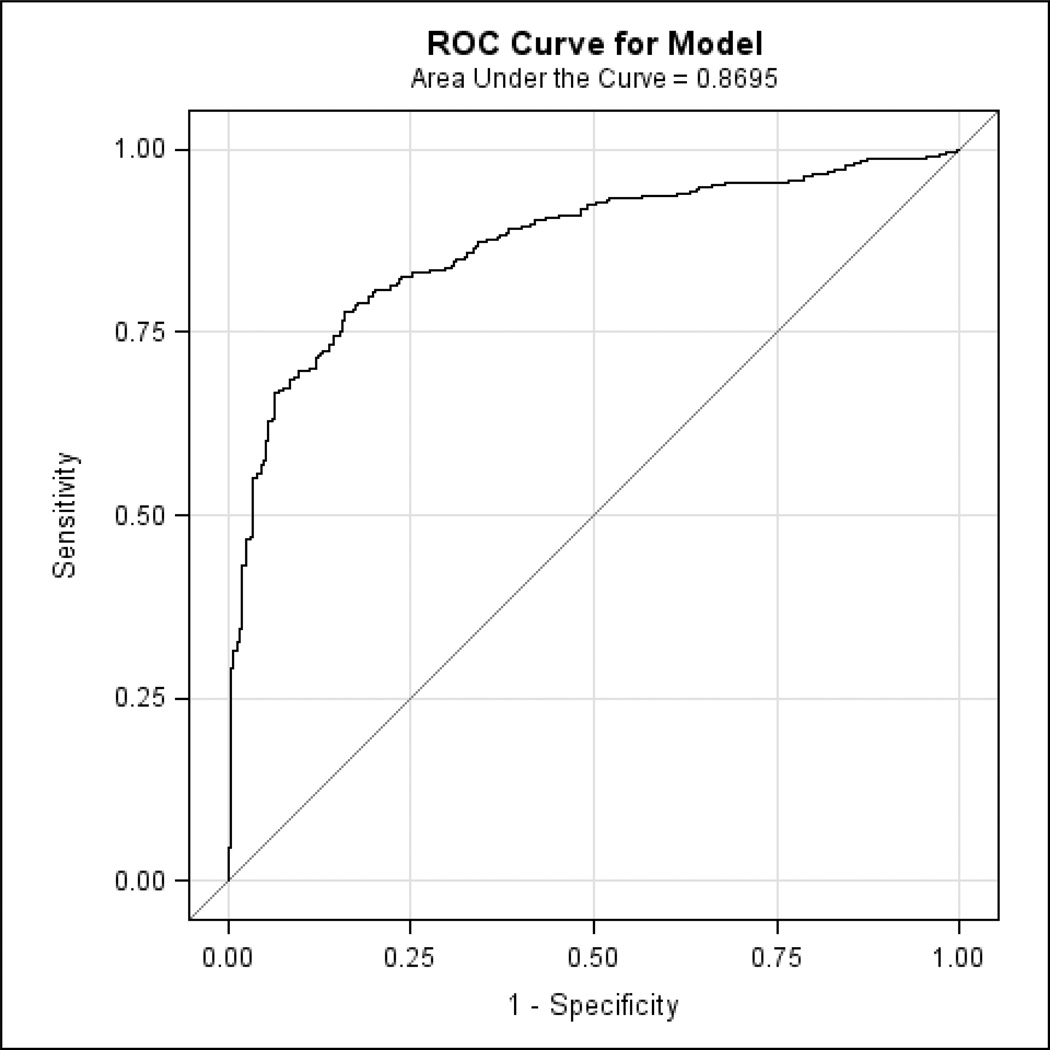
We found 3 significant informant-based ECog items, for each of which a higher score (worse IADL) predicted greater probability of being assigned a diagnosis of MCI as compared to CN: “remembering a few shopping items without a list” (p<0.0001; OR=3.51, 95% CI=2.45, 5.11), “remembering appointments, meetings, or engagements” (p<0.0001; OR=2.38, 95% CI=1.64, 3.53), and “keeping mail and papers organized” (p=0.002; OR=1.63, 95% CI=1.20, 2.24). Lower age (p<0.0001; OR for decade of age=0.43, 95% CI=0.33, 0.56) and lower estimated premorbid intelligence (AMNART IQ) (p=0.0002; OR for 10 points=0.67, 95% CI=0.54, 0.82) also predicted higher probability of MCI for this sample and were controlled for. The model as a whole was highly significant (p<0.0001). Figure 3 illustrates the relation of the optimal linear combination of logistic regression predictors (the 3 informant-based ECog items above, age, and AMNART IQ) to the probability of being assigned a diagnosis of MCI versus CN. Additionally, the specificity and sensitivity of this linear combination was high, with the area under the ROC curve equal to 0.87 (see Figure 4). When repeating the above analyses with only the 3 informant-based ECog items excluding the covariates (age and AMNART IQ), the area under the ROC curve was minimally reduced to 0.84.
