Abstract
Background
Manganese (Mn) is an essential element that can become neurotoxic through various exposure windows over the lifespan. While there is clear evidence of Mn neurotoxicity in pediatric and adult occupational populations, little is known about effects in the elderly who may exhibit enhanced susceptibilities due to compromised physiology compared to younger adults. In the province of Brescia, Italy, the Valcamonica area has been the site of three ferroalloy plants operating from 1902 to 2001. Metal emissions of Mn and to a lesser extent lead (Pb) have impacted the surrounding environment, where a high prevalence of Parkinsonism was previously observed. This study aimed to assess neurocognitive and motor functions in healthy elderly subjects residing for most of their lifetime in Valcamonica or in a reference area unimpacted by ferroalloy plant activity.
Methods
Subjects were enrolled for extensive neurobehavioral assessment of motor, cognitive and sensory functions. Exposure was assessed with 24hour personal air sampling for PM10 airborne particles, surface soil and tap water measurement at individual households, Mn levels in blood and urine and Pb in blood. Dose-response relationships between exposure indicators and biomarkers and health outcomes were analyzed with Generalized (linear and logistic) Additive Models (GAM).
Results
A total of 255 subjects (55% women) were examined; most (52.9%) were within the 65–70 years age class. Average airborne Mn was 26.41 ng/m3 (median 18.42) in Valcamonica and 20.96 ng/m3 (median 17.62) in the reference area. Average Mn in surface soil was 1026 ppm (median 923) in Valcamonica and 421 ppm (median 410) in the reference area. Manganese in drinking water was below the LDL of 1 µg/L. The GAM analysis showed significant association between airborne Mn (p=0.0237) and the motor coordination tests of the Luria Nebraska Neuropsychological Battery. The calculation of the Benchmark Dose using this dose response relationship yielded a lower level confidence interval of 22.7 ng/m3 (median 26.4). For the odor identification score of the Sniffin Stick test, an association was observed with soil Mn (p=0.0006) and with a significant interaction with blood Pb (p=0.0856). Significant dose-responses resulted also for the Raven’s Colored Progressive Matrices with the distance from exposure point source (p=0.0025) and Mn in soil (p=0.09), and for the Trail Making test, with urinary Mn (p=0.0074). Serum prolactin (PRL) levels were associated with air (p=0.061) and urinary (p=0.003) Mn, and with blood Pb (p=0.0303). In most of these associations age played a significant role as an effect modifier.
Conclusion
Lifelong exposure to Mn was significantly associated with changes in odor discrimination, motor coordination, cognitive abilities and serum PRL levels. These effects are consistent with the hypothesis of a specific mechanism of toxicity of Mn on the dopaminergic system. Lead co-exposure, even at very low levels, can further enhance Mn toxicity.
Keywords: Ferroalloy emission, heavy metals, manganese, lead, odor identification, motor coordination, cognitive abilities, prolactin control, elderly
INTRODUCTION
While it is clear that the aged are at greater risk and more susceptible to the deleterious effects from exposure to environmental agents compared to younger adults (Geller and Zenick, 2005; Risher et al., 2010), few studies have investigated manganese (Mn) pathophysiology in the elderly as a specific sensitive population. A large body of evidence, now further confirmed by meta- (Meyer-Baron et al., 2011) and pooled analyses (Meyer-Baron et al., 2013), shows that prolonged occupational exposure to Mn, even at relatively low levels causes motor neurotoxicity which may persist into retirement (Bouchard et al., 2008). Non-occupational studies with adults have shown both neuromotor and cognitive abnormalities (Kim et al. 2011; Roels et al. 2012), including increased frequency of Parkinsonism associated with Mn in airborne particles (Finkelstein and Jerrett 2007) and deposited dust (Lucchini et al. 2007). Moreover, a high prevalence of Parkinsonism has also been observed in Mn-exposed welders (Racette et al., 2012), though the symptomatology of Mn-associated Parkinsonism in welders may differ from both idiopathic Parkinson Disease and the Parkinsonism associated with environmental Mn exposure (Racette et al., 2013). This difference in symptomatology may be due to the particular type of exposure in welding operations, which are mainly characterized by fine and ultra-fine respirable particles.
Over the past decade, there has also emerged evidence of health deficits associated with elevated Mn in newborns (Claus Henn et al. 2012), and older children exposed via drinking water (Bouchard et al. 2011; Wasserman et al. 2011; Khan et al. 2012) and airborne particulates (Riojas-Rodríguez et al. 2010; Menezes-Filho et al. 2011; Lucchini et al. 2012; Zoni et al. 2012; Vivas-Carvalho et al., 2013 ). In particular, a recent study from our group showed an association between environmental Mn exposure and deficits in fine motor and olfactory discrimination in children 11–13 yrs of age (Lucchini et al., 2012) – both Mn-related health effects similar to those reported in adult studies of Mn-associated Parkinsonism (Zoni et al., 2012). Overall, these studies support the concept that exposure to Mn over different temporal windows throughout the lifespan, even at relatively low levels of exposure, may lead to similar long-lasting neurotoxic endpoints (Lucchini and Zimmerman 2009).
In light of this new evidence, we investigated the relationship between environmental Mn contamination and neurological health outcomes in aged subjects living in regions impacted by ferromanganese plant emissions in northern Italy. Previously we have reported a higher than expected prevalence of Parkinsonism in this region in relation to Mn emissions from ferroalloy plants (Lucchini et al. 2007), and Mn-related neurological deficits in both adolescents from this region and adult ferromanganese plant workers (Lucchini et al. 1999, 2012).
METHODS
Target areas
The industrial sources of Mn in the study area are three former ferromanganese plants located in ValCamonica (VC), a valley of the pre-Alps that runs for about 50 miles in the NE-SW direction with an average width of about 2 miles, and is delimited by mountains of about 10,000 feet elevation. The industries operated from 1902 to 2001 in the municipalities of Darfo (lower Valcamonica, population 13,200), Breno (mid Valcamonica, population 5,000), and Sellero (upper Valcamonica, population 1,500). The Garda Lake (GL) tourist area of the Province of Brescia, with no history of metal industry was used as a reference group community. More detailed information on the study areas were published previously (Lucchini et al., 2007, 2012). Environmental levels of Mn and other metals have been thoroughly characterized in the study regions, showing that levels of Mn are significantly higher in Valcamonica compared to the Garda Lake reference area for airborne particles (Borgese et al. 2011, 2012), deposited outdoor dust (Zacco et al. 2009), indoor house and attic dust (Pavilonis, submitted), soil (Borgese et al., 2013), and locally cultivated leafy vegetables (Ferri et al. 2012).
Study design
Elderly subjects residing in the historically exposed area of Valcamonica and in the reference area of Garda Lake were enrolled in the study. This research was part of a large project funded by the European Union 6th Frame Program called PHIME (Public Health Impact of Mixed element Exposure in susceptible populations) that targeted various age groups in the community including pregnant women, adolescents, adult workers and elderly. Based on a community approach, the PHIME study was designed with a strong collaborative interaction with various community stakeholders. Subjects were recruited through public social centers, trade unions, and cultural and religious associations, and then invited to attend ad hoc meetings where the study aims and methodology were explained in detail. Inclusion criteria included men and women aged 65–75 yrs and locally residing since at least the 1970s. Eligible participants were interviewed for the assessment of the following exclusion criteria: i) exposure to neurotoxic agents through occupation or hobbies; ii) alcohol consumption >80 g/day; iii) clinical neurologic, hepatic, or psychiatric disease; iv) medical therapies active on the nervous system; v) joint diseases of the hand and fingers; vii) visual deficits not adequately corrected. Once properly informed, participants signed an informed consent that was approved by the Ethical Committee of the Local Public Health Agency of Brescia. The health assessment was conducted on different days over 2 consecutive weeks. Trained medical doctors and neuro-psychologists conducted the testing within facilities made available by the local Public Health Agency. Socio-demographic data, consumption of alcohol and smoking habits, clinical, occupational and residential histories were collected with ad-hoc questionnaires specifically designed to assess this cohort. A questionnaire for the screening of Parkinson’s disease was also administered (Panisset et al. 1996), which included 10 items that were weighted in order to obtain a final score for the classification of “unlikely”, “possible”, or “probable” Parkinson’s disease. Anthropometric data were measured for the calculation of Body Mass Index (BMI), and a food frequency questionnaire weighted for portion sizes was administered to estimate the daily oral intake of Mn. Each participant filled a personal diary with complete records of their activities and time spent in indoor/outdoor locations during the air-sampling period. Data on atmospheric conditions during the sampling period were obtained by the online meteorological system of the local Environmental Protection Office (ARPA Lombardia).
