Abstract
The steroid hormone 17b-estradiol and the peptide hormone insulin-like growth factor (IGF)-1 independently exert neuroprotective actions in neurologic diseases such as stroke. Only a few studies have directly addressed the interaction between the two hormone systems, however, there is a large literature that indicates potentially greater interactions between the 17b-estradiol and IGF-1 systems. The present review focuses on key issues related to this interaction including IGF-1 and sex differences and common activation of second messenger systems. Using ischemic stroke as a case study, this review also focuses on independent and cooperative actions of estrogen and IGF-1 on neuroprotection, blood brain barrier integrity, angiogenesis, inflammation and post-stroke epilepsy. Finally, the review also focuses on the astrocyte, a key mediator of post stroke repair, as a local source of 17b-estradiol and IGF-1. This review thus highlights areas where significant new research is needed to clarify the interactions between these two neuroprotectants.
Introduction
Estrogen and IGF-1 share common actions on many tissues and this first noted in tumor biology. More than 40 years ago, it was recognized that while 17b-estradiol promoted tumor formation in vivo, the hormone did not show significant mitogenic activity in tumor cell cultures. A seminal study using tissue extracts from intact, ovariectomized and 17b-estradiol-replaced ovariectomized animals showed that tissues from estrogen-replete animals stimulate mitogenic activity in tumor cell lines, while tissues from estrogen-deficient (ovariectomized) animals did not (Sirbasku, 1978). This led to the hypothesis that estrogens effects on target tissue may be mediated by locally-produced growth factors. Subsequent research has confirmed that estrogens effects dependent on interaction with specific growth factor families. In the case of tumor cells, it was later shown that 17b-estradiol interacts with epidermal growth factor (Roos et al., 1986).
In the brain, 17b-estradiol has also been shown to interact with several growth factors during development and adulthood. BDNF (brain-derived neurotrophic factor), a member of the neurotrophin family of growth factors, is a good exemplar. Both estrogen and BDNF have overlapping actions in the forebrain especially in the regulation of basal forebrain cholinergic systems. BDNF-synthesizing forebrain neurons colocalize the estrogen receptors (Miranda et al., 1993), and 17b-estradiol replacement in adult, ovariectomized female rats increases BDNF expression in various forebrain regions (Singh et al., 1995; Sohrabji et al., 1995; Gibbs, 1999; Jezierski and Sohrabji, 2000; Jezierski and Sohrabji, 2001). The BDNF gene contains non-canonical estrogen response elements capable of binding estrogen-ligand complexes (Sohrabji et al., 1995). Estrogen and the neurotrophins stimulate common second messengers, such as the MAP kinases ERK1 and ERK2 (Singh et al., 1999). Estrogen may employ this growth factor as a mechanism to regulate neural cell function, in much the same way as 17b-estradiol interacts with epidermal growth factor (EGF) to regulate uterine growth and function and enhances DNA synthesis in mammary epithelial cells (Ignar-Trowbridge et al., 1992). The expanding role for estrogen in cerebrovascular disease and neurodegenerative disease focused attention on another growth factor, namely, IGF-1. Insulin-like growth factor (IGF)-1 is a potent growth-promoting factor, and its proliferative and survival actions have been reported for virtually all cell types. The main source of IGF-1 is the liver, and secreted IGF-1 affects diverse organ systems. However, deletions of hepatic IGF-1 do not affect growth and differentiation of body tissues, suggesting that locally available sources of IGF-1 are capable of exerting similar growth effects (Yakar et al., 1999). IGF-1 consists of a 70 aa polypeptide coded by the igf1 gene. The igf1 gene resides on the long arm of chromosome 12 and consists of 6 exons with long intronic sequences. In humans, two IGF-1 products are synthesized, IGF-1A which consists of exons 1,2,3,4,6 and IGF-1B which consists of exons 1,2,3,4,5. In rats, the gene consists of 6 exons, where exons 3 and 4 compose the mature IGF-1 protein (Daughaday and Rotwein 1989; Hoyt et al., 1992), also called IGF-1A. Alternate splices that contain exon 5 produce a peptide called IGF-1B. Moreover, alternate transcription start sites located in exon 1 and 2 and alternate polyadenylation sites results in several IGF-1 transcripts.
Modifications related to the N-terminal and C-terminal ends of the IGF-1 peptide produce significant biological effects. Post-translational modification of the IGF-1 peptide, where the N-terminal tripeptide is cleaved, results in a truncated IGF-1 molecule, first discovered in bovine colostrum (Francis et al., 1988). This molecule, called -3N-IGF-1, is believed to be more functionally active than the non-truncated form, possibly due to its low binding affinity for the IGF-binding protein (Szabo et al., 1988). Subsequently, the truncated tripeptide itself was demonstrated to have independent neuroactive functions. The N-terminal peptide or GPE (glycine-proline-glutamate) has been shown to inhibit glutamate binding to the NMDA receptor and potentiate the action of potassium on acetylcholine release in rat cortical slices (Sara et al., 1989). GPE has been shown to have neuroprotective properties in an experimental Parkinson's model (Guan et al., 2000) and in hypoxic injury (Svedin et al., 2007).
Formation of the mature IGF-1 peptide occurs with the cleavage of the C-terminal end of the IGF-1 propeptide. This C-terminal peptide, also called E-peptide, is coded on exon 4, 5 and 6. The E-peptide containing propeptide binds extracellular matrix with greater affinity, and this affinity is independent of the mature IGF-1 region (Hede et al., 2012). A muscle specific transgene of IGF-1E propetide significantly enhances muscle regeneration after injury, while muscle specific mature IGF-1 does not enhance muscle regeneration but increases serum levels of IGF-1 (Rabinovsky et al., 2003). These and other studies support the idea that E-peptide containing propeptides may increase the bioavailability of IGF-1 at the site of synthesis via anchorage to the ECM.
While studies have focused closely on the role of the IGF-1 mature peptide in the CNS, the actions of GPE are poorly understood and the E-peptides are virtually unstudied. Furthermore, estrogen interactions with the propeptide, the E-peptide and GPE have not been explored. In view of their critical roles in injury and regeneration in other tissue, this remains an important area for future studies. This review will therefore focus on the mature IGF-1 peptide, which is far better studied.
I: Estrogen and IGF-1 interactions
A) Sex differences and the IGF-1 system
Sex differences in several somatic systems appear to be associated with IGF-1 and provide a rich literature for understanding the interaction of estrogen and IGF-1 in normal physiology. In the case of body composition, lean body mass (LBM) decreases with age in both men and women. However, lean body mass was positively correlated with IGF-1 levels in males but not in women. In fact, LBM was greater in women on hormone replacement therapy (HRT) while IGF-1 levels were higher in women who were not on HRT (Waters et al., 2003). Interestingly, the IGF-1 binding protein IGFBP3 also decreased with age, although the ratio of IGF-1 to IGFBP3 decreased faster in males than females (Waters et al., 2003). In a study of Thai children and adolescents, serum IGF-1 levels increased with age and peaked at 13-15 years in males, and a little earlier in females (11-13 years). After the peak concentrations, IGF-1 levels declines in males but remained high in females (Jaruratanasirikul et al., 1999). Similarly, a study of young children and adolescents in Turkey showed an earlier peak of IGF-1 in female adolescents as compared to males, and a significant correlation of sex steroids with IGF-1 levels (Kanbur-Oksuz et al., 2004). Both sex steroids and IGF-1 are important for longitudinal bone growth and represent another area of synergy between these two classes of hormones. In mouse models, however, skeletal dimorphisms were found to be independently regulated by both IGF-1 and sex steroids in a time dependent manner, suggesting that the cross talk between these two hormones is not the sole mechanism (Callewaert et al., 2010).
Cardiovascular system
In cardiac biology, sex differences in cardiac contractile function may also be IGF-1 dependent, rather than sex hormone dependent. In female C57 mice, where IGF-1 levels are usually higher than males, cardiac myocytes exhibit reduced peak shortening, longer time-to-peak shortening and intracellular Ca++ clearance as compared to males. However in a severe liver IGF-1 deficient mouse (LID), where males and females have similar levels of IGF-1, sex differences in cardiac myocytes are abolished. Sex differences in Ca++ regulatory proteins were specifically attenuated in IGF-1 deficient animals (Ceylan-Isik et al., 2011). Age-related changes in the cardiovascular system are also strongly modulated by sex differences in the IGF-1 system. While IGF-1 and its receptor decline with age in both males and females, there was an 84% decline in IGF-1 and IGFR in male myocytes, while in females this loss was 40% for IGF-1 and 28-40% for IGF-1 receptors. IGF-1 reduction with age was associated with tissue injury, such as fibrosis along the left ventricular wall. However, fibrosis was significantly less in females at all ages compared to males (Leri et al., 2000). Thus the favorable cardiac function in older females, where estrogen levels are low, as compared to males may be a function of IGF-1 availability.
Skeletal System
Sex specific differences in bone mineral density (BMD) may also be tied to IGF-1 levels. Bone mass in the tibia vertebra and femur occurs earlier in females than males and BMD is higher in females than males in adolescence (5-9 weeks). This corresponds to the peak IGF-1 levels in females (3-5 weeks). In males IGF-1 levels peak at 9 weeks, which also corresponds to peak BMD. Males experience increases in IGF-1 and IGFBP3 before increases in serum testosterone levels although IGF-1 and testosterone levels are well correlated. In females, however, the correlation between serum IGF-1 and estradiol was weak (Fukuda et al., 1998). Thus sex differences in BMD and bone growth may be better associated with IGF-1 levels rather than gonadal steroids.
Hepatic System
In the liver, hepatocyte regeneration also shows an important interaction of sex and the IGF-1 system. Partial hepatectomy in the liver-specific IGFR knock out mouse significantly decreased hepatocyte regeneration in male mice, but not in females (Desbois-Mouthon et al., 2006). In the musculoskeletal system, mass and total ATPase activity reduced with age and this reduction is greater in males than females. However, circulating IGF-1 was reduced by 61% in females and only 21% in females. Paradoxically, liver IGF-1 mRNA was elevated in aging males (Severgnini et al., 1999).
Central Nervous System
Sex differences have also been noted in the IGF-1 system in the brain. In the developing cerebellum, the IGF-1 receptor IGFR-1 is elevated in the 1st postnatal week in males (P0-P7) and falls thereafter. In females, however, IGFR1 expression reaches peak expression at 2 weeks postnatally (P14) (Haghir et al., 2013). While it is not clear how this impacts cerebellar development, it is worth noting that males have more Purkinje neurons than females (Wittman and McLennan, 2011). Similarly, IGFR expression is highest in the right hippocampus of males at P7, and in the left on P14. Females, on the other hand express peak levels of IGFR at P7 in both hemispheres. Interestingly, laterality differences were seen in males and females but at different time points. These time course changes suggest that gender and laterality differences in IGF-1 may influence function and development of the hippocampus (Hami et al., 2012).
In summary, sex differences in IGF-1 function provide an important body of information relevant to interactions between these two hormone systems. Furthermore, this section also underscores variation across organ systems, with a greater impact of IGF-1 in the cardiac system and skeletal systems, as compared to the hepatic system and the CNS.
B) Estrogen and IGF-1: common second messenger systems
Cell signaling pathways such as the MAP kinases, the PI3kinase/AKT amplify the actions of growth factors and hormones, and serve as a point of synergy and interaction for the estrogen and IGF-1 systems.
Ligand-bound receptor tyrosine kinases, such as the IGF-1 receptor, recruit PI3 kinase when activated. The subsequent phosphorylation of PI3- kinase, via the Ras GTPase, activates AKT, a serine-threonine kinase, which exerts survival and proliferation via multiple pathways. The PI3K-Akt pathway is the prototypic mediator of IGF-1 effects on neurons. Initially shown to recruit glucose transporters (GLUT4) to the cell surface to internalize glucose, this pathway has also been shown to mediate IGF-1's effect on cell survival by inhibitory phosphorylation of the GSK3b, which promotes neuronal apoptosis (Hetman et al., 2000; Li et al., 2000). Similarly, 17b-estradiol has also been shown to modulate PI3K-Akt activation. One of the earliest systems where the non-nuclear effects of estrogen were recognized was the vascular system, specifically through interactions with the PI3K/Akt pathway. Estrogen receptor (ER)-alpha has been shown to bind the regulatory subunit of PI3K, and 17b-estradiol treatment increases this association resulting in Akt activation and elevation of downstream genes such as eNOS (Simoncini et al., 2000).
