Abstract
Introduction
It is believed that many of the beneficial effects of long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) are mediated by their oxidized metabolites, the oxylipins. The formation and biological role of many cytochrome P450 and lipoxygenase derived hydroxy, epoxy and dihydroxy FA, particularly of oxylipins esterified in polar lipids and triglycerides remain unclear. In this study, we compared the impact of twelve weeks of LC n-3 PUFA supplementation on the patterns of free and total (sum of esterified and free) hydroxy, epoxy and dihydroxy FAs.
Subjects and Methods
Subjects (5 male; 5 female) between 46 and 70 years were supplemented with 1.1 g/d of eicosapentaenoic acid (EPA) and 0.74 g/d docosahexaenoic acid (DHA) as ethyl esters. Blood samples were drawn before and after twelve weeks of treatment. Oxylipins in plasma were analyzed by LC-MS directly for free oxylipins and after saponification. Relative FA composition in erythrocyte membranes was analyzed by GC.
Results
LC n-3 PUFA treatment led to a significant increase in EPA (200%) and DHA (23%) in erythrocyte membranes. Of the oxylipins measured in plasma, total and free EPA-derived metabolites were highly increased (70 to 150%), while total AA-derived metabolites were decreased on average by 30%. There was no effect on DHA-metabolites. Concentrations of total hydroxy and epoxy FAs in plasma were considerably higher compared to free hydroxy and epoxy FAs (up to 350 times), while levels of most free dihydroxy FAs were in a similar range to total dihydroxy FAs. However, the individual ratios between total and free plasma oxylipins remained unchanged after LC n-3 PUFA treatment.
Discussion and Conclusions
LC n-3 PUFA supplementation causes a shift in the levels of circulating oxylipins, having the strongest impact on EPA-derived epoxy, dihydroxy and hydroxy FA. The unchanged ratio of free and esterified oxylipins in plasma indicates that both concentrations are valuable biomarkers for assessing the individual status of these lipid mediators.
Keywords: eicosanoids, epoxides, diols, EPA, DHA, PUFA, arachidonic acid, omega-3 fatty acids
INTRODUCTION
An increased intake of the long-chain omega-3 fatty acids (LC n-3 PUFAs) eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) has been associated with a reduced risk for several chronic inflammatory diseases such as rheumatoid arthritis (Miles and Calder 2012), neuropsychiatric diseases (Samieri 2008, Lin and Su 2007), atherosclerosis and cardiovascular diseases (Delgado-Lista 2012, Mozaffarian and Wu 2011). However, the underlying molecular mechanisms by which LC n-3 PUFA exert their effects are versatile and far away from being understood. It is believed that many actions of LC n-3 PUFAs are mediated by their bioactive lipid metabolites (Powell 1995, Serhan 2005, Morisseau 2010), the oxylipins. Both, LC n-6 and n-3 PUFAs undergo oxygenation in the mammalian body giving rise to a large number of active lipid mediators (Weylandt 2012, Schuchardt 2013). These oxylipins can be formed enzymatically by cyclooxygenases (Rouzer 2009), lipoxygenases (5-LOX, 12-LOX and 15-LOX) (Samuelsson 1987, Rådmark 2009), and cytochrome P450 enzymes (CYPs, e.g. CYP4, CYP2C and CYP2J2) (Roman 2002, Capdevila 2002) or are formed during FA autoxidation (Yin 2011, Spickett 2010, Niki 2008).
Oxylipins are involved in the regulation of various biological processes, for example inflammation, pain, cell proliferation, apoptosis, angiogenesis, blood coagulation and blood vessel permeability (Arnold 2010, Buczynski 2009). Compared to oxylipins derived from arachidonic acid (AA, C20:4 n-6) by cyclooxygenase and 5-lipoxygenase action (i.e. prostaglandins and leukotrienes), only limited information is available about the formation and biological role of hydroxy, epoxy and dihydroxy FA derived from LC n-3 PUFAs. Recent studies have shown that particularly epoxy FA from LC n-3 PUFAs possess highly potent anti-arrhythmic (Westphal 2011), vasodilatory (Agbor 2012) and anti-thrombotic effects (Jung 2012). Hydroxylated FAs serve as precursors for pro-resolving signaling molecules i.e. resolvins and (neuro)protectins which actively mediate the resolution of inflammation (Weylandt 2012, Serhan 2002, Serhan 2005).
Profiling of oxylipin patterns in response to LC n-3 PUFA supplementation in humans may assist to deduce their mechanisms of action. Moreover, it may provide novel biomarkers facilitating the investigation of the effects of LC n-3 PUFAs.. Several human studies documented changes of hydroxy, epoxy and dihydroxy FA levels in serum and plasma following treatment with LC n-3 PUFA regarding free oxylipins (Lundström 2013, Schuchardt 2014a) or total oxylipins (sum of both free and esterified) after conjugate cleavage by saponification (Shearer 2010, Keenan 2012, Schuchardt 2014b, Fischer 2014). Applied LC n-3 PUFA doses and treatment duration varied considerably among the long term studies: 11.0±2.0 mg/kg/d (~0.8 g/d) as ethyl esters for 4 weeks (Keenan 2012); 0.84–1.74 g/d as ethyl esters for 4–8 weeks (Fischer 2014); 3.0 g/d as re-esterified triglycerides for twelve weeks (Schuchardt 2014a); 4.0 g/d LC n-3 PUFAs as ethyl esters for 4 weeks (Shearer 2010) and 6.0 g/d EPA/DHA as ethyl esters for 3 weeks (Lundström 2013). In addition to the varying study designs, the strong inter-individual differences in the blood oxylipin levels, as discussed in (Schuchardt 2013), make it complicated to compare different studies. When comparing the reported baseline levels of free serum and plasma levels against total plasma oxylipins, it becomes apparent that the major portion of hydroxy, epoxy and dihydroxy FA are found mainly esterified in the plasma (Schuchardt 2013). Interestingly, with and without saponification most dihydroxy FA were detected in the same range indicating that no or a very low portion of these lipid mediators in plasma is found in lipids or triglycerides. LC n-3 PUFA intake causes both a change in the pattern of total oxylipins (Shearer 2010, Keenan 2012) as well as in the levels of free oxylipins (Lundström 2013, Schuchardt 2014). However, there is no information on how the changes in esterified and free oxylipins are correlated. Therefore, the aim of this study was to determine the impact of a supplementation of LC n-3 PUFAs on the patterns of hydroxy, epoxy and dihydroxy FA species and their ratio between free and total levels in plasma. Using a targeted metabolomics approach, we simultaneously quantified 50 oxylipins in human plasma before and after twelve weeks of supplementation with EPA and DHA ethyl esters.
