Abstract
Background
Altered GABA signaling in the prefrontal cortex (PFC) has been associated with cognitive dysfunction in schizophrenia and schizoaffective disorder. PFC levels of the GABA-synthesizing enzyme glutamic acid decarboxylase 67kD (GAD67) has been consistently reported to be lower in these disorders, but the status of the second GABA-synthesizing enzyme, GAD65, remains unclear.
Methods
GAD65 mRNA levels were quantified in PFC area 9 by quantitative polymerase chain reaction from 62 subjects with schizophrenia or schizoaffective disorder and 62 matched healthy comparison subjects. GAD65 relative protein levels were quantified in a subset of subject pairs by confocal immunofluorescence microscopy.
Results
Mean GAD65 mRNA levels were 13.6% lower in schizoaffective disorder subjects, but did not differ in schizophrenia subjects, relative to their matched healthy comparison subjects. In the subjects with schizoaffective disorder, mean GAD65 protein levels were 19.4% lower and were correlated with GAD65 mRNA levels. Lower GAD65 mRNA and protein measures within schizoaffective disorder subjects was not attributable to factors commonly comorbid with the diagnosis.
Conclusions
In concert with previous studies, these findings suggest that schizoaffective disorder is associated with lower levels of both GAD65 and GAD67 mRNA and protein in the PFC, whereas subjects with schizophrenia have lower mean levels of only GAD67 mRNA and protein. Because cognitive function is generally better preserved in subjects with schizoaffective disorder relative to subjects with schizophrenia, these findings may support an interpretation that GAD65 down-regulation provides a homeostatic response complementary to GAD67 down-regulation expression that serves to reduce inhibition in the face of lower PFC network activity.
Keywords: GAD65, GAD67, GABA, confocal immunofluorescence, inhibition, postmortem
Introduction
Altered GABAergic signaling in the prefrontal cortex (PFC) is thought to contribute to the cognitive impairments in schizophrenia and schizoaffective disorder (1). GABAergic neurotransmission is regulated in part by activity of the two GABA-synthesizing enzymes: glutamic acid decarboxylase 65kD (GAD65) and 67kD (GAD67). The existence of two GABA-synthesizing enzymes does not appear to be simple redundancy as these isoforms are the products of different genes (2); have distinct developmental trajectories (3–7); differentially regulate GABA synthesis and release (8–16); do not exhibit reciprocal compensatory changes in expression (9,15–18); and have distinct regional (19–21), cellular (22–25), and subcellular (26,27) patterns of distribution. These different properties of GAD65 and GAD67, and the importance of GABA synthesis in regulating inhibitory neurotransmission, highlight the importance of determining the status of each isoform in individuals with schizophrenia or schizoaffective disorder.
Lower levels of GAD67 mRNA and protein in the PFC of subjects with schizophrenia or schizoaffective disorder have been consistently replicated by different research groups (28–47). We recently showed that lower GAD67 mRNA levels are not explained by factors commonly associated with the illness (e.g., psychotropic medications or substance abuse), measures predictive of disease severity, or measures of functional outcome (28). Together, these results suggest that lower GAD67 mRNA is a conserved molecular feature of schizophrenia and schizoaffective disorder that is robust to the influence of multiple other factors. On the other hand, studies of GAD65 mRNA levels in the PFC have produced mixed results, with increases (48), decreases (39), or no differences (35,41,44) reported in cohorts composed of schizophrenia and schizoaffective disorder subjects. Although most basal cortical GABA synthesis occurs via GAD67 (15,16), GAD65 is important for GABA synthesis during sustained periods of neuronal activity (5,8,12,18,49,50) and may contribute to GABA vesicular filling (9,51–53). Thus, determining whether GAD65 expression levels are also altered in schizophrenia or schizoaffective disorder will inform which aspects of, and the degree to which, the PFC GABA system is affected in these disorders. To this end, we 1) quantified GAD65 mRNA levels in PFC area 9 from 62 subjects with schizophrenia or schizoaffective disorder and 62 matched healthy comparison subjects, 2) quantified GAD65 protein levels in a subset of these subject pairs, and 3) examined the relationship of GAD65 mRNA and protein levels to factors commonly associated with these disorders.
Methods and Materials
Human Subjects
Brain specimens (N = 124) were obtained during autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh, PA) after consent for donation was obtained from the next-of-kin. As previously described (31), an independent committee of experienced research clinicians made consensus DSM-IV (54) diagnoses for each subject, based on the results of structured interviews conducted with family members and review of medical records. The absence of psychiatric diagnoses was confirmed in comparison subjects using an identical approach. All procedures were approved by the University of Pittsburgh’s Committee for the Oversight of Research and Clinical Trials Involving the Dead and Institutional Review Board for Biomedical Research.
In order to control for experimental variance and to reduce biological variance between groups, each subject with schizophrenia (n = 39) or schizoaffective disorder (n = 23) was matched with one healthy comparison subject for sex and as closely possible for age, and tissue samples from both members of a pair were always processed together in all experiments. Subject groups did not differ in mean age, postmortem interval (PMI), RNA integrity number (RIN; Agilent Bioanalyzer, Santa Clara, CA) or tissue storage time at −80°C (Table 1; all t122 < 0.95, p > 0.34). Although brain pH significantly differed between diagnostic groups (t122 = 2.5, p = 0.01), the mean difference was very small (0.1 pH unit) and of uncertain biological significance. Each subject had a RIN ≥ 7.0, indicating excellent RNA quality. Demographic details on individual subjects are presented in Supplemental Table S1.
Table 1.
Characteristics of the subject groups for each measure of GAD65
| Characteristic | Quantitative PCR | Immunofluorescence | |||
|---|---|---|---|---|---|
| Comparison | Schizophrenia | Schizoaffective | Comparison | Schizoaffective | |
| Number | 62 | 39 | 23 | 21 | 21 |
| Sex | 47 M, 15 F | 33 M, 6 F | 14 M, 9 F | 12 M, 9 F | 12 M, 9 F |
| Race | 52 W, 10 B | 29 W, 10 B | 17 W, 6 B | 16 W, 5 B | 15 W, 6 B |
| Age (years) | 48.7 ± 13.8 | 48.8 ± 12.9 | 45.7 ± 12.5 | 48.8 ± 14.4 | 45.9 ± 12.9 |
| PMI (hours) | 18.8 ± 5.5 | 18.7 ± 8.1 | 20.2 ± 9.4 | 18.8 ± 6.0 | 18.3 ± 7.3 |
| Brain pH | 6.7 ± 0.2 | 6.6 ± 0.3 | 6.6 ± 0.3 | 6.7 ± 0.2 | 6.6 ± 0.3 |
| RIN | 8.2 ± 0.6 | 8.0 ± 0.6 | 8.1 ± 0.7 | 8.2 ± 0.7 | 8.2 ± 0.7 |
| Storage time (months) | 124.9 ± 55.3 | 121.7± 60.0 | 120.1± 60.6 | 114.7 ± 54.3 | 122.8 ± 60.3 |
PMI, postmortem interval; RIN, RNA integrity number. Values are mean ± SD.
