Abstract
Ochratoxin A (OTA) is a mycotoxin produced by several fungal species including Aspergillus ochraceus, A. carbonarius, A. niger and Penicillium verrucosum. OTA causes nephrotoxicity and renal tumors in a variety of animal species; however, human health effects are less well-characterized. Various studies have linked OTA exposure with the human diseases Balkan endemic nephropathy (BEN) and chronic interstitial nephropathy (CIN), as well as other renal diseases. This study reviews the epidemiological literature on OTA exposure and adverse health effects in different populations worldwide, and assesses the potential human health risks of OTA exposure. Epidemiological studies identified in a systematic review were used to calculate unadjusted odds ratios for OTA associated with various health endpoints. With one exception, there appears to be no statistically significant evidence for human health risks associated with OTA exposure. One Egyptian study showed a significantly higher risk of nephritic syndrome in those with very high urinary OTA levels compared with relatively unexposed individuals; however, other potential risk factors were not controlled for in the study. Larger cohort or case-control studies are needed in the future to better establish potential OTA-related human health effects, and further duplicate-diet studies are needed to validate biomarkers of OTA exposure in humans.
Keywords: Ochratoxin A, human health risks, odds ratios, risk assessment, systematic review
INTRODUCTION
Ochratoxin A (OTA) is a naturally occurring foodborne mycotoxin found in a wide variety of agricultural commodities worldwide, ranging from cereal grains to dried fruits to wine and coffee. It is produced by several different fungi including Aspergillus ochraceus, A. carbonarius, A. niger and Penicillium verrucosum. These fungi vary in their optimal growing temperatures and water activity, and contaminate various commodities as summarized in Table 1. Contamination generally occurs as a result of poor storage of commodities and suboptimal agricultural practices during the drying of foods (Moss, 1996). Ingestion is the main source of exposure to OTA, through the foods and beverages listed in Table 1.
Table 1.
Ochratoxin-producing fungi, optimal growth conditions, and commodities affected.
| OTA- producing species |
Optimal temperature range (Min- Max) °C |
Water activity |
Commodities affected |
|---|---|---|---|
| A. ochraceus | 24–31 (8–37) | 0.95–0.99 | Smoked and salted dried fish, dried beans, biltong, soya beans, chickpeas, rapeseed, pepper, dried fruit, and sesame seeds, nuts, cereals rice, barley, maize, wheat, flour, and bran, coffee beans |
| A. carbonarius | 32–25 (N/A-40) | 0.82 | Grapes and grape products, including table grapes, wines, and dried vine fruits |
| A. niger | 35–37 (6–47) | 0.77 | Nuts, apples, pears, peaches, citrus, grapes, figs, strawberries, mangoes, tomatoes, melons, onions, garlic, and yams |
| P. verrucosum | 20 (0–30) | 0.80 | Cereal crops; cheese, meat products |
Source: JECFA (Joint FAO/WHO Committee On Food Additivies (JECFA), 2001).
OTA is a chemically stable compound; hence, ordinary food processing measures fail to substantially reduce its presence in foods and beverages. OTA has been shown to be toxic and carcinogenic in animals. The kidney is the main target organ for OTA; OTA is a potent renal carcinogen in several animal species (Duarte et al., 2011; Hagelberg et al., 1989; IARC, 1993; Krogh, 1974; Kuiper-Goodman and Scott, 1989; World Health Organization (WHO), 2002). Other adverse effects of OTA include immunotoxicity (Bondy and Pestka, 2000; Pestka and Bondy, 1994), inhibition of macromolecular synthesis, increased lipid peroxidation, and inhibition of mitochondrial respiration (Kuiper-Goodman and Scott, 1989; Marquardt and Frohlich, 1992). OTA has been suspected as a cause of various human nephropathies since the 1970s including Balkan Endemic Nephropathy (BEN) (Barnes et al., 1977; Castegnaro et al., 2006; Elling and Krogh, 1977; Pfohl-Leszkowicz et al., 2002; Sattler et al., 1977) and chronic interstitial nephropathy (CIN) (Abid et al., 2003). The International Agency for Research on Cancer (IARC) has classified OTA as a Group 2B possible human carcinogen, based on demonstrated carcinogenicity in animal studies (Fazekas et al., 2005; IARC, 1993), although OTA-related carcinogenicity has not been conclusively determined in humans. A recent risk assessment on OTA (Haighton et al., 2012) states that OTA was negative in genotoxicity assays with high specificity, and that OTA-DNA adduct levels were low and not typical of genotoxic carcinogens.
Ochratoxin A has become an important topic in recent years, as Health Canada has proposed maximum limits (MLs) for OTA in a variety of foods and drinks that could have consequences for the marketability of these commodities in Canada, and could also affect nations that attempt to export food to Canada (Health Canada, 2009). Yet, little is known about population health impacts of dietary OTA exposure. Thus far, risk assessments on OTA, including those that have guided Health Canada’s recently proposed MLs, have largely been based on animal and cell culture assay studies, with relatively less focus on human studies. The goal of this study was to systematically review the epidemiological literature linking OTA exposure with adverse health effects in diverse human populations worldwide. In a discussion of risk assessments conducted on OTA in the past, we compare the state of known data with what is still missing in terms of assessing human health effects. We collected available human studies linking OTA exposure with a variety of health outcomes, and selected those studies that met predefined criteria for inclusion in the review. Odds ratios were estimated for these different studies where the data permitted these calculations. The current state of exposure assessment for OTA is discussed as well. We characterize the risk of OTA to human populations and describe limitations in the available data.
BACKGROUND: RISK ASSESSMENT OF OCHRATOXIN A
Risk assessment, the process of estimating the magnitude and the probability of a harmful effect to populations from certain agents or activities, consists of four main steps: hazard identification, hazard characterization or dose-response assessment, exposure assessment, and risk characterization (National Research Council, 1983). The four steps involved in the estimation of risk are outlined below (Liu and Wu, 2010).
Hazard identification, determining whether exposure to an agent can increase the incidence of a particular health condition, has been carried out for OTA in assessments conducted by multiple institutions; including the International Agency for Research on Cancer (IARC), Health Canada, the Joint Food and Agriculture Organization / World Health Organization Expert Committee on Food Additives (JECFA), and the European Food Safety Authority (EFSA) (European Food Safety Authority (EFSA), 2006; Health Canada, 2009; IARC, 1993; Joint FAO/WHO Committee On Food Additivies (JECFA), 1991). Ochratoxin is identified as a renal carcinogen to particular animal species (Kuiper-Goodman and Scott, 1989) and can cause nephrotoxic, teratogenic, and immunosuppressive effects in multiple animal species (Kuiper-Goodman and Scott, 1989; O'Brien and Dietrich, 2005).
For humans, however, hazard identification has been more difficult. Several adverse human health effects, including the kidney diseases Balkan Endemic Nephropathy (BEN) and chronic interstitial nephropathy (CIN), have been associated with exposure to OTA; but these associations have thus far been less conclusive than those for OTA-associated adverse effects in laboratory animal studies. The hallmark features of BEN include a familial but not inherited pattern of disease, initial manifestation after living in an endemic village for 15 years or more, and an association with upper urothelial tract cancer (Grollman et al., 2007). However, aristolochic acid (AA), a toxin produced in Aristolochia weeds commonly found in Balkan grain fields, has emerged as the most likely causative agent of BEN; as aristolactam-DNA adducts have been found in the renal cortex of BEN patients but not in patients with other chronic renal diseases (26). CIN does not appear to have the familial pattern of BEN, and may be acute or chronic with cases presenting anywhere from a few days up to 5 months. The etiology of CIN has been postulated to include infections, toxins such as OTA, or reactions to medications (Baker and Pusey, 2004).