MATERIALS AND METHODS
This investigator initiated study was designed and conducted according to the principles of the Good Clinical Practice Guidelines laid down in the Declaration of Helsinki and was approved by an independent Research Ethics Board (Freiburg Ethics Commission International).
Subjects
Ten subjects were recruited via newspaper advertisements and screened in the Institute of Food Science and Human Nutrition at the Leibniz University Hannover, Germany. The exclusion criteria were age 30 and >75 years, body mass index >35 kg/m2, type 1 and 2 diabetes, cancer, coronary heart disease, renal failure, liver disease, bleeding disorders, gastrointestinal disorders (e.g. Colitis ulcerosa, Crohn’s disease, chronic pancreatitis, pancreatic insufficiency, coeliac disease, enterocolitis, cholestasis, short bowel syndrome), surgical operation in the gastrointestinal system (e.g. gastrectomy, stomach reduction, stomach tape or balloon), hormonal disorders (e.g. hyperthyroidism and Cushing’s disease), high intake of oily fish (>2 times per week), intake of lipid-lowering drugs (statins, fibrates, ezetimibe, and niacin) or nutritional supplements (including LC n-3 PUFAs, phytosterols and polyglucosamin), intake of anticoagulant drugs. Only participants that fulfilled the criteria were included in the study population. All subjects gave their written informed consent to take part in the study.
Study design
Subjects were supplemented with LC n-3 PUFA capsules for a period of twelve weeks. Soft gelatin capsules contained re-esterified fish oil (840 mg/ capsule, Dr. Loges GmbH, Winsen, Germany). The oil contained 330 mg/g EPA and 220 mg/g DHA ethyl ester. The total amount of LC n-3 PUFA ethyl esters (C20:5, C22:6, C18:3, C18:4, C20:4, C21:5, C22:5) was 640 mg/g Moreover the oil contained 2.8 mg/g tocopheroles as antioxidant. Subjects were instructed to ingest four capsules per day (two in the morning and two in the evening together with food and a glass of water). The daily intake was 1.1 g EPA ethyl ester and 0.74 g DHA ethyl ester. Subjects were requested to abstain from eating fatty fish during the intervention period, while other exercise and dietary habits should be maintained. During the two visits, baseline (t0) and after twelve weeks of intervention (t12) fasting blood was collected. Additionally, at t0 height and weight of the subjects were measured. The subjects’ compliance was assessed by a questionnaire, capsule-intake diaries and a count of left-over capsules at the end of the intervention period.
Sample collection and analysis
Blood samples were collected by venipuncture of an arm vein into EDTA-monovettes (Sarstedt, Germany) in the morning between 6:00 and 9:30 a.m. after overnight fasting. Plasma was prepared directly by centrifugation (2000 g, 10 min, 10°C), transferred into 15 mL falcon tubes (Becton Dickinson) and immediately frozen and stored at −80°C awaiting LC-MS analysis. Other sets of EDTA-whole blood samples collected simultaneously were sent to an external laboratory (Omegametrix, Martinsried, Germany), where the FA composition in erythrocyte membranes was determined as previously described (Harris and von Schacky 2004, Park and Harris 2002). Results are presented as a percentage of the total identified FAs.
Oxylipins were analyzed in plasma samples as free oxylipins and after saponification to determine the sum of both free and esterified oxylipins as previously described (Arnold 2010b). In brief, 250 μL of plasma were mixed with an equal volume of methanol. After addition of 300 μL aqueous sodium hydroxide (10 M) the samples were incubated at 60°C for 30 min. Following neutralization with 50% acetic acid the oxylipins were extracted by solid phase extraction and quantified by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS) as previously described (Schuchardt 2013). Only oxylipins which exceeded the limit of quantification in ≥ 95% of each sample set were used for data analysis.
Data analysis and statistics
PUFA composition in erythrocyte membranes (figure 1) as well as concentrations of free and total oxylipins in plasma (table 1) is presented as mean ± standard error (SE). The sample sets were analyzed for their distribution by the Kolmogorov–Smirnov test. Differences between time points within groups were analyzed by t-tests for paired samples. Changes in the concentration (c) were calculated individually for each subject at each time point (0 and 12) as Δ %, calculated by: Δ % = 100 * (ct12 − ct0)/ct0. Results are shown as mean change ± SE (table 1, figure S1). To compare their relative distribution, the oxylipins were grouped by substrate (figure S2). For the calculation, the sum of the median values of the analyzed oxylipins from each LC PUFA (18:2, 18:3, 20:4, 20:5 and 22:6)) was divided by the sum of the medians for all oxylipins. All statistical analyses were carried out with SPSS software (Version 21, SPSS Inc., Chicago, IL, USA). Statistical significance was generally accepted at p <0.05.
Figure 1.
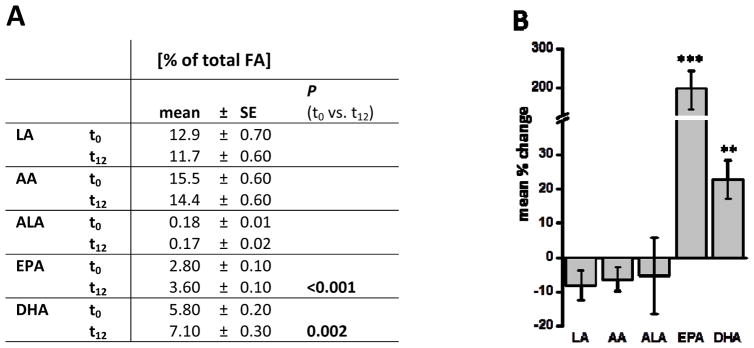
Pre- and post-treatment LC PUFA status of subjects (n=10). A) Ratio of LC PUFAs in erythrocyte membranes at baseline (t0) and after twelve weeks (t12) of treatment with LC n-3 PUFA. B) Mean relative change of LC PUFA ratios after LC n-3 PUFA treatment on individual level. All results are shown as the mean ± SE. Significant differences between the two time points were determined by dependent sample t-test.
Table 1.