All 62 pairs were used for quantification of GAD65 mRNA. For measures of GAD65 protein, only subjects with schizoaffective disorder and their matched comparison subjects were used (see Results for rationale). Two of the schizoaffective subject pairs included a subject with a PMI > 30 hours, and those subject pairs were excluded due to prior immunoblot evidence of a PMI effect on GAD65 expression (55). These subject groups (N = 21 pairs) did not differ in mean age, PMI, RIN, brain pH, or tissue storage time at −30°C (Table 1; all t40 < 1.9, p > 0.062). Because of a lack of tissue availability, different comparison subjects were utilized for subject pair 3 (Supplemental Table S1) in the mRNA and protein studies. Thus, this pair was excluded from the mRNA and protein correlation analyses.
GAD65 mRNA Levels
GAD65 mRNA levels were assessed by quantitative polymerase chain reaction (qPCR) as previously described (28). Frozen tissue blocks containing the middle portion of the right superior frontal sulcus were cut on a cryostat. The location of PFC area 9 was confirmed using Nissl-stained sections for each subject (34). Using a method that ensured minimal white matter contamination and excellent RNA preservation (31), gray matter from locations on tissue sections cut perpendicular to the pial surface was separately collected into tubes containing TRIzol reagent (Invitrogen, Grand Island, NY). Total RNA for each subject was extracted, purified with RNeasy Mini Kit (Qiagen, Valencia, CA), and converted into complementary DNA (cDNA) using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA). The cycle threshold was determined for each transcript of interest using power SYBR Green master mix (Life Technologies) and ViiA™7 Real-Time PCR system (Life Technologies) using ViiA™7 software according to the manufacturer’s instructions. cDNA samples from both subjects in a pair were processed together on the same 384-well plate. GAD65 transcript was amplified in quadruplicate with three internal reference transcripts, β-actin (ACTB), cyclophilin A (CYC), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), that were previously shown to have stable expression across subjects with schizophrenia or schizoaffective disorder and healthy comparison subjects (39). All primer sets showed ≥ 97% amplification efficiency in individual standard curve analyses and amplified a specific single product in dissociation curve analyses (Supplemental Table S2). The difference in cycle threshold for the target transcript was calculated by subtracting the geometric mean cycle threshold of the three internal reference transcripts from the mean cycle threshold of GAD65. Because the difference in cycle threshold (dCT) represents the log2-transformed expression ratio of target transcript to the reference genes, the relative expression level of the target transcript is determined as 2−dCT (39).
Total relative GAD65 protein levels
Confocal immunofluorescence microscopy was performed to quantify GAD65 relative protein levels in total gray matter from area 9. The left hemisphere of each brain was blocked coronally at ~1.2 cm intervals, fixed in cold 4% paraformaldehyde for 48 hours, immersed in a series of cold, graded sucrose solutions and stored in antifreeze solution at −30°C. Tissue blocks containing the superior frontal gyrus were sectioned coronally at 40 µm on a cryostat and stored in antifreeze solution at −30°C until processing for immunohistochemistry. Two sections spaced ~1.0 mm apart were chosen per subject, and PFC area 9 was trimmed away from the larger section. One section from each subject of a pair were processed together for all subjects in the same experiment in order to minimize experimental variance within or across subject pairs. Sections were incubated for 48 hours in guinea pig anti-GAD65 (1:500, Synaptic Systems, Goettingen, Germany), then incubated for 24 hours in goat anti-guinea pig conjugated to Alexa 568 (1:500, Invitrogen, Grand Island, NY, USA). Specificity of the GAD65 primary antibody has been shown by immunoblot (data not shown) and labeling profile (25) in our laboratory. After washing, sections were mounted (Vecatshield Hard Set Mounting Media), coded to obscure diagnosis and subject number, and stored at 4°C until imaging.
Images (768 × 1024 pixels) were collected on an Olympus BX51 microscope (Center Valley, PA) using a 10× 0.40 N.A. objective. The confocal microscope was equipped with a Hamamatsu 1394 ORCA-ER camera (Bridgewater, NJ), a high precision motorized stage with linear XYZ encoders (Ludl Electronic Products Ltd., Hawthorne, NY, USA), and controlled by SlideBook 5.0 (Intelligent Imaging Innovations, Inc., Denver, CO, USA), the same software used for image processing.
On each section, one 500 µm-wide traverse extending from the pial surface to the layer 6 – white matter border was imaged in a location where area 9 was cut perpendicular to the cortical surface. Coordinate points were set using the motorized stage such that the entire traverse was imaged. The average traverse area analyzed for comparison (1,499.7 mm2 ± 242.7) and schizoaffective disorder (1,534.0 mm2 ± 238.0) subjects did not differ (t40 = −0.462, p = 0.6). Channel exposure time was optimized for each traverse such that no pixels were saturated and the dynamic range of the camera was filled, and the exposure time remained constant for all images within a traverse.
Images were normalized for exposure time, and the Ridler-Calvard iterative thresholding method (56) was used to mask GAD65-immunoreactive objects. Mean GAD65 fluorescence intensity was calculated by dividing the total GAD65 fluorescence intensity in all masked objects by the total sampled area. A potential confound of quantitative fluorescence measures in human cortex is lipofuscin autofluorescence, which is present in all channels (57). To assess the impact of this potential confound on signal quantified in the 568 channel, lipofuscin was simultaneously imaged in the 405 channel at a constant exposure time across all sections, and masked using the Ridler-Calvard iterative thresholding method. Mean lipofuscin intensity for healthy comparison (37.0 ± 15.5 arbitrary units, a.u.) and schizoaffective disorder (35.5 ± 12.1 a.u.) subjects did not differ (t40 = 0.37, p = 0.7); and mean lipofuscin area for healthy comparison (41.7 mm2 ± 25.6) and schizoaffective disorder (40.4 mm2 ± 16.2) subjects did not differ (t40 = 0.186, p = 0.9).Thus, masked lipofuscin was not subtracted from the total GAD65 568 channel fluorescence intensity.
Statistical Analysis
Two analysis of covariance (ANCOVA) models were performed to test the effect of diagnosis on GAD65 mRNA and protein levels. The first model (paired ANCOVA) included mRNA expression level or fluorescence intensity as the dependent variable, diagnostic group as main effect, subject pair as blocking factor, and storage time, brain pH, PMI, and RIN (mRNA only) as covariates. Subject pairing may be considered an attempt to balance diagnostic groups for sex and age and to account for the parallel processing of tissue samples from a pair, and thus not a true statistical paired design. Therefore, we also used a second model (unpaired ANCOVA) without subject pair as a blocking factor that included sex, age, storage time, brain pH, PMI, and RIN (mRNA only) as covariates.