An important downstream mediator of the PI3K/AKT pathway is MTOR (mammalian target of Rapamycin). Activated AKT inhibits the formation of the tuberous sclerosis proteins TSC1 and TSC2, thus allowing activation of MTOR. Once activated, MTOR plays an important role in activating proteins that regulate translation initiation rates such as the elFBP and ribosomal protein S6 kinases (Petroulakis et al., 2006). Hence MTOR signaling integrates extracellular signals from growth factors and hormones to control protein synthesis, cell-cell progression and cell metabolism. MTOR signaling can alter critical cell processes such as A-beta and tau protein homeostasis, which impact cell death in Alzheimer's disease. IGF-1 has been closely linked to this pathway in skeletal muscle hypertrophy, and has been shown to promote muscle hypertrophy through an activated PI3K/Akt/MTOR pathway (Latres et al., 2005). While this pathway is poorly studied in the brain, IGF-1 has been shown to regulate the glucose transporter GLUT-3 in PC12 cells through the PI3K/Akt/MTOR pathway via the transcription factor HIF-1a (Yu et al., 2012). Similarly, IGF-1 regulation of MCT2 the moncarboxylase transporter that facilitates lactate usage for energy is also dependent on this pathway.In cortical cells, IGF-1 increases MCT2 but not its mRNA, suggesting that its effects are at the translational, not transcriptional level. Rapamycin, which blocks mTOR abrogated IGF-1 action on MCT2 (Chenal et al., 2008). Several studies also link estrogen with this pathway, namely in peripheral estrogen sensitive tissues such as the uterus. The PI3k/AKT/mTOR pathway is critically involved with myometrial hyperplasia during pregnancy. Estradiol treatment, which is proliferative for uterine myocytes, induces IGF-1 in ovariecxtomized rats, which is then followed by an activation of the PI3K/AKT/mTOR pathway. Rapamycin blocks this hormone action on proliferation (Jaffer et al., 2009). Similarly, in the tumor cell line MCF-7, where both estrogen and IGF-1 are known to be critical mitogens, the PI3K-AKT-mTOR pathway appears to be the principal transducer of their actions. Estrogens proliferative actions on MCF-7 cells are mediated via IGF-1, and 17b-estradiol increases IRS-1 as well as p85 (the active subunit of the PI3K). Conversely, IGF-1 is proliferative only in steroid-treated cells (Bernard et al., 2006). Synthesis of IGFBP-2, which mediates tumor growth, is activated by IGF-1 and 17b-estradiol through this pathway and may be blocked by rapamycin (Martin and Baxter, 2007). In fact IGF-1 may also employ this pathway to reduce or enhance the actions of ovarian hormones. IGF-1 decreases progresterone receptor in MCF-7 cells, via mTOR, while inducing ligand independent estrogen receptor function (Cui et al., 2003). This mechanism has not been explored in the brain and may represent a new level of interaction of and estrogen receptor systems.
A second common pathway for estrogen and IGF-1 is the MAP kinase signaling family. Mitogen-activated protein kinases (MAPKs) family consists of extracellular signal-regulated kinase (ERK), p38, and c-Jun NH2-terminal kinase (JNK), and their several isoforms. MAP kinases are serine-threonine kinases that mediate intracellular signaling for a wide variety of cellular activities, including proliferation, differentiation, survival and death (Dhillon et al., 2007). Activated MAP kinase phosphorylates a large number of proteins including transcription factors such as the estrogen receptor (reviewed in Lu and Xu, 2006). Activation of ERK1/2 has been shown to inhibit apoptosis in hypoxia (Buckley et al., 1999), growth factor withdrawal (Erhardt et al., 1999), osmotic stress (Foncea et al., 2000) and exposure to tumor necrosis factor (TNF) and Fas ligand (Tran et al., 2000). Alternately, ERK activation is also linked to cell death, especially in neuronal populations. For example, persistent activation of ERK is associated with glutamate toxicity in neurons, which can be attenuated with ERK1/2 inhibitors (Stanciu et al., 2000). Inhibition of the upstream kinase MEK1/2 protects against cell death in a 6-OHDA injury model (Kulich and Chu 2001) and intravenous injection of the MEK1 inhibitor also reduces infarct volume in a stroke injury model (Nakamura et al., 2001).
17b-estradiol treatment in vivo results in a dose-dependent activation of ERK and Akt and has a synergistic effect on IGF-1 mediated activation of the pAkt/PKB pathway (Cardona-Gomez et al., 2002). In addition to increasing ER-IGFR dimers, 17b-estradiol also promotes the association between the regulatory subunit of PI3K and the insulin receptor substrate (IRS)-1, thus increasing the possibility of activating the IGFR. The interplay between the estrogen and IGF-1 pathways may be maturation dependent. In postpubertal animals, ovariectomy and subsequent replacement with estradiol resulted in sustained activation of Akt and GSK3b, however this effect is not seen in animals ovariectomized before puberty (Sanz et al., 2008).
Estrogen and IGF-1 are both potent regulators of cellular proliferation and survival. In vivo, 17b-estradiol transiently activates the IGF-1 receptor through tyrosine phosphorylation, leading to an interaction of ER-a with IGFR-1 and a greater interaction with components of the PI3K pathway (Mendez et al., 2003). In a medial forebrain bundle injury that models Parkinson's disease, IGF-1 attenuates lesion effects and also mediates the neuroprotective effects of estrogen (Quesada et al., 2004). IGF-1 and estrogen both signal through MAPK and Akt pathways and Akt inhibitors blocked the survival effects of both 17b-estradiol and IGF-1 (Quesada et al., 2008), implicating the Akt survival pathway in estrogen and IGF-1 mediated neuroprotection. Interestingly, in the same study, a MAPK inhibitor had no effect on estrogen and IGF-1 actions. In fact the actions of estrogen and IGF-1 on ERK activation and neuroprotection appear to be controversial. For example, hippocampal cell death due to ischemia is reduced by 17b-estradiol pretreatment, and hormone treatment is shown to activate IGF-1 receptors and stimulate MAPK. Similarly, 17b-estradiols effects on memory, which are blocked by the JB-1 antagonist, is accompanied by a ERK activation as well as choline acetyltransferase, the acetylcholine-synthesizing enzyme (Witty et al., 2013), indicating that the MAP kinase pathway may be involved in the co-activation of 17b-estradiol and IGF-1 systems. In tumor cells, IGF-1 activation of the MAP kinase pathway renders the cells insensitive to antiestrogen's such as tamoxifen (Zhang et al., 2011). However, other reports have shown that interaction between these two trophic agents, while neuroprotective, appears to suppress the ERK pathway. In the damaged substantia nigra, for example, co- treatment of 17b-estradiol and IGF-1 reduces cell loss and suppresses ERK activation (Quesada et al., 2008). Similarly in an ischemic stroke model, infarct volume is reduced by estrogen and IGF-1 and is preceded by a suppression of ERK activation in the cortex (Selvamani and Sohrabji, 2010). One possible explanation to reconcile these disparate findings is that there may be a time dependent modulation of ERK; enhancement in the short term and suppression in the long term. Alternately, heterodimers of the estrogen and IGF-1 receptor, unlike ER/IGFR homodimers, may inhibiting ERK activation, which in turn has been shown to promote PI3K/AKT activation (Zhuang et al., 2007) (Figure 1).
Figure 1.
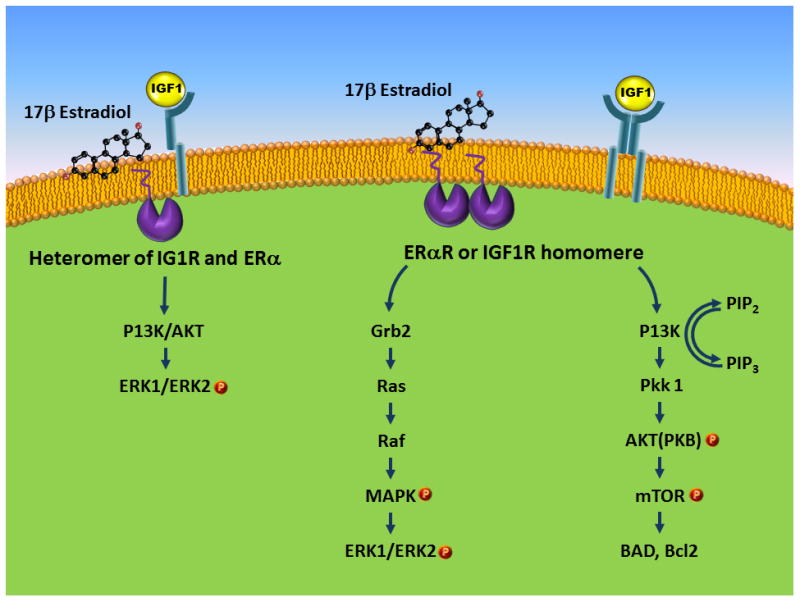
Schematic representation of the estrogen and IGF-1 receptor interaction with second messenger systems. Homodimers of ER-a and IGFR can activate the MAPK pathway and the PI3K/AKT pathway. Co-treatment with estrogen and IGF-1, which can result in ER-a/IGFR heterodimers, have been shown to inhibit ERK activation.
II: Estrogen and IGF- neuroprotection
In experimental models of neurodegenerative diseases (Garcia-Segura et al., 2000; 2006), estrogen and IGF-1 also have overlapping neuroprotective actions. Both prevent excitotoxin-induced cell death in hilar neurons (Azcoita et al., 1999), and estrogen receptor antagonists can inhibit the actions of the IGF receptor. Similarly, recovery from excitotoxic lesions of the nigostriatal dopaminergic pathway is improved by either estrogen or IGF-1, and the IGF-1 inhibitor JB-1 attenuates the protective actions of both estrogen and IGF-1 (Quesada and Micevyich, 2004). In aging ovariectomized animals where estrogen replacement has been shown to improve memory function, estrogens effects can be abrogated by treatment with JB-1 (IGFR inhibitor), indicating that estrogen may exert part of its effects through the IGFR signaling pathway (Witty et al., 2013). Similarly, in an ischemic stroke model, JB-1 treatment abolishes the neuroprotective actions of estrogen (Selvamani and Sohrabji, 2010). In fact, IGF-1 can activate estrogen receptors in the absence of estradiol (Ma et al., 1994). Both in vivo (El Bakri et al., 2004) and in vitro studies show that estrogen can regulate the IGF-1 receptor (Cardona-Gomez et al., 2002). Estrogen treatments can also transiently promote dimerization of the estrogen receptor and the IGFR (Mendes et al., 2003), providing a substrate for synergistic interactions.
A) Ischemic Stroke: Effects of Estrogen
Cerebrovascular diseases such as stroke offer an instructive example of 17b-estradiol and IGF-1 cooperative action. Stroke is one of the leading causes of adult disability and the fourth leading cause of mortality in the US. Stroke occurs disproportionately among the elderly, and among this group, more often in women than men. Stroke-related disability and institutionalization is also likely to affect women more than men (Lai et al. 2005). Newer studies suggest that the risk for stroke in middle aged women doubles that of men (Towfighi et al. 2007), and that women account for 60% of stroke-related deaths (Lloyd-Jones et al. 2010), even after normalization for age. The 5-yr stroke recurrence is disproportionately higher in females (20%) as compared to males (10%) in the 45-64 age range (Roger et al. 2011). A Canadian stroke registry study reported that 10% of women stroke patients were discharged to long term care as compared to 5% men (Kapral et al. 2005), while the Danish National Registry study reported that women have more severe strokes than men although they exhibited a survival advantage compared to men, especially at advanced ages (Olsen & Andersen 2010).
Sex differences in stroke outcome in clinical studies are also reflected in experimental data. Female rats have a smaller infarct and better cerebral blood flow than age-matched males (Alkayed et al. 1998). However, although female mice sustain a much smaller infarct as compared to age matched males (Manwani et al. 2011), aged females showed significantly more mortality and poorer stroke outcomes as compared to older males. The female advantage seen in these suggests that estrogen may be protective for stroke. Using natural variations in circulating estrogen levels across the estrous cycle, Liao and colleagues (2001) showed that the extent of ischemic damage was inversely related to circulating levels of estrogen. In fact, replacement with 17β estradiol and its inactive stereoisomer 17α estradiol (Simpkins et al. 1997) as well as the conjugate equine estrogen preparation (McCullough et al. 2001) all reduce infarct volume in female animals. Exogenous 17b-estradiol replacement is neuroprotective when given prior (Dubal et al. 1998); Selvamani and Sohrabji, 2010) or subsequent to the injury (Liu et al. 2005, Yang et al. 2003).
However, hormone effects on stroke outcomes are more complicated in the context of aging. Our studies have specifically addressed this reproductive transition phase by studying acyclic, middle-aged female animals (Johnson et al. 2006, Selvamani & Sohrabji 2010a, Selvamani & Sohrabji 2010b). Middle cerebral artery occlusion (MCAo) in this middle aged female results in a significantly larger infarct as compared to young females (Selvamani & Sohrabji 2010a), suggesting that the loss of ovarian hormones may accelerate age-related stroke severity. Paradoxically, 17b-estradiol treatment to ovariectomized reproductive senescent females increases infarct volume, indicating that the aging brain may be refractory to estrogen-dependent neuroprotection (Selvamani & Sohrabji 2010a, Selvamani & Sohrabji 2010b). This was confirmed by another study where long term 17b-estradiol replacement to aged females also failed to improve stroke (Leon et al. 2012). In aging females, the loss of estrogens is also accompanied by decreasing levels of other hormones, including IGF-1. In fact, post-stroke IGF-1 replacement to middle-aged females pretreated with 17b-estradiol abrogates the neurotoxic effects of 17b-estradiol in this group (Selvamani and Sohrabji, 2010b), indicating that collaborative actions of 17b-estradiol and IGF-1 are critical for the aging brain.
