Abstract
Background
Enzalutamide is a novel antiandrogen with proven efficacy in metastatic castration-resistant prostate cancer (mCRPC).
Objective
To evaluate enzalutamide’s effects on cancer and on androgens in blood and bone marrow, and associate these with clinical observations.
Design, setting, and participants
In this prospective phase 2 study, 60 patients with bone mCRPC received enzalutamide 160 mg orally daily and had transilial bone marrow biopsies before treatment and at 8 wk of treatment.
Outcome measurements and statistical analysis
Androgen signaling components (androgen receptor [AR], ARV7, v-ets avian erythroblastosis virus E26 oncogene homolog [ERG], cytochrome P450, family 17, subfamily A, polypeptide 1 [CYP17]) and molecules implicated in mCRPC progression (phospho-Met, phospho-Src, glucocorticoid receptor, Ki67) were assessed by immunohistochemistry; testosterone, cortisol, and androstenedione concentrations were assessed by liquid chromatography–tandem mass spectrometry; and AR copy number was assessed by real-time polymerase chain reaction. Descriptive statistics were applied.
Results and limitations
Median time to treatment discontinuation was 22 wk (95% confidence interval, 19.9–29.6). Twenty-two (37%) patients exhibited primary resistance to enzalutamide, discontinuing treatment within 4 mo. Maximal prostate-specific antigen (PSA) decline ≥50% and ≥90% occurred in 27 (45%) and 13 (22%) patients, respectively. Following 8 wk of treatment, bone marrow and circulating testosterone levels increased. Pretreatment tumor nuclear AR overexpression (>75%) and CYP17 (>10%) expression were associated with benefit (p = 0.018). AR subcellular localization shift from the nucleus was confirmed in eight paired samples (with PSA decline) of 23 evaluable paired samples. Presence of an ARV7 variant was associated with primary resistance to enzalutamide (p = 0.018). Limited patient numbers warrant further validation.
Conclusions
The observed subcellular shift of AR from the nucleus and increased testosterone concentration provide the first evidence in humans that enzalutamide suppresses AR signaling while inducing an adaptive feedback. Persistent androgen signaling in mCRPC was predictive of benefit and ARV7 was associated with primary resistance.
Patient summary
We report a first bone biopsy study in metastatic prostate cancer in humans that searched for predictors of outcome of enzalutamide therapy. Benefit is linked to a pretreatment androgen-signaling signature.
Keywords: Enzalutamide, Antiandrogens, Castration-resistant prostate cancer, Predictors of outcome, Bone metastasis, Bone tumor microenvironment, Adaptive feedback mechanism, Androgen, Androgen receptor, Primary resistance to enzalutamide, Androgen signaling inhibition, Tissue-based research, Bone marrow biopsy
1. Introduction
Persistent androgen signaling is a validated therapeutic target in metastatic castration-resistant prostate cancer (mCRPC) [1–4]. Preclinical and clinical findings confirm that transition from endocrine-dependent to intracrine androgen signaling progression is a milestone in the lethal progression of prostate cancer and resistance to standard androgen deprivation therapy [1–5]. The role of tumor-associated androgen biosynthesis and its therapeutic relevance is established [1,4,6–9]. Moreover, over the course of mCRPC progression, androgen receptor (AR) changes ensue. These include overexpression, mutation, alternate splicing, post-translational modifications, or interactions with other pathways (nonclassical AR signaling) [10–14]. The report that enzalutamide prolongs the survival of men with mCRPC demonstrates the central role of persistent AR signaling in mCRPC progression [3].
Enzalutamide is a second-generation nonsteroidal antiandrogen selected for clinical development based on unique properties [15]. Experimentally, it inhibits androgen signaling by binding to the receptor, inhibiting nuclear translocation, and by binding to androgen response elements and recruitment of coactivators [15].
We aimed to determine if the AR signaling modulation by enzalutamide in human mCRPC is in line with experimental predictions and to identify candidate predictors of benefit and resistance. The objective of this open-label, single-center, prospective, translational, phase 2 study was to assess expression of molecular components of AR signaling in bone marrow–infiltrating CRPC and associate this with clinical findings. Secondary objectives included assessing treatment efficacy, safety, and levels of circulating and bone marrow aspirate (BMA) androgens before and during treatment.
2. Methods
The M.D. Anderson Cancer Center (MDACC) institutional review board approved this prospective study. Sixty patients enrolled in the study from February 2009 to June 2011, meeting the accrual goal.
Patients had histologically confirmed prostate adenocarcinoma and castrate-resistant bone mCRPC disease progression. Patients provided informed consent and were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2, serum testosterone ≤50 ng/dl (sustained by medical or surgical castration), and adequate adrenal, renal, hepatic, and bone marrow function.
2.1. Treatment and evaluation
Patients were treated with enzalutamide 160 mg daily. Screening and pretreatment evaluations included complete medical history, physical examination, complete blood count, serum electrolytes and chemistry, prostate-specific antigen (PSA) levels, testosterone concentration, radionuclide bone scan, and tumor imaging (chest x-ray or computed tomography [CT] scans, and pelvic and abdominal CT scans). Safety assessments, using National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.3, were completed every 8 wk, along with physical examination; selected serum chemistry; and electrolyte, PSA, and testosterone evaluations. Transilial bone marrow biopsy (BMB) and BMA (≤5 ml) was performed before treatment and at week 8. Blood was collected within 2 h of BMB. Abdominal and pelvic CT and radionuclide bone scans were performed upon clinical suspicion of progression or at 6-mo intervals, whichever occurred first. Therapy was discontinued on symptomatic progression and/or imaging progression by standard Prostate Cancer Working Group 2 (PCWG2) criteria [16] at the discretion of the attending physician or if the patient revoked consent. The approach was adapted from a trial with a similar design [5]. Primary resistance was defined as treatment discontinuation within 4 mo of initiation, as a result of overt clinical progression with or without imaging progression [5].