Neuropsychological battery
The health assessment test battery aimed to assess cognitive and motor functions and was identified based on a review of specific reported in the literature for Mn neurotoxicity (Zoni et al., 2007). It included the Mini-Mental State Examination (MMSE) (Folstein et al. 1975) based on 30 simple questions and problems in a number of areas: the time and place of the test, repeating lists of words, arithmetic, language use and comprehension, and basic motor skills. The Italian version of the Story Recall Test (Spinnler and Tognoni, 1987) was used to evaluate long-term verbal memory; for this, the examiner read a short story and asked the subject to repeat it (immediate recall). The Raven’s Colored Progressive Matrices (CPM) test (Raven et al. 1983) was used to measure clear-thinking ability; it consisted of 36 items in three sets (A, AB, B), with 12 items per set. Each item contained a figure with a missing piece that the subject selected from a presented panel of options. The Trail Making test (TMT) (Reitan 1985) was used to assess the ability of spatial planning in a visual-motor track and the ability to quickly switch from a numeric stimulus to an alphabetical one. The TMT consisted of two parts, A and B; part A required adequate capacity for visual processing, recognition of numbers, knowledge and reproduction of numerical sequences and motor speed; part B required also cognitive flexibility and a shifting ability. The Digit Span (from WAIS) was used to evaluate short-time memory, attention and auditory recall; the test consisted of two different tests components: i) Digits Forward (repeating numbers forward), and ii) Digits Backward (repeating numbers backward). Motor coordination was assessed with the five subtasks from the Luria Nebraska Neuropsychological Battery (Golden et al., 1980), and the Finger Tapping test from the computerized SPES battery (Swedish Performance Evaluating System) (Iregren et al., 1996). The Digit Symbol (from WAIS) (Wechsler 1997) test was used to assess perceptual and motor speed; it consisted of digit-symbol pairs, followed by a list of digits. Under each digit the subject was asked to write down the corresponding symbol as fast as possible. The Simple Visual Reaction Time from the SPES assessed psychomotor speed measured with the time in millisecond elapsing from the presentation of a red rectangle on the computer monitor and the subject response on the space bar. Resting tremor and body sway were assessed using the Catsys Tremor 7.0 by Danish Product Development (Després et al., 2000), as previously described (Lucchini et al, 2012). The Sniffin Sticks-Olfactory Screening test (Hummel et al., 2001) was used to assess odor discrimination and identification, as described previously (Lucchini et al. 2012).
Exposure assessment
Inhalation exposure to PM10 airborne particulate matter was determined using 24h personal air monitoring (50% collection efficiency for 10 µm aerodynamic diameter particles). Airborne particles were collected on commercial filters (37 mm diameter, PTFE-Teflon) using Personal Environmental Monitors (PEM) connected to a Leland Legacy pump (SKC, Inc., Eighty-Four, PA, USA) contained within a small backpack worn by the subject. The PEM air sampler was mounted onto the backpack front strap, in or near the breathing zone, while the pump was carried in the backpack. Pumps were pre-calibrated to a flow rate of 10 L/min, using a soapless piston primary calibrator (Defender, BIOS, Butler, NJ, USA), with post-sampling flow rate confirmation. Total PM10 particulate load on the filter was determined gravimetrically, as well as chemically. Particulate metal content was determined using total reflection X-ray fluorescence (TXRF) spectroscopy, according to a methodology published elsewhere (Borgese et al., 2011, 2012). Each participant’s house was geo-referenced for spatial analysis and surface soil was analyzed for Mn and other elements with a portable X-Ray Fluorescence instrument, as previously described (Lucchini et al., 2012). Tap water was sampled from the subject’s primary residence after a 2 min flushing at a medium flow rate; water was analyzed by Total Relaxation XRF for Mn and other elements, with a water Mn detection limit of 1 µg/L.
Metal levels in blood and urine were evaluated as exposure biomarkers, as described previously (Lucchini et al., 2012). Blood samples were also used to assess complete blood counts (CBC), iron status (serum ferritin), liver and kidney function (via serum aminotransferase enzymes), and serum PRL. Each individual participant’s data was entered with anonymized identification codes accessible only to the research team, and under the responsibility of the study Principal Investigator (RL).
Statistical analysis
Preliminary graphics and stratified descriptive analyses were used to explore the distribution of the exposure-related variables and their relationship with the motor, sensory and cognitive test results in the different study sites. Non parametric Kruskal-Wallis tests was used to test the null hypothesis of no effect of the area of residence (VC sub-areas versus GL) on the tests results. Since surface soil was not measured for the entire study population we used Ordinary Kriging interpolation of measured soil Mn levels to obtain Mn concentration estimates at the missing points and gain power in the subsequent regression analyses, according to a procedure described previously (Lucchini et al., 2012).
To test the hypothesis that Mn exposure affects motor and sensory functions and to obtain an adjusted estimate of the effect, we used Generalized (linear and logistic) Additive Models (GAM) (Hastie and Tibshirani, 1990). The GAM model allows to relax the assumption of linearity between the response variable and some of the covariates, by using as a linear predictor a smooth functions of covariates. In addition to the exposure variables we always considered the effect of age, gender, self-reported alcohol consumption and smoking habits, and area of residence. Therefore we included in the GAM models the effects of the following variables, assuming additivity between them: gender, age, smoking and alcohol habits, the distance from the nearest ferromanganese plant source, the concentration of Mn in air, soil, blood, urine and the concentration of Pb in blood. All exposure variables were log-transformed before being entered in the GAMs in order to obtain a more symmetrical distribution of the model residuals. When preliminary analyses suggested a deviation from additivity in the various areas, like a different pattern in the shape of the Mn-related outcomes between the two geographic areas, we used a stratified GAM to obtain an area-specific non-parametric estimate of the Mn exposure – response relationship. We also considered the possible interaction, by means of tensor product splines, between Mn and Pb exposure when these variables were shown to influence the test outcomes. The levels of statistical significance was considered as p<0.05 and as 0.1<p<0.05 for trending observations. Data analysis and graphics were made with R (R-Core Team, 2013).
RESULTS
Socio-demographics
The study included 255 elderly subjects out of a total of 365 originally enrolled. Exclusion of 110 subjects was due to: withdrawal (n=47), not meeting age criteria (n=12), neurological diseases (n=20), previous occupational exposure to a neurotoxicant (n=10), endocrine disease (n=5), death (n=4), not meeting the residence criteria (n=4), psychiatric symptoms (n=2), visual deficiency (n=2), or other diseases (n=4). The details from participants residing in Valcamonica (n= 153) and in the GL area (n=102) are listed in table 1. The subjects from Valcamonica were further sub-classified into three sub-areas of residence according to the three sites of previous ferromanganese plant operations within Valcamonica: Darfo (lower Valcamonica, n=39), Breno (mid Valcamonica, n=65), and Sellero (upper Valcamonica, n=49). Overall, women (54.9%) were more represented than men (45.1%). The number of the study participants was representative of the population of the same age range residing in the target areas, and it varied from 2% at the Garda Lake to 26% in Sellero. Age (in years) was mostly represented by the 65–70 class (52.9) whereas the 70–75 class was 27.8%. Alcohol consumption (in g/day) was mostly represented by the zero class (37.9%), followed by the 1- 20 class (22.9%) and the 20–50 class (22.5%). Subjects were mostly never smokers (67.5%), followed by ex-smokers (24.7%) and current smokers (7.8%). Clinical values for CBC, liver and kidney function, physiological iron status and serum PRL for the 255 included subjects were all within the normal clinical range. No significant differences occurred among the study sites for educational level.