The influences of potential confounding factors on GAD65 mRNA and protein levels in subjects with schizophrenia or schizoaffective disorder were assessed with ANCOVA models, using each variable (sex; death by suicide; tobacco use at time of death; diagnosis of substance abuse or dependency at the time of death; the use of antidepressants, benzodiazepines or sodium valproate, or antipsychotics at the time of death; diagnosis of schizoaffective disorder) as the main effect, and age, tissue storage time, brain pH, PMI, and RIN (mRNA only) as covariates.
Reported ANCOVA statistics include only covariates that were statistically significant, and thus the reported degrees of freedom differ across analyses. The effects of the significant covariates are provided.
Results
GAD65 mRNA expression in subjects with schizophrenia and schizoaffective disorder
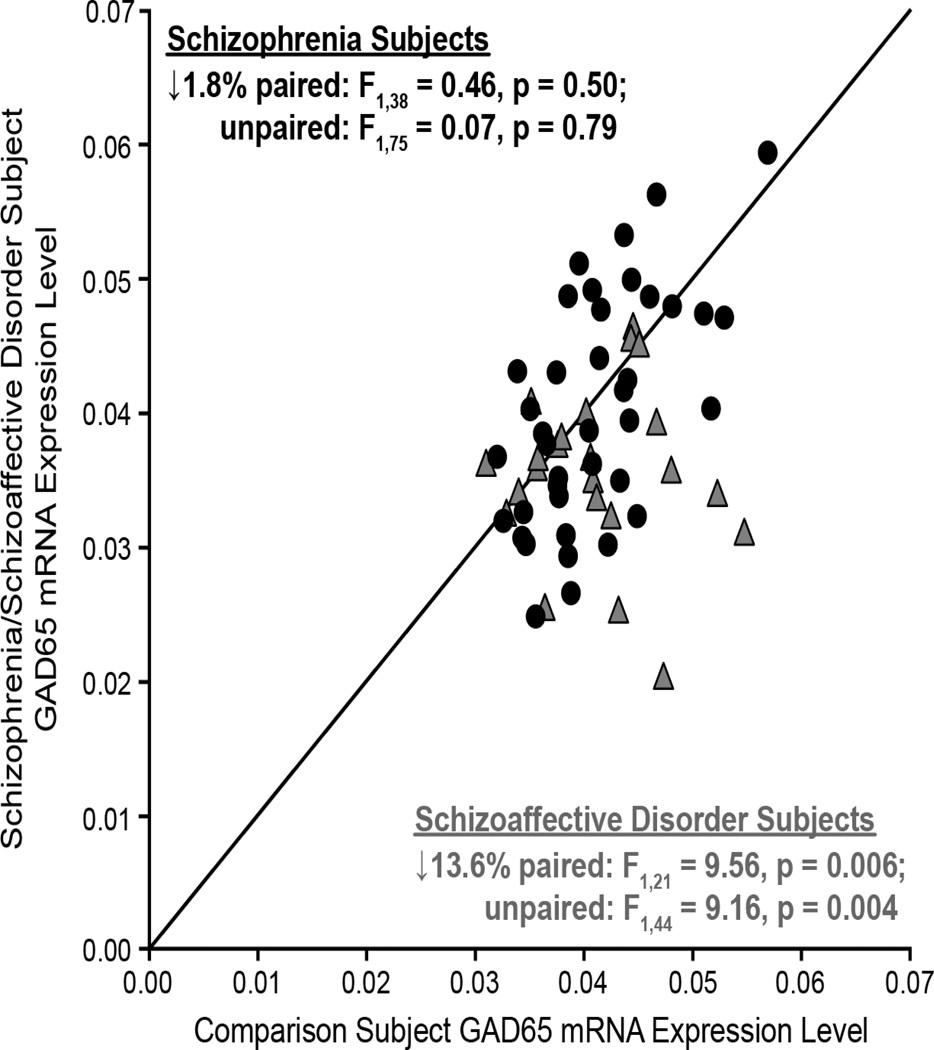
Mean GAD65 mRNA levels were 6.2% lower in the 62 subjects with schizophrenia or schizoaffective disorder relative to matched comparison subjects (Figure 1), and this difference was marginally significant (paired: F1,61 = 6.18, p = 0.016; unpaired: F1,121 = 3.88, p = 0.051). None of the covariates were significant in either of the models except for storage time in the unpaired model (F1,121 = 8.4, p = 0.004).
Figure 1.
GAD65 mRNA levels in PFC area 9 gray matter for each matched pair of healthy comparison subject and subject with schizophrenia (filled circles) or schizoaffective disorder (gray triangles). Markers below the diagonal unity line indicate lower GAD65 mRNA expression in the schizophrenia or schizoaffective subject relative to the matched comparison subject.
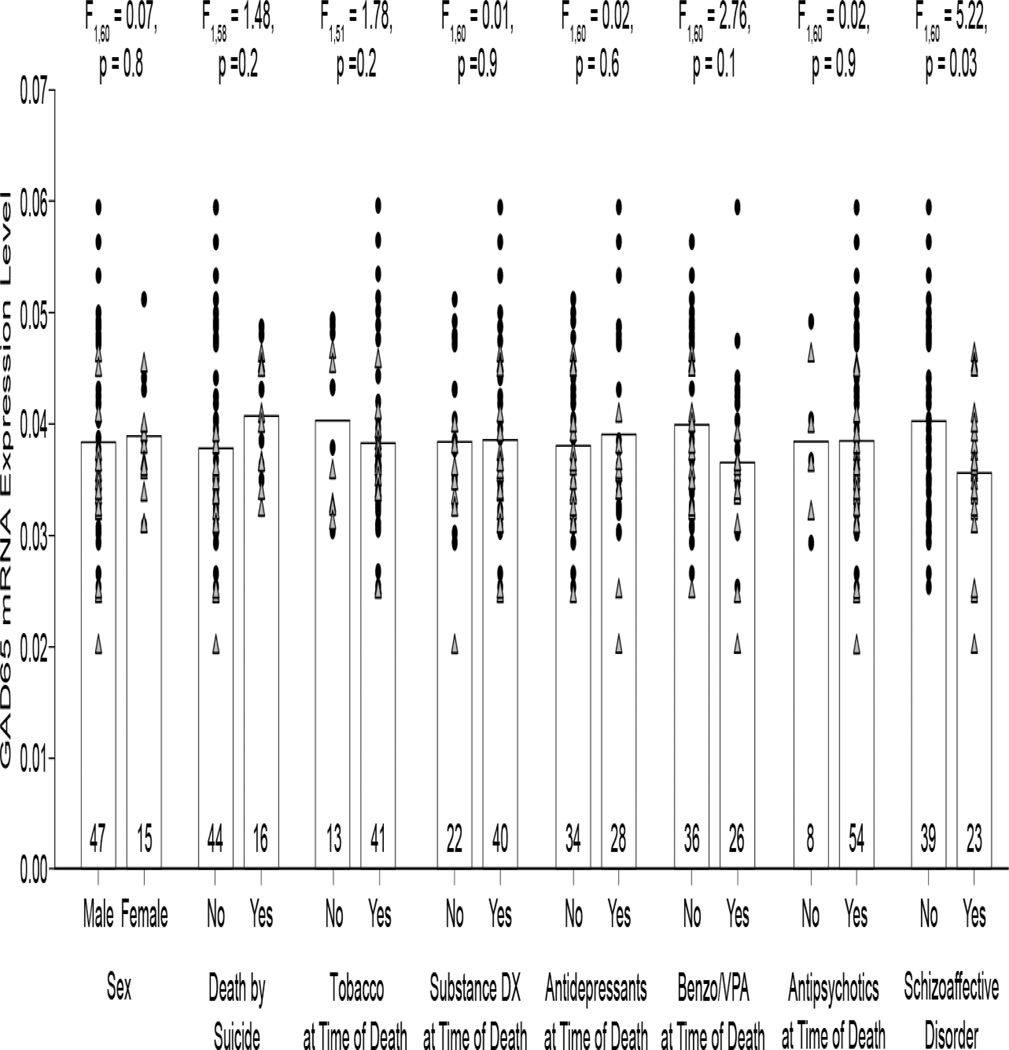
The within-pair percent differences in GAD65 mRNA levels differed substantially (range = +29.4% to −57.1%), and thus we examined factors that might contribute to this variability. An analysis of the influence of such factors showed no effect of sex; death by suicide; tobacco use at time of death; diagnosis of substance abuse or dependency at the time of death; the use of antidepressants, benzodiazepines or sodium valproate, or antipsychotics at the time of death on GAD65 mRNA levels (Figure 2). Diagnosis of schizoaffective disorder, however, did have a significant effect on GAD65 mRNA levels (F1,60 = 5.22, p = 0.03). Mean GAD65 mRNA levels in schizoaffective disorder subjects were 11.6% lower than in schizophrenia subjects.