Hazard characterization or dose-response assessment describes the relationship between different levels of exposure to a substance and associated incidence of disease in a population of animals or humans. Dose-response data from animal studies of a particular toxin are used to extrapolate an acceptable daily or weekly exposure to humans, below which no adverse effects are expected. This step usually involves a critical review of toxicological studies to set appropriate exposure metrics (Kuiper-Goodman et al., 2010), such as tolerable daily or weekly intake or negligible cancer risk intake. In the case of OTA, diverse regulatory and advisory bodies have assessed dose-response data on OTA and have set exposure metrics for tolerable exposure to OTA in humans. These are summarized in Table 2.
Table 2.
Summary of calculated tolerable human intakes of ochratoxin A (OTA) by international organization.
| Organization | Tolerable intake metric* |
Limit | Reference |
|---|---|---|---|
| European Food Safety Authority (EFSA) | PTWI | 120 ng/kg bw/week | EFSA, 2006 |
| Health Canada | PTDI | 3 ng/kg bw/day | Kuiper-Goodman et al., 2010 |
| Health Canada | NCRI | 4 ng/kg bw/day | Kuiper-Goodman et al., 2010 |
| Joint FAO/WHO Expert Committee on Food Additives (JECFA) 2007 | PTWI | 100 ng/kg bw/week | JECFA, 2007 |
| Nordic Expert Group on Food Safety | TDI | 5 ng/kg bw/day | Olson et al., 1991 |
| Scientific Committee of Food (SCF) of the European Union | PTDI | 5 ng/kg bw/day | EFSA, 2006 |
TDI - tolerable daily intake; PTDI - provisional tolerable daily intake; PTWI - provisional tolerable weekly intake; NCRI - negligible cancer risk intake
Various dose-response studies in animals were the basis for advisory groups’ determinations of safe weekly or daily OTA intakes for humans. JECFA first evaluated OTA at its 37th meeting (JECFA, 1991), setting a provisional tolerable weekly intake (PTWI) at 112 ng OTA per kg bodyweight (bw) per week based on a dose-response study of renal function deterioration in pigs, for which the lowest observed adverse effect level (LOAEL) was 8 μg/kg bw/day (Elling, 1979; Krogh, 1974). A combined uncertainty factor (UF) of 500 was applied in the calculation. JECFA re-evaluated OTA at its 44th meeting, taking into account new toxicological data. The PTWI was confirmed, but rounded down to 100 ng/kg bw/week. The most recent assessment of OTA at the 68th meeting in 2008 resulted in retaining the PTWI previously found. JECFA currently estimates OTA exposure from cereals, based on European data, to be about 8-17 ng/kg bw/week: well below the PTWI.
The European Food Safety Authority (EFSA) derived a PTWI for ochatoxin A of 120 ng/kg bw/week, based on the 8 μg/kg bw/day LOAEL used in the JECFA evaluation (European Food Safety Authority, 2006). An uncertainty factor of 450, rather than 500 used in JECFA, was applied to the LOAEL. This composite uncertainty factor was based on an intra-species factor of 10, interspecies factor of 15, and a factor of 3 for use of a LOAEL instead of a no observed adverse effect level (NOAEL). The interspecies factor of 15 was based on the longer OTA half-life in humans and monkeys rather than pigs as determined by Hagelberg et al. (1989).
A Health Canada risk assessment team (Kuiper-Goodman et al., 2010) chose to reevaluate EFSA’s PTWI for ochratoxin A, positing that the use of LOAEL rather than a NOAEL was not appropriate given the small number of animals per group, and the fact that 4 out of 9 pigs in the lowest dose group showed functional kidney changes. Rather than use a NOAEL or LOAEL, a benchmark dose corresponding to a response of 10% above background (BD10) was derived. Uncertainty factors of 10 for intra-species variability, 25 for interspecies variability, and 2 for use of a sub-chronic rather than chronic study were combined in a composite uncertainty factor of 500. Applying this composite uncertainty factor to the BD10 of 1.56 μg/kg bw/day resulted in a TDI of 3.0 ng/kg bw/day after rounding (Kuiper-Goodman et al., 2010), which in practice is considerably stricter than the JECFA or EFSA tolerable limits.
Additionally, Health Canada derived a negligible cancer risk intake (NCRI) for OTA: the exposure associated with an increased cancer risk of 1:100,000 and equivalent in units to the TDI. The tumorigenic dose at which 5% of the animals are likely to have tumors (TD05) was used to derive the NCRI for OTA (NTP, 1989). This dose was determined to be 19.6 μg/kg bw/day. The TD05 was then divided by 5000, the linear extrapolation to zero exposure, resulting in a NCRI of 4 ng/kg bw/day (Kuiper-Goodman et al., 2010).
Exposure assessment involves estimating the intensity, frequency, and duration of human exposures to toxic substances. Ochratoxin exposure is a function of the concentration of ochratoxin in foodstuffs, as well as the amount of these foodstuffs that are consumed in different populations. Depending on location, seasons, and amount of time food is kept in storage, both the amount and contamination levels of food may vary greatly even for the same population or individual (Scott, 2005). Estimating human OTA exposure may be done using food surveys combined with OTA surveillance in commodities, or biomarkers of exposure.
By determining ranges of OTA contamination of foods, OTA exposure can be estimated from the known intake levels of the given commodities (Jørgensen et al., 1996). However, the accuracy of this estimation is limited due to the large variability in OTA content in commodities, as well as variation in dietary habits (Fazekas et al., 2005). Surveillance data on OTA concentrations in different regions of the world is limited. JECFA provides extensive data on OTA exposure concentrations in commodities (JECFA, 2001); however, 85% of sampled commodities including wheat, rye, barley, oats, dried vegetables, olives, and milk came from Europe. However, it was noted that OTA occurs in coffee in countries including Brazil, Canada, Dubai, Europe, Japan, and the USA (Joint FAO/WHO Committee On Food Additivies (JECFA), 2001). In Denmark, Norway, and the UK, OTA was found in oat samples. In Germany, high OTA levels were found in unprocessed cereals, rye and buckwheat, with levels ranging from 95.6-125 ug/kg. In Africa, OTA was found in wheat, barley, cereals, dried vegetables and olives. Specifically, Maaroufi et al. (1995) found high OTA levels, with a maximum of 33,000 ug/kg in wheat, barley, mixed cereals, dried vegetables, and olives in a Tunisian population. In Nigeria, Ghana, and Burkina Faso, OTA was detected in sorghum, maize, and millet.
A more accurate method to estimate exposure, when possible, is through measuring human biomarkers of OTA exposure, as reviewed in Duarte et al. (Duarte et al., 2011). Although neither serum OTA nor urinary OTA has been validated, they have been used in multiple studies to estimate OTA exposure. OTA is found in serum due to its long elimination half-life of about 35 days (Studer-Rohr et al., 2000), and is excreted in urine as both unchanged OTA and its derivatives.