Concentration of total (sum of esterified and free) and free oxylipins in plasma of healthy human subjects (n=10) at baseline (t0) and after twelve weeks (t12) of treatment with LC n-3 PUFA. Mean relative change from t0 after twelve weeks of treatment with LC n-3 PUFA was calculated individually for each subject. All results are shown as the mean ± SE. Significant differences between the two time points were determined by dependent sample t-test.
| Total oxylipins | Free oxylipins | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| concentration [nM] | relative change from t0 [%] | concentration [nM] | relative change from t0 [%] | ||||||
| mean ± SE | mean ± SE | P (t0 vs. t12) | mean ± SE | mean ± SE | P (t0 vs. t12) | ||||
| LA-derived oxylipins | Hydroxy FA | 9-HODE | t0 | 470 ± 63.4 | 8.50 ± 1.34 | ||||
| t12 | 347 ± 55.1 | −23.6 ± 9.9 | 10.0 ± 0.98 | 10.9 ± 12.1 | |||||
|
| |||||||||
| Epoxy FA | 9(10)-EpOME | t0 | 62.9 ± 7.60 | 1.56 ± 0.34 | |||||
| t12 | 37.9 ± 3.76 | −33.2 ± 7.9 | 0.010 | 1.20 ± 0.27 | −18.8 ± 19.4 | ||||
| 12(13)-EpOME | t0 | 67.2 ± 7.76 | 2.02 ± 0.42 | ||||||
| t12 | 44.8 ± 4.87 | −26.3 ± 9.3 | 0.026 | 1.98 ± 0.31 | 5.0 ± 19.3 | ||||
|
| |||||||||
| Dihydroxy FA | 9,10-DiHOME | t0 | 8.35 ± 0.99 | 1.32 ± 0.31 | |||||
| t12 | 6.68 ± 0.68 | −13.3 ± 10.3 | 1.40 ± 0.26 | 24.9 ± 33.8 | |||||
| 12,13-DiHOME | t0 | 6.03 ± 0.67 | 3.04 ± 0.71 | ||||||
| t12 | 4.98 ± 0.80 | −14.8 ± 9.4 | 3.75 ± 0.60 | 23.5 ± 17.6 | |||||
|
| |||||||||
| AA-derived oxylipins | Hydroxy FA | 5-HETE | t0 | 79.5 ± 7.52 | 0.82 ± 0.14 | ||||
| t12 | 44.6 ± 4.93 | −41.3 ± 6.6 | 0.002 | 0.74 ± 0.05 | −13.2 ± 9.0 | ||||
| 8-HETE | t0 | 68.6 ± 5.83 | ≤ LOQ | ||||||
| t12 | 43.3 ± 5.71 | −35.4 ± 7.7 | 0.004 | ≤ LOQ | −12.3 ± 11.0 | ||||
| 9-HETE | t0 | 112 ± 8.70 | 0.27 ± 0.05 | ||||||
| t12 | 70.3 ± 9.65 | −36.7 ± 7.8 | 0.002 | 0.22 ± 0.02 | −11.3 ± 9.0 | ||||
| 11-HETE | t0 | 102 ± 8.58 | 0.79 ± 0.14 | ||||||
| t12 | 66.4 ± 8.48 | −33.6 ± 7.6 | 0.003 | 0.44 ± 0.05 | −43.5 ± 11.4 | ||||
| 12-HETE | t0 | 100 ± 9.44 | 0.72 ± 0.13 | ||||||
| t12 | 69.3 ± 10.1 | −28.8 ± 9.4 | 0.021 | 0.40 ± 0.05 | −38.3 ± 10.2 | ||||
| 15-HETE | t0 | 252 ± 23.8 | 1.09 ± 0.18 | ||||||
| t12 | 139 ± 18.5 | −43.6 ± 6.7 | <0.001 | 1.04 ± 0.17 | −10.0 ± 12.0 | ||||
|
| |||||||||
| Epoxy FA | 8(9)-EpETrE | t0 | 11.5 ± 1.20 | ≤ LOQ | |||||
| t12 | 6.54 ± 0.95 | −33.2 ± 15.9 | 0.018 | ≤ LOQ | |||||
| 11(12)-EpETrE | t0 | 21.0 ± 1.86 | 0.24 ± 0.04 | ||||||
| t12 | 17.3 ± 2.23 | −10.8 ± 14.1 | 0.20 ± 0.05 | −24.2 ± 16.7 | |||||
| 14(15)-EpETrE | t0 | 18.4 ± 1.44 | 0.24 ± 0.05 | ||||||
| t12 | 11.3 ± 1.16 | −34.6 ± 8.1 | 0.005 | 0.22 ± 0.06 | −12.2 ± 18.6 | ||||
|
| |||||||||
| Dihydroxy FA | 8,9-DiHETrE | t0 | 4.12 ± 0.24 | 0.32 ± 0.05 | |||||
| t12 | 2.53 ± 0.17 | −38.4 ± 3.2 | <0.001 | 0.30 ± 0.03 | −16.3 ± 6.7 | ||||
| 11,12-DiHETrE | t0 | 1.47 ± 0.09 | 0.51 ± 0.08 | ||||||
| t12 | 0.89 ± 0.06 | −39.1 ± 2.3 | <0.001 | 0.43 ± 0.06 | −27.2 ± 5.9 | ||||
| 14,15-DiHETrE | t0 | 1.01 ± 0.06 | 0.81 ± 0.12 | ||||||
| t12 | 0.60 ± 0.05 | −40.6 ± 4.2 | <0.001 | 0.72 ± 0.06 | −21.1 ± 5.8 | ||||
|
| |||||||||
| ALA-derived oxylipins | Hydroxy FA | 9-HOTrE | t0 | 5.26 ± 0.81 | 0.41 ± 0.07 | ||||
| t12 | 4.60 ± 0.98 | −14.5 ± 7.8 | 0.42 ± 0.04 | −1.6 ± 11.3 | |||||
| 13-HOTrE | t0 | 19.1 ± 2.23 | 0.33 ± 0.05 | ||||||
| t12 | 17.1 ± 3.30 | −6.3 ± 16.4 | 0.32 ± 0.03 | −15.7 ± 7.9 | |||||
|
| |||||||||
| Epoxy FA | 9(10)-EpODE | t0 | 2.07 ± 0.37 | 0.17 ± 0.04 | |||||
| t12 | 1.42 ± 0.16 | −21.5 ± 9.9 | 0.13 ± 0.03 | −16.3 ± 23.6 | |||||
| 12(13)-EpODE | t0 | 1.22 ± 0.24 | 0.11 ± 0.02 | ||||||
| t12 | 0.85 ± 0.11 | −15.9 ± 14.1 | 0.10 ± 0.02 | −17.7 ± 20.8 | |||||
| 15(16)-EpODE | t0 | 8.65 ± 1.99 | 1.74 ± 0.41 | ||||||
| t12 | 4.45 ± 0.69 | −33.3 ± 15.6 | 0.041 | 0.92 ± 0.05 | −33.7 ± 11.7 | ||||
|
| |||||||||