2.2. Assay methodologies
2.2.1. Tissue and derivatives banking and immunohistochemistry
Bone marrow specimens were obtained by transilial BMB and samples were processed according to MDACC decalcification and fixation procedures. Following pathologic evaluation, samples were stored in the MDACC Prostate Cancer Tissue Bank with matching BMA. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections for N-terminal AR (dilution 1:50) and Ki67 (dilution 1:50) (Dako, Carpinteria, CA, USA); cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17) (dilution 1:175) (Novus, Littleton, CO, USA); phospho-Src (dilution 1:50) (Novus, Littleton, CO, USA); phospho-Met (dilution 1:175) (Novus, Littleton, CO, USA); glucocorticoid receptor [GR] (dilution 1:300) (BD Biosciences, San Jose, CA, USA); v-ets avian erythroblastosis virus E26 oncogene homolog [ERG] (dilution 1:50) (Biocare Medical, Concord, CA, USA); and ARV7 (dilution 1:200) (A&G Pharmaceutical, Columbia, MD, USA). AR and Ki67 antibodies are standard diagnostics validated for clinical use; accepted quality control measures were used (Supplement 1) [17]. Marker expression was assessed by scoring two or more fields containing ≥100 tumor cells (marker heterogeneity dependent) and expressed as a percentage. Pathologists were blind to outcome. We applied involvement scoring per standard proliferation index (Ki67) assessment methodology previously described [5], providing a continuous variable for statistical analysis purposes. Involvement cut-offs for AR overexpression (>75%, high intensity) and CYP17 expression (>10%) were prespecified based on prior findings. Intensity was scored as low, intermediate, or high. Subcellular distribution of biomarker expression was recorded.
2.2.2. Androgen receptor copy numbers
AR copy number methods were assessed as previously described [5].
2.2.3. Mass spectrometry
Liquid chromatography–tandem mass spectrometry (LCMS) analysis of androgens was performed as described previously [18].
2.3. Statistical considerations
This study was designed to enroll 60 patients anticipating (baseline and week 8) adequate BMB and BMA harvest from ≥40% of patients for endpoint evaluation. Descriptive statistics were used. The Wilcoxon signed-rank test was used to assess biomarker change. The Wilcoxon rank-sum test was used to assess treatment duration between samples with and without CYP17 expression and AR overexpression, and BMA testosterone levels between samples with and without CYP17 expression. A sample size of 30 paired BMAs would provide 82% power to detect at least a 0.55 change in standard deviation in BMA testosterone levels using a two-sided Wilcoxon signed-rank test at a 0.05 significance level. Correlations between blood and BMA testosterone by mass spectrometry were assessed using Spearman methods. The Fisher exact test was used to assess significance of associations between two categorical variables. Overall survival and time to treatment discontinuation from treatment initiation were estimated by the Kaplan-Meier method.
3. Results
3.1. Clinical outcomes
Table 1 summarizes demographic, clinical, and tumor characteristics. The median age of the patients was 71 yr (range: 40–89 yr).
Table 1.
Patient and tumor characteristics
| No. (%)* | |
|---|---|
| Evaluable patients | 60 |
| Race | |
| White | 53 |
| Black | 4 |
| Hispanic | 2 |
| Asian | 1 |
| Age, yr, median (range) | 71 (40–89) |
| Performance status | |
| 0 | 15 (25) |
| 1 | 40 (67) |
| 2 | 5 (8) |
| Gleason score at diagnosis | |
| ≥8 | 36 (68) |
| 7 | 16 (30) |
| 6 | 1 (2) |
| Not available | 7 |
| Metastatic disease at diagnosis (data not available for two patients) | 19 (33) |
| Prior radical prostatectomy or/and prostatic bed radiation therapy | 39 (65) |
| Time to CRPC >1 yr | 39 (65) |
| Time to CRPC >2 yr | 28 (47) |
| Prior therapies for prostate cancer | |
| Chemotherapy | 48 (80) |
| Two or more regimens | 16 (27) |
| Docetaxel-based regimen(s) | 47 (78) |
| Radiopharmaceuticals | 6 (10) |
| Salvage hormonal therapies | 37 (62) |
| Ketoconazole | 21 (35) |
| Abiraterone acetate | 2 (3) |
| Estrogens and/or ketoconazole and/or abiraterone acetate | 29 (48) |
| Prior experimental treatments/novel agents | 7 (12) |
| (Thalidomide [n = 2], tasquinimod [n = 1] | |
| dasatinib [n = 1], | |
| sunitinib [n = 1], experimental vaccine [n = 2]) | |
| Tumor characteristics | |
| Median PSA level, ng/d (25th–75th percentile) | 57.1 (16.1–140.3) |
| >20 bone metastases | 43 (72) |
| Lymph nodes | 20 (33) |
| Visceral metastases | 7 (12) |
| Bone marrow involvement (any time point) | 32 (53) |
| At baseline | 28 (47) |
| At week 8 | 27 (45) |
| Both time points | 23 (38) |
CRPC = castration-resistant prostate cancer; PSA = prostate-specific antigen.
Data given as no. (%) unless otherwise indicated.
All patients had bone mCRPC, 20 (33%) patients had lymph node metastases, and 7 (12%) had visceral metastases. All patients were evaluable for safety and benefit. Most had received prior chemotherapy and several lines of hormonal manipulation. Median ECOG PS was 1 (range: 0–2).