B) Ischemic Stroke: Effects of IGF-1
Independently of estrogen, IGF-1 is also positively associated with stroke outcomes. IGF-1and IGF-BP3 levels are reduced in stroke patients as compared to those reported in the literature for healthy individuals. Within the stroke population, plasma IGF-1 levels were lower in individuals with a large infarct (Schwab et al., 1997), and stroke-associated mortality was lower in patients with higher IGF-1 levels (Denti-et al., 2004). Conversely, higher IGF-1 levels as associated with improved stroke outcomes. In a study of 255 stroke patients, higher IGF-1 levels or a higher IGF-1/IGFBP3 ratio was associated with better functional outcome at 3 months. Interestingly, baseline severity was not different between high and low IGF-1 groups, suggesting that IGF-1 modulates stroke sequelae and not the immediate phase (De Smedt et al., 2011). In a case cohort of older individuals in the Cardiovascular Health Study, neither IGF-1 nor IGFBP-1 predicted MI or stroke, but low IGFBP-3 levels were associated with higher levels of coronary events. Analyzing non-fatal MI only, suggested that low IGF-1 and IGFBP-3 levels elevated the risk of these events (Kaplan et al., 2007).
In experimental models, also, exogenous IGF-1 treatment is neuroprotective and has been shown to reduce ischemic stroke injury in many species including rats, mice and sheep (Gluckman et al., 1992; Lee and Bondy, 1993; Johnston et al., 1996; Guan et al., 2001). IGF-1 promotes post stroke neuronal survival, myelination and angiogenesis (Smith, 2005; Wang et al., 2000), as well as stroke-induced neurogenesis (Yan et al., 2006) and progenitor cell proliferation (Dempsey et al,. 2003). The peptide hormone is effective for stroke when delivered intravenously (Rizk et al., 2007), intracerebroventricularly or intranasally (Lin et al., 2009), as also peripheral administration of the N-terminal tripeptide of IGF-1 (GPE) (Shapira et al., 2009). Additionally, treatment with IGF-1, IGF-2 and IGF binding protein (IGFBP) ligand inhibitors, which displace and therefore increase bioactive IGFs from their binding protein, all improved infarct volume in MCAo models (Mackay et al., 2003).
Cerebral injury upregulates IGF-1 and its receptors, and this is believed to be a protective response. In the Mongolian gerbil, IGF-1 positive cells were detected in GABAergic interneurons in CA1 and in pyramidal and non-pyramidal cells of CA2/3, 12h and 24 h after ischemia. Four days later, this neuronal IGF-1 disappears and is replaced in astrocytes and microglia. The temporal pattern suggests that these support cells may act to prevent delayed cell loss mechanisms (Hwang et al., 2004). In a head contusion model, IGF-1 mRNA levels increased at the site of the injury during the first 72h after trauma (Sandberg-Nordqvist et al., 1996), underscoring the fact that IGF-1 systems are mobilized during cerebral injury. In asphyxia, which is a transient neural injury, IGF-1 and IGFBP-2 and -3 are elevated in glial cells (Gluckman et al., 1998), while in a hypo-ischemic model where animals were placed in 10% oxygen for 4 days, IGF-1 and IGFR mRNA was significantly reduced (Gaddipati et al., 1999). In the MCAo suture model, IGF-1 was increased in brain and plasma, whereas IGFBP-2 decreased in the brain. In brain microvessel endothelial cells, IGF-1 and IGFBP-2 were both elevated by OGD. However, exposing neurons to conditioned media from OGD exposed BMEC increased neuronal apoptosis, while conditioned media from untreated (normal) BMEC increased neuronal survival (Wang et al., 2013). Thus increased expression of IGF-1 per se may not always be protective. In rats, cerebral ischemia using a 2-vessel occlusion model increased IGFR in the CA1 CA3 and dentate gyrus of the hippocampus, however IGF-1 treatment, pre or post stroke was not protective in this model (Bergstedt and Wieloch, 1993).
C) Ischemic Stroke: Estrogen and IGF-1 interactions
Evidence supports the hypothesis that IGF-1 may mediate estrogen's neuroprotective effects. In a Parkinson's disease model, inhibiting IGFR abrogated the protective response to 17b-estradiol, suggesting that steroid-mediated neuroprotection involves the IGF-1 signaling pathway (Quesada et al., 2004). In an experimental stroke model, JB-1 induced blockade of the IGF receptor in young females completely abolished the protective effect of 17b-estradiol (Selvamani and Sohrabji, 2010b). In vitro studies have also confirmed that estrogen mediated neuroprotection is mediated by IGFR, such that the addition of JB-1 to the media of human fetal neuroblast cells abolished the protective effects of 17b-estradiol on peroxide and amyloid-induced toxicity (Luciani et al., 2012).
Interestingly, in a bilateral carotid artery occlusion model of stroke, loss of hippocampal cells in middle-aged females was reduced by either 17b-estradiol or IGF-1, with no additional benefit from combining the two hormones (Traub et al., 2009). In the case of the sodium calcium exchanger in cortical neurons, however, combining the two compounds enhanced the independent action of 17b-estradiol and IGF-1. Furthermore, this action was independent of the classical receptors, since neither estrogen nor IGF-1 receptor antagonists could prevent these effects (Sanchez et al., 2011). Similarly, both estrogen and IGF-1 enhance neurogenesis in the dentate gyrus of the hippocampus and the hormones appear to have a synergistic effect. The ER antagonist, ICI 182,780 blocked the actions of IGF-1 in the presence or absence of 17b-estradiol implicating the estrogen receptor in IGF-1 mediated effects (Perez Martin et al., 2003). Conversely, in a kainate acid injury model, where both 17b-estradiol and IGF-1 protected hippocampal hilar cells, an antagonist to either the estrogen receptor or the IGF receptor blocked estrogen's effects. Reciprocally, IGF-1's protective actions were inhibited by the ICI 182,780 compound, suggesting that IGF-1 acts through the estrogen receptor (Azcoitia et al., 1999).
Overall, the published studies support the conclusion that estrogen and IGF-1 are neuroprotective independently and collaboratively. An important area in need to greater study is the interaction of estrogen and IGF-1 during aging, where the cells that synthesize these compounds and respond to these compounds undergo metabolic and functional changes.
III: Estrogen and IGF-1 interactions: Post-stroke repair processes
Thus far, most studies examining the interactions of estrogen and IGF-1 have focused directly on neurons, however, in stroke, as in other neurovascular and neurodegenerative diseases, neuronal survival is directly impacted by changes in the vascular and immune systems. Within minutes of ischemic stroke, neurons deprived of oxygen and glucose undergo anoxic depolarization and succumb to cell death. Excitotoxicity caused by hypoperfusion also results in microvascular damage and damage to the blood brain barrier, initiating post stroke inflammation. The role of estrogen and IGF-1 has been investigated on some of these post stroke processes, although the interaction between the two systems is poorly understood.
A: Post-stroke repair processes: Blood brain barrier
The blood brain barrier consists of closely aligned endothelial cells, encased in basement membrane and supported by astrocytic endfeet and pericytes (Risau & Wolburg 1990, Abbott et al. 2006, Kulik et al. 2008, Haseloff et al. 2005) (Figure 2). The principal role of the blood brain barrier is to regulate the influx and efflux of cells, molecules and ions thus maintaining the homeostatic environment of the brain. Alterations in the blood brain barrier are thus likely to elevate the risk for stroke and severity, by increasing the influx of cytotoxic cells and proteins. Age increases blood brain barrier permeability (Kalaria 1999; Abbott 2000; Bake and Sohrabji, 2004; Farrall and Wardlaw 2007; Grammas et al., 2011), and is further increased with neurologic disease such as vascular dementia and Alzheimer's disease (Farrall & Wardlaw 2007). Blood brain barrier disruption enhances infiltration of serum proteins into brain parenchyma (Loftspring et al. 2006) and triggers an inflammatory response. The presence of tight junctions between adjacent endothelial cells that act as a gatekeeper to blood borne toxins (Brightman & Reese 1969). Following stroke, blood brain permeability is enhanced in older animals and tPA, the thrombolytic compound approved for stroke treatment, further increases barrier permeability (Kaur et al. 2011).
Figure 2.
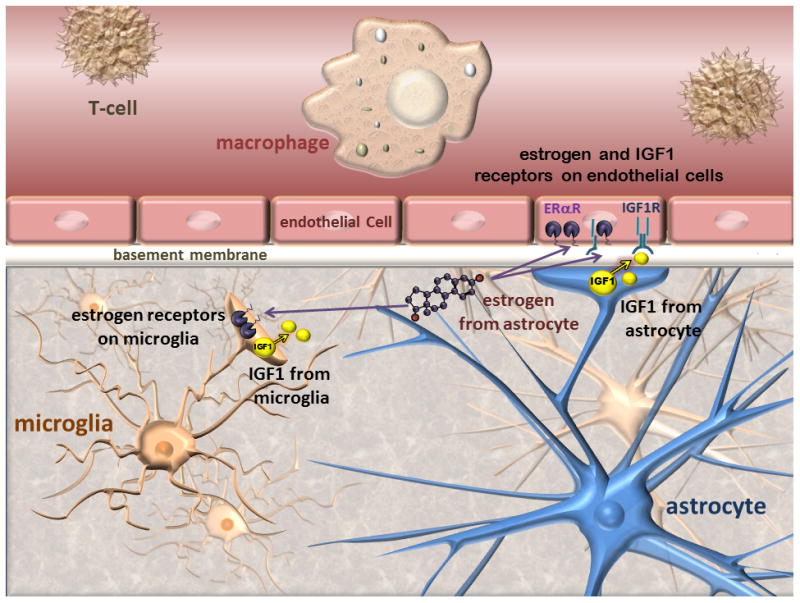
Schematic representation of the blood brain barrier: The blood brain barrier consists of closely arranged endothelial cells surrounded by basement membrane.Circulating immune cells are shown in the lumen of microvessels, while microglia are located in the brain parenchyma. Astrocytic endfeet contact the endothelial layer and provide physical and growth factor-mediated support to the barrier. Estrogen receptors and the IGF-1 receptor are located on endothelial cells, astrocytes and microglia. Astrocytes also synthesize IGF-1 and neurosteroids including estrogen. Activated microglia can also synthesize IGF-1. In cerebral ischemia, this morphological arrangement ensures steroid and growth factor support for endothelial cells.
While there is clear evidence of age-associated disruption of blood brain barrier, alterations in barrier function in estrogen-deficient conditions such as menopause or reproductive senescence are relatively understudied. Low estrogen levels associated with menopause or reproductive senescence will negatively impact barrier and endothelial function due to the known role of estrogen on this cell type. In most cases, estrogen's effects on blood brain barrier permeability appear to be protective. Specifically, 17b-estradiol reduces barrier permeability following injury in young female rodents (Liu et al. 2005), reduces ischemic- (Chi et al. 2002, Chi et al. 2005) and VEGF-induced (Chi et al. 2004) leakiness of the blood-brain barrier in the cortex (Chi et al. 2002), and attenuates VEGF-mediated blood-retina barrier (Chen et al., 2011) and tPA mediated barrier breakdown and hemorrhagic transformation in male rats (Li et al., 2011). 17b-estradiol treatment also increases glucose uptake and transport (Bishop & Simpkins 1995, Shi et al. 1997), reduces edema in an experimental stroke model by reducing the activity (O'Donnell et al. 2006) and abundance (Chang et al. 2008) of the Na-K-Cl cotransporter. Oral conjugate estrogens also reduce vascular lesions and leukocyte permeability caused by A-beta 40 and A beta-42 infusions (Rhodin et al. 2003). The ER-beta agonist DPN has been shown to reduce surrogate markers for blood brain barrier permeability in a stroke injury such as vasogenic edema and extravasation of IgG, although tight junction proteins and the water channel aquaporin 4 was not affected (Shin et al., 2013). However, the synthetic estrogen ethinyl estradiol is shown to increase the endothelial permeability to albumin (Gammal & Zuk 1980) and 17b-estradiol acts synergistically with myelin basic protein to cause mast cell infiltration into the brain parenchyma (Theoharides et al. 1993). Moreover, in estrogen-deficient middle aged female rats (reproductive senescence), there is a constitutive increase in the permeability of the blood brain barrier in the forebrain as compared to estrogen-sufficient young females (Bake & Sohrabji 2004), accompanied by increased perivascular IgG expression in hippocampus, a commonly used marker to assess barrier integrity in aging and disease dysregulation of junctional proteins in cerebral (Bake et al. 2009). In fact, cerebral microvessels from a small sample of pre and post-menopausal women also confirmed this reproductive age-related loss of junctional localization (Bake et al. 2009).