| Dihydroxy FA | 9,10-DiHODE | t0 | 0.50 ± 0.04 | 0.11 ± 0.03 | |||||
| t12 | 0.40 ± 0.02 | −12.4 ± 6.0 | 0.024 | 0.10 ± 0.01 | −7.8 ± 9 | ||||
| 12,13-DiHODE | t0 | 0.39 ± 0.05 | 0.12 ± 0.03 | ||||||
| t12 | 0.33 ± 0.03 | −5.1 ± 5.1 | 0.11 ± 0.01 | −4.7 ± 9.3 | |||||
| 15,16-DiHODE | t0 | 7.95 ± 0.52 | 8.39 ± 1.50 | ||||||
| t12 | 5.01 ± 0.36 | −33.7 ± 7.4 | 0.003 | 7.51 ± 0.58 | −11.5 ± 12.8 | ||||
|
| |||||||||
| EPA-derived oxylipins | Hydroxy FA | 5-HEPE | t0 | 7.14 ± 0.78 | 0.17 ± 0.03 | ||||
| t12 | 14.7 ± 3.48 | 115 ± 40.5 | 0.048 | 0.23 ± 0.02 | 26.4 ± 12.8 | ||||
| 8-HEPE | t0 | 6.59 ± 0.75 | 0.19 ± 0.02 | ||||||
| t12 | 16.0 ± 4.35 | 149 ± 49.8 | 0.049 | 0.32 ± 0.02 | 46.4 ± 10.1 | 0.01 | |||
| 12-HEPE | t0 | 11.3 ± 1.40 | ≤ LOQ | ||||||
| t12 | 30.0 ± 9.20 | 172 ± 60.3 | ≤ LOQ | ||||||
| 15-HEPE | t0 | 8.65 ± 1.03 | 0.43 ± 0.06 | ||||||
| t12 | 23.1 ± 6.71 | 176 ± 59.7 | 0.64 ± 0.06 | 32.9 ± 9.2 | 0.019 | ||||
|
| |||||||||
| Epoxy FA | 8(9)-EpETE | t0 | 2.31 ± 0.34 | ≤ LOQ | |||||
| t12 | 4.86 ± 1.01 | 154 ± 76.4 | 0.019 | ≤ LOQ | |||||
| 11(12)-EpETE | t0 | 2.87 ± 0.32 | ≤ LOQ | ||||||
| t12 | 5.19 ± 0.81 | 102 ± 44.6 | 0.007 | ≤ LOQ | |||||
| 14(15)-EpETE | t0 | 2.42 ± 0.27 | ≤ LOQ | ||||||
| t12 | 4.97 ± 0.93 | 140 ± 67.3 | 0.016 | ≤ LOQ | |||||
| 17(18)-EpETE | t0 | 3.06 ± 0.29 | 0.11 ± 0.02 | ||||||
| t12 | 6.59 ± 1.38 | 124 ± 46.9 | 0.019 | 0.20 ± 0.03 | 78.7 ± 37.0 | 0.038 | |||
|
| |||||||||
| Dihydroxy FA | 11,12-DiHETE | t0 | 0.34 ± 0.04 | 0.05 ± 0.01 | |||||
| t12 | 0.57 ± 0.05 | 83.0 ± 23.2 | 0.001 | 0.13 ± 0.02 | 132 ± 37.0 | 0.006 | |||
| 14,15-DiHETE | t0 | 0.27 ± 0.05 | 0.68 ± 0.16 | ||||||
| t12 | 0.43 ± 0.04 | 87.0 ± 27.5 | 0.005 | LOQ | |||||
| 17,18-DiHETE | t0 | 0.61 ± 0.07 | 0.64 ± 0.12 | ||||||
| t12 | 1.00 ± 0.15 | 68.9 ± 20.3 | 0.011 | 1.58 ± 0.23 | 123 ± 29.1 | 0.012 | |||
|
| |||||||||
| DHA-derived | Epoxy FA | 10(11)-EpDPE | t0 | 4.41 ± 0.44 | 1.84 ± 0.36 | ||||
| t12 | 4.77 ± 0.52 | 14.4 ± 12.7 | 2.43 ± 0.40 | 17.9 ± 21.6 | |||||
| 13(14)-EpDPE | t0 | 2.89 ± 0.28 | 0.24 ± 0.05 | ||||||
| t12 | 3.10 ± 0.46 | 19.8 ± 23.4 | 0.30 ± 0.05 | 48.5 ± 30.6 | |||||
| 16(17)-EpDPE | t0 | 3.08 ± 0.33 | 0.12 ± 0.02 | ||||||
| t12 | 3.31 ± 0.57 | 19.9 ± 21.9 | 0.17 ± 0.04 | 15.0 ± 31.1 | |||||
| 19(20)-EpDPE | t0 | 4.64 ± 0.45 | 0.33 ± 0.06 | ||||||
| t12 | 5.12 ± 1.05 | 19.8 ± 23.9 | 0.39 ± 0.06 | 15.9 ± 26.7 | |||||
|
| |||||||||
| Dihydroxy FA | 4,5-DiHDPE | t0 | 3.74 ± 0.35 | 0.24 ± 0.05 | |||||
| t12 | 3.98 ± 0.47 | 9.4 ± 12.6 | 0.33 ± 0.05 | 44.9 ± 40.4 | |||||
| 7,8-DiHDPE | t0 | 1.95 ± 0.22 | 0.12 ± 0.02 | ||||||
| t12 | 1.73 ± 0.24 | −2.2 ± 14.5 | 0.16 ± 0.02 | 20.8 ± 13.2 | |||||
| 10,11-DiHDPE | t0 | 0.72 ± 0.08 | 0.21 ± 0.04 | ||||||
| t12 | 0.62 ± 0.09 | −7.4 ± 14.0 | 0.27 ± 0.02 | 23.2 ± 12.0 | |||||
| 13,14-DiHDPE | t0 | 0.37 ± 0.03 | 0.20 ± 0.03 | ||||||
| t12 | 0.33 ± 0.04 | −6.7 ± 13.7 | 0.26 ± 0.03 | 14.7 ± 6.9 | |||||
| 16,17-DiHDPE | t0 | 0.73 ± 0.06 | 0.38 ± 0.06 | ||||||
| t12 | 0.78 ± 0.11 | 18.4 ± 23.1 | 0.54 ± 0.07 | 25.4 ± 16.1 | |||||
| 19,20-DiHDPE | t0 | 2.79 ± 0.29 | 2.58 ± 0.38 | ||||||
| t12 | 2.14 ± 0.25 | −19.9 ± 9.7 | 0.043 | 3.59 ± 0.39 | 21.5 ± 13.4 | ||||
≤LOQ, below limit of quantification
RESULTS
Included subjects (5 male; 5 female) had an average age of 63 years (range: 46–70 years) and were slightly overweight with a body mass index of 26 kg/m2 (range: 21–35 kg/m2). No changes in body weight and body mass index as well as dietary and lifestyle habits were documented after the intervention period. The LC n-3 PUFA capsules were well tolerated. Compliance was high (>90%) and all subjects completed the study. No obvious sex specific differences in the oxylipin level and in the response to LC n-3 PUFA supplementation were observed (table S1), thus all analyses were carried out for the whole study collective.