Figure 3.
The relation of the optimal linear combination of logistic regression predictors to the probability of being assigned a diagnosis of MCI versus CN is illustrated. Age, AMNART IQ and 3 of the informant-based ECog items were retained in the final model. The y-axis signifies the probability of MCI with 1 = MCI and 0 = CN. The circles represent actual subjects whereas the sigmoid curve is the predicted probability of being assigned a diagnosis of MCI as opposed to CN. AMNART IQ (American National Adult Reading Test intelligence quotient), CN (clinically normal), ECog (Everyday Cognition), MCI (mild cognitive impairment).
Figure 4.
The ROC curve corresponding to the logistic regression model indicates the specificity and sensitivity of the optimal linear combination of 3 informant-based ECog items (and the covariates of age and AMNART IQ) retained in the final model discriminating MCI from CN. AMNART IQ (American National Adult Reading Test intelligence quotient), CN (clinically normal), ECog (Everyday Cognition), MCI (mild cognitive impairment), ROC (receiver operating characteristic).
Primary Longitudinal Analyses
After a mean of 1.0±0.4 years, 11 out of 219 participants (5.0%) qualifying for the analyses progressed from a diagnosis of CN to MCI. For participant-based ECog items, we found that worse performance on “keeping mail and papers organized” (Hazard Ratio (HR)=2.27, p=0.07, 95% CI for HR=0.88, 5.34) marginally predicted greater hazard of progressing from CN to MCI (see Figure 5). None of the covariates were significant in this analysis. The proportional hazard assumption of the Cox model was found to be questionable, but a non-parametric survival analysis of the retained ECog item verified its significance (Wilcoxon p=0.0004) due to a significantly greater hazard of transitioning to MCI when the ECog item equaled 3 than when it was 1 (p=0.003).
Figure 5.
Kaplan-Meier survival curves for values of the only participant-based ECog item retained in the model (ECog item “keeping mail and papers organized”). “Survival” means maintenance of a stable diagnosis of CN as opposed to progression from CN to MCI. Different ECog item scores are illustrated (the score range is 1–4, but the highest score for this item in the current analysis was 3). CN (clinically normal), ECog (Everyday Cognition), MCI (mild cognitive impairment).
For informant-based ECog items, none of the ECog items or covariates were significant in this analysis.
Discussion
Our results indicate that a few simple questions targeting early functional changes, addressed either to the individual or an informant who knows them well, can effectively distinguish between CN elderly and individuals with MCI. Additionally, one of those questions marginally predicted progression from CN to MCI. These findings were significant after adjusting for participant demographics, among which lower age and an estimate of premorbid intelligence were associated with greater likelihood of having a diagnosis of MCI versus CN.
Prior studies utilizing the total ECog score have shown that it can help effectively differentiate not only between MCI and AD dementia, but also between MCI and CN elderly [5]. In our preliminary analyses looking separately at each of the 17 IADL-related ECog items for both participant-based and informant-based reports, worse IADL performance was noted for participants with MCI when compared to CN elderly for all items regardless of the method of correction for multiple comparisons that was employed. However, when looking at all the items together, statistically adjusting each for correlations with the others and with demographics, 5 ECog items were found to best distinguish between CN and MCI: “remembering a few shopping items without a list”, “remembering appointments, meetings, or engagements”, “developing a schedule in advance of anticipated events”, “balancing the checkbook without error”, and “keeping mail and papers organized”. These results are in line with a similar study using the ADNI database, which found that the Functional Activities Questionnaire items that best distinguished between CN and MCI were “remembering appointments, family occasions, holidays, and medications” and “assembling tax records, business affairs, or other papers” [2]. These everyday activities depend primarily on memory and executive function. Early changes in IADL have been shown to be associated with early global cognitive impairment, as well as impairment in specific domains, of which executive function has been implicated most often [3,9,19].