The effect of IGF-1 on blood brain barrier permeability is less studied and more controversial. In the spinal cord, topical applications of high doses of IGF-1 reduced the breakdown of the blood-spinal cord barrier and spinal edema and improve axonal repair in a traumatic injury to the T10-T11 segments (Sharma, 2005), while low dose IGF-1 was ineffective. In an acute demyelinating EAE model of multiple sclerosis, intravenous IGF-1 given over 8 days reduced permeability of the blood-spinal cord barrier and improved motor outcomes. Higher doses of IGF-1 were more effective than lower doses (Liu et al., 1995). However, other evidence indicates that IGF-1 treatment in an acute inflammatory may actually increase blood brain barrier permeability. Intraventricular injections of LPS in a neonate rat, which is an experimental model of periventricular leukomalacia, causes rapid dysregulation of the blood brain barrier, activation of microglia and astrocytes and recruitment of leukocytes to the brain. Low dose IGF-1 treatment in this model prevented cell death associated with this injury, however, IGF-1 also increased permeability of the blood brain barrier, recruitment of leukocytes and caused intracerebral hemorrhage (Pang et al., 2010). High doses of IGF-1 in conjunction with LPS increased mortality, indicating that the peptide hormone may anomalous effects in acute inflammation.
Cerebral edema due to cirrhotic liver disease and portal vein occlusion was not improved by IGF-1 treatment (Odena et al., 2012). In fact, in peripheral tissues, IGF-1 treatment is known to cause mild generalized and reversible edema. In a small study with 8 health participants, IGF-1 treatment resulted in greater vascular permeability in skin and retina as compared to controls (Hussain et al., 1995). In view of the limited number of studies examining IGF-1's actions at the blood brain barrier, it is not surprising that there is virtually no data on the interactions of estrogen and IGF-1 on this unique structure. This remains an important and understudied area of investigation for stroke neuroprotection. Since both estrogen and IGF-1 promote survival and proliferation of target cells, it is likely that dual treatment may improve survival of endothelial cells in microvessels, thus preserving barrier function.
B: Post-stroke repair processes: Angiogenesis
The formation of new vessels is an adaptative response to ischemic injury (del Zoppo & Milner 2006, Sonntag et al. 2007), usually initiated by hypoxia-induced upregulation of HIF-1 and VEGF which promote endothelial cell growth (Kanaan et al., 2006; Pugh and Ratcliffe, 2003). While usually devoid of blood, new vessels formed post-stroke provide an angiogenic niche that promotes neurogenesis (Palmer et al., 2000; Zhang et al., 2004; Chopp et al., 2007; Arai et al., 2011). Neurons and astrocytes within the neurovascular unit also secrete trophic factors such as IGF-1 (Li et al. 2010), VEGF (Ferrara et al. 2003), and PDGF-b (Marti et al. 2000, Jin et al. 2002, Beck & Plate 2009) to induce angiogenesis, which in turn enhances proliferation and differentiation of neuronal precursor cells to promote neurogenesis (Sun et al. 2003).
Estrogen promotes angiogenesis in many vascular beds (Rubanyi et al., 2002), and has also been shown to decrease free radical production, increase cell survival, and stimulate angiogenesis in cerebral endothelial cells (Krause et al. 2006). However much less is known about the effects of estrogen on stroke-induced angiogenesis. In a study examining the effect of estrogen on angiogenesis and stroke, 17b-estradiol treatment increased Ang-1 and microvessel density prior to stroke (Ardelt et al. 2005), suggesting that the hormone may enhance angiogenic potential. In the peri-infarct area, 17b-estradiol increased microvessel density at 10 days post stroke in ovariectomized female rats, although this was not associated with functional recovery (Ardelt et al. 2007). Subsequent studies also showed that this effect was dose dependent, in that post-stroke vessel density was greater with a 0.72mg estradiol pellet versus a low dose (0.18mg) or control pellet. The higher dose was also associated with functional recovery (Ardelt et al., 2012). Interestingly, in a study using male rats, 17b-estradiol treatment failed to increase microvessel density in the peri-infarct area, but preserved motor function in this group (Ulbrich et al., 2012). Thus estrogen-mediated angiogenesis may be dose and sex restricted.
The effect of IGF-1 on angiogenesis is well known although its role in injury-induced angiogenesis is limited. More than 20 years ago, it was shown that IGF receptors are present on vascular endothelia cells (Bar et al., 1988) and that IGF-1 increased endothelial cell migration and tube formation in collagen matrices (Nakao-Hayashi et al., 1992; Nicosia et al., 1994). IGFR is poorly expressed in peripheral tissue beds in the adult as compared to the neonate, however, tissue damage increased IGFR in endothelial cells (Hannson et al., 1989) and in microembolic injury in the heart (Kluge et al., 1995). Evidence shows that IGF-1 increases VEGF levels, the prototypic angiogenic growth factor (Bermont et al., 2000; Smith et al., 1999). In aging, angiogenesis is impaired and sprout formation of microvessels grown in collagen gels was impaired in aged mice as compared to young animals. Treatment with minimal serum media and IGF-1 improved sprout formation in aged microvessels, obliterating the difference between young and aged microvessels (Arthur et al., 1998). Among the earliest studies to examine the effect of IGF-1 on angiogenesis focused on the ischemic retina. IGF-1 injected intravitreally, in the ischemic eye, resulted in proliferation of blood vessels (Grant et al., 1993). In the IGF-1 homozygous knockout, the number of capillaries per muscle fiber in the diaphragm is reduced (Fournier and Lewis, 2000), while blockade of the IGFR reduced angiogenesis in a colon cancer model (Reinmuth et al., 2002). IGF-1 induces migration of endothelial cells and promotes tubular formation in combination with high glucose levels (Shigematsu et al., 1999). This latter finding is particularly worrisome since ocular neovascularization in diabetic retinopathy is a major public health problem and also cautions the wide application of IGF-1 as a therapeutic in disease such as stroke, where diabetes or metabolic disease is a frequent comorbidity.
The problem of post stroke angiogenesis is complicated by the fact that stroke occurs most often in older populations, where both steroid hormone levels and IGF-1 levels are deficient. The mechanics of angiogenesis in this group are poorly studied. In aging, there is a constitutive decline in the number/density of vessels in the cerebral cortex, and this is partly attributed to the decline of growth hormone and IGF-1 in this group (Sonntag et al., 1997). Moreover, ischemia-induced capillary angiogenesis is impaired in older animals (Ingraham et al., 2008). Interestingly, IGF-1 mRNA levels are not shown to decrease with aging, however IGF-1 protein levels do decrease (Sonntag et al., 1999) suggesting that there may be translational repression of IGF-1. In elderly ischemic patients, well established angiogenesis is positively correlated with higher IGF-1 levels (Schwab et al., 1997). In animal models, systemic IGF-1 increases vessel density, while exercise, which is known to promote angiogenesis, does not do so if animals have low serum IGF-1 levels. Similarly, brain injury stimulated angiogenesis is abrogated in the presence of an IGF-1 antagonist (Lopez-Lopez et al., 2004).
Thus while estrogen and IGF-1 both influence angiogenesis, there are very few studies that have directly examined this interaction either in vivo or in vitro. An example of such an interaction is seen in hormone-induced prostate cancer, where 17b-estradiol or testosterone pellets to the tumor-prone Noble rat causes prostatic cancer. This treatment also induces VEGF and IGF-1 receptor expression in epithelial and endothelial cells (Wang and Wong 1998). Similarly, in breast cancer tissue, IGF-1 elevates urokinase plasminogen activator (uPA-1), which increases angiogenesis (Dunn et al., 2000).
C: Post-stroke repir processes: Inflammation
Neuroinflammation is a critical aspect of stroke and responsible for secondary cascades of cell death. Anti-inflammatory compounds such as minocycline, for example, have been shown to improve infarct volume in experimental stroke (Koistinho et al., 2007). Activation of microglial and astrocytes locally mediate the early inflammatory events in stroke, followed by recruitment of activated splenocytes to the ischemic brain. Subsequent to this early phase of immune activation is a later immunosuppressive phase that affects somatic health and recovery. Both estrogen and IGF-1 have direct and indirect actions on inflammatory cells, which are reviewed here, although the combined actions of both hormones have not been studied in the context of neuroinflammation.
Estrogen has pleiotrophic effects on the inflammatory response. In specific tissues such as uveitis of the anterior chamber of the eye, (Miyamoto et al., 1999), carrageenan-induced pleurisy in the lungs (Cuzzocrea et al., 2000; 2001) and adjuvant-induced arthritis (Badger et al., 1999), estrogen appears to decrease inflammation. On the other hand, estrogen promotes prostatitis (Naslund et al., 1988), stimulates edema (Tchernitchin and Galand, 1983) and increases vascular permeability and influx of macrophages in the uterus (De and Wood, 1990; Kaushic et al., 1998). In in vitro models, 17b-estradiol pretreatment attenuates LPS-induced superoxide release, phagocytic activity and an increase in iNOS in microglia (Bruce-Keller et al., 2000, Drew and Chavis, 2000; Vegeto et al., 2000; Vegeto et al., 2001). 17b-estradiol pretreatment in vivo reduces activation of local microglia and peripheral monocyte expression following intraventricular injections of the bacterial pathogen, lipopolysaccharide (LPS; Vegeto et al., 2003) and subcutaneous injections of proteolipid protein in an experimental autoimmune encephalomyelitis model (Subramanian et al., 2003) in young adult female mice, and suppresses the inflammatory response in influenza A pathogenesis (Robinson et al., 2011). Similarly, estrogen receptor specific ligands such as tamoxifen and raloxifen are also able to suppress LPS-activation of microglial cultures and LPS activation of microglia in vivo in both males and females (Arevalo et al., 2012).
Both these compounds, as well as other selective estrogen receptor modulators (SERMS) also suppress LPS-induced cytokine release in astrocytes (Arevelo et al., 2012). Similarly, pre-treatment with 17beta-estradiol, HPTE (ER-a agonist/ER-b antagonist), PPT (ERa agonist) and DPN (ER-b agonist) suppressed IL-1β and TNFα in astrocyte cultures derived from young adult (3-4 mos.) and middle aged females (9-11 mos., acyclic) (Lewis et al., 2008), suggesting that activation of either estrogen receptor in this cell type was anti-inflammatory.
However, estrogens action in ex vivo microglial cultures derived from either young adult or middle aged females are not uniformly immunoprotective. LPS treatment increased nitric oxide production in microglia derived from young females, as well as cytokines. However 17b-estradiol treatment attenuated NO production, but failed to attenuate cytokine expression and MMP-9 activity. In contrast, 17b-estradiol failed to suppress cytokine expression and NO in microglia from middle aged females, and paradoxically, increased MMP-9 lytic activity in these cultures (Johnson and Sohrabji, 2004). These studies indicate that estrogen effect on the inflammatory response is modulated by other aging-related events in microglia. One possibility is the loss of IGF-1 stimulation with aging, which may alter the actions of estrogen.
IGF-1 also exhibits anti-inflammatory actions in non-neuronal cells. In muscle regeneration, IGF-1 mediated repair is associated with suppression of the anti-inflammatory response. In a transgenic mouse where local IGF-1 expression was restricted to muscle, IGF-1 reduced key components of the inflammatory pathway including high mobility protein group -1, the transcription factor NFkB, and cytokines such as macrophage migration inhibitory factor (MIF), TNF-a and IL-1b (Pelosi et al., 2007). In the brain, IGF-1 is produced by microglial cells and activation of human brain-derived microglia by LPS, poly I:C or interferon suppressed IGF-1 synthesis. Th2 cytokines did not effect IGF-1 production and a cAMP analog increased expression of this growth factor (Suh et al., 2013). Similar to estrogen treatment, IGF-1 treatment to astrocytes also suppresses the response to LPS. In vitro, IGF-1 gene therapy or exogenous IGF-1 decreased TLR4 receptor expression on astrocytes and attenuated nuclear translocation of the p65 NFkB protein (Bellini et al., 2011). In vivo administration of IGF-1 also downregulates glial activation. In an experimental model of depression caused by ICV injections of LPS, animals experience sickness behaviors, loss of weight and feed intake and reduced social exploration, as well as elevation of prototypic inflammatory mediators IL-1b and TNF-a. Pretreatment with IGF-1 however, reduced measures of depression and decreased inflammatory cytokines, as well as GFAP (Park et al., 2011). These data support the idea that IGF-1 exerts an anti-inflammatory effect in the brain and improves behavioral outcomes, such as depression, associated with neural inflammation. Compelling evidence of the protective role of microglial IGF-1 in stroke comes from a mouse model where microglial cells were destroyed using a mutant HSV and the antiviral ganciclovir. Ablation of proliferating microglia cells after induction of ischemic stroke increased infarct volume, decreased stroke-induced IGF-1 and elevated inflammatory cytokines in the infarcted hemisphere. Stimulating microglial cells with macrophage colony stimulating factor, on the other hand, increased IGF-1 synthesis (Lalancette-Hebert et al., 2007). Similarly, antagonists to microRNA, a class of translational repressors, that target the IGF-1 gene also increases IGF-1 in microglia (Selvamani et al., 2012). Besides its direct actions on local inflammatory cells, IGF-1 also reduces the inflammatory cascade by preventing the binding of monocytes to endothelial cells (Motani et al., 1996), which precedes their entry through the endothelial barrier.