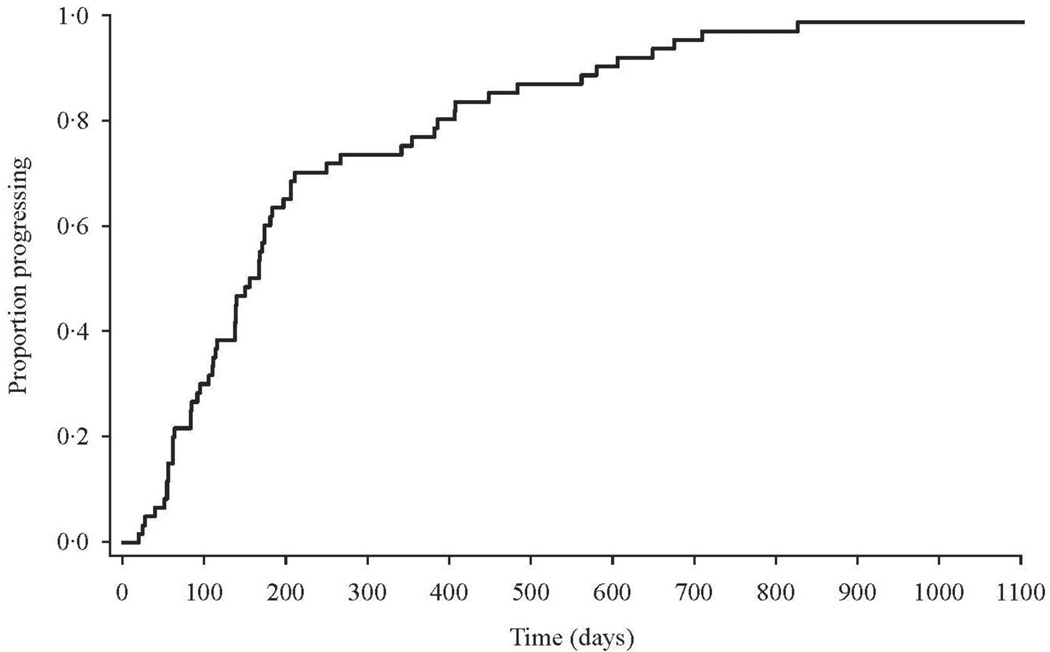
Enzalutamide was received for a median of 22 wk (95% confidence interval [CI], 19.9–29.6 wk) (Fig. 1). Therapy was well tolerated, with most adverse events categorized as grade 1/2 (NCI-CTCAE) and a safety profile consistent with that previously reported [3]. Seven grade 3 events, possibly related to the study drug, occurred. All but two patients have discontinued treatment; 33 had evidence of clinical/symptomatic progression. Sixteen patients discontinued treatment as a result of imaging progression by PCWG2 criteria. Four patients discontinued based on investigator decision and one progressed to small cell/neuroendocrine differentiated cancer. Two patients discontinued treatment as a result of adverse events (grade 3 depression and facial swelling, respectively). Of the remaining three patients who discontinued therapy, one withdrew consent and two withdrew due to progression of preexisting comorbidities unrelated to the study drug.
Fig. 1.
Proportion of patients progressing to treatment discontinuation over time.
Twenty patients experienced prolonged benefit (on treatment >6 mo). The remainder had moderate benefit by clinical criteria.
A maximal decline in PSA level ≥50% occurred in 27 of 60 (45%) evaluable patients, with 13 (22%) having ≥90% decline. A decline in PSA level ≥30% occurred in 31 (52%) patients.
Twenty-two patients exhibited primary resistance to enzalutamide, determined by symptomatic and/or imaging progression within 4 mo of study entry.
Median overall survival for the entire cohort was 21.7 mo (95% CI, 16.6 to ≥35 mo). Median survival for primarily resistant patients was 11.3 mo (95% CI, 9.1–16.7 mo) and ≥35 mo for the remaining patients (95% CI, 29.5 to ≥35 mo).
3.2. Clinical/tumor characteristics and outcome
Table 2 depicts post hoc univariate analyses of BMB infiltration status and outcome with select clinical/tumor characteristics. Extensive bone metastases (>20) were associated with bone marrow infiltration.
Table 2.
Univariate analyses in search of association of bone marrow biopsy outcome and treatment benefit with tumor and prior treatment characteristics
| BMB: tumor infiltration, no./total (%) |
BMB: No tumor infiltration, no./total (%) |
p value* |
Treatment benefit, no./total (%) |
No treatment benefit, no./total (%) |
p value * |
Patients with no available data, no. |
|
|---|---|---|---|---|---|---|---|
| >20 bone lesions | 28/32 (88) | 15/28 (54) | 0.004 | 25/38 (66) | 18/22 (88) | 0.2 | 0 |
| Metastases in lymph nodes | 10/32 (31) | 10/28 (36) | 0.8 | 14/38 (37) | 6/22 (27) | 0.4 | 0 |
| Visceral metastases | 5/32 (16) | 2/28(7) | 0.4 | 4/38 (11) | 3/22 (14) | 1 | 0 |
| Gleason score ≥8 | 21/29 (72) | 15/24 (63) | 0.6 | 20/31 (65) | 16/22 (73) | 0.2 | 7 |
| Metastatic at diagnosis | 7/31 (23) | 12/27 (44) | 0.06 | 14/36 (42) | 5/22 (23) | 0.1 | 2 |
| Treatment of primary with RPS or/and RT | 24/32 (75) | 15/28 (55) | 0.1 | 21/38 (55) | 18/22 (82) | 0.05 | 0 |
| Prior chemotherapy | 26/32 (81) | 22/28 (79) | 1 | 27/38 (71) | 21/22 (95) | 0.041 | 0 |
| Prior chemotherapy >1 line | 10/32 (31) | 6/28 (21) | 0.6 | 8/38 (21) | 8/22 (36) | 0.06 | 0 |
| Prior ketoconazole | 7/32 (22) | 14/28 (50) | 0.03 | 15/38 (39) | 6/22 (27) | 0.8 | 0 |
| Prior multiple hormonal manipulations** | 14/32 (44) | 20/28 (71) | 0.04 | 24/38 (63) | 10/22 (45) | 0.8 | 0 |
| Time to CRPC >1 yr | 21/31 (68) | 18/26 (69) | 1 | 25/37 (68) | 14/20 (70) | 1 | 3 |
| Time to CRPC >2 yr | 17/31 (55) | 11/26 (42) | 0.4 | 18/37 (49) | 10/20 (50) | 0.8 | 3 |
BMB = bone marrow biopsy;; CRPC = castration-resistant prostate cancer; RT = radiation therapy.