The percentage of total FAs for LA, AA, ALA, EPA and DHA in erythrocyte membranes (figure 1) as well as concentration of total and free hydroxy FA, epoxy FA and dihydroxy FA in plasma (table 1) were quantified in blood samples of all ten study participants before and after twelve weeks of treatment with LC n-3 PUFA. All variables were normally distributed.
Relative fatty acid composition of erythrocyte membranes
After twelve weeks of LC n-3 PUFA treatment the relative EPA and DHA content was significantly increased (figure 1). Correspondingly, the relative LA, AA and ALA levels were slightly decreased, although not significantly. At time point t12, a significant change of EPA (200±39% of baseline) and DHA (23±5.7% of baseline) content was calculated on the individual subject level.
Total and free oxylipin levels at baseline
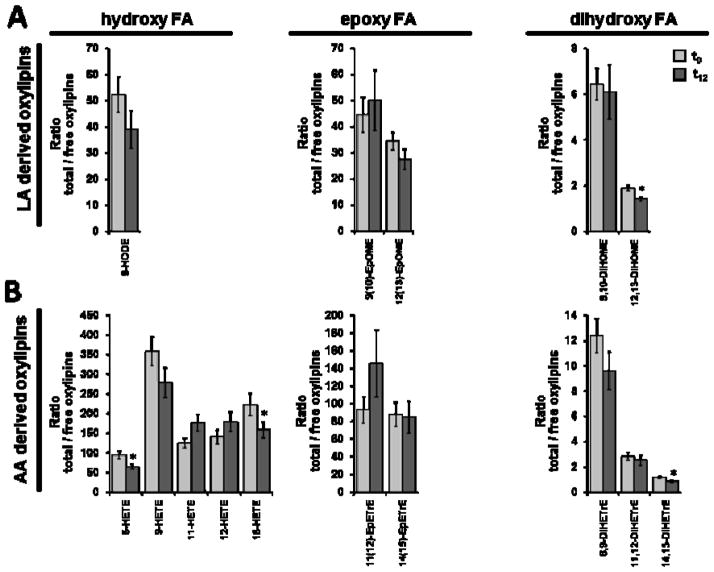
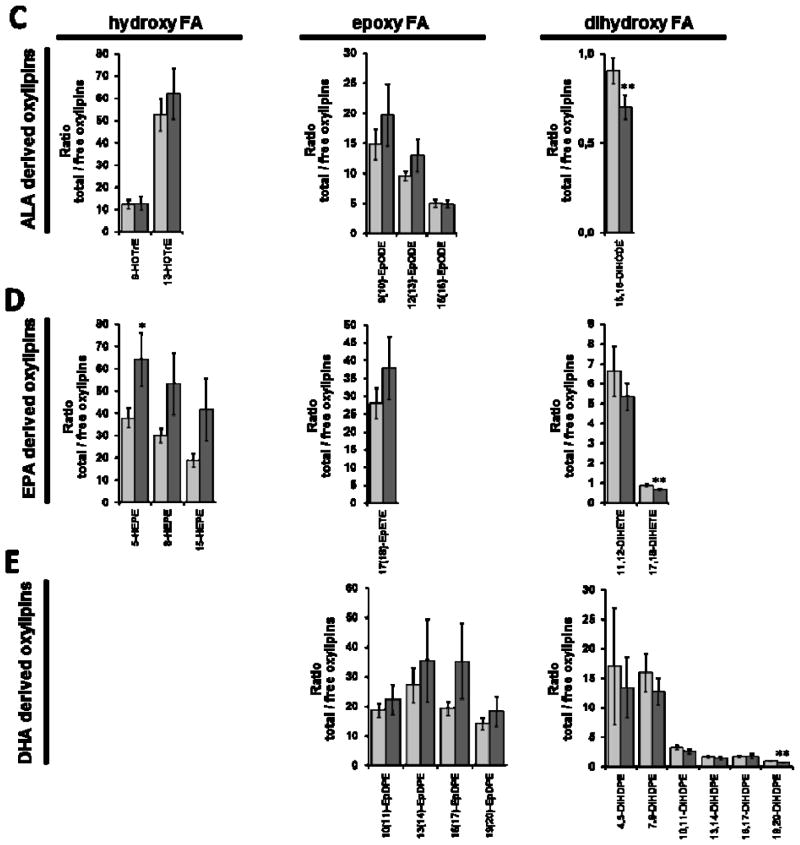
Among the 50 analyzed oxylipins, 46 could be detected in the samples after saponification representing the total oxylipins in plasma and 40 were quantified as free oxylipins. Interindividual variation for both total and free oxylipins in plasma was high ( mean SE 14±7%). Concentrations of total plasma oxylipins are, for most analytes, considerably higher compared to free plasma oxylipins (table 1) leading to a high total:free-ratio (figure 2). Particularly, the concentrations of total LA and AA derived hydroxy and epoxy FAs were 50- to 350-times higher than those of the free oxylipins. The total:free plasma oxylipin ratio for hydroxy FAs ranged between 10 to 40. In contrast, levels of several dihydroxy FAs (12,13-DiHOME, 11,12-DiHETrE, 14,15-DiHETrE, 15,16-DiHODE, 17,18-DiHETE, 10,11-DiHDPE, 13,14-DiHDPE, 16,17-DiHDPE, 19,20-DiHDPE) were in a similar range with and without conjugate cleavage (table 1) resulting in total:free-ratios of 1 to 3 (figure 2). This is consistent with earlier findings that dihydroxy FA are only or dominantly present non-esterified in plasma (Schuchardt 2013).
Figure 2.
Ratio between total (sum of esterified and free) and free oxylipins (total:free-ratio) in plasma at baseline (t0) and after twelve weeks (t12) of treatment with LC n-3 PUFA. Ratios of hydroxy, epoxy and dihydroxy fatty acids derived from A) linoleic acid (LA C18:2 n-6), B) arachidonic acid (AA C20:4 n-6), C) alpha-linolenic acid (ALA C18:3 n-3), D) eicosapentaenoic acid (EPA C20:5 n-3) and E) docosahexaenoic acid (DHA C22:6 n-3) are shown. Significant differences were determined by dependent sample t-test. * p<0.05, ** p<0.005, *** p<0.001.