OTA in serum was first detected in 1977 and has been one of the most widely used biomonitoring approaches for human OTA exposure. Renal absorption, enterohepatic circulation and binding to plasma proteins results in a long half-life for OTA in the body, allowing it to be detected in human blood (Dahlmann et al., 1998; Roth et al., 1988). Epidemiological studies conducted in multiple countries, including Romania (Voo et al., 2002), Spain (Jimenez et al., 1998), the Czech Republic (Malir et al., 2001a; Malir et al., 2001b), Turkey (Ozcelik et al., 2001), Italy (Breitholtz-Emanuelsson et al., 1994), Egypt (Wafa et al., 1998), Algeria (Khalef et al., 1993), and Tunisia (Abid et al., 2003; Hassen et al., 2004), have associated higher serum or plasma OTA levels in patients with kidney and urinary disorders compared to healthy controls, although the associations may not be causal (Scott, 2005).
Although many studies have used serum OTA as a human biomarker of OTA exposure, considerable intra-subject variation has been noted. Levels of serum OTA have been noted to vary up to tenfold in one subject when tested over a ten-year period (Radic et al., 1997) and in repeatedly tested subjects over one year in Tuscany (Palli et al., 1999). Furthermore, studies in Germany (Martlbauer et al., 1996), Switzerland (Studer-Rohr et al., 2000), Czech Republic (Ruprich and Ostry, 1993), Japan (Kawamura et al., 1993), and Bulgaria (Petkova-Bocharova et al., 2003) all showed high intra-subject variability in human subjects tested over time. This variability is likely due to the decreases in plasma concentrations based on the half-life of OTA (Scott, 2005).
Urinary OTA, another potential biomarker of OTA exposure, is often found in very low amounts compared to those in blood; however, new technologies have increase the sensitivity and accuracy of detection (Duarte et al., 2011). Pascale & Visconti (2000) detected OTA in 37 out of 55 healthy individuals in Italy with levels ranging from 0.012-0.046 ng/ml. In Hungary, Fazekas et al. (Fazekas et al., 2005) detected OTA in 54 out of 88 samples from healthy individuals at levels of 0.006-0.065 mg/ml. Patients with end stage renal disease or nephritic syndrome in Egypt had significantly higher levels of urinary OTA than two reference groups (Wafa et al., 1998). The highest incidence of detectable OTA exposures in urine, 100%, were found by Petkova-Bocharova et al. (Petkova-Bocharova et al., 2003) in a BEN-endemic region and in non-endemic regions including Portugal (Duarte et al., 2010b) and Italy (Breitholtz-Emanuelsson et al., 1994). The highest recorded levels of OTA in urine, 367 ng/ml and 1801 ng/ml, were found in two French siblings with renal failure (Godin et al., 1996). Petkova-Bocharova et al. (2003) and Castegnaro et al. (2006) applied similar methodology as Gilbert et al. (2001) when studying 16 human participants (Castegnaro et al., 2006). Increases of OTA intake resulted in an increase of OTA elimination a week after ingestion, not immediately (Castegnaro et al., 2006). Table 3 summarizes urinary OTA levels in populations from different world regions. The table includes OTA levels from Duarte et al. (Duarte et al., 2011) and more recent studies.
Table 3.
OTA occurrence in human urine.
| Country | Year collected |
Number of positive samples (%) for OTA |
Range of urinary OTA levels (ng/ml) |
Sampled population |
Reference |
|---|---|---|---|---|---|
| Bulgaria | 1984–1990 | 44/127 (35) | 0.005–0.604 | Healthy humans in Balkan Endemic Nephropathy (BEN) areas, Non-BEN areas and BEN patients | (Nikolov et al., 2002) |
| Bulgaria | 2003 | 16/16 (100) | 0.016–0.860 | Patients from BEN areas | (Petkova-Bocharova et al., 2003) |
| Bulgaria | Not Available | 61/152 (40) | n.d.-0.03 | BEN and Urothelial Tract Tumor (UTT) patients | (Castegnaro et al., 1990) |
| Croatia | 2000 | 24/63 (38) | 0.005–0.086 | BEN and Non-BEN areas | (Domijan et al., 2009) |
| Croatia | 2005 | 9/63 (14) | 0.005–0.015 | BEN and Non-BEN areas | (Domijan et al., 2009) |
| Egypt | 1998 | 19/122 (16) | 0–8.19 | Healthy controls, kidney donors, patients with End Stage Renal Disease (ESRD), transplant recipients, nephritic syndrome patients, and UTT patients | (Wafa et al., 1998) |
| Germany | 2008 | 13/13 (100) | 0.02–0.13 | Healthy volunteers | (Munoz et al., 2010) |
| Hungary | 2003 | 54/88 (61) | 0.006–0.065 | Healthy volunteers | (Fazekas et al., 2005) |
| Italy | 2001 | 25/41 (61) | 0.012–0.140 | Healthy individuals and karyomegalic interstitial nephritis patients | (Pascale and Visconti, 2000) |
| Italy | Not Available | 10/10 (100) | 0.02–0.25 | Healthy volunteers | (Solfrizzo et al., 2011) |
| Korea | Not Available | 12/12 (100) | 0.012–0.093 | Healthy volunteers | (Ahn et al., 2010) |
| Portugal | 2007 | 174/198 (88) | n.d.-0.071 | Healthy volunteers | (Duarte et al., 2010a; Duarte et al., 2009) |
| Portugal | 2004 | 42/60 (70) | n.d.-0.105 | Healthy volunteers | (Pena et al., 2006) |
| Portugal | 2005 | 13/30 (43) | n.d.-0.208 | Healthy volunteers | (Manique et al., 2008) |
| Sierra Leone | 1992–93 | 63/434 (25) | 0.06–148 | Healthy child volunteers | (Jonsyn-Ellis, 2001) |
| Spain | 2005 | 25/31 (81) | n.d.-0.124 | Healthy volunteers | (Manique et al., 2008) |
| Spain | 2011 | 9/72 (13) | 0.057–0.562 | Healthy volunteers | (Coronel et al., 2011) |
| Spain | 2011 | 3/27 (11) | n.d.-<1.5 | Healthy volunteers | (Rubert et al., 2011) |
| United Kingdom | 2001 | 46/50 (92) | <0.01–0.058 | Healthy volunteers | (Gilbert et al., 2001) |
A 2001 study on both serum and urinary biomarkers of OTA exposure revealed a stronger correlation between dietary OTA intake and urinary OTA than serum OTA. Gilbert et al. (Gilbert et al., 2001) examined OTA levels in urine and plasma as a function of dietary OTA intake in 50 subjects in the United Kingdom. The volunteers kept a daily food diary and provided blood samples once per week, urine samples daily, and duplicate diet samples daily, for one month. Baseline samples were taken at the beginning of the study. OTA was detected in all but four urine samples, with levels ranging from <0.01-0.058 ng/ml. OTA was detected in all plasma samples with baseline sample levels ranging from 0.15-2.17 ng/ml and composited plasma samples ranging from 0.4-3.11 ng/ml. A statistically significant correlation between urine OTA levels (R2 = 0.52) and dietary OTA consumption was found. However, the authors caution that this relationship is too weak to be used in a predictive manner (Gilbert et al., 2001). In plasma, no significant correlation was found between the two (R2 = 0.29). For the purpose of establishing a valid biomarker of OTA exposure in the future, urinary OTA appears a stronger candidate.
Risk characterization integrates the dose-response and exposure assessments to determine the probability of an adverse effect to human populations by an agent. To estimate risk associated with OTA exposure and various health effects, it was not possible to estimate a population attributable risk due to a lack of available epidemiological data. Instead, a systematic review was performed in this study, and unadjusted odds ratios (ORs) were calculated for various health effects associated with OTA exposure based on data from existing studies.