Individually, IGF-1 and estrogen both appear to promote an anti-inflammatory phenotype acting on both microglia and astrocytes. Thus it could be assumed that both hormones would promote synergistic effects, however, little direct evidence of this interaction is available for inflammation. In skin injury model, investigators were able to dissociate the contribution of each hormone by examining the effects of IGF-1 replacement in ovariectomized WT and ER-a KO mice. IGF-1 replacement repaired skin wounds by suppressing inflammation and promoting reepithelialization. However, in the ER-a KO mouse, IGF-1 continued to promote reepithelialization, but did not promote healing and increased local inflammation (Emmerson et al., 2012), suggesting that the inflammatory response may require the combined action of IGF-1 and ovarian steroids.
IV: Estrogen and IGF-1 interactions: Post stroke neurologic disease
Stroke can result in other neurologic diseases such as post-stroke depression and epilepsy. Stroke is a leading cause of epilepsy and factors that affect stroke severity may also contribute to epilepsy. In an animal model of temporal lobe epilepsy, IGF-1 treatment has been shown to attenuate seizure severity induced by kainic acid injections. IGF-1 also increased hippocampal neurogenesis and protected against kainic acid induced neurodegeneration (Miltiadous et al., 2011). In late gestation fetal sheep subject to cerebral ischemia by occlusion of the carotid artery, IGF-1 attenuated neuronal loss and also delayed the onset of seizures and reduced their frequency (Johnston et al., 1996).
In kindling, which is widely used as a model for epilepsy, neuronal excitability is linked to structural changes and synaptic reorganization within the hippocampus. In situ binding assays for the insulin and IGF receptors showed that I125 IGF-1 binding was significantly reduced in animals that received 100 kindling stimuli (Kalynchuk et al., 2002). The authors propose that this reduction in binding sites may be due to receptor occupation by increased availability of IGF-1 from local or peripheral sources, as has been shown in the case of other peptides (Roder et al., 1996). Interestingly, there appears to be no sex difference in the emotional response to kindling (Wintink et al., 2003), although sex differences have been noted in seizure models. Males display greater susceptibility to convulsants such as pilocarpine and kainic acid as compared females. Furthermore, gonadectomized males replaced with testosterone had increased seizure susceptibility, stronger and more frequent seizures as compared to those not replaced with testosterone. Interestingly, while testosterone enhances seizure occurences, kainic acid-induced seizures decrease plasma testosterone, while elevating plasma corticosterone levels (Mejias-Aponte et al., 2002). Sex differences in epileptogenesis may also be linked to developmental/early stressors such as cortical developmental abnormalities, neonatal hypoxia, etc. Male and female rat pups subject to a two-hit model consisting of a freeze-induced cortical lesion at P1, and a prolonged hyperthermic seizure at P10 revealed striking sex differences. All males subject to this protocol developed mesial temporal lobe epilepsy when measured 3-4 months of age, while none of the females showed epilepsy (Desgent et al., 2012). While IGF-1 and IGFR expression was not reported in this study, peak IGFR expression in the hippocampus occurs earlier in females than males (Hami et al., 2012), indicating the availability of an early protective substrate in females. Similarly, sex differences are also evident in seizure behavior following ethanol withdrawal. Male and female mice subject to 4 cycles of ethanol vapor and subsequent withdrawal were tested for handling induced seizures. At the initial withdrawl, males and females showed a similar degree of seizure behavior, however, on subsequent withdrawal bouts, males had robust increases in seizure severity while females did not. Interestingly, this was not associated with estrogen levels or with sex differences in blood alcohol levels Veatch et al., 2007), although sex differences in blood IGF-1 levels have been reported (Chaler et al., 2009; Gupta et al., 2011).
Overall, the evidence supports the hypothesis that both estrogen and IGF-1 affect seizures and underscore their role in post stroke therapies for specific subsets of patients, although direct evidence for such actions is lacking. Besides epilepsy, other neurological consequences of stroke include depression and dementia, and neural targets for these diseases are also sensitive to estrogens and IGF-1. However, the interaction effects of IGF-1 and 17b-estradiol on these post-stroke diseases are also poorly understood.
V: Estrogen and IGF-1: Astrocytes as critical cellular mediator
Although the largest amount of estrogen and IGF-1 is synthesized by peripheral sources such as the ovary and the liver respectively, local production of both hormones has been reported for several tissues, including the brain. The astrocyte is known to synthesize both 17b-estradiol and IGF-1, and in view of its critical role in ischemic injury, and its proximity to ischemic neurons and the blood brain barrier (Figure 2), astrocytic secretions may be crucial for brain repair and recovery.
In the case of 17b-estradiol, production of aromatase is central to synthesis of local hormone. Early studies established that aromatase is expressed in neurons in the rat and quail hypothalamus (Canick et al., 1986; Schlinger and Callard, 1989), areas essential for neuroendocrine function. However, the discrepancy between high aromatase activity and low neuronal expression of aromatase in non-hypothalamic areas pointed to another source for this enzyme, namely glia. Aromatase was detected in astroglial cells of the zebra finch forebrain (Schlinger et al, 1994), as well as radial glia in the rainbow trout (Menuet et al., 2003) and glioblastomas (Yague et al., 2004). Subsequently, aromatase expression as well as estradiol synthesis was confirmed in cortical astrocytes from neonatal rats (Zwain et al., 1997). In the hippocampus, no aromatase expression was detected in astrocytes in the uninjured animal, however, systemic injections of kainic acid or stab wound injury in the brain caused an injury-induced upregulation of aromatase in hippocampal astrocytes (Garcia-Segura et al., 1999). Similarly, cerebral ischemia results in rapid upregulation of aromatase in astrocytes in the rat (Carswell et al., 2005) and nitrosative stress upregulates aromatase in cultured fetal sheep astrocytes (Lepore et al., 2011). Pharmacological inhibition of aromatase exacerbates neuronal death following mild excitotoxic stimuli, suggesting that the induction of aromatase and consequent estradiol synthesis is neuroprotective (Azcoitia et al., 2003). Greater expression of aromatase in astrocytes from females is also associated with neuroprotective advantage in the case of ischemic stress or anti-oxidant induced cell death, although not when combined with inflammatory mediators (Liu et al., 2007). Glial aromatase is also thought to promote injury-induced neurogenesis in the zebra finch brain (Peterson et al., 2007).
Similarly, IGF-1 is also synthesized by astrocytes. In late embryonic development, IGF-1 is synthesized neurons, astrocytes and oligodendroglial precursors (Baron-Van Evercooren et al., 1991; Ballotti et al., 1987), underscoring the role of this growth factor in brain development. In a transgenic mouse where IGF-1 is overexpressed in astrocytes, there is marked overgrowth in the brain, including increased brain weight, DNA and protein concentrations, and neuron number (Ye et al., 2004). In glial tumors, IGF-1 expression appears to be correlated with tumor malignancy related to aberrant angiogenesis (Hirano et al., 1999). In a cuprizone demyelinating model, IGF-1 is induced in astrocytes located in degenerating white matter (Komoly et al., 1992) and in the spinal cord in experimental autoimmune encephalitis (Liu et al., 1994). A similar increase in astrocytic IGF-1 is seen 3 days after ischemic injury in neonatal rats (Gluckman et al., 1992). In adult male rats, IGF-1 expression is seen in astrocytes in the areas surrounding the infarcted tissue, and in microglia within the infarcted region (O'Donnell et al., 2001). Since astrocytes are capable of synthesizing both 17b-estradiol and IGF-1 and are positioned at critical targets such as the blood brain barrier and in proximity with ischemic neurons, cell-specific therapies actively targeting this cell can be exploited for new stroke pharmacotherapies. Specifically, epigenetic modifiers to increase transcription of the aromatase and IGF-1 gene and microRNA technologies to enhance translational of these proteins would be critical for development of the next generation of stroke therapies.
In summary
While the cooperative actions of 17b-estradiol and IGF-1 have been demonstrated in vivo in stroke models, the cellular and molecular site of action and the second messengers required for these actions remain poorly understood. While evidence shows that both compounds promote neuronal survival by acting directly on neurons, there is no direct evidence for such actions in vivo. No doubt neuronal survival is enhanced by this process, however, it is likely that estrogen and IGF-1 actions converge on non-neuronal cells to promote neuronal survival. As this review shows, the endothelium may be one such site of convergence, promoting blood barrier integrity, angiogenesis and reduced inflammation.
Highlights.
Estrogen and IGF-1 interactions are critical for normal development and response to neural injury
Estrogen/IGF-1 co-treatment reduces infarct volume and improves recovery in experimental stroke
Estrogen and IGF-1 separately influence post-stroke angiogenesis and inflammation
New research should focus on estrogen-IGF-1 interaction on critical post stroke events
Astrocytes may serve as a critical cellular source of local IGF-1 and estrogen in the ischemic brain
Acknowledgments
The author wishes to thank Ms Tiffany Heard for her assistance with literature searches and Ms Annette Fincher for creating the schematics in Figures 1 and 2. FS is supported by AG042189, and NS074895.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Arai K, Lok J, Guo S, Hayakawa K, Xing C, Lo EH. Cellular mechanisms of neurovascular damage and repair after stroke. J Child Neurol. 2011;26:1193–1198. doi: 10.1177/0883073811408610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelt AA, Carpenter RS, Lobo MR, Zeng H, Solanki RB, Zhang A, Kulesza P, Pike MM. Estradiol modulates post-ischemic cerebral vascular remodeling and improves long-term functional outcome in a rat model of stroke. Brain Res. 2012;1461:76–86. doi: 10.1016/j.brainres.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelt AA, Anjum N, Rajneesh KF, Kulesza P, Koehler RC. Estradiol augments peri-infarct cerebral vascular density in experimental stroke. Exp Neurol. 2007;206:95–100. doi: 10.1016/j.expneurol.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke. 2005;36:337–341. doi: 10.1161/01.STR.0000153795.38388.72. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. J Neuroendocrinol. 2012;24:183–90. doi: 10.1111/j.1365-2826.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Vernon RB, Sage EH, Reed MJ. Growth factors reverse the impaired sprouting of microvessels from aged mice. Microvasc Res. 1998;55:260–70. doi: 10.1006/mvre.1998.2078. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58:815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Badger AM, Blake SM, Dodds RA, Griswold DE, Swift BA, Rieman DJ, Stroup GB, Hoffman SJ, Gowen M. Idoxifene, a novel selective estrogen receptor modulator, is effective in a rat model of adjuvant-induced arthritis. J Pharmacol Exp Ther. 1999;291:1380–1386. [PubMed] [Google Scholar]
- Bake S, Friedman J, Sohrabji F. Reproductive age-related changes in the blood brain barrier: Expression of IgG and tight junction proteins. Microvasc Res. 2009;78(3):413–24. doi: 10.1016/j.mvr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17b-estradiol differentially regulates blood brain barrier permeability in young and aging female rats. Endocrinol. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Ballotti R, Nielsen FC, Pringle N, Kowalski A, Richardson WD, Van Obberghen E, Gammeltoft S. Insulin-like growth factor I in cultured rat astrocytes: expression of the gene, and receptor tyrosine kinase. EMBO J. 1987;6:3633–9. doi: 10.1002/j.1460-2075.1987.tb02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar RS, Boes M, Dake BL, Booth BA, Henley SA, Sandra A. Insulin, insulin-like growth factors, and vascular endothelium. Am J Med. 1988;85:59–70. doi: 10.1016/0002-9343(88)90398-1. [DOI] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Olichon-Berthe C, Kowalski A, Visciano G, Van Obberghen E. Expression of IGF-I and insulin receptor genes in the rat central nervous system: a developmental, regional, and cellular analysis. J Neurosci Res. 1991;28:244–53. doi: 10.1002/jnr.490280212. [DOI] [PubMed] [Google Scholar]