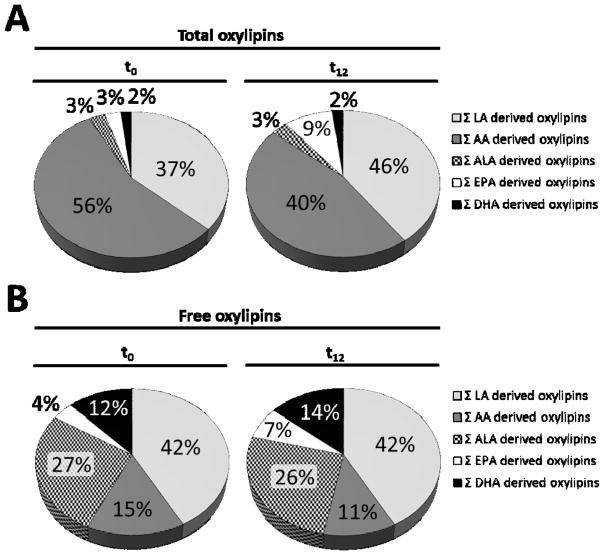
Comparing the relative distribution of total analyzed oxylipin concentrations grouped by substrate, the AA-derived oxylipins are the most prominent fraction followed by LA-derived metabolites (56% and 37% at t0, respectively). The LC n-3 PUFA metabolites together summed up to less than 10% of total of quantified analytes (figure 3A). Looking at the same set of analytes, this distribution is completely different among the free oxylipins (figure 3B). The percentage of oxylipins from the five different LC PUFAs was more evenly distributed, with the LA- and AA-derived metabolites being most abundant. The LC n-3 PUFA-derived oxylipins together summed up to 43% of total at t0.
Figure 3.
Relative distribution of all analyzed oxylipins with respect to their precursor LC PUFA. Shown is the pattern for A) total and B) free plasma oxylipins at baseline (t0) and after twelve weeks (t12) of treatment with LC n-3 PUFA. The relative distribution was calculated based on the sum of median concentrations of measured LA-, AA-, ALA-, EPA- and DHA- derived oxylipins.
In the fraction of total plasma oxylipins, hydroxy FAs were generally present in highest concentrations followed by epoxy and dihydroxy FAs (table 1). When comparing the concentrations of the lipid mediators derived from each PUFA individually, large differences in the concentrations of the most abundant oxylipins and the lowest ones were found, spanning e.g. over a range of more than 3 orders of magnitude for AA (15-HETE vs. 14,15-DiHETrE). The plasma levels of free oxylipins were in a more narrow concentration range, e.g. for AA-derived oxylipins between 0.2–1.1 nM. As a consequence the pattern of the relative concentration of the free and total plasma oxylipins of each PUFA showed pronounced differences (figure S2). For AA the relative pattern of the analyzed oxylipins was similar, except for the relative low concentration of DiHETrEs in fractions after conjugate cleavage. The most abundant hydroxy FA in total and free plasma was 15-HETE, while all three analyzed epoxides of AA were found in the same concentration range (table 1, figure S1). The relative low concentration of dihydroxy FAs in the total oxylipin fraction changed the observed pattern between free and total plasma oxylipins of ALA and EPA (figure 2). Whereas 15,16-DiHODE and 17,18-DiHETE dominate the pattern of free oxylipins, the hydroxy FAs 13-HOTrE and 12-HEPE were found most abundantly after conjugate cleavage. The plasma concentrations of free oxylipins were generally below 1 nM, except for the LA-derived metabolite levels and a few other oxylipins (15-HETE, 15,16-EpODE, 15,16-DiHODE, 10(11)-EpDPE, 19,20-DiHDPE). Because of these low concentrations, several free oxylipins (8-HETE, 8(9)-EpETrE, 12-HEPE, 8(9)-EpETE, 11(12)-EpETE, 14(15)-EpETE), which were present in the saponificated samples in detectable amounts, could not be quantified.
Changes in total and free oxylipin levels following LC n-3 PUFA supplementation
Because of the high interindividual differences in the oxylipin levels, the changes in oxylipin patterns were additionally calculated as mean percent change based on individual changes in plasma oxylipin concentrations (table 1, figure S1). The average absolute concentrations before and after intervention are also shown in table 1 and gender specific in table S1. The total plasma concentrations of EPA-derived oxylipins were highly increased with a mean increase of 70 to 150% (table 1, figure S1). Simultaneously, decreased levels of AA-derived oxylipins were observed with a mean change of about −30% (table 1, figure S1). Changes in EPA- and AA-metabolites were significant with a few exceptions (12-HEPE, 15-HEPE, 11(12)-EpETrE). The massive change in the total plasma oxylipins derived from LC n-3 PUFA supplementation becomes even more apparent regarding the relative distribution of all analyzed oxylipins (figure 3A). The EPA oxylipins increased from 3 to 9% and AA-derived oxylipins decreased from 54 to 40% of the sum of total plasma oxylipins (figure 3A). The concentration of total plasma LA- and ALA-derived oxylipins decreased in response to treatment and were significant for 9(10)-EpOME, 12(13)-EpOME, 15(16)-EPODE, 15,16-DiHODE (table 1). No effect of LC n-3 PUFA supplementation was observed on total plasma epoxy and dihydroxy FA from DHA.
Similar effects of n-3 PUFA treatment were observed on free plasma oxylipin levels, though the absolute changes in concentrations were lower and fewer reached significance (table 1, figure S1). The EPA-derived oxylipins were highly increased, up to 150%, while AA-derived oxylipins were decreased by 10–40% (table 1). A slight decrease in ALA-metabolites – although not significant – was observed, while no effect was seen for LA- and DHA-metabolites (table 1, figure S1).
Changes in the total:free plasma oxylipin ratio following LC n-3 PUFA supplementation
The similar change in total and free plasma oxylipins is also reflected by a constant total:free plasma oxylipin ratio after LC n-3 PUFA treatment (figure 2). A slight - but significant - lower total:free ratio in plasma following LC n-3 PUFA supplementation was observed for 12,13-DiHOME, 5-HETE, 15-HETE, 14,15-DiHETrE, 15,16-DiHODE, 17,18-DiHETE and 19,20-DiHDPE). For EPA-derived hydroxy FAs about 1.7 fold higher ratios were observed post n-3 PUFA treatment. Thus, the ratios of free and esterified hydroxy, epoxy and dihydroxy FAs are overall not affected by the change in the oxylipin patterns caused by LC n-3 PUFA intake.