SYSTEMATIC REVIEW
The purpose of this systematic review was to attempt to reconcile the human and animal study results on OTA toxicity, or lack thereof. A literature search was performed on PubMed until October 4th, 2011. Search terms used without restriction included combinations of: (ochratoxin A), (human), (population), (disease), (urinary ochratoxin), (urinary OTA), and (urinary biomarker). Additionally, we searched reference lists from retrieved articles and searched ochratoxin review papers for any additional epidemiological studies on adverse effects associated with OTA exposure that may not have been retrieved in the initial search.
Eligibility criteria for inclusion in the review were as follows: (1) epidemiological studies; (2) case-control or cohort study design; (3) ochratoxin A as the exposure of interest; (4) OTA exposure measured either in terms of dietary intake or urinary OTA levels; and (5) relative risk (RR) or odds ratio (OR) estimates with 95% confidence intervals (CIs) reported, or data to calculate these. Studies using serum OTA as a marker for exposure were excluded because of the poor correlation between dietary OTA intake and serum OTA as measured in Gilbert et al. (2001).
Data on the following were extracted from each study: authors, publication year, study design and sample size, study location, study period, participants’ gender and age, range of ochratoxin exposure, health effect under investigation, and data necessary to calculate ORs for each health effect if the OR was not already calculated. From these data, we calculated unadjusted ORs and 95% confidence intervals for each ochratoxin-related health effect examined in each of the studies (several studies examined more than one adverse effect).
RESULTS
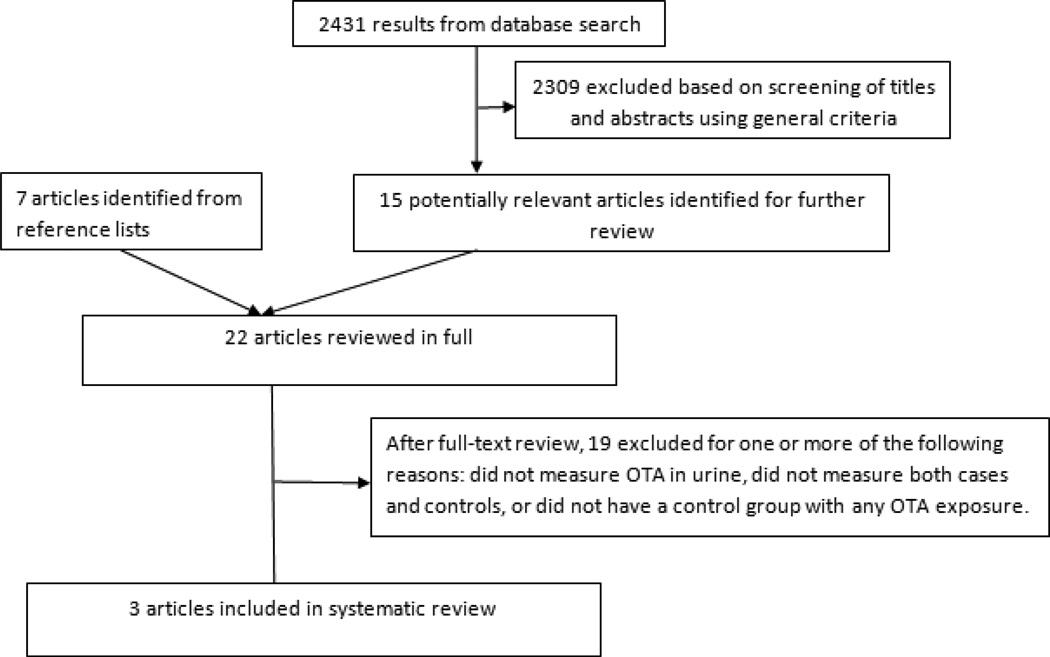
The step-by-step process of our literature search is presented in Figure 1. From 2431 results, only those studies that met the criteria listed above were included. Fifteen studies were selected based on information in the title and abstract, and seven more were added based on reference lists in those selected studies. A full-text review of all 22 articles resulted in 19 being excluded because they did not measure urinary OTA or dietary OTA, or did not include both diseased and healthy individuals. Three studies contained the relevant information needed to calculate unadjusted ORs for different OTA health effects.
Figure 1.
Selection of studies for inclusion in systematic review of adverse health effects associated with ochratoxin A (OTA) exposure as measured by urinary OTA. Format of figure: Liu et al., 2012.
Table 4 provides an overview of the three eligible studies. Based on the data needed to calculate ORs, three studies were included. Due to the lack of similar health endpoints across the different studies, data could not be combined for meta-analysis. The three eligible studies included two in the Balkans (Croatia and Bulgaria) and one in Egypt. All studies measured urinary OTA levels and associated these levels with several different adverse health effects. Each study also had at least one corresponding control group. Domijan et al. (2009) compared individuals in a BEN-endemic village to those in a non-BEN-endemic village, whereas Nikolov et al. (2002) and Wafa et al. (1998) study used healthy human controls. Epidemiological studies were not included in the review if they did not examine both cases (i.e., those with confirmed disease) and controls. For example, Petkova-Bocharova et al. (Petkova-Bocharova et al., 2003) and Castegnaro et al. (Castegnaro et al., 2006) examined OTA in serum and urine in human subjects living in BEN-endemic vs. non-BEN-endemic villages, but all subjects involved in the study were healthy.
Table 4.
Eligible studies and Unadjusted Odds Ratios (ORs).
| Location | Population characteristics |
Proportion positive for urinary OTA (%) |
Mean urinary OTA (ng/ml) (Range) |
OR (95% CI) | Reference |
|---|---|---|---|---|---|
| Egypt | End Stage Renal Disease (ESRD) patients w/ treatment | 4/11 (36.4%) | 1.85 (nd*-6.70) | 2.74 (0.402–18.69) | (Wafa et al., 1998) |
| ESRD dialysis patients | 1/11 (9.1%) | 0.36 (nd-4.0) | 1.23 (0.115–13.14) | ||
| ESRD Totals | 5/22 (22.7%) | Not Available | 1.94 (0.357–10.52) | ||
| Renal transplant recipients | 2/15 (13.3%) | 0.12 (nd-1.36) | 1.89 (0.28–12.65) | ||
| Nephritic Syndrome patients | 8/15 (53.3%) | 3.09 (nd-8.19) | 10.79 (2.28–50.91) | ||
| Patients with urothelial tract tumors (UTT) | 1/15 (6.7%) | 0.36 (nd-4.64) | 0.88 (0.085–9.14) | ||
| Potential kidney donors | 2/15 (13.3%) | 0.26 (nd-3.42) | Not Available | ||
| Controls | 3/40 (7.5%) | 0.01 (nd-0.31) | Not Available | ||
| Bulgaria | BEN/UTT patients | 14/36 (38.9%) | Not Available (0.005–0.604) | 1.29 (0.58–2.87) | (Nikolov et al., 2002) |
| Healthy persons from BEN families | 12/25 (48%) | Not Available (nd-0.033) | Not Available | ||
| Healthy persons from non-BEN families in BEN villages | 14/32 (43.8%) | Not Available (nd-0.043) | Not Available | ||
| Healthy persons from non-BEN villages in BEN area | 4/31 (12.9%) | Not Available (nd-0.041) | Not Available | ||
| Healthy persons from non-BEN area | 0/3 (0%) | Not Available | Not Available | ||
| Croatia 2000 | Endemic BEN village | 19/45 (43%) | 0.007 (nd-0.086) | 1.90 (0.58–6.23) | (Domijan et al., 2009) |
| Healthy control village | 5/18 (28%) | 0.003 (nd-0.02) | Not Available | ||
| Croatia 2005 | Endemic BEN village | 8/45 (18%) | 0.001 (nd-0.015) | 3.68 (0.43–31.78) | (Domijan et al., 2009) |
| Healthy control village | 1/18 (6%) | 0.005 (0–0.01) | Not Available | ||
| Combined BEN Villages | 27/90 (30%) | Not Available | 2.14 (0.80–5.74) |
nd: non-detect
Mean urinary OTA levels were not provided for all sampled groups and were therefore labeled as ‘Not Available’. Odds ratios labeled ‘Not Available’ were for control groups, hence, no odds ratio could be calculated.