Table 1.
| a: Socio-demographic nominal variables of
the elderly subjects. The Valcamonica (VC) communities of Sellero, Breno, and Darfo
correspond to the upper, mid, and lower VC, respectively. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Levels | Garda | % Garda |
Lower VC |
%Lower VC |
Medium VC |
Upper VC |
%Upper VC |
all | % all |
Σ %all |
| Gender | f | 60 | 58.8 | 29 | 59.2 | 31 | 20 | 51.3 | 140 | 54.9 | 54.9 |
| m | 42 | 41.2 | 20 | 40.8 | 34 | 19 | 48.7 | 115 | 45.1 | 100.0 | |
| all | 102 | 100.0 | 49 | 100.0 | 65 | 39 | 100.0 | 255 | 100.0 | ||
| Age | [60,65] | 8 | 7.8 | 3 | 6.1 | 12 | 1 | 2.6 | 24 | 9.4 | 9.4 |
| (65,70] | 40 | 39.2 | 33 | 67.3 | 38 | 24 | 61.5 | 135 | 52.9 | 62.3 | |
| (70,75] | 34 | 33.3 | 12 | 24.5 | 13 | 12 | 30.8 | 71 | 27.8 | 90.2 | |
| (75,80] | 20 | 19.6 | 1 | 2.0 | 2 | 2 | 5.1 | 25 | 9.8 | 100.0 | |
| all | 102 | 100.0 | 49 | 100.0 | 65 | 39 | 100.0 | 255 | 100.0 | ||
| Alch | 0 | 46 | 45.5 | 17 | 35.4 | 18 | 15 | 38.5 | 96 | 37.9 | 37.9 |
| (0, 20] | 24 | 23.8 | 10 | 20.8 | 16 | 8 | 20.5 | 58 | 22.9 | 60.9 | |
| (20, 50] | 19 | 18.8 | 15 | 31.2 | 13 | 10 | 25.6 | 57 | 22.5 | 83.4 | |
| >50 | 12 | 11.9 | 6 | 12.5 | 18 | 6 | 15.4 | 42 | 16.6 | 100.0 | |
| all | 101 | 100.0 | 48 | 100.0 | 65 | 39 | 100.0 | 253 | 100.0 | ||
| Smoke | never | 71 | 69.6 | 27 | 55.1 | 45 | 29 | 74.4 | 172 | 67.5 | 67.5 |
| actual | 5 | 4.9 | 7 | 14.3 | 5 | 3 | 7.7 | 20 | 7.8 | 75.3 | |
| ex | 26 | 25.5 | 15 | 30.6 | 15 | 7 | 17.9 | 63 | 24.7 | 100.0 | |
| all | 102 | 100.0 | 49 | 100.0 | 65 | 39 | 100.0 | 255 | 100.0 | ||
| b: Socio-demographic continuous variables
of the elderly subjects. The Valcamonica (VC) communities of Sellero, Breno, and Darfo
correspond to the upper, mid, and lower VC, respectively. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Levels | n | Min | 1st q.le | Mean | Median | 2rd q.le | Max | #NA | ||
| age | Garda | 102 | 63.0 | 69.0 | 71.3 | 71.0 | 74.0 | 79.0 | 0 | ||
| Lower VC | 49 | 63.0 | 68.0 | 69.3 | 69.0 | 71.0 | 77.0 | 0 | |||
| Medium VC | 65 | 63.0 | 66.0 | 68.6 | 68.0 | 70.0 | 80.0 | 0 | |||
| Upper VC | 39 | 65.0 | 68.0 | 70.1 | 70.0 | 71.5 | 80.0 | 0 | |||
| all | 255 | 63.0 | 67.0 | 70.0 | 69.0 | 72.5 | 80.0 | 0 | |||
| weight | Garda | 101 | 51.4 | 67.3 | 78.0 | 75.2 | 87.4 | 128.5 | 1 | ||
| Lower VC | 45 | 54.2 | 67.0 | 75.9 | 75.6 | 84.4 | 104.7 | 4 | |||
| Medium VC | 51 | 54.7 | 64.2 | 73.6 | 73.3 | 81.5 | 103.5 | 14 | |||
| Upper VC | 36 | 44.5 | 59.1 | 71.5 | 71.4 | 76.5 | 105.0 | 3 | |||
| all | 233 | 44.5 | 65.6 | 75.6 | 74.5 | 84.2 | 128.5 | 22 | |||
| School years | Garda | 102 | 5.0 | 5.0 | 7.8 | 5.0 | 11.8 | 18.0 | 0 | ||
| Lower VC | 49 | 5.0 | 5.0 | 6.0 | 5.0 | 8.0 | 13.0 | 0 | |||
| Medium VC | 64 | 5.0 | 5.0 | 6.4 | 5.0 | 6.5 | 16.0 | 1 | |||
| Upper VC | 39 | 5.0 | 5.0 | 7.5 | 5.0 | 9.5 | 17.0 | 0 | |||
| all | 254 | 5.0 | 5.0 | 7.1 | 5.0 | 8.0 | 18.0 | 1 | |||
Exposure assessment
The descriptive statistics for environmental and biological exposure indicators are reported in table 2. The average Mn concentration in 24h PM10 airborne particles for the entire group of subjects was 24.2 ng/m3 (median 18.3, range 2.44–103), with higher levels in Valcamonica (mean 26.4, median 18.4, range 1.99–103 ng/m3) compared to Garda Lake (mean 21.0, median 17.6, range 2.44–68.1 ng/m3). The median concentration of PM10 airborne particulates was 29.5 µg/m3 (31.2 in Valcamonica and 28.1 in Garda Lake). The highest levels of PM10 airborne particulates were measured in the mid valley area of Breno, where the ferromanganese plant closed most recently. The subjects’ diaries kept over the 24h air sampling period showed an average of 5h outdoor and 19h indoor, with no variation across the different study areas. Data on rain precipitation showed that 16% of samples were collected over 24h periods that contained some rain, while 84% were collected with no rain during the sampling time, with slight differences between the Valcamonica (10% rain and 90% no rain) and Garda Lake (26% rain and 74% no rain) areas. In surface soil, the overall average Mn concentration was 784 µg/g (median 786, range 313–1724), with significantly higher levels in Valcamonica (mean 1026, median 923, range 473–1724) compared to Garda Lake (mean 421, median 410, range 313–549). Soil levels showed an increasing gradient from the lower to the upper valley, consistent with an underlying geological presence of higher Mn levels in deep soil and bedrock. The concentrations of dissolved metals in tap water, reflecting the public water supply from either study area, were not elevated. In fact, water Mn levels were below the LDL of 1 µg/L in all water samples. Regarding the exposure biomarkers, there were no measurable differences in the subjects’ blood or urine Mn concentrations between study sites, in contrast to the site-based differences in the environmental samples (surface soil and airborne particulates) noted above. Total dietary oral Mn intake calculated with the food frequency questionnaire was 4.76 ± 2.17 mg/day (4.69 ± 2.29 for women and 4.85± 2.0 for men), with no significant differences between the study sites. The main sources of Mn intake were cereals and legumes (combined 3.58 ± 1.97 mg/day), with lower intake from fruit (0.65 ± 0.36 mg/day) and vegetables (0.53± 0.40 mg/day).
Table 2.