DISCUSSION
In this study, we present quantitative data on 40 free and 46 total oxylipin levels in plasma from a cohort of ten subjects before and after supplementation with LC n-3 PUFAs for a period of twelve weeks. The increase of relative EPA and DHA concentration in erythrocyte membranes in the treatment group demonstrated that subjects responded to the LC n-3 PUFA supplementation. The pattern of hydroxy, epoxy and dihydroxy FAs in both free and total plasma oxylipins are significantly modulated by LC n-3 PUFA treatment. The strongest changes were observed in EPA-oxylipins, where particularly CYP-derived epoxides were consistently elevated. This is consistent with all previous studies investigating the effects of dietary LC n-3 PUFA supplementation on the human plasma oxylipin pattern (Shearer 2010; Keenan 2012, Lundström 2013, Schuchardt 2014a, Schuchardt 2014b, Fischer 2014). Because of this massive increase and the biological activity of EPA epoxides on the cardiovascular system (Westphal 2011, Agbor 2012, Jung 2012), it is reasonable to expect that these mediators might be at least in part responsible for the cardio-protective effects described for LC n-3 PUFA (Delgado-Lista 2012, Mozaffarian and Wu 2011, Arnold 2010b). Though the subjects received an equal daily dose of DHA, which is also confirmed by the significantly elevated relative DHA content in erythrocyte membranes, no changes in EpDPEs and its sEH hydrolyses products were observed. This is also well in line with previous studies describing unchanged or slightly elevated levels of CYP derived EpDPEs and DiHDPEs (Schuchardt 2014, Lundström 2013, Keenan 2012). Fischer and Konkel et al., however, observed a significant increase in DHA epoxides and diols, which was also less pronounced than that of EPA derived metabolites (Fischer 2014). With respect to HEPEs, we found with an increase of 100–250% of all measured total HEPEs similar results as Fischer and Konkel, while the increase was less pronounced for free HEPEs (table 1). It should be noted that hydroxy-DHAs were not monitored in this study and thus a potential increase in these metabolites could not be detected. However, the massive increase in EPA metabolites makes it reasonable to speculate that - in case hydroxy, epoxy, or dihydroxy FAs are a driving force for the effects of LC n-3 PUFA action on human health - EPA-derived oxylipins play a major role.
Our results could also substantiate another common explanation for the effects of LC n-3 PUFA, which is the displacement of AA and thereby inhibiting the formation of its lipid mediators. Though the treatment did not lead to a change in the relative AA content in erythrocytes, the mean plasma concentration of AA-oxylipins, as well as the individual plasma concentration of the subjects were decreased (figure 1, table 1). The change of AA-metabolites in the total plasma oxylipin fraction was much more pronounced indicating particularly a displacement of esterified AA-derived oxylipins after twelve weeks of LC n-3 PUFA supplementation (figure 1). This reduction in AA-derived oxylipin concentrations may be explained by a competition of the LC n-3 PUFA for the same enzymes as it has already been described for COX and LOX (Weylandt and Kang 2005) or CYP pathways (Fischer 2014). However, since the FA pattern in plasma was not determined it could also be possible that the shift in plasma oxylipins is caused by a shift in the plasma FA levels.
Taken together the increase of EPA-derived oxylipins and the decrease in AA-derived oxylipins after twelve weeks of LC n-3 PUFA treatment led to a pronounced shift in the overall pattern of oxylipins (table 1, figure S2–S3). However, the key question is in which tissue and which cellular compartment the changes have to arise to cause biological effects. Generally, it is believed that oxylipins act largely in an autocrine or paracrine fashion and thus locally in the tissue were they are formed (Shearer and Newman 2009). With exception of direct effects on the endothelia in the vascular system, the quantification of oxylipins in plasma or serum can therefore largely be regarded as a proxy reflecting the situation in the tissue of interest (which is not or difficult to obtain for analysis). Having said that, one must ask: Are none-esterified (free) oxylipins, total plasma oxylipins or even another specific fraction, such as oxylipins in lipoproteins (Shearer and Newman 2008, Newman 2007), or specific classes of lipids best suited to investigate the biology of oxylipins? The answer will be different dependent on the physiological or pathophysiological parameter investigated as well as for the class of lipid mediator regarded. However, with respect to the investigation of the impact of LC n-3 PUFA treatment on the levels of hydroxy, epoxy, and dihydroxy FAs in plasma, our study shows that both free and total plasma oxylipins support overall similar conclusions. The modulation of the oxylipin profile caused by twelve weeks of LC n-3 PUFA supplementation – i.e. an increase of EPA-derived oxylipins and a decrease in AA-derived oxylipins – is reflected by both parameters with an overall constant ratio between free and total plasma oxylipins. Thus, both parameters are comparable biomarkers to assess the effect of nutrition on this class of lipid mediators.
Nevertheless, for each study aiming to investigate biological effects of LC n-3 PUFA it should be carefully decided whether it is more appropriate to focus on free or total (after saponification) oxylipins in plasma. The total hydroxy, epoxy, and dihydroxy FAs are present in concentrations which can be well detected and changes in EPA and AA levels are more pronounced. Though this fraction appears to be a more suitable biomarker for LC n-3 PUFA treatment, it should be kept in mind that the biological role of esterified oxylipins is not clear and that they may reflect a pool of lipid mediators which have to be released to elicit biological effects (Newman and Shearer 2009). Unbound, i.e. “free” oxylipins, reflect the biological active form, though the molecular target of most hydroxy, epoxy, and dihydroxy FAs is not clear up to now. The free plasma concentration, particularly of LC n-3 PUFA derived oxylipins, is so low that analysis is challenging, making it difficult to assess the effect of LC n-3 PUFA treatment on several hydroxy, epoxy, and dihydroxy FAs (table 1). Ultimately, only a correlation of the oxylipin concentration in the tissue of interest with the targeted biological/physiological effect will answer the question whether the free or the bound/total fraction of oxylipins in plasma is the most suitable analytical parameter assessing modulation of lipid mediators by LC n-3 PUFA.
Limitations of the study
The effects were only analyzed in 10 human subjects making it possible that the high variation of the oxylipin levels might mask biologically significant differences in the oxylipin pattern or its ratios. The regarded hydroxy, epoxy- and dihydroxy-FAs do not reflect all possibly formed oxylipins, because of limited coverage of the analytical method used. Particularly, the levels of hydroxyDHA-metabolites, resolvins, protectins and maresins as found in human plasma (Colas 2014) were not regarded in the present study. Because of the saponification used to assess total oxylipins several lipid mediators, e.g. prostanoids, are degraded making it impossible to compare the levels of these oxylipins (Glandine 2014). Analytical methods utilizing improved techniques to liberate esterified oxylipins, e.g. by enzymatic treatment together with a comprehensive detection would allow a more complete picture on oxylipin levels and their biology in clinical and experimental studies.