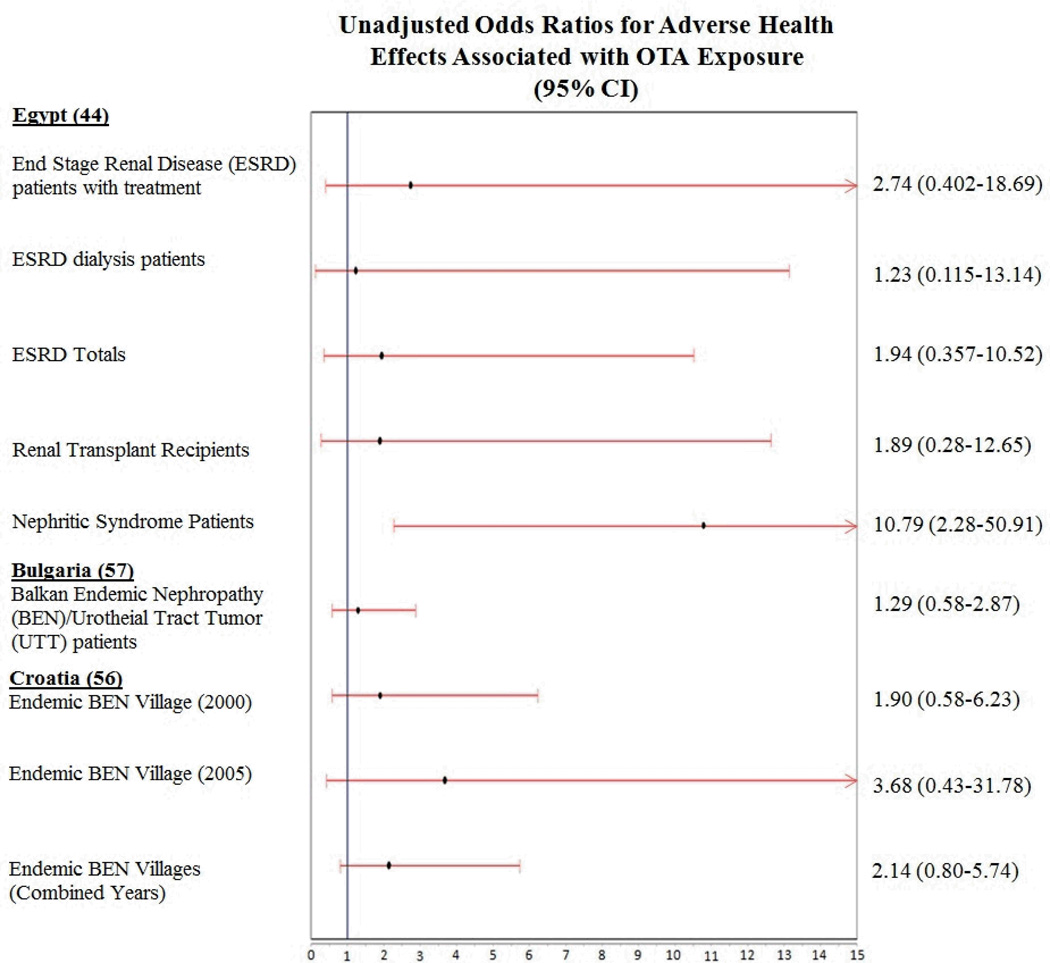
The calculated unadjusted odds ratios are summarized by study and health effect in Table 4. The table includes the three studies used to calculate unadjusted ORs organized by location. Information on each disease assessed, the proportion of subjects with each disease who had detectable urinary OTA, the levels (mean and range) of measured urinary OTA, and unadjusted odds ratio with 95% confidence interval are included in the table. The highest level of measured urinary OTA was in nephritic syndrome patients in Egypt, followed by patients being treated for ESRD in Egypt. Patients mean levels of urinary OTA were 3.09±3.4 ng/ml and 1.85±2.8 ng/ml respectively. Unadjusted ORs ranged from 0.88-10.79 for all adverse health endpoints. While these ORs were unadjusted, demographics including age, sex, socio-economic status, and other lifestyle factors, were similar across both cases and controls in each accepted study.
No statistically significant associations between OTA exposure and any human disease were found in the Bulgarian or Croatian study populations. Only one adverse health effect, nephritic syndrome, was found to have a statistically significant association with OTA exposure, in the Egyptian study population (Wafa et al., 1998). The OR of OTA-related nephritic syndrome in this population was 10.79 (95% CI: 2.28-50.91). However, it is worth noting that the sample size of this particular study group was 15: relatively small. Figure 2 summarizes ORs and 95% confidence intervals for each of the adverse health outcomes associated with OTA exposure as measured by urinary OTA in the three study populations.
Figure 2.
Unadjusted odds ratios and 95% confidence intervals for various health endpoints of OTA exposure identified through a systematic review.
Urinary OTA levels obtained by Wafa et al. (1998) were compared to those obtained by Gilbert et al. (Gilbert et al., 2001) and the urinary OTA studies summarized in Table 3. Upon comparison, the levels of OTA found in urine in the Gilbert study ranged from non-detectable to 0.06 ng/ml: much lower than the 3.09 ng/ml mean urinary OTA level found in the nephritic syndrome patients in Wafa et al. (1998). In both the Wafa et al. (1998) and Gilbert et al. (2001) studies, as well as the Croatian study in the systematic review (Domijan et al. 2009), OTA presence was determined and analyzed by HPLC. Urinary OTA levels in humans from several different world regions, summarized in Table 3, range from <0.01-148 ng/ml. The extremely high end of this range comes from a study in Sierra Leone (Jonson-Ellis 2001). When this study and the Egyptian study are excluded, the urinary OTA levels measured in different world regions ranges from non-detectable to 0.860 ng/ml: much lower than the levels found in the study populations in Egypt and Sierra Leone.
CONCLUSIONS
The review of the epidemiological data suggests that, with one exception, there appears to be no statistically significant evidence for human health risks associated with OTA exposure. The one exception concerns an increased risk of nephritic syndrome at very high exposures to OTA, based on case-control studies assessing multiple potential adverse health effects in an Egyptian population (Wafa et al., 1998). However, the sample size of this studied population was very small, and the urinary OTA levels associated with nephritic syndrome were much higher than urinary OTA levels measured in multiple other world regions, with the exception of Sierra Leone.