Environmental and biological Mn exposure parameters grouped by study area (VC= Valcamonica; GL=GardaLake) (* Kruskal–Wallis test)
| Variable | Area | n | Min | 1st a.le | Mean | Median | 3rd a.le | Max |
|---|---|---|---|---|---|---|---|---|
| Mn soil (ppm) |
VC-Sellero | 39 | 1313.8 | 1455.1 | 1472.1 | 1463.0 | 1497.5 | 1724.0 |
| VC-Breno | 65 | 534.7 | 832.7 | 953.9 | 982.5 | 1150.9 | 1233.3 | |
| VC-Darfo | 49 | 472.8 | 774.5 | 766.6 | 807.7 | 834.6 | 1006.2 | |
| VC | 153 | 472.8 | 811.8 | 1026.0 | 922.9 | 1313.8 | 1724.0 | |
| GL | 102 | 313.3 | 389.1 | 421.0 | 410.0 | 447.1 | 548.9 | |
| P <0.0001 * | all | 255 | 313.3 | 419.2 | 784.0 | 786.3 | 1008.1 | 1724.0 |
| Mn air (ng/m3) |
VC-Sellero | 39 | 2.0 | 13.6 | 23.4 | 16.3 | 31.4 | 58.4 |
| VC-Breno | 65 | 4.0 | 19.8 | 39.3 | 30.0 | 55.2 | 103.0 | |
| VC-Darfo | 49 | 4.1 | 10.0 | 21.1 | 18.0 | 27.6 | 68.5 | |
| VC | 153 | 2.0 | 13.9 | 29.4 | 22.2 | 37.6 | 103.0 | |
| GL | 101 | 2.4 | 15.6 | 21.2 | 20.5 | 26.1 | 68.1 | |
| p < 0.0001 | all | 254 | 2.0 | 14.8 | 26.1 | 21.2 | 30.1 | 103.0 |
| Mn blood (µg/L) |
VC-Sellero | 34 | 4.3 | 7.7 | 9.1 | 9.1 | 10.3 | 15.6 |
| VC-Breno | 64 | 4.3 | 6.4 | 8.0 | 7.5 | 9.8 | 19.5 | |
| VC-Darfo | 48 | 3.6 | 6.3 | 8.4 | 8.2 | 9.6 | 14.8 | |
| VC | 146 | 3.6 | 6.5 | 8.4 | 8.1 | 10.0 | 19.5 | |
| GL | 92 | 3.6 | 7.2 | 10.2 | 9.5 | 12.8 | 21.6 | |
| p = 0.0011 | all | 238 | 3.6 | 6.8 | 9.1 | 8.5 | 10.4 | 21.6 |
| Mn urine (µg/L) |
VC-Sellero | 34 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 3.7 |
| VC-Breno | 64 | 0.1 | 0.1 | 0.3 | 0.1 | 0.2 | 5.9 | |
| VC-Darfo | 48 | 0.1 | 0.1 | 0.4 | 0.1 | 0.3 | 2.9 | |
| VC | 146 | 0.1 | 0.1 | 0.3 | 0.1 | 0.2 | 6 | |
| GL | 93 | 0.1 | 0.1 | 0.4 | 0.1 | 0.3 | 9.4 | |
| p = 0.95 | all | 239 | 0.1 | 0.1 | 0.3 | 0.1 | 0.2 | 9.4 |
| Pb Blood (µg/dL) |
VC-Sellero | 34 | 0.8 | 2.8 | 4.1 | 3.3 | 5.5 | 12.8 |
| VC-Breno | 64 | 1.1 | 2.5 | 4.1 | 3.2 | 5.3 | 11.5 | |
| VC-Darfo | 48 | 0.5 | 2.4 | 4.7 | 4.5 | 6.2 | 17.3 | |
| VC | 146 | 0.5 | 2.5 | 4.3 | 3.6 | 5.7 | 17.3 | |
| GL | 92 | 0.5 | 2.1 | 4.0 | 3.5 | 5.1 | 15.3 | |
| p = 0.48 | all | 238 | 0.5 | 2.3 | 4.2 | 3.5 | 5.5 | 17.3 |
GAM multivariate analysis
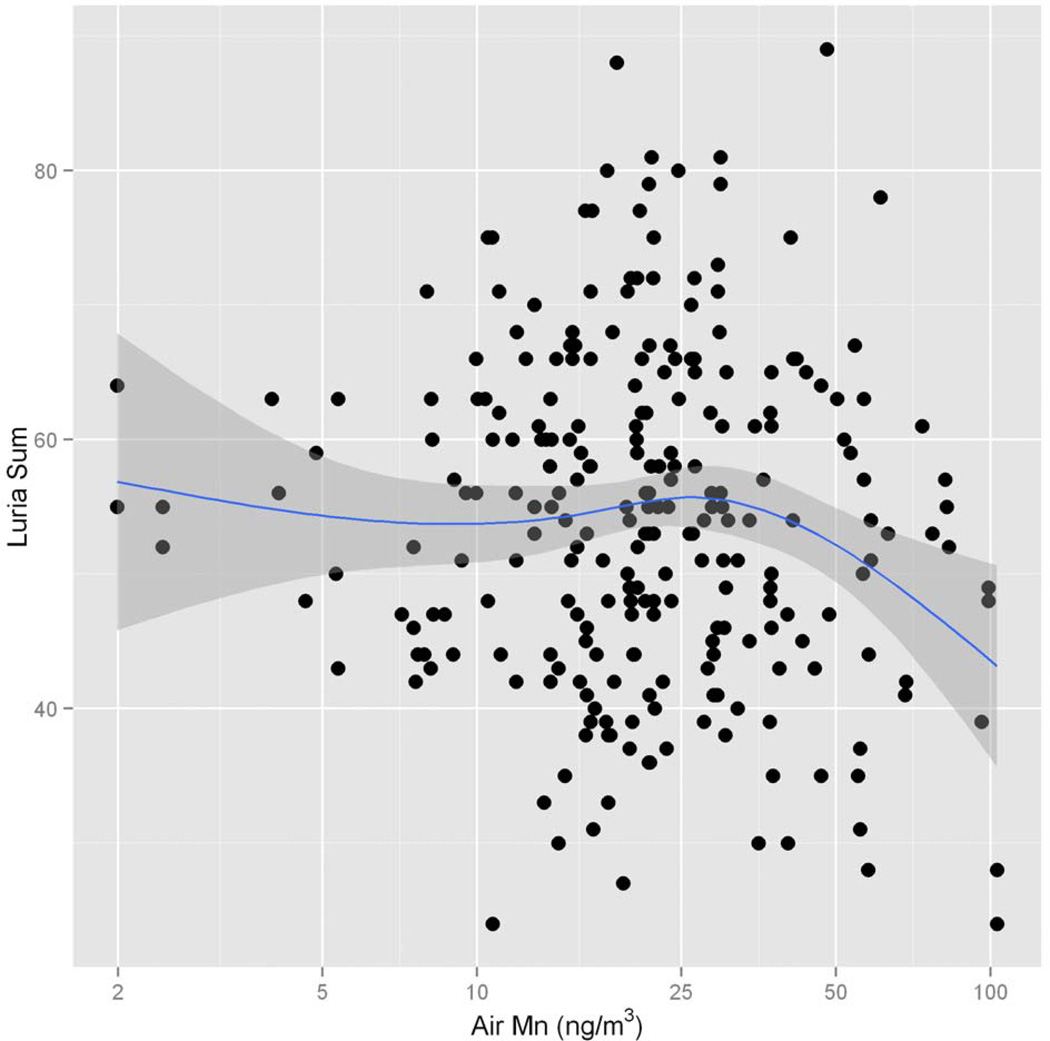
The descriptive statistics of all neurobehavioral testing is reported in the supplementary material (tables S1–S7). The GAM multivariate analysis yielded significant Mn dose-effect associations with a number of test outcomes. The sum of the five motor coordination subtests of the Luria Nebraska Neuropsychological Battery was significantly negatively associated with the distance from the nearest exposure point of historical ferromanganese plant emission (table 3). Gender (M versus F) was also significantly associated with the Luria Nebraska test score (p=0.0036), with men exhibiting better motor coordination scores compared to women. The non-parametric component of the GAM analysis showed a negative association between the Luria Nebraska motor coordination score and the concentration of Mn in airborne particles (p=0.0237) and blood lead (p=0.0201), age (p=0.0123) and the distance from the nearest ferromanganese plant point source (p=0.0035). No interaction was observed between Mn and Pb. The negative association between air Mn and the adjusted motor coordination score is shown in figure 1. Removing potentially influential values of air Mn >100 ng/m3 did not modify the model estimates.
Table 3.
Multivariate analysis using GAM models and the sum score of the five motor coordination subtests of the Luria Nebraska test score as the dependent variable. Sellero, Breno, and Darfo are ferromanganese plant communities in the upper, mid, and lower Valcamonica, respectively. GL=GL., e.d.f.= estimated degrees of freedom
| Generalized (Linear) Additive
Model Dependent variable: Luria Nebraska sum | ||
|---|---|---|
|
Parametric
part Variable |
Estimate (S.E.) | P-value |
| (Intercept) | 56.6 (3.28) | <0.0001 |
| Plant (Darfo Vs GL) | −8.18 (5.05) | 0.1071 |
| Plant (Breno Vs GL) | −6.61 (5.30) | 0.2138 |
| Plant (Sellero Vs GL) | −12.4 (6.11) | 0.0426 |
| Gender M Vs F | 5.60 (1.90) | 0.0036 |
| Smoke (actual Vs never) | 1.10 (2.93) | 0.7076 |
| Smoke (ex Vs never) | 3.33 (1.80) | 0.0657 |
| Alcohol (0,20] Vs no | −2.07 (1.94) | 0.2873 |
| Alcohol (20,50] Vs no | −2.93 (2.10) | 0.1639 |
| Alcohol (>50) Vs no | 0.35 (2.70) | 0.89551 |
|
Non parametric (smooth)
part Variable |
e.d.f. | p-value |
| log10 (Distance from nearest plant) | 3.95 | 0.0035 |
| log10 (Soil Mn) | 0.81 | 0.2054 |
| log10 (Air Mn) | 0.80 | 0.0237 |
| log10 (Blood Mn) | 0.00 | 0.8238 |
| log10 (Urinary Mn) | 0.00 | 0.3654 |
| log10 (Blood Pb) | 1.57 | 0.0201 |
| Age | 1.45 | 0.0123 |
Figure 1.