Fisher exact test.
Beyond luteinizing hormone-releasing hormone agonist/antagonist and bicalutamide.
3.3. Tissue and aspirate harvest
Twenty-eight (47%) patients had pretreatment tumor-infiltrated bone marrow and 32 (53%) had infiltration at any time point. Paired samples were available for 23 (38%) patients, 11 from men with cancers exhibiting primary resistance to enzalutamide.
BMAs were harvested from 56 (93%) patients pretreatment and 48 (80%) patients at week 8. Four patients did not yield BMAs at either time point, as a result of extensive bone marrow infiltration (so-called dry tap). Paired BMAs were available for measurements from 44 (73%) patients. Blood samples were taken from 59 (98%) patients before treatment and from 56 (93%) patients at week 8.
3.4. Molecular characterization of bone marrow metastases
3.4.1. Androgen signaling
Table 3 depicts AR overexpression (>75%), CYP17 expression >10%, and presence of ARV7 and ERG in bone marrow metastases at pretreatment, at 8 wk, and at either time point in the overall study population and according to benefit and resistance.
Table 3.
Molecular marker expression at baseline, 8 wk, and at either of these time points in the overall study population and according to benefit and resistance
| Patients, no./total evaluable (%) | p value | ||||||
|---|---|---|---|---|---|---|---|
| Primary resistance |
Moderate benefit 4–6 mo |
Prolonged benefit >6 mo |
Benefit | All patients | Primary resistant vs benefit |
Primary resistant vs prolonged benefit |
|
| Molecular marker expression (baseline) | |||||||
| Combined androgen signaling signature | 3/12 (25) | 4/6 (67) | 6/6 (100) | 10/12 (83) | 13/24 (54) | 0.012 | 0.009 |
| N-terminal AR overexpression (>75% expression plus high intensity) | 8/13 (62) | 5/6 (83) | 6/6 (100) | 11/12 (92) | 19/25 (76) | 0.2 | 0.1 |
| CYP17 presence (>10% expression) | 5/12 (42) | 4/6 (67) | 6/6 (100) | 10/12 (83) | 15/24 (62) | 0.09 | 0.038 |
| ARV7 presence | 6/12 (50) | 1/6 (17) | 0/5 (0) | 1/11 (9) | 6/23 (26) | 0.07 | 0.1 |
| ERG presence | 1/11 (9) | 2/6 (33) | 2/5 (40) | 4/11 (36) | 5/22 (23) | 0.3 | 0.2 |
| GR presence | 5/13 (38) | 2/5 (40) | 1/6 (17) | 3/11 (27) | 8/24 (33) | 0.68 | 0.6 |
| Ki67 (>30% expression) | 9/12 (75) | 2/5 (40) | 1/6 (17) | 3/11 (27) | 12/23 (52) | 0.039 | 0.043 |
| Phospho-Src (>30%) | 12/13 (92) | 4/5 (80) | 2/5 (40) | 6/10 (60) | 18/23 (78) | 0.1 | 0.044 |
| Phospho-Met (>30%) | 10/11 (91) | 2/5 (40) | 3/5 (60) | 5/10 (50) | 15/21 (71) | 0.06 | 0.21 |
| Molecular marker expression (week 8) | |||||||
| N-terminal AR overexpression | 8/11 (73) | 5/6 (83) | 4/7 (57) | 9/13 (69) | 17/24 (71) | 1 | 0.6 |
| CYP17 presence (>10% expression) | 6/10 (60) | 3/6 (50) | 4/6 (67) | 7/12 (58) | 13/22 (59) | 1 | 1 |
| ARV7 presence | 7/10 (70) | 2/6 (33) | 0/7 (0) | 2/13 (15) | 9/23 (39) | 0.013 | 0.01 |
| ERG presence | 1/9 (11) | 1/5 (20) | 2/3 (67) | 3/8 (38) | 4/17 (24) | 0.3 | 0.1 |
| GR presence | 6/9 (67) | 2/6 (33) | 1/6 (17) | 3/12 (25) | 9/21 (43) | 0.09 | 0.12 |
| Ki67 (>30%) | 5/11 (45) | 1/6 (17) | 0/5 (0) | 1/11 (9) | 6/22 (27) | 0.2 | 0.1 |
| Phospho-Src (>30%) | 10/11 (91) | 6/6 (100) | 2/6 (33) | 8/12 (67) | 18/23 (78) | 0.3 | 0.028 |
| Phospho-Met (>30%) | 6/9 (67) | 4/5 (80) | 2/5 (40) | 6/10 (60) | 12/19 (63) | 1 | 0.6 |
| Molecular marker expression (any time point) | |||||||
| ARV7 presence* | 8/14 (57) | 3/7 (43) | 0/7 (0) | 3/14 (21) | 11/28 (39) | 0.1 | 0.018 |
| ERG (presence)* | 1/12 (8) | 3/7 (43) | 3/7 (43) | 6/14 (43) | 7/26 (27) | 0∙08 | 0.1 |
| GR (presence)* | 9/13 (69) | 4/7 (57) | 2/7 (29) | 6/14 (43) | 15/27 (56) | 0.3 | 0.2 |
| Ki67 (>30%)** | 10/14 (71) | 3/7 (43) | 1/8 (12) | 4/15 (27) | 14/29 (48) | 0.027 | 0.024 |
| Phospho-Src (>30%)** | 12/13 (92) | 4/6 (67) | 2/7 (29) | 6/13 (46) | 18/26 (69) | 0.03 | 0.007 |
| Phospho-met (>30%)** | 11/13 (85) | 4/7 (57) | 4/7(57) | 8/14 (57) | 19/27 (70) | 0.1 | 0.3 |
AR = androgen receptor; GR = glucocorticoid receptor.