Nephritic syndrome, also known as glomerulonephritis, is a disorder of the glomeruli characterized by body tissue swelling (edema), high blood pressure, and the presence of red blood cells in the urine. The cause of nephritic syndrome is multifactorial, and the term “nephritic syndrome” itself describes a condition with multiple symptoms. As no other risk factors were controlled for in Wafa et al. (Wafa et al., 1998), it is possible that OTA is not the only etiologic factor in all the cases of nephritic syndrome in this study population. Moreover, the OTA exposures measured in Wafa et al. (Wafa et al., 1998) were over three orders of magnitude higher than the highest exposures measured in Gilbert et al. (Gilbert et al., 2001) and the vast majority of other urinary biomarker studies. Populations in which OTA exposures are extremely high (such as those studied in Egypt and Sierra Leone) may experience a significantly increased risk of nephritic syndrome. However, because this extremely high level of OTA exposure is not expected in most other parts of the world as evidenced by urinary OTA levels collected in multiple other world regions, the risk of OTA-related nephritic syndrome on a global scale is not expected to be significant.
Several limitations exist with our analysis of epidemiological studies on OTA. The main limitation is lack of validated markers of exposure in human populations. While multiple studies examined urinary and serum OTA levels in humans in different regions of the world, none of the studies for which odds ratios were calculated measured actual OTA exposure in the diet. Ideally, the urinary OTA biomarker of exposure should be validated by repeated associations with dietary OTA intake, with reasonable statistical significance, in other populations worldwide. Another limitation of our analysis concerns the small number of studies assessed, and the relatively small sample population sizes in these few case-control studies. It was not feasible to conduct a meta-analysis of OTA-related health disease, because each study assessed measured a different health endpoint. Finally, it was not possible to calculate adjusted odds ratios, because the studies did not provide sufficient data on potential confounders; instead, unadjusted odds ratios were calculated.
For the purposes of establishing appropriate regulatory policies regarding human exposure to ochratoxin A, it is critical to gain a better understanding of OTA’s impacts on human health. To improve our understanding of possible effects of OTA exposure on human health, two types of further studies would be useful. First, larger cohort or case-control studies in different parts of the world, which control for sociodemographic and other potential risk factors, are needed to better establish potential OTA-related human health effects. Second, further duplicate-diet studies are needed to validate biomarkers of OTA exposure in humans. Ideally, these studies would be replicated in different parts of the world; and, similar to (56), would assess OTA intake and biomarker levels over at least one month to account for the long serum half-life and renal elimination of OTA. Such studies would allow for improved exposure assessment, as well as improved correlation with human diseases and conditions, to better inform human health risk assessment of OTA.
Acknowledgement
TB was responsible for literature and data gathering and the calculations, while FW was responsible for data analysis.
Sources of support: This work was funded by the National Cancer Institute (NCI) of the National Institutes of Health (NIH), Grant No. 5R01CA153073-2; and the US Department of Agriculture (USDA) Agriculture and Food Research Initiative (AFRI) Grant No. 20011-67005-30018. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI, NIH, or USDA.
Footnotes
Both authors contributed to the writing of the manuscript. The authors declare no conflicts of interest.
References
- Abid S, Hassen W, Achour A, Skhiri H, Maaroufi K, Ellouz F, Creppy E, Bacha H. Ochratoxin A and chronic human nephropathy in Tunisia: is the situation endemic? Human and Experimental Toxicology. 2003;22:77–84. doi: 10.1191/0960327103ht328oa. [DOI] [PubMed] [Google Scholar]
- Ahn J, Kim D, Kim H, Jahng KY. Quantitative determination of mycotoxins in urine by LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27:1674–1682. doi: 10.1080/19440049.2010.505201. [DOI] [PubMed] [Google Scholar]
- Baker RJ, Pusey CD. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2004;19:8–11. doi: 10.1093/ndt/gfg464. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Austwick PK, Carter RL, Flynn FV, Peristianis GC, Aldridge WN. Balkan (endemic) nephropathy and a toxin-producing strain of Penicillium verrucosum var cyclopium: An experimental model in rats. Lancet. 1977;1:671–675. doi: 10.1016/S0140-6736(77)92115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy GS, Pestka JJ. Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev. 2000;3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- Breitholtz-Emanuelsson A, Minervini F, Hult K, Visconti A. Ochratoxin A in human serum samples collected in southern Italy from healthy individuals and individuals suffering from different kidney disorders. Natural Toxins. 1994;2:366–370. [PubMed] [Google Scholar]
- Castegnaro M, Canadas D, Vrabcheva T, Petkova-Bocharova T, Chernozemsky IN, Pfohl-Leszkowicz A. Balkan endemic nephropathy: Role of ochratoxins A through biomarkers. Mol Nutr Food Res. 2006;50:519–529. doi: 10.1002/mnfr.200500182. [DOI] [PubMed] [Google Scholar]
- Castegnaro M, Maru V, Maru G, Ruiz-Lopez MD. High-performance liquid chromatographic determination of ochratoxin A and its 4R-4-hydroxy metabolite in human urine. Analyst. 1990;115:129–131. doi: 10.1039/an9901500129. [DOI] [PubMed] [Google Scholar]
- Coronel MB, Marin S, Tarrago M, Cano-Sancho G, Ramos AJ, Sanchis V. Ochratoxin A and its metabolite ochratoxin alpha in urine and assessment of the exposure of inhabitants of Lleida, Spain. Food Chem Toxicol. 2011;49:1436–1442. doi: 10.1016/j.fct.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Dahlmann A, Dantzler WH, Silbernagl S, Gekle M. Detailed mapping of ochratoxin A reabsorption along the rat nephron in vivo: the nephrotoxin can be reabsorbed in all nephron segments by different mechanisms. J Pharmacol Esp Ther. 1998;286:157–162. [PubMed] [Google Scholar]
- Domijan AM, Peraica M, Markov K, Fuchs R. Urine ochratoxin A and sphinganine/sphingosine ratio in residents of the endemic nephropathy area in Croatia. Arh Hig Rada Toksikol. 2009;60:387–393. doi: 10.2478/10004-1254-60-2009-1938. [DOI] [PubMed] [Google Scholar]
- Duarte S, Bento J, Pena A, Lino CM, Delerue-Matos C, Oliva-Teles T, Morais S, Correia M, Oliveira MB, Alves MR, Pereira JA. Monitoring of ochratoxin A exposure of the Portuguese population through a nationwide urine survey--Winter 2007. Sci Total Environ. 2010a;408:1195–1198. doi: 10.1016/j.scitotenv.2009.11.048. [DOI] [PubMed] [Google Scholar]