The ECog is administered to both the participant and an informant, which is especially important when assessing early cognitive and IADL changes in individuals at risk for AD [10]. Participants and informants reporting cognitive and IADL performance on the ECog have been shown to be in agreement when rating CN elderly and those with MCI, but participants with dementia tended to under-report their deficits when compared to their informants’ report [20]. In the current study, the two memory dependent ECog items (“remembering a few shopping items without a list” and “remembering appointments, meetings, or engagements”) best discriminated between CN and MCI for both participant and informant-based reports. However, the effect size (either in the preliminary univariate analyses measured by the percent variance or in the primary multivariate analyses measured by the odds ratio) were larger for the informant-based items. On the other hand, the executive function dependent ECog items did not line up for participant and informant: “developing a schedule in advance of anticipated events” and “balancing the checkbook without error” stood out for participant-based reports, while “keeping mail and papers organized” stood out for informant-based reports. This is in agreement with a prior study that showed that individuals with MCI reported performing better on financial tasks when compared to their informants’ reports; they also tended to report better performance in driving [21]. On the other hand CN elderly or individuals with subjective cognitive concerns not meeting criteria for MCI are acutely aware of changes in their cognition and IADL and may even be more aware of these changes than their informants [22]. Therefore, it appears that new subjective IADL scales that target individuals with preclinical or early prodromal AD need to consist of self-report and not just informant-report, which has been the standard for most IADL scales.
In the current study, one participant-based ECog item marginally predicted progression from CN to MCI: “keeping mail and papers organized”. As noted above, the same item reported by informants stood out in discriminating between CN and MCI at baseline. Therefore, it could be that when a normal individual notes this type of everyday difficulty, it is a sign of impending decline in the future, but when it is noted by somebody who knows the individual well, that individual already has cognitive impairment consistent with MCI. Two other scales (the Activities of Daily Living Prevention Instrument, which is a subjective IADL scale, and the Structured Interview and Scoring Tool—Massachusetts Alzheimer’s Disease Research Center Informant Report, which combines subjective report of IADL and cognitive performance) have been shown to predict future cognitive decline in CN, as well as progression from CN to MCI [6,7]. Of note, participants in the current study were followed up to 2 years but the mean follow-up period was 1 year and only 5% of CN elderly progressed to MCI over the course of the study. Therefore, future studies with longer follow-up periods will be necessary to better determine the potential of the various ECog items to predict disease progression. These studies could be augmented by adding biomarker information that could help designate CN individuals as having different stages of preclinical AD based on presence or absence of amyloid and markers of neurodegeneration.
There were some limitations to the current study. The ADNI sample used for our analyses consisted of highly educated and intelligent individuals willing to undergo extensive assessments. Therefore, this sample does not necessarily generalize to the rest of the population. That said, the primary analyses were adjusted for level of education and an estimate of premorbid intelligence. Moreover, this sample is representative of natural history biomarker studies and clinical trials and therefore our results can certainly inform future clinical research endeavors. However, they would need to be replicated in the general population before more widely implementing them in clinical practice as potentially sensitive screening questions. As mentioned above, the longitudinal follow-up was limited, not allowing a sufficient number of individuals to progress from CN to MCI. Therefore, longer follow-up is needed. That said, one ECog item was found to have marginal significance, and that item was also identified in the cross-sectional analyses, suggesting that the finding may not be spurious.
Conclusions
In the current study, we were able to demonstrate that 5 questions addressing everyday functioning can effectively distinguish between those who are aging normally and those with cognitive deficits consistent with MCI. Moreover, one of those questions suggested future decline to MCI in normal elderly individuals. Once this study is replicated in the general population, these questions can serve as a useful, straight-forward screening tool for early AD. IADL impairment in early AD has been associated with greater amyloid burden and various markers of neurodegeneration [23–30]. In order to better validate the utility of the sensitive questions identified here, future studies could explore their association with AD biomarkers. Moreover, these questions can be used to develop more sensitive subjective IADL scales targeting preclinical AD that could be used as outcome measures to detect IADL changes in individuals who decline in upcoming secondary prevention trials. As we shift toward earlier detection and treatment of AD, improved assessment of IADL changes is vital to assure that the voices of our patients and caregivers, who care most about their daily functioning, are represented.