Conclusion
Twelve weeks of LC n-3 PUFA supplementation induced changes in both free and total oxylipin patterns. Especially hydroxy, epoxy, and dihydroxy EPA were increased, while the corresponding AA derivatives were decreased. The modulation of total plasma oxylipins was more pronounced compared to free oxylipins. Thus, the majority of hydroxy, epoxy and dihydroxy EPA formed during twelve weeks of supplementation are found in the pool of esterified oxylipins in blood lipids. Despite these changes, the free:total ratio remained overall unchanged indicating an equilibrium between the free and esterified oxylipins of LC PUFA. Accordingly, both direct analysis and analysis after saponification are suitable analytical techniques assessing the individual status of hydroxy, epoxy and dihydroxy LC PUFA leading overall to similar results.
Supplementary Material
Highlights.
12-week LC-n3-PUFA supplementation led to changes in the overall pattern of both free and total plasma oxylipins.
EPA-derived hydroxy, epoxy und dihydroxy fatty acids were significantly elevated, while AA-derived metabolites were lowered.
Changes in concentrations of DHA- and other PUFA-derived oxylipins were minor.
The ratio between total and free plasma oxylipins remained unchanged
Both free and total plasma oxylipins concentrations support similar conclusions regarding the biological effects of LC-n3-PUFA.
Acknowledgments
This study was supported by a Marie Curie Career Integration Grant of the European Union and a Grant of the German Research Foundation (DFG)to NHS, and grants from US National Institutes of Health (NIH), Environmental Health (NIEHS, P42 ES004699 and R01 ES002710) and Diabetes and Digestive and Kidney Diseases (NIDDK, U24 DK097154) and the West Coast Central Comprehensive Metabolomics Resource Core (WC3MRC) to BDH. BDH is a George and Judy Marcus Senior Fellow of the American Asthma Association. The provision of the fish oil supplement by Dr. Loges and Co. GmbH (Winsen, Germany) is kindly acknowledged. The authors are solely responsible for the design and conduct of the study, collection, management, analysis, and interpretation of the data, as well as preparation of the manuscript. We would like to thank the participants of the study who contributed their time to this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agbor LN, Walsh MT, Boberg JR, Walker MK. Elevated blood pressure in cytochrome P4501A1 knockout mice is associated with reduced vasodilation to omega-3 polyunsaturated fatty acids. Toxicol Appl Pharmacol. 2012;264(3):351–60. doi: 10.1016/j.taap.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010a;62(3):536–47. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- Arnold C, Markovic M, Blossey K, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010b;285(43):32720–33. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50(6):1015–38. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 2002:68–69. 325–44. doi: 10.1016/s0090-6980(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014 doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr. 2012;107 (Suppl 2):S201–13. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- Fischer R, Konkel A, Mehling H, et al. Dietary Omega-3 Fatty Acids Modulate the Eicosanoid Profile in Man Primarily via the CYP-epoxygenase Pathway. J Lipid Res. 2014 doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladine C, Newman JW, Durand T, et al. Lipid profiling following intake of the omega 3 fatty acid DHA identifies the peroxidized metabolites F4-neuroprostanes as the best predictors of atherosclerosis prevention. PloS one. 2014;9(2):e89393. doi: 10.1371/journal.pone.0089393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS, Von Schacky C. The Omega-3 Index. a new risk factor for death from coronary heart disease? Preventive medicine. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Jung F, Schulz C, Blaschke F, Muller DN, Mrowietz C, Franke RP, et al. Effect of cytochrome P450-dependent epoxyeicosanoids on Ristocetin-induced thrombocyte aggregation. Clin Hemorheol Microcirc. 2012;52(2–4):403–16. doi: 10.3233/CH-2012-1614. [DOI] [PubMed] [Google Scholar]
- Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res. 2012;53(8):1662–9. doi: 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Su K. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056–61. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Lundström SL, Yang J, Brannan JD, Haeggström JZ, Hammock BD, Nair P, et al. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol Nutr Food Res. 2013;57(8):1378–89. doi: 10.1002/mnfr.201200827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107 (Suppl 2):S171–84. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–67. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Newman JW, Kaysen GA, Hammock BD, Shearer GC. Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J Lipid Res. 2007 Aug;48(8):1792–800. doi: 10.1194/jlr.M700146-JLR200. Epub 2007 May 11. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid peroxidation products as oxidative stress biomarkers. BioFactors. 2008;34(2):171–80. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- Park YS, Harris WS. Eicosapentaenoic acid, but docosahexaenoic acid, decreases mean platelet volume in normal subjects. Arterioscl Throm Vas. 2002;22:A61–A61. [Google Scholar]
- Powell WS, Gravel S, Gravelle F. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J Lipid Res. 1995;36(12):2590–2598. [PubMed] [Google Scholar]
- Rådmark O, Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. J Lipid Res. 2009;50 (Suppl):S40–5. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82(1):131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50 (Suppl):S29–34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samieri C, Féart C, Letenneur L, Dartigues JF, Pérès K, Auriacombe S, et al. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008;88(3):714–21. doi: 10.1093/ajcn/88.3.714. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlén SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237(4819):1171–76. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, et al. Resolvins. a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. The Journal of experimental medicine. 2002;196(8):1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8(2):115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Schuchardt JP, Schmidt S, Kressel G, Dong H, Willenberg I, Hammock BD, Hahn A, Schebb NH. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot Essent Fatty Acids. 2013;89:19–29. doi: 10.1016/j.plefa.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt JP, Schmidt S, Kressel G, Willenberg I, Hammock BD, Hahn A, Schebb NH. Modulation of blood oxylipin levels by long-chain omega-3 fatty acid supplementation in hyper- and normolipidemic men. Leukot Essent Fatty Acids. 2014a;90:27–37. doi: 10.1016/j.plefa.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt JP, Schneider I, Willenberg I, et al. Increase of EPA-derived hydroxy, epoxy and dihydroxy fatty acid levels in human plasma after a single dose of long-chain omega-3 PUFA. Prostaglandins & other lipid mediators. 2014b doi: 10.1016/j.prostaglandins.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids. 2008;79(6):215–22. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer GC, Newman JW. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr Atheroscler Rep. 2009;11(6):403–10. doi: 10.1007/s11883-009-0061-3. [DOI] [PubMed] [Google Scholar]
- Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51(8):2074–81. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickett CM, Wiswedel I, Siems W, Zarkovic K, Zarkovic N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic Res. 2010;44(10):1172–202. doi: 10.3109/10715762.2010.498476. [DOI] [PubMed] [Google Scholar]
- Westphal C, Konkel A, Schunck W. CYP-eicosanoids--a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011;96(1–4):99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Weylandt KH, Kang JX. Rethinking lipid mediators. Lancet. 2005;366(9486):618–20. doi: 10.1016/S0140-6736(05)67119-X. [DOI] [PubMed] [Google Scholar]
- Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2011:73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Yin H, Xu L, Porter NA. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem Rev. 2011;111(10):5944–72. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.