Figure 2.
The effect of potential confounding factors on GAD65 mRNA expression levels in subjects with schizophrenia (filled circles) or schizoaffective disorder (gray triangles). GAD65 mRNA expression levels were significantly 11.6% lower in subjects with a diagnosis of schizoaffective disorder relative to subjects with schizophrenia. Bars indicate mean values for a category, and markers represent mean values for individual subjects. Numbers at the bottom of bars indicate number of schizophrenia or schizoaffective disorder subjects per group. Two subjects had an undetermined manner of death, and were not included in the death by suicide analysis. Eight subjects had unknown tobacco status at the time of death, and were not included in the tobacco analysis.
To further investigate the effect of schizoaffective diagnosis on GAD65 mRNA levels, we analyzed the 23 pairs of schizoaffective disorder and healthy comparison subjects separately. Mean GAD65 mRNA levels were significantly 13.6% lower (paired: F1,21 = 9.56, p = 0.006; unpaired: F1,44 = 9.161, p = 0.004) in schizoaffective disorder subjects (0.036 ± 0.006) relative to their matched comparison subjects (0.041 ± 0.006) (Figure 1). None of the covariates were significant in either of the models except for RIN in the paired model (F1,21 = 4.89, p = 0.038). We next determined whether lower GAD65 mRNA in schizoaffective subjects could be a consequence of factors which may be more commonly associated with that diagnosis than with schizophrenia (i.e., death by suicide (58–62); use of antidepressants, benzodiazepines or sodium valproate at time of death (63,64); diagnosis of substance abuse or dependence at time of death (65,66)). None of these factors were disproportionally represented in schizoaffective disorder subjects relative to schizophrenia subjects (all χ2 < 3.6, p > 0.06). Thus, lower GAD65 mRNA levels in PFC area 9 appear to be specifically associated with a diagnosis of schizoaffective disorder.