- Bellini MJ, Heren CB, Goya RG, Garcia-Segura LM. Insulin-like growth factor-I gene delivery to astrocytes reduces their inflammatory response to lipopolysaccharide. J Neuroinflam. 2011;8:21. doi: 10.1186/1742-2094-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermont L, Lamielle F, Fauconnet S, Esumi H, Weisz A, Adessi GL. Regulation of vascular endothelial growth factor expression by insulin-like growth factor-I in endometrial adenocarcinoma cells. Int J Cancer. 2000;85:117–123. doi: 10.1002/(sici)1097-0215(20000101)85:1<117::aid-ijc21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bernard L, Legay C, Adriaenssens E, Mougel A, Ricort JM. Estradiol regulates the insulin-like growth factor-I (IGF-I) signalling pathway: A crucial role of phosphatidylinositol 3-kinase (PI 3-kinase) in estrogens requirement for growth of MCF-7 human breast carcinoma cells. Biochem and Biophys Res Commun. 2006;350:916–921. doi: 10.1016/j.bbrc.2006.09.116. [DOI] [PubMed] [Google Scholar]
- Bishop J, Simpkins J. Estradiol enhances brain glucose uptake in ovariectomized rats. Brain Res Bull. 1995;36:315–320. doi: 10.1016/0361-9230(94)00208-i. [DOI] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keelink JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinol. 2000;41:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Buckley S, Driscoll B, Barsky L, Weinberg K, Anderson K, Warburton D. ERK activation protects against DNA damage and apoptosis in hyperoxic rat AEC2. Am J Physiol. 1999;277:L159–166. doi: 10.1152/ajplung.1999.277.1.L159. [DOI] [PubMed] [Google Scholar]
- Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, Vanderschueren D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res. 2010;25:617–626. doi: 10.1359/jbmr.090828. [DOI] [PubMed] [Google Scholar]
- Canick JA, Vaccaro DE, Livingston EM, Leeman SE, Ryan KJ, Fox TO. Localization of aromatase and 5 alpha-reductase to neuronal and non-neuronal cells in the fetal rat hypothalamus. Brain Res. 1986;372:277–82. doi: 10.1016/0006-8993(86)91135-2. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2002;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, Li Q, Ren J. Insulin-like growth factor I (IGF-1) deficiency ameliorates sex difference in cardiac contractile function and intracellular Ca(2+) homeostasis. Toxicol Lett. 2011;206:130–138. doi: 10.1016/j.toxlet.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaler EA, Meazza C, Guercio G, Maceiras M, Rivarola MA, Laarej K, Pagani S, Areny G, Albertini R, Llinares V, Belgorosky A, Bozzola M. Serum IGF-I and IGFBP-3 reference values from a chemiluminescent assay in normal children and adolescents of hispanic and italian origin: presence of sexual dimorphism in IGF-I values. J Pediatr Endocrinol Metab. 2009;22:1127–1135. doi: 10.1515/jpem.2009.22.12.1127. [DOI] [PubMed] [Google Scholar]
- Chang E, O'Donnell ME, Barakat AI. Shear stress and 17beta-estradiol modulate cerebral microvascular endothelial Na-K-Cl cotransporter and Na/H exchanger protein levels. Am J Physiol Cell Physiol. 2008;294:C363–371. doi: 10.1152/ajpcell.00045.2007. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen S, Chen Y, Zhang C, Wang J, Zhang W, Liu G, Zhao B. Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: possible implications in cerebral ischemic damage. Am J Physiol Endocrinol Metab. 2011;301:E62–71. doi: 10.1152/ajpendo.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenal J, Pierre K, Pellerin L. Insulin and IGF-1 enhance the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin pathway. Eur J Neurosci. 2008;27:53–65. doi: 10.1111/j.1460-9568.2007.05981.x. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Hunter C, Liu X, Weiss HR. Effects of 17beta-estradiol on blood-brain barrier disruption in focal ischemia during GABA(A) receptor inhibition. Horm Metab Res. 2005;37:209–213. doi: 10.1055/s-2005-861379. [DOI] [PubMed] [Google Scholar]
- Chi O, Barsoum S, Wen Y, Liu X, Weiss H. 17beta-estradiol prevents blood-brain barrier disruption induced by VEGF. Horm Metab Res. 2004;36:272–276. doi: 10.1055/s-2004-814478. [DOI] [PubMed] [Google Scholar]
- Chi O, Liu X, Weiss H. Effects of 17beta-estradiol on blood-brain barrier disruption during focal ischemia in rats. Horm Metab Res. 2002;34:530–534. doi: 10.1055/s-2002-34794. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- Cui X, Lazard Z, Zhang P, Hopp TA, Lee AV. Progesterone crosstalks with insulin-like growth factor signaling in breast cancer cells via induction of insulin receptor substrate-2. Oncogene. 2003;22:6937–41. doi: 10.1038/sj.onc.1206803. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Sautebin L, Serraino I, Dugo L, Calabro G, Caputi AP, Maggi A. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med. 2001;7:478–87. [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Santagati S, Sautebin L, Mazzon E, Calabro G, Serraino I, Caputi AP, Maggi A. 17beta-estradiol anti-inflammatory activity in carrageenan-induced pleurisy. Endocrinol. 2000;141:1455–63. doi: 10.1210/endo.141.4.7404. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- De M, Wood GW. Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol. 1990;126:417–24. doi: 10.1677/joe.0.1260417. [DOI] [PubMed] [Google Scholar]
- De Smedt A, Brouns R, Uyttenboogaart M, De Raedt S, Moens M, Wilczak N, Luijckx DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87:586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- Denti L, Scoditti U, Tonelli C, Saccavini M, Caminiti C, Valcavi R, Benatti M, Ceda GP. The poor outcome of ischemic stroke in very old people: a cohort study of its determinants. J Am Geriatr Soc. 2010;58:12–17. doi: 10.1111/j.1532-5415.2009.02616.x. [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J. 2006;20:773–5. doi: 10.1096/fj.05-4704fje. [DOI] [PubMed] [Google Scholar]
- Desgent S, Duss S, Sanon NT, Lema P, Lévesque M, Hébert D, Rébillard RM, Bibeau K, Brochu M, Carmant L. Early-life stress is associated with gender-based vulnerability to epileptogenesis in rat pups. PLoS One. 2012;7:e42622. doi: 10.1371/journal.pone.0042622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon SA, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Dubal D, Kashon M, Pettigrew L, Ren J, Finklestein S, Rau S, Wise P. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Torres JV, Nihei N, Barrett JC. The insulin-like growth factor-1 elevates urokinase-type plasminogen activator-1 in human breast cancer cells: a new avenue for breast cancer therapy. Mol Carcinog. 2000;27:10–7. doi: 10.1002/(sici)1098-2744(200001)27:1<10::aid-mc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Suliman I, Lindgren U, Winblad B, Adem A. Ovariectomy and gonadal hormone treatment: effects on insulin-like growth factor-1 receptors in the rat brain. Growth Horm IGF Res. 2004;14:38–93. doi: 10.1016/j.ghir.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Emmerson E, Campbell L, Davies FC, Ross NL, Ashcroft GS, Krust A, Chambon P, Hardman MJ. Insulin-like growth factor-1 promotes wound healing in estrogen-deprived mice: new insights into cutaneous IGF-1R/ER-alpha cross talk. J Invest Dermatol. 2012;132:2838–48. doi: 10.1038/jid.2012.228. [DOI] [PubMed] [Google Scholar]
- Erhardt P, Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease - systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–52. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Foncea R, Galvez A, Perez V, Morales MP, Calixto A, Melendez J, Gonzalez-Jara F, Diaz-Araya G, Sapag-Hagar M, Fournier M, Lewis MI. Influences of IGF-I gene disruption on the cellular profile of the diaphragm. Am J Physiol Endocrinol Metab. 2000;278:E707–15. doi: 10.1152/ajpendo.2000.278.4.E707. [DOI] [PubMed] [Google Scholar]
- Francis GL, Upton FM, Ballard FJ, McNeil KA, Wallace JC. Insulin-like growth factors 1 and 2 in bovine colostrum. Sequences and biological activities compared with those of a potent truncated form. Biochem J. 1988;251:95–103. doi: 10.1042/bj2510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Usuki S, Mukai N, Amagai H, Hayashi K, Takamatsu K. Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, sex steroids, osteocalcin and bone mineral density in male and female rats. Gynecol Endocrinol. 1998;12:297–305. doi: 10.3109/09513599809012830. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Kawano T. Akt is a molecular target for signal transduction therapy in brain ischemic insult. J Pharmacol Sci. 2003;92:317–327. doi: 10.1254/jphs.92.317. [DOI] [PubMed] [Google Scholar]
- Gaddipati JP, Mani H, Banaudha KK, Sharma SK, Kulshreshtha DK, Maheshwari RK. Picroliv modulates the expression of insulin-like growth factor (IGF)-I, IGF-II and IGF-I receptor during hypoxia in rats. Cell Mol Life Sci. 1999;56:348–55. doi: 10.1007/s000180050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammal E, Zuk A. Effect of ethinyl estradiol on endothelial permeability to 125I-labeled albumin in female rats. Exp Mol Pathol. 1980;32:91–101. doi: 10.1016/0014-4800(80)90046-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Cardona-Gomez GP, Chowen JA, Azcoitia I. Insulin-like growth factor-1 receptors and estrogen receptors interact in the promotion of neuronal survival and neuroprotection. J Neurocytol. 2000;29:425–437. doi: 10.1023/a:1007125626308. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Sanz A, Mendez P. Cross-talk between IGF-I and estradiol in the brain: focus on neuroprotection. Neuroendocrinol. 2006;84:275–279. doi: 10.1159/000097485. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neurosci. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- De Smedt A, Brouns R, Uyttenboogaart M, De Raedt S, Moens M, Wilczak N, Luijckx GJ, De Keyser J. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42:2180–5. doi: 10.1161/STROKEAHA.110.600783. [DOI] [PubMed] [Google Scholar]
- Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–9. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–9. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Guan J, Williams C, Scheepens A, Zhang R, Bennet L, Gunn A. Asphyxial brain injury--the role of the IGF system. Mol Cell Endocrinol. 1998;140:95–9. doi: 10.1016/s0303-7207(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- Grant MB, Mames RN, Fitzgerald C, Ellis EA, Aboufriekha M, Guy J. Insulin-like growth factor I acts as an angiogenic agent in rabbit cornea and retina: comparative studies with basic fibroblast growth factor. Diabetologia. 1993;36:282–91. doi: 10.1007/BF00400229. [DOI] [PubMed] [Google Scholar]
- Guan J, Bennet L, George S, Wu D, Waldvogel HJ, Gluckman PD, Faull RL, Crosier PS, Gunn AJ. Insulin-like growth factor-I reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21:493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Guan J, Krishnamurthi R, Waldvogel HJ, Faull RL, Clark R, Gluckman P. N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res. 2000;859:286–292. doi: 10.1016/s0006-8993(00)01988-0. [DOI] [PubMed] [Google Scholar]
- Gupta N, Lustig RH, Kohn MA, McCracken M, Vittinghoff E. Sex differences in statural growth impairment in Crohn's disease: Role of IGF-1. Inflamm Bowel Dis. 2011;17:2318–2325. doi: 10.1002/ibd.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghir H, Rezaee AA, Nomani H, Sankian M, Kheradmand H, Hami J. Sexual dimorphism in expression of insulin and insulin-like growth factor-I receptors in developing rat cerebellum. Cell Mol Neurobiol. 2013;33:369–377. doi: 10.1007/s10571-012-9903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hami J, Sadr-Nabavi A, Sankian M, Haghir H. Sex differences and left-right asymmetries in expression of insulin and insulin-like growth factor-1 receptors in developing rat hippocampus. Brain Struct Funct. 2012;217:293–302. doi: 10.1007/s00429-011-0358-1. [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hede MS, Salimova E, Piszczek A, Perlas E, Winn N, Nastasi T, Rosenthal N. E-Peptides Control Bioavailability of IGF-1. PLoS ONE. 2012;7(12):e51152. doi: 10.1371/journal.pone.0051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H, Lopes MB, Laws ER, Jr, Asakura T, Goto M, Carpenter JE, Karns LR, VandenBerg SR. Insulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomas. Neuro Oncol. 1999;1:109–19. doi: 10.1093/neuonc/1.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt EC, Hepler JE, Van Wyk JJ, Lund PK. Structural characterization of exon 6 of the rat IGF-I gene. DNA Cell Biol. 1992;11:433–41. doi: 10.1089/dna.1992.11.433. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Studer K, Messmer EP, Froesch ER. Treatment with insulin-like growth factor I alters capillary permeability in skin and retina. Diabetes. 1995;44:1209–12. doi: 10.2337/diab.44.10.1209. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Park SK, An SJ, Lee JY, Choi SY, Kang JH, Kwon YG, Kang TC, Won MH. Expression and changes of endogenous insulin-like growth factor-1 in neurons and glia in the gerbil hippocampus and dentate gyrus after ischemic insult. Neurochem Int. 2004;45:149–156. doi: 10.1016/j.neuint.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge DK, Nelson M, Bidwell S, Curtis T, Washburn J, McLachlan JA, Korach KS. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci USA. 1992;89:4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63:12–20. doi: 10.1093/gerona/63.1.12. [DOI] [PubMed] [Google Scholar]
- Jaffer S, Shynlova O, Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinol. 2009;150:4672–80. doi: 10.1210/en.2009-0419. [DOI] [PubMed] [Google Scholar]
- Jandó G, Carpi D, Kandel A, Urioste R, Horvath Z, Pierre E, Vadi D, Vadasz C, Buzsák IG. Spike-and-wave epilepsy in rats: sex differences and inheritance of physiological traits. Neurosci. 1995;64:301–317. doi: 10.1016/0306-4522(94)00329-4. [DOI] [PubMed] [Google Scholar]