Relationship of Luria Nebraska sum score and Mn in air represented as a smooth function. The blue line and the shaded area represent the smoothing spline estimate and its 95% interval. Note the log-transformation of the x-axis.
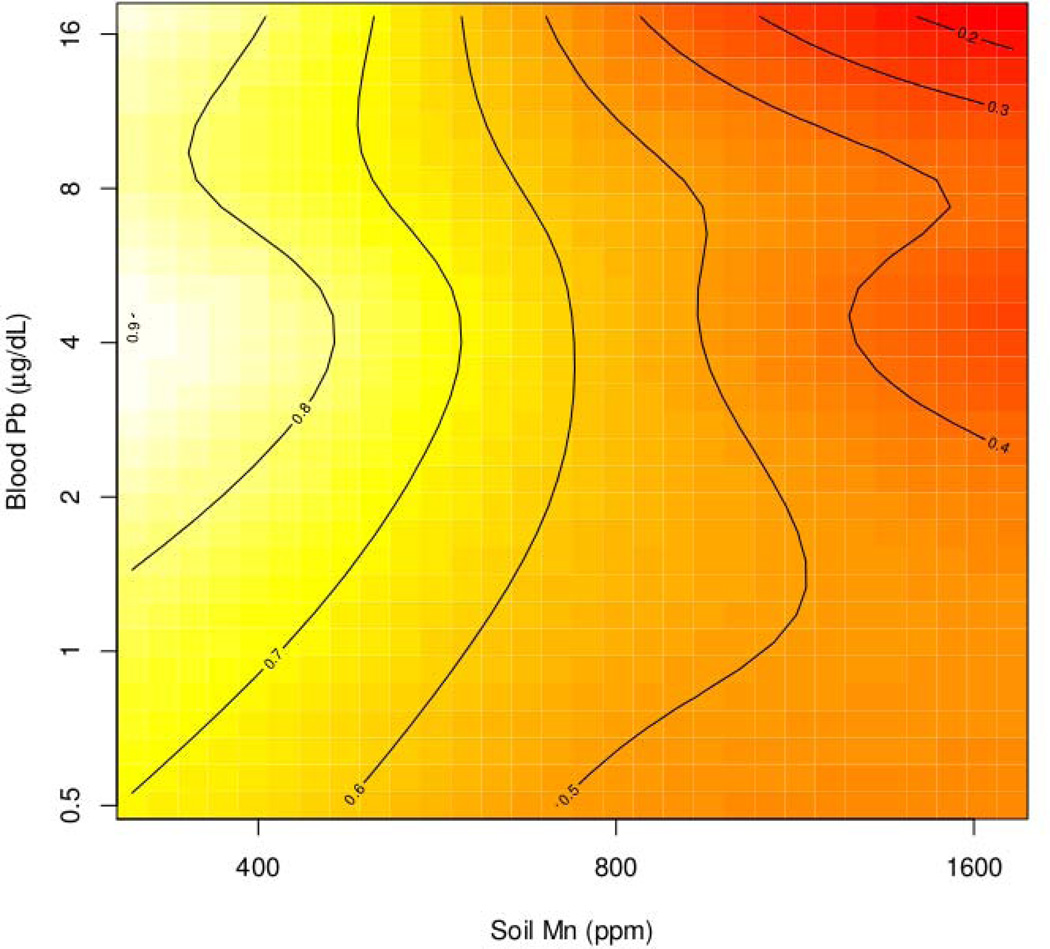
The GAM analysis showed also significant associations of the Sniffin Stick Test-proportion of correct answers with gender (p=0.0009, with men exhibiting poorer odour identification), and alcohol use (p=0.0335 ) (table 4). The non-parametric analysis showed a significant association between the Sniffin Stick Test and Mn levels in air (p<0.0001), soil (p=0.0006 ) and blood (p=0.0100), Pb in blood (p=0.009), the distance from the nearest point source (p<0.0001), and age (p<0.0001). Therefore we considered the multiple interaction between these variables by means of tensor product splines. The analyses showed that the Sniffin Stick Test score was influenced by the interaction between soil Mn + blood Pb (p=0.0856), which is represented in figure 2 by tensor product smooth. The graph shows the interaction effect of Soil Mn and blood Pb on the proportion of correct answers on the Sniffing test. The dark colors represent worse performance in correct odor discrimination. Lines represent points with the model based expected performance level. Increased Mn is associated with worse performance whereas the independent effect of Pb is minimal. Increased Mn and Pb levels determine an interactive effect as shown by the dark color in the right upper corner of the graph. The GAM analysis assumes that these effects are additive and not exactly interactive, therefore both Mn and Pb modify the effect on odor discrimination, although maintaining separate influences. This may be interpreted in view of two different cognitive and sensorial component of olfactory discrimination that may be differently affected by Pb (cognitive) and Mn (sensorial).
Table 4.
Multivariate analysis using GAM models and the Sniffin Stickin test score (proportion of correct answers) as dependent variable (Alcohol consumption expressed in g/day), e.d.f.= estimated degrees of freedom
| Generalized (Logistic) Additive
Model Dependent variable: score of correct answers at the Sniffin Stick test | ||
|---|---|---|
|
Parametric
part Variable |
Estimate (S.E.) | p-value |
| (Intercept) | 0.91 (0.25) | 0.0004 |
| Plant (Darfo Vs GL) | −0.02 (0.36) | 0.9558 |
| Plant (Breno Vs GL) | 0.47 (0.40) | 0.2430 |
| Plant (Sellero Vs GL) | 0.83 (0.57) | 0.1474 |
| Gender M Vs F | −0.40 (0.12) | 0.0009 |
| Smoke (actual Vs never) | −0.22 (0.18) | 0.2168 |
| Smoke (ex Vs never) | −0.01 (0.11) | 0.8780 |
| Alcohol (0,20] Vs no | 0.27 (0.12) | 0.0335 |
| Alcohol (20,50] Vs no | 0.22 (0.13) | 0.1011 |
| Alcohol (>50) Vs no | 0.11 (0.17) | 0.5186 |
|
Non parametric (smooth)
part Variable |
e.d.f. | p-value |
| log10 (Soil Mn) | 1.38 | 0.0006 |
| log10 (Air Mn) | 3.86 | <0.0001 |
| log10 (Blood Mn) | 1.37 | 0.0100 |
| log10 (Urinary Mn) | 0.24 | 0.2385 |
| log10 (Blood Pb) | 1.44 | 0.0090 |
| Age | 2.35 | <0.0001 |
| log10(Distance from the nearest plant) | 4.69 | <0.0001 |
| log10 (Soil Mn)*log10 (Blood
Pb) interaction |
0.85 | 0.0856 |
| log10 (Soil Mn)*log10(Air Mn) interaction | 3.66 | 0.0060 |
| log10 (Soil Mn)*log10(Air
Mn) interaction*log10(Blood Pb) interaction |
6.53 | 0.0061 |
Figure 2.
The interaction between soil Mn and blood Pb levels on Sniffin Stick test score, represented by tensor product smooth.