GAD65 protein expression in subjects with schizoaffective disorder
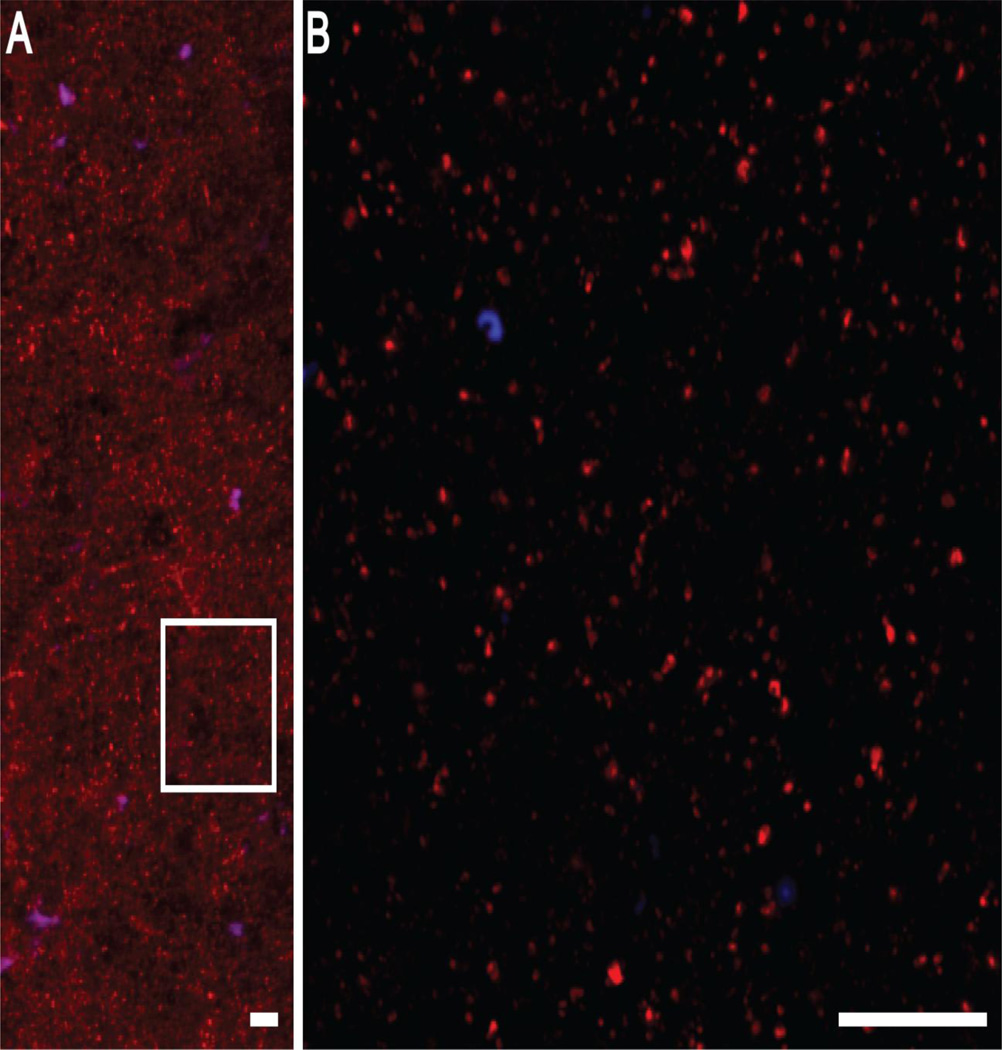
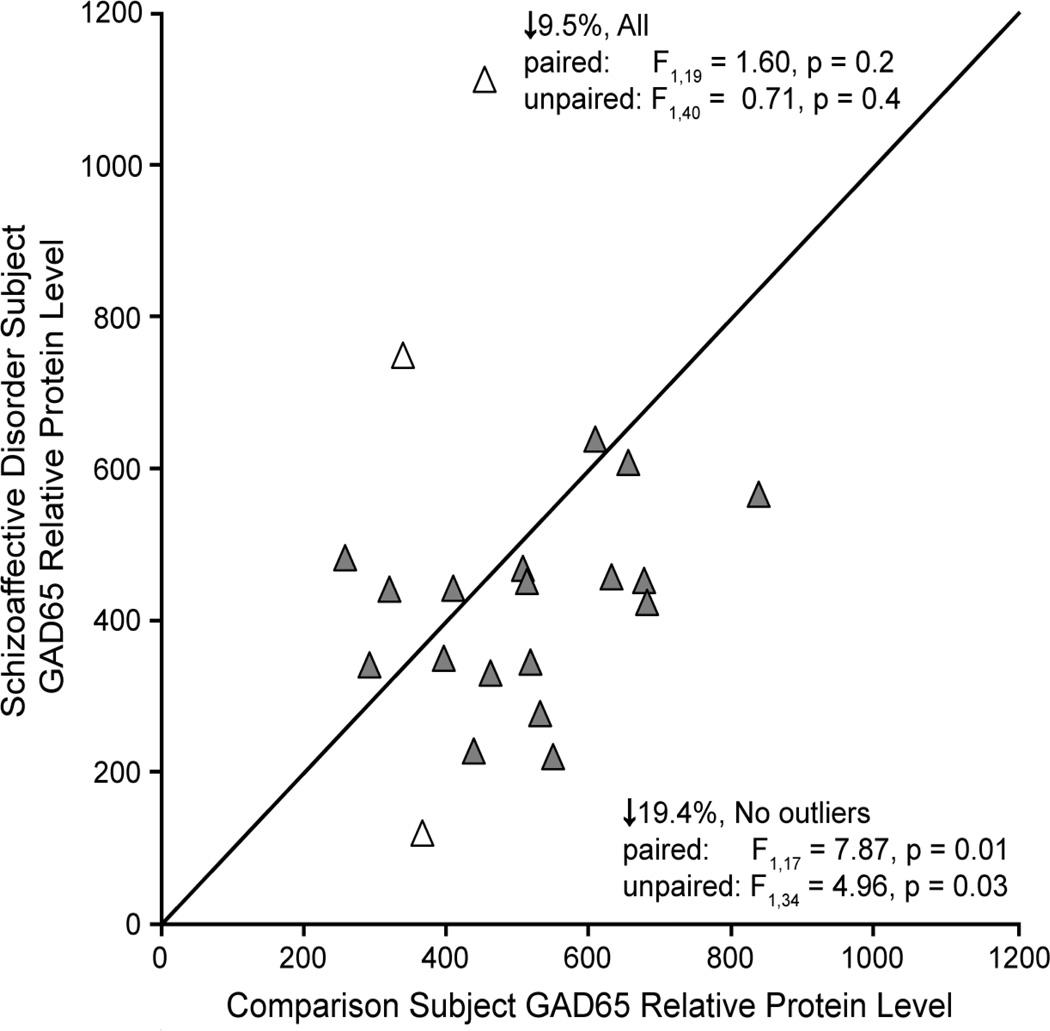
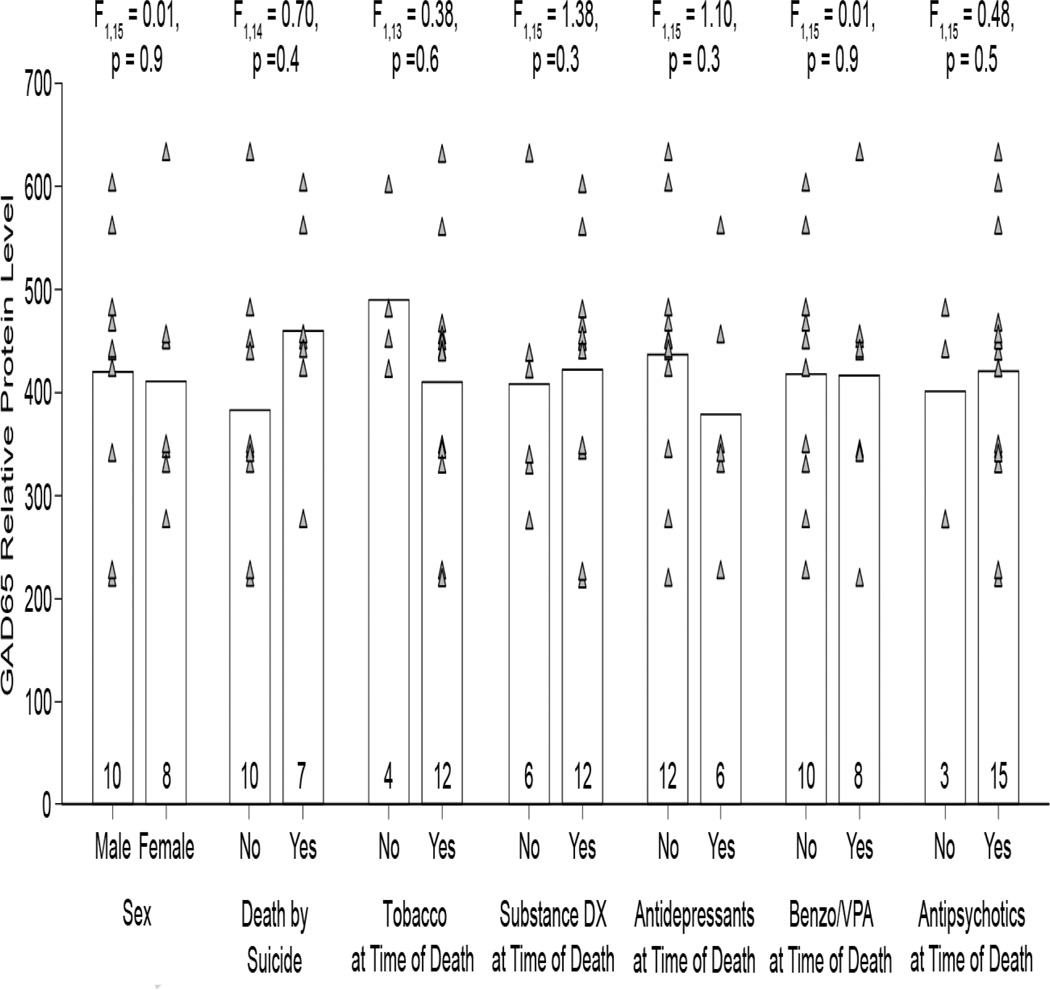
Next we determined whether lower mean GAD65 transcript levels in schizoaffective disorder subjects resulted in less GAD65 protein translation. As expected, GAD65 labeling appeared primarily as punctate structures consistent with the localization of GAD65 primarily in axon terminals (Figure 3). Mean GAD65 relative protein levels were non-significantly 9.5% lower in schizoaffective disorder subjects relative to comparison subjects (Figure 4; paired: F1,19 = 1.60, p = 0.2; unpaired: F1,40 = 0.71, p = 0.4). However, values for three subject pairs (Figure 4, open triangles) were outliers by Tukey’s Outlier test (67). Exclusion of these subject pairs revealed that mean GAD65 relative protein levels were significantly 19.4% lower (paired: F1,17 = 7.87, p = 0.01; unpaired: F1,34 = 4.96, p = 0.03) in the schizoaffective disorder subjects (416.1 ± 116.7 a.u.) relative to their matched comparison subjects (516.5 ± 151.5 a.u.). An analysis of the influences of potential confounding factors showed no effect of sex; death by suicide; tobacco use at time of death; diagnosis of substance abuse or dependency at the time of death; or the use of antidepressants, benzodiazepines or sodium valproate, or antipsychotics at the time of death on mean GAD65 relative protein levels (Figure 5; all F1,15 < 1.38, all p > 0.26).