Any time point with presence (>5–10% tumor cell expression).
Any time point with >30% expression.
Nuclear AR expression was invariably present, but varied in involvement within and among samples in pretreatment BMBs (range: 50–100% involvement) and was of moderate to high intensity. Cytoplasmic CYP17 expression in tumor cells was heterogeneous in involvement and intensity. CYP17 tumor expression >10% in the background of intense homogeneous nuclear AR expression (expression in >75% of tumor cells and high intensity) was associated with longer time to treatment discontinuation (Wilcoxon signed-rank test, p = 0.012). The combined expression (named the androgen signaling signature) was more prominent in patients with prolonged benefit versus primarily resistant to enzalutamide (p = 0.009) (Table 2). Pretreatment CYP17 expression in the tumor correlated with increased BMA plasma testosterone concentration (Spearman ρ: 0.59; p = 0.018) in evaluable, paired BMB and BMA samples, as previously reported [5].
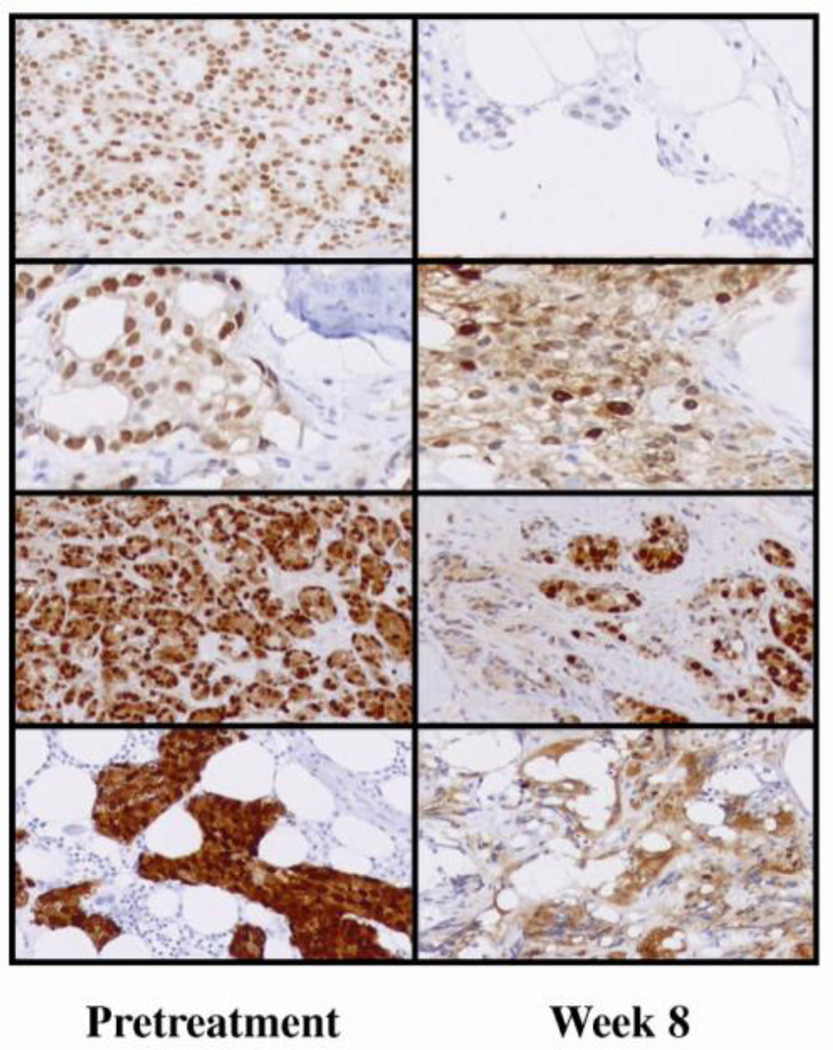
A shift from dominantly nuclear to cytoplasmic AR subcellular localization following 8 wk of treatment was confirmed in eight paired specimens (Fig. 2), seven of which pertained to patients with benefit, and all were associated with PSA decline.
Fig. 2.
Androgen receptor subcellular localization at pretreatment and following 8 wk of treatment in four patients (paired specimens).