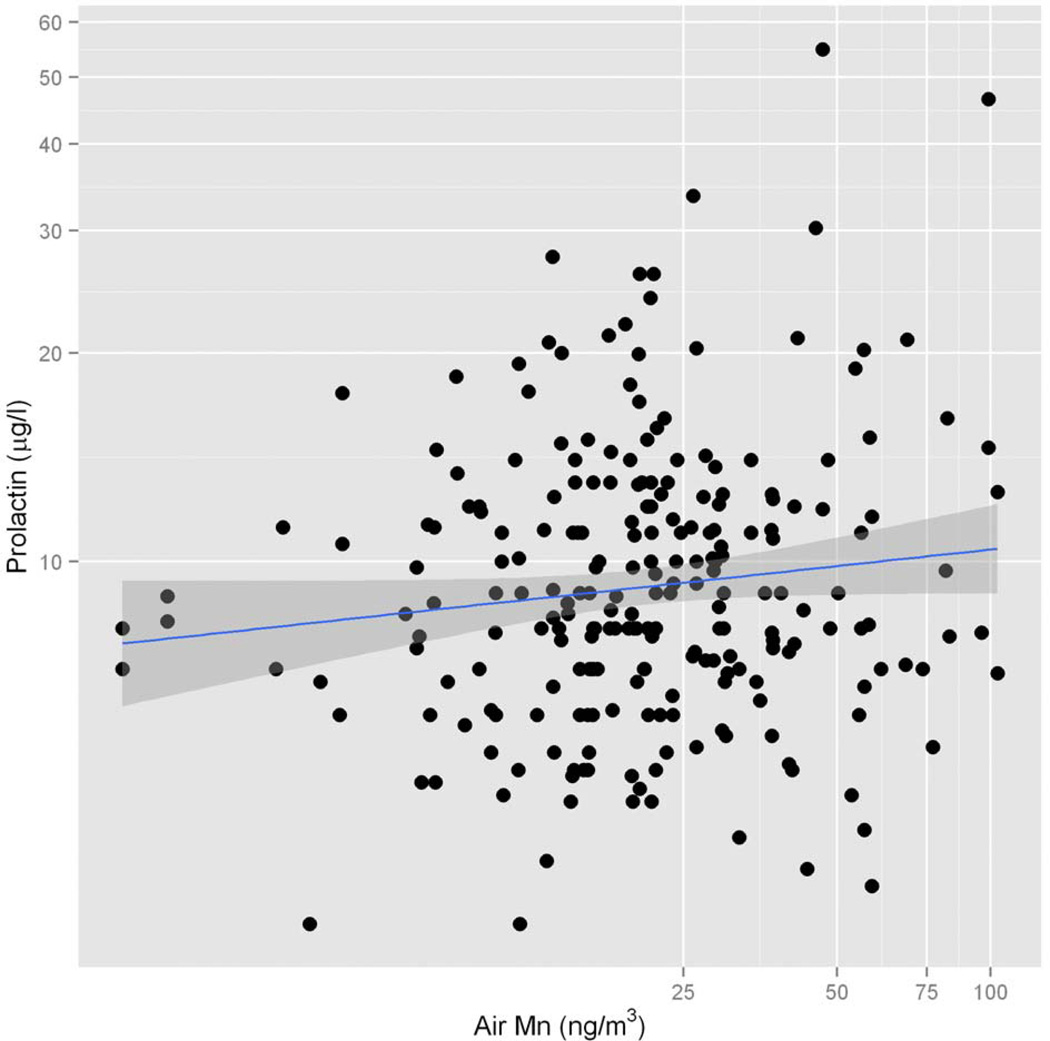
Regardless the high significance, the influence of air Mn on the test score was not clearly explained by the model in the interaction with the other exposure variables. Manganese dose-response associations were observed for the two cognitive tests CPM and TMT (data shown in supplementary material, table S8 and S9 respectively). For the CPM test, gender was a predictor of performance with women exhibiting poorer performance compared to men (p=0.0003). The non-parametric part of GAM analysis shows a negative association of CMP performance with age (p<0.0001) and with the distance from the nearest ferromanganese plant exposure point source (p=0.0025, figure S12), as well as a border line negative association with soil Mn levels (p=0.09, figure S13). For the TMT, urinary Mn (p=0.0074, figure S14) and age (p=<0.0001) were significantly negatively associated with TMT test performance. Finally, the GAM analysis revealed positive associations between serum PRL levels and residing in the vicinity of a ferromanganese plant (p=0.0085), cigarette smoking (p=0.0079), air (p=0.061) and urinary Mn (p=0.003) and blood Pb (p=0.0303). The positive association between air Mn and the adjusted serum PRL level is shown in figure 3. Removing the potentially influential lowest serum PRL values did not modify significantly the model estimates. No other associations were observed with the GAM analysis considering the other neurobehavioral testing and symptom questionnaires with the parameters of Mn and Pb exposure.
Figure 3.
Relationship between air Mn levels and serum prolactin (PRL). The blue line represents the linear regression between the variables (the GAM analysis suggested a linear relationship between log-transformed PRL and Air Mn) and the shaded area the 95% confidence interval of the estimate. Note the log-transformation of both axes.
Benchmark Dose calculation
For the Luria Nebraska test, in order to obtain an estimate of a Benchmark Dose (BMD) for air Mn levels, defined as the air Mn level expected to cause a decrease of one point of the Luria Nebraska sum score in respect to the geometric mean air Mn level in the reference area of Garda Lake, we reduced the first GAM model to a more parsimonious one using the Akaike's Information Criterion as guidance and, in absence of evidence of a non-linear relationship between the (log-transformed) air Mn levels and the Luria Nebraska Sum score after considering the other covariates, we moved the air Mn effect estimation to the parametric part of the GAM analysis.
The BMD was calculated according to Budtz-Jørgensen et al. (2001), using a bootstrapping approach to take into account the variability in the estimates of the geometric mean air Mn levels in the Garda Lake reference area. The lower level of the BMD confidence interval (BMDL_01) was defined as the level of the internal confidence limit lower than 95% of the estimated dose expected to decrease the score of one point. The bootstrap estimate of the BMDL_01 was 22.7 ng/m3 and 26.4 ng/m3 for the mean and median air Mn levels, respectively.
DISCUSSION
This study of elderly subjects with environmental exposure to Mn has revealed associations between Mn exposure and deficits in motor coordination, odor identification, and cognitive abilities, in addition to increased serum PRL. These findings are particularly noteworthy as they occur in a geographical area characterized by high environmental impact of prolonged metal emission from ferromanganese industries. In this area a high prevalence of parkinsonism has been observed in relation to the ferromanganese emission point sources and the Mn levels in deposited dust (Lucchini et al., 2007). Thus, the observation of a Mn dose-dependent impairment of motor coordination in elderly subjects residing for most of their life in this area suggests the presence of preclinical effects that, in the future, may progress to symptomatic neurodegenerative illness. Age was found to be a significant predictor of test performance in all models, further suggesting an overlapping effect of exposure on physiological aging of neurological function. Although age is also a surrogate of the duration of exposure, the effect of normal aging and deterioration in performance due to age-related decline in function is likely a predominant component that can be exacerbated by cumulative exposure to Mn. The alteration of the Luria Nebraska motor coordination subtest, as well as the odor identification Sniffin Sticks test, were observed also in adolescents (Lucchini et al., 2012) and ferromanganese workers (Lucchini et al., 1999) from the same geographical area. Alteration of both olfactory discrimination and sensitivity, as examined with the complete Sniffin Stick test battery, was seen in 30 adults residing in the Mn mining district of Molango, Mexico, compared to controls (Guarneros et al., 2013). Notably, cognitive functions were also related to Mn exposure, further supporting the hypothesis that a common mechanism of neurotoxicity of Mn underlying many of the observed neurological deficits is dopaminergic system dysregulation (Guilarte, 2013).
The positive association of serum PRL with Mn exposure was observed previously among ferromanganese workers in this area (Mutti et al., 1996), and more recently among Mn exposed welders (Tutkun et al., 2013). It has been observed also among Mexican school children in the Molango mining district (Montes et al., 2011), and thus may be considered as a biomarker of Mn toxicity (Marreilha Dos Santos et al., 2011).
Deficits in dopamine system function as a result of chronic environmental Mn exposure is an attractive hypothesis that may help explain the effects observed here, as the dopaminergic system plays a major role in the regulation of motor (Groenewegen, 2003), olfactory (Scherfler et al., 2013), and pituitary PRL secretion, with the latter being regulated by the inhibitory activity of dopaminergic neurons in the hypothalamus (Fitzgerald and Dinan, 2008).