- Jaruratanasirikul S, Leethanaporn K, Pradutkanchana S, Sriplung H. Serum insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) in healthy Thai children and adolescents: relation to age, sex, and stage of puberty. J Med Assoc Thai. 1999;82:275–283. [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Mol Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Johnson AB, Sohrabji F. Estrogen's inflammatory effects on central and circulating immune cells vary with reproductive age. Neurobiol Aging. 2005;26:1365–1374. doi: 10.1016/j.neurobiolaging.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Johnson AB, Bake S, Lewis DL, Sohrabji F. Temporal Expression of IL-1 β Protein and mRNA in the Brain after Systemic LPS Injection is Affected by Age and Estrogen. J Neuroimmunol. 2006;174:82–91. doi: 10.1016/j.jneuroim.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Johnson AB, Bake S, Lewis DK, Sohrabji F. Temporal expression of IL-1beta protein and mRNA in the brain after systemic LPS injection is affected by age and estrogen. J Neuroimmunol. 2006;174:82–91. doi: 10.1016/j.jneuroim.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Mallard EC, Williams CE, Gluckman PD. Insulin-like growth factor-1 is a potent neuronal rescue agent after hypoxic-ischemic injury in fetal lambs. J Clin Invest. 1996;97:300–308. doi: 10.1172/JCI118416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul A, Dalgaard P, Blum WF, Bang P, Hall K, Michaelsen KF, Müller J, Skakkebaek NE. Serum levels of insulin-like growth factor (IGF)-binding protein- 3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab. 1995;80:2534–2542. doi: 10.1210/jcem.80.8.7543116. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. The blood-brain barrier and cerebrovascular pathology in Alzheimer's disease. Ann N Y Acad Sci. 1999;893:113–125. doi: 10.1111/j.1749-6632.1999.tb07821.x. [DOI] [PubMed] [Google Scholar]
- Kalynchuk LE, Meaney MJ, Kar S. Amygdala kindling decreases insulin-like growth factor-I receptor binding sites in the rat hippocampus. Brain Res. 2002;935:118–123. doi: 10.1016/s0006-8993(02)02459-9. [DOI] [PubMed] [Google Scholar]
- Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1105–1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- Kanbur-Oksüz N, Derman O, Kinik E. Correlation of sex steroids with IGF-1 and IGFBP-3 during different pubertal stages. Turk J Pediatr. 2004;46:315–321. [PubMed] [Google Scholar]
- Kaplan RC, McGinn AP, Pollak MN, Kuller LH, Strickler HD, Rohan TE, Cappola AR, Xue X, Psaty BM. Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J Clin Endocrinol Metab. 2007;92:1319–25. doi: 10.1210/jc.2006-1631. [DOI] [PubMed] [Google Scholar]
- Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- Kaur J, Tuor UI, Zhao Z, Barber PA. Quantitative MRI reveals the elderly ischemic brain is susceptible to increased early blood-brain barrier permeability following tissue plasminogen activator related to claudin 5 and occludin disassembly. J Cereb Blood Flow Metab. 2011;31:1874–1885. doi: 10.1038/jcbfm.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR. Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 1998;39:209–16. doi: 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Kluge A, Zimmermann R, Munkel B, Mohri M, Sack S, Schaper J, Schaper W. Insulin-like growth factor I is involved in inflammation linked angiogenic processes after microembolisation in porcine heart. Cardiovasc Res. 1995;29:407–15. [PubMed] [Google Scholar]
- Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, Opdenakker G, Koistinaho J. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–7. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- Komoly S, Hudson LD, Webster HD, Bondy CA. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc Natl Acad Sci U S A. 1992;89:1894–8. doi: 10.1073/pnas.89.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101:1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signalregulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacol. 2008;55:281–288. doi: 10.1016/j.neuropharm.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SM, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis. 2005;2:A13. [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 2005;280:2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- Le Grevès M, Le Grevès P, Nyberg F. Age-related effects of IGF-1 on the NMDA-, GH- and IGF-1-receptor mRNA transcripts in the rat hippocampus. Brain Res Bull. 2005;65:369–374. doi: 10.1016/j.brainresbull.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Lee WH, Bondy C. Insulin-like growth factors and cerebral ischemia. Ann N Y Acad Sci. 1993;679:418–422. doi: 10.1111/j.1749-6632.1993.tb18332.x. [DOI] [PubMed] [Google Scholar]
- Leon RL, Li X, Huber JD, Rosen CL. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinol. 2012;153:3386–3393. doi: 10.1210/en.2011-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore G, Gadau S, Peruffo A, Mura A, Mura E, Floris A, Balzano F, Zedda M, Farina V. Aromatase expression in cultured fetal sheep astrocytes after nitrosative/oxidative damage. Cell Tissue Res. 2011;344(3):407–13. doi: 10.1007/s00441-011-1160-3. [DOI] [PubMed] [Google Scholar]
- Leri A, Kajstura J, Li B, Sonnenblick EH, Beltrami CA, Anversa P, Frishman WH. Cardiomyocyte aging is gender-dependent: the local IGF-1-IGF-1R system. Heart Dis. 2000;2:108–15. [PubMed] [Google Scholar]
- Lewis DK, Woodin HR, Sohrabji F. Astrocytes from Acyclic Female Rats Exhibit Lowered Capacity for Neuronal Differentiation. Aging Cell. 2008;7:836–849. doi: 10.1111/j.1474-9726.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DK, Stohlgren S, Harms A, Sohrabji F. Effects of Estrogen Receptor Agonists on Regulation of the Inflammatory Response in Astrocytes from Young Adult and Middle-Aged Female Rats. J Neuroimmunol. 2008;195:47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DK, Thomas K, Selvamani A, Sohrabji F. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiol, Aging. 2012;33:1123.e1–1123.e16. doi: 10.1016/j.neurobiolaging.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang Z, Sun W, Koehler RC, Huang J. 17b-estradiol attenuates breakdown of blood-brain barrier and hemorrhagic transformation induced by tissue plasminogen activator in cerebral ischemia. Neurobiol Dis. 2011;44:277–83. doi: 10.1016/j.nbd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH, Carmichael ST. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Chen W, Kuo J, Chen C. Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci Lett. 2001;297:159–162. doi: 10.1016/s0304-3940(00)01704-3. [DOI] [PubMed] [Google Scholar]
- Lin S, Fan LW, Rhodes PG, Cai Z. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 2009;217:361–370. doi: 10.1016/j.expneurol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27(1):135–41. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- Liu X, Yao DL, Bondy CA, Brenner M, Hudson LD, Zhou J, Webster HD. Astrocytes express insulin-like growth factor-I (IGF-I) and its binding protein, IGFBP-2, during demyelination induced by experimental autoimmune encephalomyelitis. Mol Cell Neurosci. 1994;5:418–30. doi: 10.1006/mcne.1994.1052. [DOI] [PubMed] [Google Scholar]
- Liu X, Yao DL, Webster H. Insulin-like growth factor I treatment reduces clinical deficits and lesion severity in acute demyelinating experimental autoimmune encephalomyelitis. Mult Scler. 1995;1:2–9. doi: 10.1177/135245859500100102. [DOI] [PubMed] [Google Scholar]
- Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang SH. 17beta-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Loftspring MC, Beiler S, Beiler C, Wagner KR. Plasma proteins in edematous white matter after intracerebral hemorrhage confound immunoblots: an ELISA to quantify contamination. J Neurotrauma. 2006;23:1904–1911. doi: 10.1089/neu.2006.23.1904. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–8. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–31. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- Luciani P, Deledda C, Benvenuti S, Cellai I, Modi G, Fibbi B, Danza G, Vannelli GB, Peri A. Relationship between the neuroprotective effects of insulin-like growth factor-1 and 17Œ≤-oestradiol in human neuroblasts. J Neuroendocrinol. 2012;24:1304–10. doi: 10.1111/j.1365-2826.2012.02343.x. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZQ, Santagati S, Patrone C, Pollio G, Vegeto E, Maggi A. Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human neuroblastoma cell line SK-ER3. Mol Endocrinol. 1994;8:910–918. doi: 10.1210/mend.8.7.7984152. [DOI] [PubMed] [Google Scholar]
- Mackay KB, Loddick SA, Naeve GS, Vana AM, Verge GM, Foster AC. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J Cereb Blood Flow Metab. 2003;23:1160–1167. doi: 10.1097/01.WCB.0000087091.01171.AE. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Baxter RC. Expression of insulin-like growth factor binding protein-2 by MCF-7 breast cancer cells is regulated through the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway. Endocrinol. 2007;148:2532–41. doi: 10.1210/en.2006-1335. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- Mejías-Aponte CA, Jiménez-Rivera CA, Segarra AC. Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res. 2002;944:210–218. doi: 10.1016/s0006-8993(02)02691-4. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Sequra LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: Comparison with estrogen receptor alpha. J Comp Neurol. 2003;462:180–93. doi: 10.1002/cne.10726. [DOI] [PubMed] [Google Scholar]
- Miltiadous P, Stamatakis A, Koutsoudaki PN, Tiniakos DG, Stylianopoulou F. IGF-I ameliorates hippocampal neurodegeneration and protects against cognitive deficits in an animal model of temporal lobe epilepsy. Exp Neurol. 2011;231:223–235. doi: 10.1016/j.expneurol.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for the neurotrophins and their receptors in the developing CNS suggest the potential for autocrine interactions. Pro Natl Acad Sci USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Mandai M, Suzuma I, Suzuma K, Kobayashi K, Honda Y. Estrogen protects against cellular infiltration by reducing the expressions of E-selectin and IL-6 in endotoxin-induced uveitis. J Immunol. 1999;163:374–9. [PubMed] [Google Scholar]
- Motani A, Forster L, Tull S, Anggard EE, Ferns GA. Insulin-like growth factor-I modulates monocyte adhesion to EAhy 926 endothelial cells. Int J Exp Pathol. 1996;77:31–5. doi: 10.1046/j.1365-2613.1996.960098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao-Hayashi J, Ito H, Kanayasu T, Morita I, Murota S. Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells. Atheroscler. 1992;92:141–149. doi: 10.1016/0021-9150(92)90273-j. [DOI] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049–53. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Nicosia SV, Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol. 1994;145:1023–9. [PMC free article] [PubMed] [Google Scholar]
- O'Donnell SL, Frederick TJ, Krady JK, Vannucci SJ, Wood TL. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia. 2002;39:85–97. doi: 10.1002/glia.10081. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26:1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- Odena G, Miquel M, Serafin A, Galan A, Morillas R, Planas R, Bartoli R. Rifaximin, but not growth factor 1, reduces brain edema in cirrhotic rats. World J Gastroenterol. 2012;18:2084–2091. doi: 10.3748/wjg.v18.i17.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto OK, Janjoppi L, Bonone FM, Pansani AP, da Silva AV, Scorza FA, Cavalheiro EA. Whole transcriptome analysis of the hippocampus: toward a molecular portrait of epileptogenesis. BMC Genomics. 2010;11:230. doi: 10.1186/1471-2164-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto OK, Janjoppi L, Bonone FM, Pansani AP, da Silva AV, Scorza FA, Cavalheiro EA. Whole transcriptome analysis of the hippocampus: toward a molecular portrait of epileptogenesis. BMC Genomics. 2010;11:230. doi: 10.1186/1471-2164-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pang Y, Zheng B, Campbell LR, Fan LW, Cai Z, Rhodes PG. IGF-1 can either protect against or increase LPS-induced damage in the developing rat brain. Pediatr Res. 2010;67:579–84. doi: 10.1203/PDR.0b013e3181dc240f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SE, Dantzer R, Kelley KW, McCusker RH. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J Neuroinflamm. 2011;8:12. doi: 10.1186/1742-2094-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musaro A. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21:1393–402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Azcoitia I, Trejo JL, Sierra A, Garcia-Segura LM. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur J Neurosci. 2003;18:923–930. doi: 10.1046/j.1460-9568.2003.02830.x. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Fernando G, Day L, Allen TA, Chapleau JD, Menjivar J, Schlinger BA, Lee DW. Aromatase expression and cell proliferation following injury of the adult zebra finch hippocampus. Dev Neurobiol. 2007;67:1867–78. doi: 10.1002/dneu.20548. [DOI] [PubMed] [Google Scholar]
- Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2006;94:95–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxydopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, Shenaq SM, Schwartz RJ. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J. 2003;17:53–55. doi: 10.1096/fj.02-0183fje. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Fan F, Jung YD, Ahmad SA, Stoeltzing O, Bucana CD, Radinsky R, Ellis LM. Blockade of insulin-like growth factor I receptor function inhibits growth and angiogenesis of colon cancer. Clin Cancer Res. 2002;8:3259–69. [PubMed] [Google Scholar]