Figure 3.
Representative images of the GAD65 immunolabeling (red) used to quantify relative protein levels. A) An image collected at 10× magnification showing the predominantly punctate pattern of GAD65 labeling. B) A 60× magnification image of the boxed region in A. GAD65 labeling is confined to small, discrete punctate structures, presumed axonal boutons, in agreement with previous reports. Lipofuscin autofluorescence (blue) was predominately identified in larger structures, presumed cell bodies. Scale bars equal 10 µm.
Figure 4.
Relative GAD65 protein levels as measured by fluorescence intensity of GAD65 immunoreactivity in PFC area 9 for each matched pair of healthy comparison and schizoaffective disorder subjects. Markers below the diagonal unity line indicate lower GAD65 protein levels in the schizoaffective disorder subject relative to the comparison subject. Open triangles indicate outlier subject pairs.
Figure 5.
No effect of potential confounding factors on GAD65 protein levels in subjects with schizoaffective disorder. Bars indicate mean values for a category, and gray triangles represent mean values for individual subjects. Numbers at the bottom of bars indicate number of schizoaffective disorder subjects per group. One subject had an undetermined manner of death, and was not included in the death by suicide analysis. Two subjects had unknown tobacco status at the time of death, and were not included in the tobacco analysis.
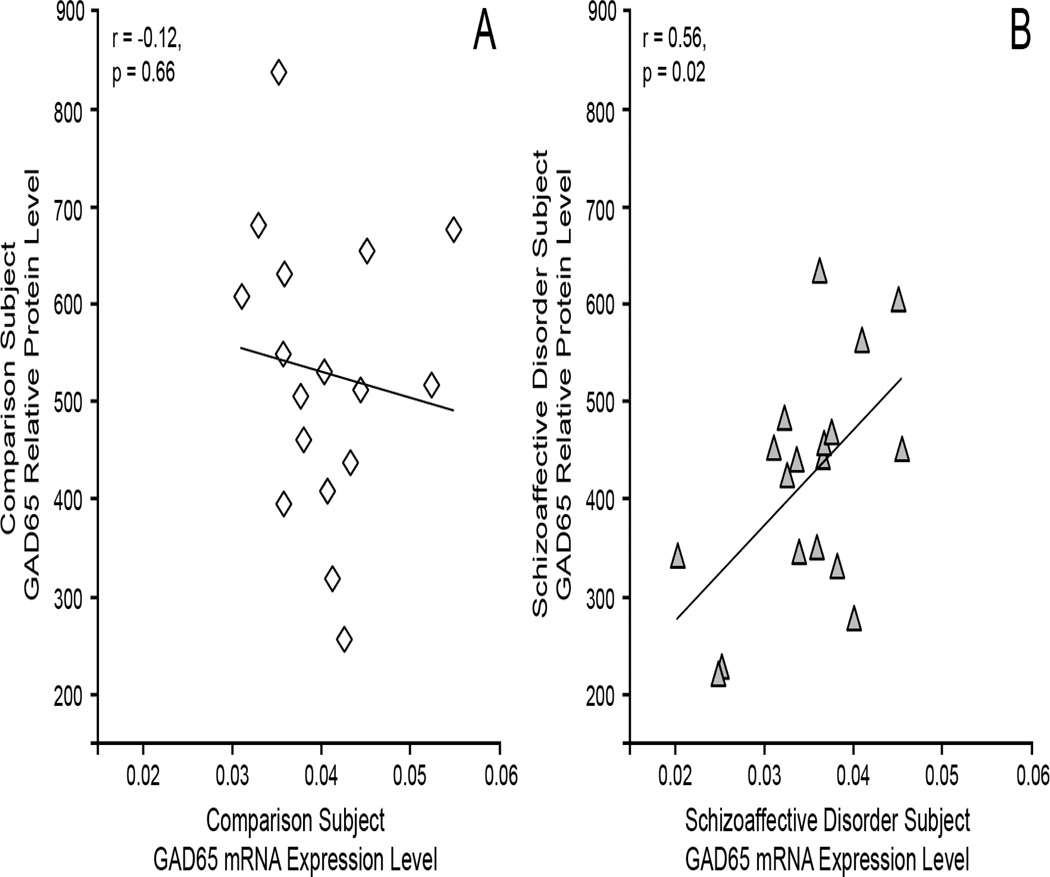
Correlation of GAD65 mRNA and protein levels in subjects with schizoaffective disorder
As shown in Figure 6, GAD65 mRNA and protein levels were strongly positively correlated for schizoaffective disorder subjects (r = 0.56, p = 0.02) and not for comparison subjects (r = −0.12, p = 0.66).
Figure 6.
Correlation of GAD65 mRNA and protein levels in A) healthy comparison subjects and B) subjects with schizoaffective disorder.
Discussion
Using the largest cohort studied to date, the current study shows that mean GAD65 mRNA levels in PFC area 9 are not altered in subjects with schizophrenia, but are lower in subjects with schizoaffective disorder. In these schizoaffective disorder subjects, measures of GAD65 protein were also significantly lower in the opposite hemisphere, indicating that the reduction in GAD65 is present bilaterally in the PFC. The significant correlation between GAD65 mRNA and protein across subjects with schizoaffective disorder suggests that lower GAD65 mRNA expression results in less translation of GAD65 protein.
Lower levels of GAD65 mRNA and protein in schizoaffective disorder subjects appear to reflect the disease process and not to be a consequence of factors frequently comorbid with this diagnosis, including medications. First, each of these factors was also present in the schizophrenia subjects, whose mean GAD65 mRNA levels did not differ from healthy comparison subjects. Second, subjects with factors that may be more commonly associated with schizoaffective disorder than schizophrenia (i.e., death by suicide (58–62); use of antidepressants, benzodiazepines or sodium valproate medications (63,64); and diagnosis of substance abuse or dependence (65,66)), and thus could underlie the finding of lower mean GAD65 levels, were not disproportionally present in the schizoaffective disorder subjects relative to the schizophrenia subjects. Finally, GAD65 mRNA and protein levels did not differ as a function of any these factors across the subjects with schizoaffective disorder.