Another relevant finding of this study is also related to the observed interaction between Mn and Pb on neurobehavioral outcomes that has already been shown measures of fetal exposure in pregnancy (Lin et al., 2013), early childhood (Claus Henn et al., 2012) and school aged children (Kim et al., 2009). As Pb is ubiquitous and there is no known exposure level without deleterious health effects, potential interactions with Pb exposure should always be verified in human studies on Mn neurotoxicity. The interaction between the two metals observed here implies also a separate influence by each component. As Pb targets cognitive functions, it may play a specific role on the cognitive component of odor recognition, which is necessary for the identification process. The mechanism of Mn toxicity on the olfactory function may target instead the dopaminergic control of olfaction. Emerging literature indicates that Mn neurotoxicity can target also cognitive domains that are controlled by frontal cortex and other brain subareas (Roels et al., 2012; Guilarte, 2013). This study confirms this hypothesis showing Mn-related impairment of cognitive functions assessed by CMP and TMT tests.
The BMD calculation on the dose-response between Mn concentrations in PM10 airborne particles and the Luria Nebraska motor coordination score, has led to a BMDL_01 value of 22.7 ng/m3 (mean value) and 26.4 ng/m3 (median value). This level is lower than the Rfc level of 50 ng/m3 indicated by the U.S. EPA (1999) and Health Canada (2010), although a direct comparison is not entirely practical, since the Rfc is based on exposure to a smaller particle size (PM2.5) and annualized Mn exposure levels extrapolated from occupational studies. The BMDL result reported here may be considered as more accurate, since it is based on the direct analysis of the dose-response relationship in this presumably more susceptible elderly target population, without the application of additional Uncertainty Factors as used in the Rfc calculation process. In addition, the personal data on airborne particle exposure may more accurately represent individual exposures compared to the air monitoring data from a stationary air monitor station, since the 24h personal sampling better captures inhalation exposure from the indoor (home) environment, where the elderly typically spend a greater proportion of their day than younger subjects.
This study had several limitations. There was incomplete coverage of all subjects with personal sampling of airborne particles and surface soil measurements, though the use of statistical data interpolation provided reliable estimates of missing data and helped with the shortcomings of this limitation. Another possible limitation of the study is that the Mn exposure biomarkers did not reflect the differences in air and soil Mn levels across sites. This is likely due to the fact that blood and urine Mn levels are homeostatic regulated, such that they do not appear to capture differences in environmental exposure. Other biomarkers like hair may have better reflected differences in Mn absorptions, but hair was not available from these subjects.
Similarly to other environmental health studies targeting the nervous system, this study presents multiple statistical comparisons that may generate non plausible results. To account for this possibility we retained in the GAM models all the terms that could have affected the response on an “a priori” basis, even if non-significantly associated. However, the consistency of some findings, namely the Mn-related impairment on motor and odor functions observed in adolescents residing in the same geographical areas (Lucchini et. Al., 2012), supports the outcomes reported here and reinforces the hypothesis of biologically plausible exposure-related effects in a coherent picture across different age groups. Finally, we observed (multi) collinearity and concurvity in the model, which is expected because these variables are correlated (Table S11). In this study the collinearity effect was judged as moderate and the results were fairly stable and resistant to adding or removing from the model a certain variable.
In light of the operational history of the ferromanganese plants in the Valcamonica, the last of which (Breno) ceased ferromanganese alloy operations in 2001, air Mn levels were undoubtedly higher in the past, though significant burdens of environmental Mn persist in these communities as evidenced by the elevated surface soil Mn levels. The subjects’ age and residence histories indicate they likely suffered greater environmental exposures over the majority of their lifespan than has likely occurred over the past decade or so since the ferromanganese operations ceased. Nevertheless, the airborne Mn levels measured in this study are likely to represent the exposure levels since 2001 in Breno, 1985 in Sellero and 1995 in Darfo, since much of the airborne Mn may be attributed to resuspended dusts that have been cumulatively impacted by the ferromanganese plant emissions over decades. These periods of time cover the old age span of this cohort, which is a more sensitive exposure window. In addition, since Mn deposited to surface soils is relatively immobile and slowly accumulates with time, the soil Mn levels available in this study reflect spatially local differences in environmental Mn deposition integrated over the timeframe of decades. Therefore the soil values measured during the health assessment are not expected to differ significantly from when the plants were active. While air Mn levels to a great extent reflect airborne particles resuspended from surface soils, these levels were likely higher during the period when the plants were active, particularly within 0.5 – 1 km from the plants; but outside that close range they are expected to reflect large resuspension of surface soil and dusts, which reflect cumulative emission deposition over decades, as noted above. In any case, further research is warranted in this area for a more in depth characterization of cumulative exposure, aimed to define exposure lifeline able to incorporate all available historical data.
Supplementary Material
Highlights.
We assessed elderly subjects for manganese related neurofunctional changes
We examined various dopaminergic domains
Soil and air manganese predicts dysfunction of odor discrimination and motor control
Increased prolactin levels are related to manganese exposure
Cognitive domains may also be a target of manganese toxicity
ACKNOWLEDGMENTS
This study was supported by funding from the European Union through its Sixth Framework Programme for RTD (contract no FOOD-CT-2006- 016253). It reflects only the authors' views, and the European Commission is not liable for any use that may be made of the information contained therein. The project was also supported by Award Number R01ES019222 from the National Institute Of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Borgese L, Zacco A, Pal S, Bontempi S, Lucchini R, Zimmerman N, et al. A new nondestructive method for chemical analysis of particulate matter filters: the case of manganese air pollution in Vallecamonica (Italy) Talanta. 2011;84:192–198. doi: 10.1016/j.talanta.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese L, Salmistraro M, Gianoncelli A, Zacco A, Zimmerman N, Lucchini R, et al. Airbone particulate matter (PM) filter analysis and modeling by total reflection Xray fluorescence (TXRF) and X-ray standing wave (XSW) Talanta. 2012;89:99–104. doi: 10.1016/j.talanta.2011.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese L, Federici S, Zacco A, Gianoncelli A, Rizzo L, Smith DR, Donna F, Lucchini R, Depero LE, Bontempi E. Metal fractionation in soils and assessment of environmental contamination in Vallecamonica, Italy. Environ Sci Pollut Res Int. 2013 doi: 10.1007/s11356-013-1473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin ME, Panisset M. Manganese cumulative exposure and symptoms: a follow-up study of alloy workers. Neurotoxicology. 2008;29(4):577–5783. doi: 10.1016/j.neuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur MÈ, Bouffard T, Limoges E, Bellinger DC, Mergler D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119(1):138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jørgensen E, Keiding N, Grandjean P. Benchmark dose calculation from epidemiological data. Biometrics. 2001;57:698–706. doi: 10.1111/j.0006-341x.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernández-Avila M, Amarasiriwardena C, Hu H, Bellinger DC, Wright RO, Téllez-Rojo MM. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect. 2012;120(1):126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després C, Lamoureux D, Beuter A. Standardization of a neuromotor test battery: The CATSYS system. Neurotoxicology. 2000;21:1–11. [PubMed] [Google Scholar]
- EPA. U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Manganese. Washington, DC: National Center for Environmental Assessment, Office of Research and Development; 1999. [Google Scholar]