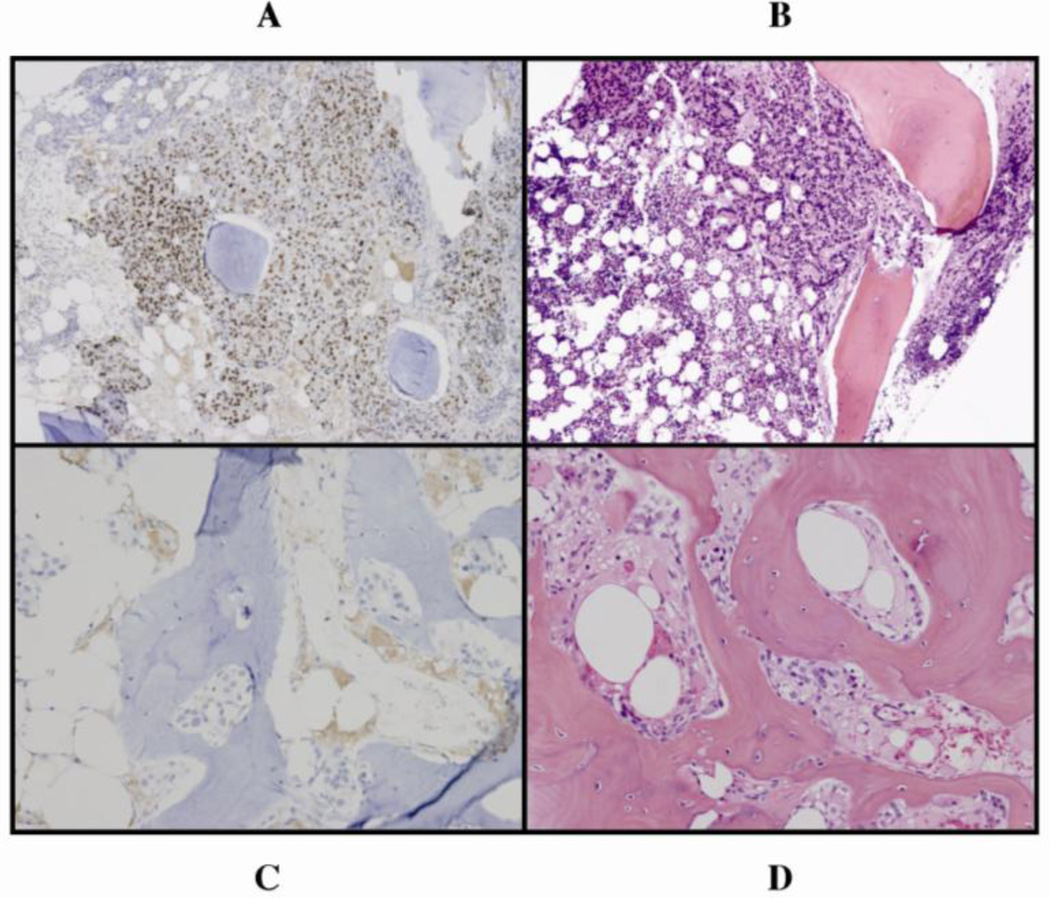
Splice variant ARV7 presence at any time point was more common in patients with primary resistance to enzalutamide (p = 0.018) (Fig. 3, Table 2). ARV7 expression was not found in tumor specimens from patients with prolonged benefit (>6 mo).
Fig. 3.
(a, b) Nuclear ARV7 expression in bone marrow-infiltrating tumor cells, with corresponding hematoxylin and eosin (H&E) staining, primarily resistant to enzalutamide versus (c, d) absence of ARV7 expression in bone marrow–infiltrating tumor cells, with corresponding H&E staining, sensitive to enzalutamide treatment.
AR copy numbers were assessed in 14 evaluable paired samples, eight from tumors primarily resistant to enzalutamide. No associations with outcome were identified (data not shown).
3.4.2. Assessment of non–androgen-receptor candidate markers of primary resistance
Table 3 depicts presence of GR and expression >30% of phospho-Met, phospho-Src, and Ki67. Increased proliferation index (Ki67 >30%) was more prominent in tumors primarily resistant to enzalutamide.
3.5. Bone marrow aspirate and blood androgen and steroid measurements
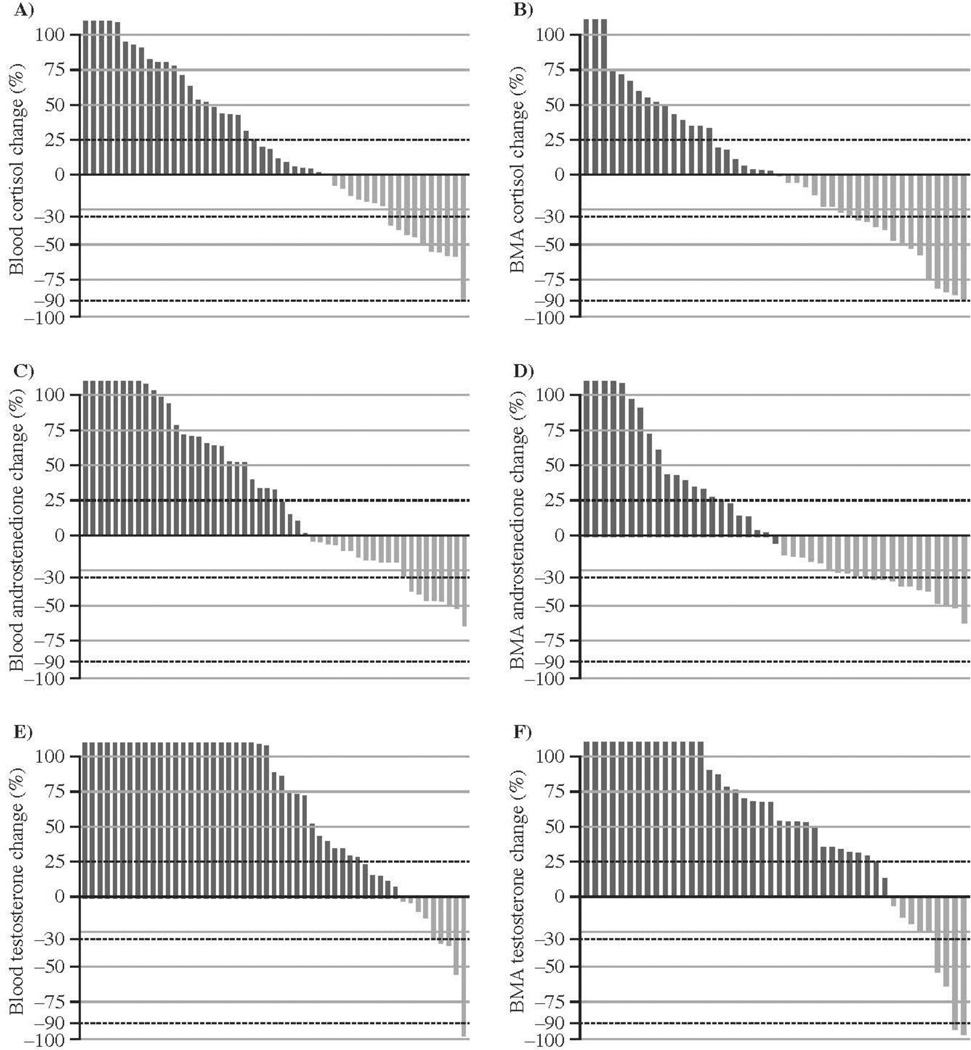
Figure 4 depicts changes in cortisol, androstenedione, and testosterone assessed by LCMS. Testosterone increased following 8 wk of treatment in the majority of patients with evaluable paired samples in both blood (40 of 51, 78%) and BMA plasma (34 of 44, 77%). There is a correlation in metabolite concentrations between the two compartments, as previously reported [5] (Supplemental Table 1, Supplemental Fig. 1).