Most cellular and/or molecular studies that have examined both schizophrenia and schizoaffective disorder subjects have found no differences by diagnosis. For example, levels of somatostatin (44,68), parvalbumin (44,47), GABAA receptor subunits (69–71), and cannabinoid 1 receptor (72,73) have been reported to be similarly altered in both schizophrenia and schizoaffective disorder subjects. However, the sample size in some of these studies may not have had sufficient power to detect a difference. Interestingly, a small number of studies have reported molecular or cellular differences in PFC between these two diagnoses: 1) lower expression of arrestin and G-protein coupled receptor kinases was found selectively in schizophrenia subjects (74), 2) lower expression of neuropeptide Y mRNA was found selectively in the white matter of schizoaffective disorder subjects (75), and 3) a laminar-specific increased density of pyramidal cells and decreased density of putative interneurons was found selectively in schizoaffective disorder subjects (76). Thus, in concert with the current study, the existing postmortem literature provides some support for the disease process of schizoaffective disorder as having both distinct differences and substantial similarities with the disease process operative in schizophrenia.
The major difference in diagnostic criteria between schizophrenia and schizoaffective disorder is the extent of mood dysregulation (54), suggesting that the reduction in PFC GAD65 levels in schizoaffective disorder subjects might be shared with other mood disorders. However, the existing literature does not strongly support this interpretation. Two studies of GAD65 mRNA levels in PFC area 9 found no difference between healthy comparison and bipolar disorder subjects (35,77), although a smaller study did find evidence of fewer GAD65-labeled puncta in PFC areas 10 and 24 (78). Studies in subjects with major depressive disorder have also found no differences in GAD65 mRNA or protein expression in PFC area 9 (35,77,79), though lower levels of cortical GABA have been reported by magnetic resonance imaging (80,81). Although a definitive study of sufficient sample size is needed, together these data suggest that lower PFC levels of GAD65 might distinguish schizoaffective disorder from both schizophrenia and mood disorders. However, it is also important to recognize (as suggested by the finding of lower GAD65 mRNA levels in some individual subjects with schizophrenia relative to their matched comparison subjects; see Figure 1) that lower PFC GAD65 expression may not be unique to schizoaffective disorder. Furthermore, evaluation of GAD65 levels within other brain regions (e.g. limbic structures) may be needed to determine whether lower GAD65 is unique to schizoaffective disorder or is also present in other mood disorders. Thus, examination of multiple brain regions in a large cohort of subjects with psychotic and/or mood symptoms will be needed to determine whether lower GAD65 expression identifies a unique subset of affected individuals independent of DSM diagnosis.
Along with previous reports (28), the current data indicate that schizoaffective disorder subjects have a central tendency towards lower levels of both GAD65 and GAD67 mRNA and protein in the PFC. Therefore, in addition to less GAD67-synthesized GABA, schizoaffective disorder subjects might also have reductions in putative GAD65-dependent functions such as GABA synthesis during periods of high GABA demand (5,8,12,18,49,50), packaging of GABA into synaptic vesicles (9,51–53), and basal GABA synthesis (11,16). However, subjects with schizoaffective disorder generally have better preserved cognitive function than subjects with schizophrenia (reviewed in 82,83). Taken together with the current results, these data suggest that lower GAD67 and GAD65 in the PFC of subjects with schizoaffective disorder may not be pathological deficits in the GABA system, but instead might reflect a homeostatic response that lowers inhibition in order to compensate for lower excitatory activity of PFC pyramidal neurons (reviewed in 84). Indeed, long-term reductions in excitation are associated with reductions in GAD67 and GAD65 protein expression that serve to maintain balanced levels of excitation and inhibition within perturbed neuronal networks (17,85–88). Thus, under this scenario, compensatory reductions of both GAD67 and GAD65 expression in subjects with schizoaffective disorder, but of GAD67 only in subjects with schizophrenia, might contribute to the generally better preserved cognitive function in individuals with schizoaffective disorder relative to those with schizophrenia. If so, than understanding the processes that lead to lower GAD65 expression selectively in subjects with schizoaffective might not only further inform the nature of this disease process but also open new windows for therapeutic interventions in schizophrenia.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the excellent technical assistance of Holly Bazmi and Mary Brady. This work was supported by NIH grants R01 MH043784 (DAL) and R01 MH096985 (KNF), and Nara Medical University (SK).
Financial Disclosures
Dr. David A. Lewis currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and in 2012–2014 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Glausier, Kimoto, and Fish report no biomedical financial interests or potential conflicts of interest.
Reference List
- 1.Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 3.Kiser PJ, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 1998;402:62–74. [PubMed] [Google Scholar]
- 4.Dupuy ST, Houser CR. Prominent expression of two forms of glutamate decarboxylase in the embryonic and early postnatal rat hippocampal formation. J Neurosci. 1996;16:6919–6932. doi: 10.1523/JNEUROSCI.16-21-06919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y, Kaplan IV, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of the cat visual cortex. Brain Res Dev Brain Res. 1997;103:127–141. doi: 10.1016/s0165-3806(97)81789-0. [DOI] [PubMed] [Google Scholar]
- 8.Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci U S A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Jin Y, Buddhala C, Osterhaus G, Cohen E, Jin H, et al. Role of glutamate decarboxylase (GAD) isoform, GAD65, in GABA synthesis and transport into synaptic vesicles-Evidence from GAD65-knockout mice studies. Brain Res. 2007;1154:80–83. doi: 10.1016/j.brainres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Walls AB, Eyjolfsson EM, Smeland OB, Nilsen LH, Schousboe I, Schousboe A, et al. Knockout of GAD65 has major impact on synaptic GABA synthesized from astrocyte-derived glutamine. J Cereb Blood Flow Metab. 2011;31:494–503. doi: 10.1038/jcbfm.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls AB, Nilsen LH, Eyjolfsson EM, Vestergaard HT, Hansen SL, Schousboe A, et al. GAD65 is essential for synthesis of GABA destined for tonic inhibition regulating epileptiform activity. J Neurochem. 2010;115:1398–1408. doi: 10.1111/j.1471-4159.2010.07043.x. [DOI] [PubMed] [Google Scholar]