- Ferri R, Donna F, Smith DR, Guazzetti S, Zacco A, Rizzo L, Bontempi E, Zimmerman NJ, Lucchini RG. Heavy Metals in Soil and Salad in the Proximity of Historical Ferroalloy Emission. Journal of Environmental Protection. 2012;5:374–385. doi: 10.4236/jep.2012.35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007 Jul;104(3):420–432. doi: 10.1016/j.envres.2007.03.002. Epub 2007 Apr 18. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol. 2008;22(2 Suppl):12–19. doi: 10.1177/0269216307087148. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ““Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician”. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geller AM, Zenick H. Aging and the environment: a research framework. Environ Health Perspect. 2005 Sep;113(9):1257–1262. doi: 10.1289/ehp.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ, Hammeke T, Purish A. Manual for the Luria Nebraska Neuropsychological battery. Los Angeles: Western Psychological Services; 1980. [Google Scholar]
- Golden CJ, Hammeke T, Purish A. Manual for the Luria Nebraska Neuropsychological battery. Los Angeles: Western Psychological Services; 1980. [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10(1–2):107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros M, Ortiz-Romo N, Alcaraz-Zubeldia M, Drucker-Colín R, Hudson R. Nonoccupational environmental exposure to manganese is linked to deficits in peripheral and central olfactory function. Chem Senses. 2013;38(9):783–791. doi: 10.1093/chemse/bjt045. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci. 2013;5:23. doi: 10.3389/fnagi.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Generalized Additive Models. Chapman & Hall: Health Canada. Human Health Risk Assessment for Inhaled Manganese; 1990. 2010. Available on Internet at: www.hc-sc.gc.ca/ewh-semt/pubs/air/manganese-eng.php. [Google Scholar]
- Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function using a 4 minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110:976–981. doi: 10.1177/000348940111001015. [DOI] [PubMed] [Google Scholar]
- Iregren A, Gamberale F, Kjellberg A. SPES: a psychological test system to diagnose environmental hazards. Neurotoxicol Teratol. 1996;18:485–491. doi: 10.1016/0892-0362(96)00033-5. [DOI] [PubMed] [Google Scholar]
- Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, Levy D, Mey J, van Geen A, Graziano JH, Factor-Litvak P. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology. 2012 Jan;33(1):91–97. doi: 10.1016/j.neuro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kim BN, Hong YC, Shin MS, Yoo HJ, Kim JW, Bhang SY, Cho SC. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology. 2009;30(4):564–571. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bowler RM, Abdelouahab N, Harris M, Gocheva V, Roels HA2011. Motor function in adults of an Ohio community with environmental manganese exposure. Neurotoxicology. 2011;32(5):606–614. doi: 10.1016/j.neuro.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH, Hsieh WS, Jeng SF, Su YN, Chen PC. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–57. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D, Sassine MP, Palmi S, Alessio L. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20(2–3):287–297. [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med. 2007 Nov;50(11):788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Zimmerman N. Lifetime cumulative exposure as a threat for neurodegeneration: need for prevention strategies on a global scale. Neurotoxicology. 2009 Nov;30(6):1144–1148. doi: 10.1016/j.neuro.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, Salmistraro M, Bontempi E, Zimmerman NJ, Smith DR. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33(4):687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreilha Dos Santos AP, Lopes Santos M, Batoréu MC, Aschner M. Prolactin is a peripheral marker of manganese neurotoxicity. Brain Res. 2011;1382:282–290. doi: 10.1016/j.brainres.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011;111(1):156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, de Carvalho-Vivas CF, Viana GF, Ferreira JR, Nunes LS, Mergler D, Abreu N. Elevated manganese exposure and school-aged children’s behavior: A gender-stratified analysis. Neurotoxicology. 2013 doi: 10.1016/j.neuro.2013.09.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Meyer-Baron M, et al. Statistical means to enhance the comparability of data within a pooled analysis of individual data in neurobehavioral toxicology. Toxicology Letters. 2011;206:144–151. doi: 10.1016/j.toxlet.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Meyer-Baron M, Schäper M, Knapp G, Lucchini R, Zoni S, Bast-Pettersen R, Ellingsen DG, Thomassen Y, He S, Yuan H, Niu Q, Wangg XL, Yang YJ, Iregren A, Sjögren B, Blond M, Laursen P, Netterstromm B, Mergler D, Bowler R, van Thriel C. The neurobehavioral impact of manganese: Results and challenges obtained by a meta-analysis of individual participant data. Neurotoxicology. 2013;36:1–9. doi: 10.1016/j.neuro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes S, Schilmann A, Riojas-Rodriguez H, Rodriguez-Agudelo Y, Solis-Vivanco R, Rodriguez-Dozal SL, Tristan-López LA, Rios C. Serum prolactin rises in Mexican school children exposed to airborne manganese. Environ Res. 2011;111(8):1302–1308. doi: 10.1016/j.envres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Mutti A, Bergamaschi E, Alinovi R, Lucchini R, Vettori MV, Franchini I. Serum prolactin in subjects occupationally exposed to manganese. Ann Clin Lab Sci. 1996;26(1):10–17. [PubMed] [Google Scholar]
- Panisset M, Lucchini R, Bélanger S, Mergler D. Permanent parkinsonism in workers formerly exposed to manganese. Movement disorders. 1996;11:599–600. [Google Scholar]
- Pavilonis BT, Lioy PJ, Guazzetti S, Bostick BC, Donna F, Peli M, Zimmerman NJ, Bertrand P, Lucas E, Smith DR, Georgopoulos PG, Mi Z, Royce SG, Lucchini RG. Manganese concentrations in soil and settled dust in an area with historic ferroalloy production. J Exposure Sci Environ Epidemiol. doi: 10.1038/jes.2014.70. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, Criswell SR, Lundin JI, Hobson A, Seixas N, Kotzbauer PT, Evanoff BA, Perlmutter JS, Zhang J, Sheppard L, Checkoway H. Increased risk of parkinsonism associated with welding exposure. NeuroToxicology. 2012;33:1356–1361. doi: 10.1016/j.neuro.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA. Manganism in the 21st century: The Hanninen lecture. Neurotoxicology. 2013 Oct 19;:ii. doi: 10.1016/j.neuro.2013.09.007. S0161-813X(13)00157-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Standard Progressive Matrices. London, England: Lewis; 1983. Manual for Raven’s Progressive Matrices and Vocabularycales. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Riojas-Rodríguez H, Solís-Vivanco R, Schilmann A, Montes S, Rodríguez S, Ríos C, Rodríguez-Agudelo Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118(10):1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher JF, Todd GD, Meyer D, Zunker CL. The elderly as a sensitive population in environmental exposures: making the case. Rev Environ Contam Toxicol. 2010;207:95–157. doi: 10.1007/978-1-4419-6406-9_2. [DOI] [PubMed] [Google Scholar]
- Roels HA, Bowler RM, Kim Y, Claus Henn B, Mergler D, Hoet P, Gocheva VV, Bellinger DC, Wright RO, Harris MG, Chang Y, Bouchard MF, Riojas-Rodriguez H, Menezes-Filho JA, Téllez-Rojo MM. Manganese exposure and cognitive deficits: A growing concern for manganese neurotoxicity. Neurotoxicology. 2012;33(4):872–880. doi: 10.1016/j.neuro.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C, Esterhammer R, Nocker M, Mahlknecht P, Stockner H, Warwitz B, Spielberger S, Pinter B, Donnemiller E, Decristoforo C, Virgolini I, Schocke M, Poewe W, Seppi K. Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson’s disease. Brain. 2013;136(Pt 10):3028–3037. doi: 10.1093/brain/awt234. [DOI] [PubMed] [Google Scholar]
- Spinnler H, Tognoni G. Italian standardization of neuropsychological test. Ital J Neurol Sc. 1987;6(supp l8):12–120. [Google Scholar]
- Tutkun E, Abuşoğlu S, Yılmaz H, Gündüzöz M, Gıynas N, Bal CD, Unlü A. Prolactin levels in manganese-exposed male welders. Pituitary. 2013 Dec 13; doi: 10.1007/s11102-013-0545-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Vivas-Carvalho CF, Menezes-Filho JA, Matos VP, Bessa JR, Coelho-Santos J, Viana GF, Argollo N, Abreu N. Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology. 2013 Dec 3;:ii. doi: 10.1016/j.neuro.2013.11.006. S0161-813X(13)00178-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, Kline J, van Geen A, Mey J, Slavkovich V, Siddique AB, Islam T, Graziano JH. Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology. 2011;32(4):450–457. doi: 10.1016/j.neuro.2011.03.009. Epub 2011 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III & WMS-III technical manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Zacco A, Resola S, Lucchini R, Albini E, Zimmerman N, Guazzetti S, Bontempi E. Analysis of settled dust with X-ray Fluorescence for exposure assessment of metals in the province of Brescia, Italy. J Environ Monit. 2009;11(9):1579–1585. doi: 10.1039/b906430c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni S, Albini E, Lucchini R. Neuropsychological testing for the assessment of manganese neurotoxicity: a review and a proposal. Am J Ind Med. 2007;50(11):812–830. doi: 10.1002/ajim.20518. [DOI] [PubMed] [Google Scholar]
- Zoni S, Bonetti G, Lucchini R. Olfactory functions at the intersection between environmental exposure to manganese and Parkinsonism. Trace Elem Med Biol. 2012 Jun 2; doi: 10.1016/j.jtemb.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.