Fig. 4.
Changes in blood and bone marrow aspirate (BMA) following 8 wk of enzalutamide treatment: (a) blood cortisol (n = 48); (b) BMA cortisol (n = 44); (c) blood androstenedione (n = 51); (d) BMA androstenedione (n = 43); (e) blood testosterone (n = 51), increase observed in 40 of 51 samples (78%); (f) BMA testosterone (n = 44), increase observed in 34 of 44 (77%) paired samples.
4. Discussion
Our findings provide the first evidence in human tumors that the therapeutic benefit of enzalutamide can be attributed to AR inhibition manifested by relocalization of the nuclear N-terminal AR to the cytoplasm. The results confirm AR as a driver of bone mCRPC and an important therapy target, even in the presence of biologically meaningful tissue androgen concentration. Furthermore, the results point to a feedback loop between AR and androgen biosynthesis in men with mCRPC analogous to, yet more consistent than, that reported with first-generation antiandrogens [19].
Estimating the benefit of any therapy in men with bone mCRPC is challenging because of largely nonmeasurable metastases and the absence of validated surrogate markers. Therefore, we applied clinical criteria and PCWG2 imaging criteria [16] to determine treatment discontinuation, and used duration of therapy as a measure of benefit. We dichotomized patients based on duration of therapy and linked this to the characterization of signaling pathways and steroid hormones in contemporaneously collected tissues, as described previously [5]. Serial tumor biopsying in this context presents well-documented difficulties. We previously established the feasibility of bone marrow sampling, although it comes with limitations concerning yield of specimens, application of multiple characterization approaches, and enrichment for patients who may have a larger disease volume. Tumors do not respond and hence show no regression of tumor in marrow or, alternatively, may stay on treatment for a protracted period of time. Tumors with nuclear AR overexpression (>75% involvement and high intensity) and presence of CYP17 are likely to respond to enzalutamide, as was the case for abiraterone acetate (Table 3). We confirmed the reported correlation between BMA testosterone levels and CYP17 expression [5]. These results support the idea that therapeutically relevant androgen signaling persists in men with mCRPC and ascribe functionality to CYP17 expression. The association between duration of therapy and the androgen signaling signature previously presented [5] indirectly supports optimal enzalutamide treatment outcome in the presence of wild-type natural ligand-binding AR. The absence of ARV7 expression in any tumors with prolonged response to enzalutamide is in line with recent preclinical reports suggesting that alterations in AR-mediated androgen signaling may account for resistance to enzalutamide in some cancers [20–22]. Given a small sample size and a p value of limited relevance due to multiple comparisons, these findings are hypothesis generating but consistent with experimental and clinical observations reported [1,2,23]. We screened for associations with clinical and tumor characteristics depicted in Table 1 and 2 and were unable to identify trends of importance [5]. A prospective study in larger numbers is warranted to determine the significance of these observations.
In contrast to the effects of androgen biosynthesis inhibition on mCRPC [5], we observed an increase in testosterone levels in blood and bone marrow and a nuclear-to-cytoplasmic shift of the AR following 8 wk of enzalutamide therapy (Fig. 2 and 4), which is consistent with the anticipated mechanism of action and has subsequently been observed in hormone-sensitive disease [19,24]. This increase suggests a physiologic feedback mechanism. It is unknown whether this androgen signaling adaptive effort may contribute to treatment resistance and, if so, to what extent.
We also screened for candidate predictors of outcome with enzalutamide, based on available data in the literature. The association observed between increased proliferation index (Ki67) and primary resistance, along with trends for increased phospho-Met and phospho-Src in this context are consistent with reports that altered cell cycle is inherent to androgen signaling inhibition resistance [13,14,23,25]. GR expression was not associated with primary resistance, although patients progressing in ≤6 mo are enriched for GR tumor expression, as noted in our recent collaborative report [26]. The screen for resistance in this small sample is limited to distinguishing the extremes of benefit from primary resistance; further study is required to elucidate the roles of phospho-Src, phospho-Met, and GR in enzalutamide-treated CRPC.
5. Conclusions
Our findings build on studies with androgen biosynthesis inhibitors in this setting [5,27] and provide the first clinical data on the mechanism of action of enzalutamide. Although patient and tumor characteristics, overall benefit, duration of treatment, and survival are similar in both studies, the inverse androgen receptor and biosynthesis alterations following treatment with the two respective reagents point to a two-compartment adaptive system driven by altered androgen biosynthesis and AR. Increased testosterone following enzalutamide AR inhibition and increased AR copy numbers following abiraterone acetate androgen depletion [5] suggest combined AR and androgen biosynthesis inhibition may block the feedback and improve efficacy in a subset of mCRPC patients [5]. This hypothesis is being explored (NCT01650194) [27]. The candidate androgen-signaling predictive signature comprised of but not limited to AR overexpression and CYP17 presence warrants further enhancement and is currently being pursued (NCT01254864).