- Duarte SC, Bento JM, Pena A, Lino CM. Ochratoxin A exposure assessment of the inhabitants of Lisbon during winter 2007/2008 through bread and urine analysis. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009;26:1411–1420. doi: 10.1080/02652030903107914. [DOI] [PubMed] [Google Scholar]
- Duarte SC, Pena A, Lino CM. Ochratoxin a in Portugal: a review to assess human exposure. Toxins (Basel) 2010b;2:1225–1249. doi: 10.3390/toxins2061225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte SC, Pena A, Lino CM. Human ochratoxin A biomarkers--From exposure to effect. Critical Reviews in Toxicology. 2011;41:187–212. doi: 10.3109/10408444.2010.529103. [DOI] [PubMed] [Google Scholar]
- Elling F. Ochratoxin A-induced mycotoxic porcine nephropathy: Alterations in enzyme activity in tubular cells. Acta Pathol. Microbiol. Scand. 1979;87:237–243. doi: 10.1111/j.1699-0463.1979.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Elling F, Krogh P. Fungal toxins and Balkan (endemic) nephropathy. Lancet. 1977;1(1213) doi: 10.1016/s0140-6736(77)92761-1. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ochratoxin A in food. 2006;365:1–56. [Google Scholar]
- Fazekas B, Tar A, Kovacs M. Ochratoxin a content of urine samples of healthy humans in Hungary. Acta Vet Hung. 2005;53:35–44. doi: 10.1556/AVet.53.2005.1.4. [DOI] [PubMed] [Google Scholar]
- Gilbert J, Brereton P, MacDonald S. Assessment of dietary exposure to ochratoxin A in the UK using a duplicate diet approach and analysis of urine and plasma samples. Food Addit Contam. 2001;18:1088–1093. doi: 10.1080/02652030110070030. [DOI] [PubMed] [Google Scholar]
- Godin M, Francois A, Le Roy M, Morin J-P, Creppy E, Hemet J, Fillastre JP. Karyomegalic interstitial nephritis. American Journal of Kidney Diseases. 1996;27:166. doi: 10.1016/s0272-6386(96)90047-5. [DOI] [PubMed] [Google Scholar]
- Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, Suzuki N, Fernandes A, Rosenquist T, Medverec Z, Jakovina K, Brdar B, Slade N, Turesky RJ, Goodenough AK, Rieger R, Vukelic M, Jelakovic B. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci U S A. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelberg S, Hult K, Fuchs R. Toxicokinetics of ochratoxin A in several species and its plasma-binding properties. J Appl Toxicol. 1989;9:91–96. doi: 10.1002/jat.2550090204. [DOI] [PubMed] [Google Scholar]
- Haighton LA, Lynch BS, Magnuson BA, Nestmann ER. A reassessment of risk associated with dietary intake of ochratoxin A based on a lifetime exposure model. Critical Reviews in Toxicology. 2012;42:147–168. doi: 10.3109/10408444.2011.636342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassen W, Abid S, Achour A, Creppy E, Bacha H. Ochratoxin A and beta2-microglobulinuria in healthy individuals and in chronic interstitial nephrppathy patients in the centre of Tunisia: a hot spot of Ochratoxin A exposure. Toxicology. 2004;199:185–193. doi: 10.1016/j.tox.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Safety B. o. C, editor. Health Canada. Information Document on Health Canada’s Proposed Maximum Limits (Standards) for the Presence of the Mycotoxin Ochratoxin A in Foods. Ottawa: 2009. [Google Scholar]
- IARC. IARC Monographs on the evaluation of carcinogenic risks to humans: some naturally. Lyon: International Agency for Research on Cancer; 1993. pp. 489–521. [Google Scholar]
- Jimenez AM, Lopez de Cerain A, Gonzalez-Penas E, Bello J, Betebeder AM, Creppy EE. Exposure to ochratoxin A in Europe: comparison with a region of northern Spain. Journal of Toxicology-Toxin Reviews. 1998;17:479–491. [Google Scholar]
- Joint FAO/WHO Committee On Food Additivies (JECFA) Evaluation of certain food additives and contaminants (Thirty-seventh report) WHO Technical Report Series. 1991 [PubMed]
- Joint FAO/WHO Committee On Food Additivies (JECFA) Safety Evaluations of Certain Mycotoxins in Food. JECFA Food Additives Series. 2001
- Joint FAO/WHO Committee On Food Additivies (JECFA) Evaluation of certain food additives and contaminants (Sixty-eighth report) of the Joint FAO/WHO Expert Committee on Food Additives) WHO Technical Report Series. 2007
- Jonsyn-Ellis FE. Seasonal variation in exposure frequency and concentration levels of aflatoxins and ochratoxins in urine samples of boys and girls. Mycopathologia. 2001;152:35–40. doi: 10.1023/a:1011950512675. [DOI] [PubMed] [Google Scholar]
- Jørgensen K, Rasmussen G, Thorup I. Ochratoxin A in Danish cereals 1986–1992. Food Addit. Contam. 1996;13:95–104. doi: 10.1080/02652039609374384. [DOI] [PubMed] [Google Scholar]
- Kawamura O, Maki S, Sato S, Ueno Y. Ochratoxin A in livestock and human sera in Japan quantified by a sensitive ELISA. In: Creppy EE, Castegnaro M, Dirheimer G, editors. Human ochratoxicosis and its pathologies. France: Montrouge; 1993. pp. 123–127. [Google Scholar]
- Khalef A, Zidane C, Charef A, Gharbi A, Tadjerouna M, Betbeder AM, Creppy EE. Ochratoxicose humaine en Algerie. In: Creppy EE, Castegnaro M, Dirheimer G, editors. Human ochratoxicosis and its pathologies. France: Montrouge; 1993. pp. 167–174. [Google Scholar]
- Krogh P. Mycotoxic porcine neprophathy: A possible model for Balkan endemic nephropathy. In: Puhlev A, editor. Endemic Nephropathy. Sofia: Publishing House of the Bulgarian Academy of Science; 1974. pp. 266–270. [Google Scholar]
- Kuiper-Goodman T. Risk assessment and risk management of mycotoxins in food. In: Magan N, Olsen M, editors. Mycotoxins in Food. Detection and Control. Boca Raton (FL): CRC Press LLC; 2004. pp. 3–31. [Google Scholar]
- Kuiper-Goodman T, Hilts C, Billiard SM, Kiparissis Y, Richard IK, Hayward S. Health risk assessment of ochratoxin A for all age-sex strata in a market economy. Food Additives and Contaminants. 2010;27:212–240. doi: 10.1080/02652030903013278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper-Goodman T, Scott PM. Risk assessment of the mycotoxin ochratoxin A. Biomed Environ Sci. 1989;2:179–248. [PubMed] [Google Scholar]
- Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: Systematic review and meta-analysis. European Journal of Cancer. 2012 Mar 8; doi: 10.1016/j.ejca.2012.02.009. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Env Health Perspec. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaroufi K, Achour A, Betbeder AM, Hammami M, Ellouz F, Creppy EE, Bacha H. Foodstuffs and human blood contamination by the mycotoxin ochratoxin A: Correlation with chronic interstitial nephropathy in Tunisia. Archives of Toxicology. 1995;69:552–558. doi: 10.1007/s002040050211. [DOI] [PubMed] [Google Scholar]
- Malir F, Brndiar M, Roubal T, Severa J, Fixa P, Kacerovsky J, Zahradnik J, Osterreicher J, Knizek H, Cerna M. A study of the accumulation of ochratoxin A (OTA) in patients with chronic renal insufficiency (CHRI) in the Czech Republic. Mycotoxin Research. 2001a;17:39–44. doi: 10.1007/BF03036420. [DOI] [PubMed] [Google Scholar]