Acknowledgements
This study was supported by ADNI (U01 AG024904), DOD ADNI (W81XWH-12-2-0012), R01 AG027435, K23 AG033634, K24 AG035007, the Harvard Aging Brain Study (P01 AGO36694), and the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The authors are site investigators and research staff for ADNI at Brigham and Women’s Hospital and Massachusetts General Hospital. The other site investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/ uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Conflicts of Interest
The authors have received research salary support from Janssen Alzheimer Immunotherapy (GAM, REA), Wyeth/Pfizer Pharmaceuticals (GAM, REA), Eisai Inc. (GAM), Eli Lilly and Company (GAM), and Bristol-Myers-Squibb (RAS).
References
- 1.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA, et al. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2011;7(3):300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris JC. Revised Criteria for Mild Cognitive Impairment May Compromise the Diagnosis of Alzheimer Disease Dementia. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farias ST, Mungas D, Harvey DJ, Simmons A, Reed BR, DeCarli C. The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement. 2011:7593–7601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006;20(4) Suppl 3:S152–S169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- 7.Okereke OI, Pantoja-Galicia N, Copeland M, Hyman BT, Wanggaard T, Albert MS, et al. The SIST-M: predictive validity of a brief structured clinical dementia rating interview. Alzheimer Dis Assoc Disord. 2012;26(3):225–231. doi: 10.1097/WAD.0b013e318231cd30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall GA, Amariglio RE, Sperling RA, Rentz DM. Activities of daily living: Where do they fit in the diagnosis of Alzheimer’s disease. Neurodegener Dis Manag. 2012;2(5):483–491. doi: 10.2217/nmt.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–S68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7(5):486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh JI, Yesavage JA. Clinical Gerontology : A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. WMS-R Wechsler Memory Scale Revised Manual. New York: The Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1987. [Google Scholar]
- 17.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 18.Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14(2):234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- 19.Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ, et al. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19(3):249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 20.Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20(9):827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okonkwo OC, Griffith HR, Vance DE, Marson DC, Ball KK, Wadley VG. Awareness of functional difficulties in mild cognitive impairment: a multidomain assessment approach. J Am Geriatr Soc. 2009;57(6):978–984. doi: 10.1111/j.1532-5415.2009.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caselli RJ, Chen K, Locke DE, Lee W, Roontiva A, Bandy D, et al. Subjective cognitive decline: Self and informant comparisons. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Gois Vasconcelos L, Jackowski AP, Oliveira MO, Flor YM, Bueno OF, Brucki SM. Voxel-based morphometry findings in Alzheimer's disease: neuropsychiatric symptoms and disability correlations - preliminary results. Clinics (Sao Paulo) 2011;66(6):1045–1050. doi: 10.1590/S1807-59322011000600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall GA, Lorius N, Locascio JJ, Hyman BT, Rentz DM, Johnson KA, et al. Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer disease spectrum. J Alzheimers Dis. 2014;41(3):719–728. doi: 10.3233/JAD-132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall GA, Olson LE, Frey MT, Maye J, Becker JA, Rentz DM, et al. Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord. 2011;31(6):443–450. doi: 10.1159/000329543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadkarni NK, Levy-Cooperman N, Black SE. Functional correlates of instrumental activities of daily living in mild Alzheimer's disease. Neurobiol Aging. 2012;33(1):53–60. doi: 10.1016/j.neurobiolaging.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okonkwo OC, Alosco ML, Griffith HR, Mielke MM, Shaw LM, Trojanowski JQ, et al. Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol. 2010;67(6):688–696. doi: 10.1001/archneurol.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy K, Pepin LC, Philiossaint M, Lorius N, Becker JA, Locascio JJ, et al. Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2014;42(1):291–300. doi: 10.3233/JAD-131796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer's disease. J Alzheimers Dis. 2010;19(2):517–527. doi: 10.3233/JAD-2010-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]