Our tissue-based research contributes to the effort to identify, test, and validate predictors of outcome that will allow refinement of current clinical practice in advanced prostate cancer. The plethora of reagents with different mechanisms of actions provides a unique opportunity to optimize benefit through appropriate selection timing and address concerns with regard to sequencing and potential negative interactions [28,29]. Despite limitations, serial biopsy is an approach that allows for tumor microenvironment molecular characterization and that accounts for temporal heterogeneity of prostate cancer [30].
Supplementary Material
Take-home message.
This bone biopsy study confirms the experimentally defined enzalutamide mechanism in human metastatic castrate-resistant prostate cancer. We provide the first evidence in humans associating wild-type androgen receptor (AR) signaling with benefit and ARV7 with primary resistance, and we identify adaptive feedback between AR and androgen biosynthesis.
Acknowledgments
Acknowledgment statement: The authors would like to acknowledge Craig Berman for providing support in the development of the study concept and input on manuscript content.
Funding/Support and role of the sponsor: This study was supported in part by Medivation, Inc. (San Francisco, CA, USA) and Astellas (Northbrook, IL, USA) and by a Prostate Cancer Foundation Young Investigator Award and Career Development Award (E. Efstathiou), a National Cancer Institute Cancer Center Support Grant 5P30 CA16672-35 (E. Efstathiou), a US Department of Defense grant award (W81XWH-10-1-0273 MT), the Prostate Cancer Foundation Therapy Consortium, the Stanford Alexander Tissue Derivatives Laboratory, and the David H. Koch Center for Applied Research of Genitourinary Cancers. The sponsors reviewed and approved the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the American Society of Clinical Oncology Annual Meeting, June 4–8, 2011, Chicago, IL, USA, and at the American Association for Cancer Research Annual Meeting, March 31–April 4, 2012, Chicago, IL, USA.
Author contributions: Christopher J. Logothetis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Efstathiou, Logothetis.
Acquisition of data: Efstathiou, Logothetis, Ashe, Karlou, Hoang, Titus, Tu, Aparicio.
Analysis and interpretation of data: Efstathiou, Logothetis, Mohler, Troncoso.
Drafting of the manuscript: Efstathiou, Logothetis.
Critical revision of the manuscript for important intellectual content: Efstathiou, Logothetis.
Statistical analysis: Wen.
Obtaining funding: Logothetis.
Administrative, technical, or material support: Efstathiou, Logothetis, Hoang, Ashe.
Supervision: Efstathiou, Logothetis.
Other (specify): None.
Financial disclosures: Christopher J. Logothetis certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Eleni Efstathiou and Christopher J. Logothetis have provided consultancy for Medivation. All other authors declare they have no conflicts of interest.
References
- 1.De Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castrationresistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geller J. Prolonging survival in metastatic prostate cancer: the case for adrenal androgens—overview and summary of therapeutic controversies in prostatic cancer. J Clin Endocrinol Metab. 1995;80:1074–1078. doi: 10.1210/jcem.80.4.7714070. [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL, Gregory CW, Ford OH, III, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 8.Penning TM. New frontiers in androgen biosynthesis and metabolism. Curr Opin Endocrinol Diabetes Obes. 2010;17:233–239. doi: 10.1097/MED.0b013e3283381a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 10.Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. J Endocrinol. 2012;215:221–237. doi: 10.1530/JOE-12-0238. [DOI] [PubMed] [Google Scholar]
- 11.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. doi: 10.1038/onc.2013.284. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waltering KK, Urbanucci A, Visakorpi T. Androgen receptor (AR) aberrations in castration-resistant prostate cancer. Mol Cell Endocrinol. 2012;360:38–43. doi: 10.1016/j.mce.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Drake JM, Graham NA, Stoyanova T, et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci U S A. 2012;109:1643–1648. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varkaris A, Corn PG, Gaur S, Dayyani F, Logothetis CJ, Gallick GE. The role of HGF/c- Met signaling in prostate cancer progression and c-Met inhibitors in clinical trials. Expert Opin Investig Drugs. 2011;20:1677–1684. doi: 10.1517/13543784.2011.631523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efstathiou E, Troncoso P, Wen S, et al. Initial modulation of the tumor microenvironment accounts for thalidomide activity in prostate cancer. Clin Cancer Res. 2007;13:1224–1231. doi: 10.1158/1078-0432.CCR-06-1938. [DOI] [PubMed] [Google Scholar]
- 18.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 19.Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID. Casodex 10–200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. An overview of the efficacy, tolerability and pharmacokinetics from three phase II dose-ranging studies. Casodex Study Group. Eur Urol. 1998;33:39–53. doi: 10.1159/000019526. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Chen S, Sowalsky AG, et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20:1590–1600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan X, Cai C, Chen S, et al. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. doi: 10.1038/onc.2013.235. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith M, Borre M, Rathenborg P, et al. Enzalutamide (ENZA) monotherapy in hormone naive prostate cancer (HNPC): complete analysis of a phase 2 study [abstract 2852]. Presented at: European Cancer Congress 2013; September 27–October 1, 2013; Amsterdam, The Netherlands. [Google Scholar]
- 25.Pollack A, DeSilvio M, Khor LY, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22:2133–2140. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- 26.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Efstathiou E, Titus M, Wen AS, et al. The effects of enzalutamide (ENZA) in combination with abiraterone acetate (AA) in patients with bone metastatic castration resistant prostate cancer (mCRPC) [abstract 285]. Presented at: European Cancer Congress 2013; September 27–October 1, 2013; Amsterdam, The Netherlands. [Google Scholar]
- 28.Schmid SC, Geith A, Böker A, et al. Enzalutamide after docetaxel and abiraterone therapy in metastatic castration-resistant prostate cancer. Adv Ther. 2014;31:234–241. doi: 10.1007/s12325-014-0092-1. [DOI] [PubMed] [Google Scholar]
- 29.Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65:30–36. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 30.Efstathiou E, Logothetis CJ. A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res. 2010;16:1100–1107. doi: 10.1158/1078-0432.CCR-09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.