- Malir F, Roubal T, Brndiar M, Osterreicher J, Severa J, Knizek H, Kacerovsky J, Tmejova M, Bebeder AM, Baudrimont I, Creppy EE. Ochratoxin A in the Czech Republic. Journal of Toxicology-Toxin Reviews. 2001b;20:261–274. [Google Scholar]
- Manique R, Pena A, Lino CM, Molto JC, Manes J. Ochratoxin A in the morning and afternoon portions of urine from Coimbra and Valencian populations. Toxicon. 2008;51:1281–1287. doi: 10.1016/j.toxicon.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Marquardt RR, Frohlich AA. A review of recent advances in understanding ochratoxicosis. J Anim Sci. 1992;70:3968–3988. doi: 10.2527/1992.70123968x. [DOI] [PubMed] [Google Scholar]
- Martlbauer E, Usleber E, Straka M. Mycotoxin Workshop. Germany: Kulmbach; 1996. Ochratoxin A im Serum eines Lehrstuhlkollektivs: 1990–1995; pp. 97–101. [Google Scholar]
- Moss MO. Mode of formation of ochratoxin A. Food Addit Contam. 1996;13 Suppl:5–9. [PubMed] [Google Scholar]
- Munoz K, Blaszkewicz M, Degen GH. Simultaneous analysis of ochratoxin A and its major metabolite ochratoxin alpha in plasma and urine for an advanced biomonitoring of the mycotoxin. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2623–2629. doi: 10.1016/j.jchromb.2009.11.044. [DOI] [PubMed] [Google Scholar]
- Council NR, editor. National Research Council (NRC) Risk assessment in the Federal Government: Managing the process. Washington, DC: National Academy Press; 1983. [PubMed] [Google Scholar]
- Services., D. o. H. a. H., editor. National Toxicology Program (NTP) Toxicology and carcinogenesis studies of ochratoxin A (CAS No. 303-47-9) in F344/N rats (gavage studies) Research Triangle Park (NC): 1989. [PubMed] [Google Scholar]
- Nikolov IG, Chernozemsky IN, Petkova-Bocharova T, Vrabcheva T, Stojchev I, Bankov B, Castegnaro M, Pfohl-Leszkowicz A, Dragacci S, Day N, Gill C, Useleber E, Dietrich R, Martlbauer E. Review of the cooperative studies of the national oncological centre in Bulgaria on Balkan endemic nephropathy and associated urinary tract tumors. Medicine and Biology. 2002;9:119–122. [Google Scholar]
- O'Brien E, Dietrich DR. Ochratoxin A: the continuing enigma. Crit Review Toxicol. 2005;35:33–60. doi: 10.1080/10408440590905948. [DOI] [PubMed] [Google Scholar]
- Olsen M, Thorup I, Knudsen I, Larsen JJ, Hald B, Olsen J. Health evaluation of ochratoxin A in food products. Copenhagen: Nordic Council of Ministers; 1991. [Google Scholar]
- Ozcelik N, Kosar A, Sosal D. Ochratoxin A in human serum samples collected in Isparta-Turkey from healthy individuals and individuals suffering from different urinary disorders. Toxicology Letters. 2001;121:9–13. doi: 10.1016/s0378-4274(00)00291-5. [DOI] [PubMed] [Google Scholar]
- Palli D, Miraglia M, Saieva C, Masala G, Cava E, Colatosti M, Corsi AM, Russo A, Brera C. Serum levels of ochratoxin A in healthy adults in Tuscany: Correlation with individual characteristics and between repeat measurements. Cancer Epidemiology, Biomarkers, Prevention. 1999;8:265–269. [PubMed] [Google Scholar]
- Pascale M, Visconti A. Rapid method for the determination of ochratoxin A in urine by immunoaffinity column clean-up and high performance liquid chromatography. Mycopathologia. 2000;152:91–95. doi: 10.1023/a:1012463227948. [DOI] [PubMed] [Google Scholar]
- Pena A, Seifrtova M, Lino C, Silveira I, Solich P. Estimation of ochratoxin A in portuguese population: new data on the occurrence in human urine by high performance liquid chromatography with fluorescence detection. Food Chem Toxicol. 2006;44:1449–1454. doi: 10.1016/j.fct.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Bondy GS. Mycotoxin-induced immunomodulation. In: Dean JH, Luster MI, Munson AE, Kimber I, editors. Immunotoxicology and immunopharmacology. New York: Raven Press; 1994. pp. 163–182. [Google Scholar]
- Petkova-Bocharova T, Castegnaro M, Pfohl-Leskowicz A, Garren L, Grosso F, Nikolov I, Vrabcheva T, Dragacci S, Chernozemsky IN. Analysis of ochratoxin A in serum and urine of inhabitants from an area with Balkan endemic nephropathy: a one-month follow-up study. J Agric Food Chem. 2003;52:2404–2410. doi: 10.1021/jf030498z. [DOI] [PubMed] [Google Scholar]
- Pfohl-Leszkowicz A, Petkova-Bocharova T, Chernozemsky IN, Castegnaro M. Balkan endemic nephropathy and associated urinary tract tumours: a review on aetiological causes and the potential role of mycotoxins. Food Addit Contam. 2002;19:282–302. doi: 10.1080/02652030110079815. [DOI] [PubMed] [Google Scholar]
- Radic B, Fuchs R, Peraica M, Lucic A. Ochratoxin A in human sera in the area with endemic nephropathy in Croatia. Toxicology Letters. 1997;91:105–109. doi: 10.1016/s0378-4274(97)03877-0. [DOI] [PubMed] [Google Scholar]
- Roth A, Chakor K, Creppy EE, Kane A, Roschenthaler R, Dirheimer G. Evidence for an enterohepatic circulation of ochratoxin A in mice. Toxicology. 1988;48:293–308. doi: 10.1016/0300-483x(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Rubert J, Soriano JM, Manes J, Soler C. Rapid mycotoxin analysis in human urine: a pilot study. Food Chem Toxicol. 2011;49:2299–2304. doi: 10.1016/j.fct.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Ruprich J, Ostry V. Health risk assessment of the mycotoxin ochratoxin A to humans: Czech Republic - Brno - 1991/92. Central European Journal of Public Health. 1993;1:86–93. [PubMed] [Google Scholar]
- Sattler TA, Dimitrov T, Hall PW. Relation between endemic (Balkan) nephropathy and urinary-tract tumours. Lancet. 1977;1:278–280. doi: 10.1016/s0140-6736(77)91824-4. [DOI] [PubMed] [Google Scholar]
- Scott PM. Biomarkers of human exposure to ochratoxin A. Food Addit Contam. 2005;22(Suppl 1):99–107. doi: 10.1080/02652030500410315. [DOI] [PubMed] [Google Scholar]
- Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S, Visconti A. Simultaneous LC-MS/MS determination of aflatoxin M1, ochratoxin A deoxynivalenol, de-epoxydeoxynivalenol, alpha and beta-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem. 2011;401:2831–2841. doi: 10.1007/s00216-011-5354-z. [DOI] [PubMed] [Google Scholar]
- Studer-Rohr I, Schlatter J, Dietrich DR. Kinetic parameters and intraindividual fluctuations of ochratoxin A plasma levels in humans. Arch. Toxicol. 2000;74:499–510. doi: 10.1007/s002040000157. [DOI] [PubMed] [Google Scholar]
- Voo S, Drugarin D, Biriescu A, Margineanu F, Olariu RT, Koreck IA. Ochratoxin A serum levels in patients with Balkan nephropathy. Central European Journal of Occupational and Environmental Medicine. 2002;8:178–182. [Google Scholar]
- Wafa EW, Yahya RS, Sobh MA, Eraky I, El-Baz M, El-Gayar HA, Betbeder AM, Creppy EE. Human ochratoxicosis and nephropathy in Egypt: a preliminary study. Human and Experimental Toxicology. 1998;17:124–129. doi: 10.1177/096032719801700207. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva: World Health Organization; 2002. Evaluation of certain mycotoxins in food; pp. 27–35. [PubMed] [Google Scholar]