- Rhodin JA, Thomas TN, Clark L, Garces A, Bryant M. In vivo cerebrovascular actions of amyloid beta-peptides and the protective effect of conjugated estrogens. J Alzheimers Dis. 2003;5:275–286. doi: 10.3233/jad-2003-5403. [DOI] [PubMed] [Google Scholar]
- Riikonen RS, Jääskeläinen J, Turpeinen U. Insulin-like growth factor-1 is associated with cognitive outcome in infantile spasms. Epilepsia. 2010;51:1283–1289. doi: 10.1111/j.1528-1167.2009.02499.x. [DOI] [PubMed] [Google Scholar]
- Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17b-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder C, Schwarzer C, Vezzani A, Gobbi M, Mennini T, Sperk G. Autoradiographic analysis of neuropeptide Y receptor binding sites in the rat hippocampus after kainic acid-induced limbic seizures. Neurosci. 1996;70:47–55. doi: 10.1016/0306-4522(95)00332-d. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos W, Fabbro D, Kung W, Costa SD, Eppenberger U. Correlation between hormone dependency and the regulation of epidermal growth factor receptor by tumor promoters in human mammary carcinoma cells. Proc Natl Acad Sci U S A. 1986;83:991–5. doi: 10.1073/pnas.83.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- Sanchez JC, Lopez-Zapata DF, Francis L, De Los Reyes L. Effects of estradiol and IGF-1 on the sodium calcium exchanger in rat cultured cortical neurons. Cell Mol Neurobiol. 2011;31:619–627. doi: 10.1007/s10571-011-9657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg Nordqvist AC, von Holst H, Holmin S, Sara VR, Bellander BM, Schalling M. Increase of insulin-like growth factor (IGF)-1, IGF binding protein-2 and -4 mRNAs following cerebral contusion. Brain Res Mol Brain Res. 1996;38:285–93. doi: 10.1016/0169-328x(95)00346-t. [DOI] [PubMed] [Google Scholar]
- Sanz A, Carrero P, Pernia O, Garcia-Segura LM. Pubertal maturation modifies the regulation of insulin-like growth factor-I receptor signaling by estradiol in the rat prefrontal cortex. Dev Neurobiol. 2008;68:1018–28. doi: 10.1002/dneu.20641. [DOI] [PubMed] [Google Scholar]
- Sara VR, Carlsson-Skwirut C, Bergman T, Jornvall H, Roberts PJ, Crawford M, Hakansson LN, Civalero I, Nordberg A. Identification of Gly-Pro-Glu (GPE), the aminoterminal tripeptide of insulin-like growth factor 1 which is truncated in brain, as a novel neuroactive peptide. Biochem Biophys Res Commun. 1989;165:766–71. doi: 10.1016/s0006-291x(89)80032-4. [DOI] [PubMed] [Google Scholar]
- Saura J, Curatolo L, Williams CE, Gatti S, Benatti L, Peeters C, Guan J, Dragunow M, Post C, Faull RL, Gluckman PD, Skinner SJ. Neuroprotective effects of Gly-Pro-Glu, the N-terminal tripeptide of IGF-1, in the hippocampus in vitro. Neuroreport. 1999;10:161–164. doi: 10.1097/00001756-199901180-00031. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Amur-Umarjee S, Shen P, Campagnoni AT, Arnold AP. Neuronal and non-neuronal aromatase in primary cultures of developing zebra finch telencephalon. J Neurosci. 1994;14:7541–7552. doi: 10.1523/JNEUROSCI.14-12-07541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Estrogen receptors in quail brain: a functional relationship to aromatase and aggressiveness. Biol Reprod. 1989;40:268–75. doi: 10.1095/biolreprod40.2.268. [DOI] [PubMed] [Google Scholar]
- Schober ME, Ke X, Xing B, Block BP, Requena DF, McKnight R, Lane RH. Traumatic brain injury increased IGF-1B mRNA and altered IGF-1 exon 5 and promoter region epigenetic characteristics in the rat pup hippocampus. J Neurotrauma. 2012;29:2075–2085. doi: 10.1089/neu.2011.2276. [DOI] [PubMed] [Google Scholar]
- Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulinlike growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28:1744–8. doi: 10.1161/01.str.28.9.1744. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS ONE. 2012;7(2):e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of IGF-1. J Neurosci. 2010;30:6852–61. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2008.08.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severgnini S, Lowenthal DT, Millard WJ, Simmen FA, Pollock BH, Borst SE. Altered IGF-I and IGFBPs in senescent male and female rats. J Gerontol A Biol Sci Med Sci. 1999;54:B111–5. doi: 10.1093/gerona/54.3.b111. [DOI] [PubMed] [Google Scholar]
- Shapira S, Mathai S, Zhang R, Guan J. Delayed peripheral administration of the N-terminal tripeptide of IGF-1 (GPE) reduces brain damage following microsphere induced embolic damage in young adult and aged rats. Neurosci Lett. 2009;454:53–7. doi: 10.1016/j.neulet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Sharma HS. Neuroprotective effects of neurotrophins and melanocortins in spinal cord injury: an experimental study in the rat using pharmacological and morphological approaches. Ann N Y Acad Sci. 2005;1053:407–421. doi: 10.1111/j.1749-6632.2005.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhang Y, Simpkins J. Effects of 17beta-estradiol on glucose transporter 1 expression and endothelial cell survival following focal ischemia in the rats. Exp Brain Res. 1997;117:200–206. doi: 10.1007/s002210050216. [DOI] [PubMed] [Google Scholar]
- Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J. 1999;46(Suppl):S59–62. doi: 10.1507/endocrj.46.suppl_s59. [DOI] [PubMed] [Google Scholar]
- Shin JA, Yang SJ, Jeong SI, Park HJ, Choi YH, Park EM. Activation of estrogen receptor alpha reduces blood-brain barrier breakdown following ischemic injury. Neurosci. 2013;235:165–73. doi: 10.1016/j.neuroscience.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins J, Rajakumar G, Zhang YQ, Simpkins C, Greenwald D, Yu C, Bodor N, Day A. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Guan X, Warren M, Toran-Allerand C. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Meyer E, Simpkins J. The effect of ovariectomy and estradiol replacement on brain derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinol. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Sirbasku DA. Hormone-responsive growth in vivo of a tissue culture cell line established from the MT-W9A rat mammary tumor. Cancer Res. 1978;38:1154–65. [PubMed] [Google Scholar]
- Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- Smith PF. Neuroprotection against hypoxia-ischemia by insulin-like growth factor-1 (IGF-1) Drugs. 2003;6:1173–1177. [PubMed] [Google Scholar]
- Sohrabji F, Miranda R, Toran-Allerand CD. Identification of a putative estrogen response element in the gene coding for BDNF. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, McShane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neurosci. 1999;88:269–79. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Eckman DM, Ingraham J, Riddle DR. Regulation of Cerebrovascular Aging. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press; 2007. Chapter 12. [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinol. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, Subramaniam S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003;170:1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- Sugden PH, LeRoith D, Lavandero S. Extracellular regulated kinase, but not protein kinase C, is an antiapoptotic signal of insulin-like growth factor-1 on cultured cardiac myocytes. Biochem Biophys Res Commun. 2000;273:736–744. doi: 10.1006/bbrc.2000.3008. [DOI] [PubMed] [Google Scholar]
- Suh HS, Zhao ML, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflamm. 2013;10:37. doi: 10.1186/1742-2094-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedin P, Guan J, Mathai S, Zhang R, Wang X, Gustavsson M, Hagberg H, Mallard C. Delayed peripheral administration of a GPE analogue induces astrogliosis and angiogenesis and reduces inflammation and brain injury following hypoxia-ischemia in the neonatal rat. Dev Neurosci. 2007;29:393–402. doi: 10.1159/000105480. [DOI] [PubMed] [Google Scholar]
- Szabo L, Mottershead DG, Ballard FJ, Wallace JC. The bovine insulin-like growth factor (IGF) binding protein purified from conditioned medium requires the N-terminal tripeptide in IGF-1 for binding. Biochem Biophys Res Commun. 1988;151:207–14. doi: 10.1016/0006-291x(88)90580-3. [DOI] [PubMed] [Google Scholar]
- Tchernitchin AN, Galand P. Oestrogen levels in the blood, not in the uterus, determine uterine eosinophilia and oedema. J Endocrinol. 1983;99:123–30. doi: 10.1677/joe.0.0990123. [DOI] [PubMed] [Google Scholar]
- Theoharides T, Dimitriadou V, Letourneau R, Rozniecki J, Vliagoftis H, Boucher W. Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain myelin changes resembling early stages of demyelination. Neurosci. 1993;57:861–871. doi: 10.1016/0306-4522(93)90030-j. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurol. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- Tran SE, Holmstrom TH, Ahonen M, Kahari VM, Eriksson JE. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J Biol Chem. 2001;276:16484–16490. doi: 10.1074/jbc.M010384200. [DOI] [PubMed] [Google Scholar]
- Traub ML, De Butte-Smith M, Zukin RS, Etgen AM. Oestradiol and insulin-like growth factor-1 reduce cell loss after global ischaemia in middle-aged female rats. J Neuroendocrinol. 2009;21:1038–1044. doi: 10.1111/j.1365-2826.2009.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich C, Zendedel A, Habib P, Kipp M, Beyer C, Dang J. Long-term cerebral cortex protection and behavioral stabilization by gonadal steroid hormones after transient focal hypoxia. J Steroid Biochem Mol Biol. 2012;131:10–6. doi: 10.1016/j.jsbmb.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Res. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Pollio G, Ciana P, Maggi A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35:1309–1316. doi: 10.1016/s0531-5565(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Tang Y, Zhang W, Zhao H, Wang R, Yan Y, Xu L, Li P. Insulin-like growth factor-1 secreted by brain microvascular endothelial cells attenuates neuron injury upon ischemia. FEBS J. 2013;280:3658–68. doi: 10.1111/febs.12359. [DOI] [PubMed] [Google Scholar]
- Wang JM, Hayashi T, Zhang WR, Sakai K, Shiro Y, Abe K. Reduction of ischemic brain injury by topical application of insulin-like growth factor-I after transient middle cerebral artery occlusion in rats. Brain Res. 2000;859:381–5. doi: 10.1016/s0006-8993(00)02008-4. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Wong YC. Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-I (IGF-I) and vascular endothelial growth factor (VEGF) in the development of prostate cancer. Prostate. 1998;35:165–77. doi: 10.1002/(sici)1097-0045(19980515)35:3<165::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Waters DL, Yau CL, Montoya GD, Baumgartner RN. Serum Sex Hormones, IGF-1, and IGFBP3 Exert a Sexually Dimorphic Effect on Lean Body Mass in Aging. J Gerontol A Biol Sci Med Sci. 2003;58:648–652. doi: 10.1093/gerona/58.7.m648. [DOI] [PubMed] [Google Scholar]
- Wintink AJ, Young NA, Davis AC, Gregus A, Kalynchuk LE. Kindling-induced emotional behavior in male and female rats. Behav Neurosci. 2003;117:632–640. doi: 10.1037/0735-7044.117.3.632. [DOI] [PubMed] [Google Scholar]
- Wittmann W, McLennan IS. The male bias in the number of Purkinje cells and the size of the murine cerebellum may require Mullerian inhibiting substance/anti-Mullerian hormone. J Neuroendocrinol. 2011;23:831–8. doi: 10.1111/j.1365-2826.2011.02187.x. [DOI] [PubMed] [Google Scholar]
- Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinol. 2013;154:842–52. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- Yague JG, Lavaque E, Carretero J, Azcoitia I, Garcia-Segura LM. Aromatase, the enzyme responsible for estrogen biosynthesis, is expressed by human and rat glioblastomas. Neurosci Lett. 2004;368:279–84. doi: 10.1016/j.neulet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–9. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-I is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann N Y Acad Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- Ye P, Popken GJ, Kemper A, McCarthy K, Popko B, D'Ercole AJ. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. J Neurosci Res. 2004;78:472–484. doi: 10.1002/jnr.20288. [DOI] [PubMed] [Google Scholar]
- Yu J, Li J, Zhang S, Xu X, Zheng M, Jiang G, Li F. IGF-1 induces hypoxia-inducible factor 1alpha-mediated GLUT3 expression through PI3K/Akt/mTOR dependent pathways in PC12 cells. Brain Res. 2012;1430:18–24. doi: 10.1016/j.brainres.2011.10.046. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Moerkens M, Ramaiahgari S, de Bont H, Price L, Meerman J, van de Water B. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13:R52. doi: 10.1186/bcr2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007;292:F440–7. doi: 10.1152/ajprenal.00170.2006. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Arroyo A, Amato P, Yen SS. A role for hypothalamic astrocytes in dehydroepiandrosterone and estradiol regulation of gonadotropin-releasing hormone (GnRH) release by GnRH neurons. Neuroendocrinol. 2002;75:375–83. doi: 10.1159/000059434. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS, Cheng CY. Astrocytes cultured in vitro produce estradiol-17beta and express aromatase cytochrome P-450 (P-450 AROM) mRNA. Biochem Biophys Acta. 1997;1334:338–348. doi: 10.1016/s0304-4165(96)00115-8. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinol. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]