- 12.Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh SN, Martin DL. Elevation of brain GABA levels with vigabatrin (gamma-vinylGABA) differentially affects GAD65 and GAD67 expression in various regions of rat brain. J Neurosci Res. 1998;52:736–741. doi: 10.1002/(SICI)1097-4547(19980615)52:6<736::AID-JNR12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, et al. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD(67) protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- 15.Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, et al. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Comm. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 17.Lau CG, Murthy VN. Activity-dependent regulation of inhibition via GAD67. J Neurosci. 2012;32:8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, et al. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- 20.Esclapez M, Tillakaratne NJ, Tobin AJ, Houser CR. Comparative localization of mRNAs encoding two forms of glutamic acid decarboxylase with nonradioactive in situ hybridization methods. J Comp Neurol. 1993;331:339–362. doi: 10.1002/cne.903310305. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh SN, Martin SB, Martin DL. Regional distribution and relative amounts of glutamate decarboxylase isoforms in rat and mouse brain. Neurochem Int. 1999;35:73–80. doi: 10.1016/s0197-0186(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda T, Aika Y, Heizmann CW, Kosaka T. GABAergic axon terminals at perisomatic and dendritic inhibitory sites show different immunoreactivities against two GAD isoforms, GAD67 and GAD65, in the mouse hippocampus: A digitized quantitative analysis. J Comp Neurol. 1998;395:177–194. doi: 10.1002/(sici)1096-9861(19980601)395:2<177::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, et al. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, et al. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Fish KN, Sweet RA, Lewis DA. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 2011;21:2450–2460. doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: Clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibanez JM, Crespo C, Nacher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci Lett. 2012;530:97–102. doi: 10.1016/j.neulet.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Bharadwaj R, Jiang Y, Mao W, Jakovcevski M, Dincer A, Krueger W, et al. Conserved chromosome 2q31 conformations are associated with transcriptional regulation of GAD1 GABA synthesis enzyme and altered in prefrontal cortex of subjects with schizophrenia. J Neurosci. 2013;33:11839–11851. doi: 10.1523/JNEUROSCI.1252-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi D, Fung SJ, Rothwell A, Weickert CS. Higher gamma-aminobutyric Acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biol Psychiatry. 2012;72:725–733. doi: 10.1016/j.biopsych.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 34.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 35.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 36.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 37.Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: A preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- 38.Woo T-U, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 41.Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo TU, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon WC. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatry Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. J Neurosci Res. 2004;76:581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- 49.Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, et al. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- 50.Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic Acid decarboxylase-65 knock-out mice. J Neurosci. 2002;22:5271–5276. doi: 10.1523/JNEUROSCI.22-13-05271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buddhala C, Hsu CC, Wu JY. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem Int. 2009;55:9–12. doi: 10.1016/j.neuint.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Buddhala C, Suarez M, Modi J, Prentice H, Ma Z, Tao R, et al. Calpain cleavage of brain glutamic acid decarboxylase 65 is pathological and impairs GABA neurotransmission. PloS One. 2012;7:e33002. doi: 10.1371/journal.pone.0033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, et al. Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Psychiatric Association. DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 55.Curley AA, Arion D, Lewis DA. GAD67 and GAD65 protein levels in the dorsolateral prefrontal cortex of subjects with schizophrenia. Society for Neuroscience. Abstract 340.5/N26. 10-19-2009. [Google Scholar]
- 56.Ridler TW, Calvard S. Picture thresholding using an iterative selection method. IEEE Trans. System, Man and Cybernetics SMC-8. 1978:630–632. [Google Scholar]
- 57.Billinton N, Knight AW. Seeing the wood through the trees: a review of techniques for distinguishing green fluorescent protein from endogenous autofluorescence. Anal Biochem. 2001;291:175–197. doi: 10.1006/abio.2000.5006. [DOI] [PubMed] [Google Scholar]
- 58.Laursen TM, Munk-Olsen T, Nordentoft M, Bo MP. A comparison of selected risk factors for unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia from a danish population-based cohort. J Clin Psychiatry. 2007;68:1673–1681. doi: 10.4088/jcp.v68n1106. [DOI] [PubMed] [Google Scholar]
- 59.Goldstein G, Haas GL, Pakrashi M, Novero AM, Luther JF. The cycle of schizoaffective disorder, cognitive ability, alcoholism, and suicidality. Suicide Life Threat Behav. 2006;36:35–43. doi: 10.1521/suli.2006.36.1.35. [DOI] [PubMed] [Google Scholar]
- 60.Potkin SG, Alphs L, Hsu C, Krishnan KR, Anand R, Young FK, et al. Predicting suicidal risk in schizophrenic and schizoaffective patients in a prospective two-year trial. Biol Psychiatry. 2003;54:444–452. doi: 10.1016/s0006-3223(03)00178-1. [DOI] [PubMed] [Google Scholar]
- 61.Radomsky ED, Haas GL, Mann JJ, Sweeney JA. Suicidal behavior in patients with schizophrenia and other psychotic disorders. Am J Psychiatry. 1999;156:1590–1595. doi: 10.1176/ajp.156.10.1590. [DOI] [PubMed] [Google Scholar]
- 62.Walsh E, Harvey K, White I, Higgitt A, Fraser J, Murray R. Suicidal behaviour in psychosis: prevalence and predictors from a randomised controlled trial of case management: report from the UK700 trial. Br J Psychiatry. 2001;178:255–260. doi: 10.1192/bjp.178.3.255. [DOI] [PubMed] [Google Scholar]
- 63.Ndetei DM, Khasakhala L, Meneghini L, Aillon JL. The relationship between schizoaffective, schizophrenic and mood disorders in patients admitted at Mathari Psychiatric Hospital, Nairobi, Kenya. Afr J Psychiatry (Johannesbg) 2013;16:110–117. doi: 10.4314/ajpsy.v16i2.14. [DOI] [PubMed] [Google Scholar]
- 64.Murru A, Pacchiarotti I, Nivoli AM, Grande I, Colom F, Vieta E. What we know and what we don't know about the treatment of schizoaffective disorder. Eur Neuropsychopharmacol. 2011;21:680–690. doi: 10.1016/j.euroneuro.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58–64. doi: 10.2174/1874941001104010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saarni SI, Viertio S, Perala J, Koskinen S, Lonnqvist J, Suvisaari J. Quality of life of people with schizophrenia, bipolar disorder and other psychotic disorders. Br J Psychiatry. 2010;197:386–394. doi: 10.1192/bjp.bp.109.076489. [DOI] [PubMed] [Google Scholar]
- 67.Tukey JW. Exploratory data analysis. Reading, Massachusetts: Addison-Wesley Publishing Company, Inc.; 1977. [Google Scholar]
- 68.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: Illness progression vs developmental disturbance. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharm. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharm. 2010;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bychkov ER, Ahmed MR, Gurevich VV, Benovic JL, Gurevich EV. Reduced expression of G protein-coupled receptor kinases in schizophrenia but not in schizoaffective disorder. Neurobiol Dis. 2011;44:248–258. doi: 10.1016/j.nbd.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris HM, Stopczynski RE, Lewis DA. NPY mRNA expression in the prefrontal cortex: Selective reduction in the superficial white matter of subjects with schizoaffective disorder. Schizophr Res. 2009;115:261–269. doi: 10.1016/j.schres.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 77.Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 79.Karolewicz B, Maciag D, O'Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–420. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 81.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 82.Cheniaux E, Landeira-Fernandez J, Lessa TL, Lessa JL, Dias A, Duncan T, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209–217. doi: 10.1016/j.jad.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- 87.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hendry SHC, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.