To the Editor:
Restrictive dermopathy (RD) is a rare lethal multiple congenital anomaly syndrome characterized by severely taut skin that restricts the growth and movement of the affected fetuses and newborns (OMIM 275210). Skin that appears tight, translucent and partially eroded is associated with joint contractures, rocker bottom feet and characteristic craniofacial abnormalities including micrognathia, a facial expression with the mouth fixed in an “O” position, a small pinched nose and low-set ears [Moulson et al., 2005]. Mutations in two genes responsible for the proper functioning of lamin A, a component of the nuclear lamina and nuclear matrix, have been identified as causative, leading to the classification of RD as a laminopathy. RD is primarily inherited as an autosomal recessive disorder due to homozygous or compound heterozygous mutations in ZMPSTE24, but when mutations in LMNA are responsible it is a sporadic autosomal dominant condition. ZMPSTE24 mutations abolish normal functioning of a zinc metalloproteinase responsible for the correct processing and maturation of lamin A, and mutations in LMNA produce a truncated precursor to lamin A [Morais et al., 2009]. Soon after the initial modern description of RD in the literature [Toriello et al., 1983], one Mennonite family and two Hutterite sibships with RD were identified in Alberta [Lowry et al., 1985]. The Mennonites and Hutterites are distinct genetic isolates today, however, both groups originated during the Protestant Reformation in 16th century Europe as Anabaptist groups [Hostetler, 1985 and Orton et al., 2008]. The Hutterite brethren was established in Austria and moved extensively due to religious persecutions eventually residing in Ukraine before settling in North America in 1874 [Hostetler, 1985]. The Mennonites living in North America are divided into two ancestral groups from different parts of Europe. One group, Old Colony (Dutch-German) Mennonites (OCMs) originated from Dutch and Northern-German ancestry and arrived in North America via Russia and the Ukraine [Orton et al, 2008]. The other group, Old Order Mennonites (OOMs) arrived in North America in the 1700s from Switzerland and Southern Germany via Alsace [Puffenberger, 2003]. Because each population is descended from a small number of founders, many rare alleles were likely introduced by one or a few common ancestors, and their continued isolation has resulted in a high level of certain Mendelian disorders within each population [Boycott et al., 2008, Puffenberger 2003, and Orton et al., 2008]. Lowry and colleagues hypothesized that the Hutterites and OCMs shared a founder mutation for RD. The mutation was thought to have originated in the Mennonites and later introduced into the Hutterite population when some Mennonites joined the Hutterites in 1783, some of which were potential ancestors of the original Hutterite patients identified [Lowry et al., 1985 and Orton et al., 2008]. Recently, we had the opportunity to study a number of Old Colony Mennonite (OCM) and Hutterite patients or their obligate carrier parents in an attempt to identify the causative mutations in these populations.
Prior to this present study, a c.54dupT mutation in ZMPSTE24 was reported in a Mennonite kindred [Moulson et al., 2005]. This led us to speculate that this mutation was the common founder mutation in Hutterite and OCM patients, but it soon became clear through mutational analysis that although the c.54dupT mutation was common in OOM patients (personal observation), it was not causative in OCM and Hutterite families. In the course of us completing this work, an OCM patient with RD was reported to be homozygous for the c.1085dupT mutation in ZMPSTE24 [Li, 2010a], a mutation that has been reported previously in the literature in other populations. This raised the possibility of two distinct mutations causing RD in Mennonites [Miner, 2010; Li, 2010b]. Herein we report our findings identifying the c.1085dupT mutation in OCM and Hutterite patients from Alberta, Manitoba, Ontario and South Dakota, and present data supporting this as a shared founder mutation in OCM and Hutterites.
Blood from four patients with RD or their obligate carrier parents was collected from clinical centres across North America, and relevant ethics approval for genetic study and carrier control studies in the Schmiedeleut was in place.
The first patient was a female child delivered in Alberta at 33 weeks to a 23-year-old G3P2 OCM mother and her 23-year-old husband with no known close consanguinity. Multiple dysmorphic features consistent with a diagnosis of RD were noted including thin, tense and translucent skin, a narrow nose, short palpebral fissures, closely spaced eyes, a narrow mouth and arthrogryposis. Given the lethal prognosis, palliative care was initiated, and the child died at one day of age. Consent for autopsy was provided, and microscopy of the skin displayed abnormal areas of hyperkeratosis and a paucity of elastic fibers, further supporting a diagnosis of RD. PCR amplification followed by sequencing of the coding regions and flanking intronic regions of ZMPSTE24, revealed a homozygous c.1085dupT mutation in this patient.
The second patient was a male child born in Ontario to a 22-year-old primigravida mother of Scottish, Irish and Finnish descent and an OCM father. The baby was delivered at 31 weeks and 5 days after placental abruption and polyhydramnios following an otherwise uneventful pregnancy. Typical features of RD were evident in the child: very tight skin, multiple joint contractures, a narrow mouth with a fixed open position, small and very stiff ears, narrow nostrils, with a wide nasal bridge. The baby had a birth weight at the 50th centile and was macrocephalic with very large anterior and posterior fontanelles and a widely split sagittal suture. There were flexion contractures at the elbows, wrists, fingers, hips, knees, and feet. The skin was eroded on the chest and abdomen, and areas in the axillae displayed cracked skin. Blood vessels were not easily visible below the skin of the chest, but were evident under the skin over the lower extremities. A full thickness skin punch biopsy was consistent with the clinical diagnosis of RD. Palliative care was initiated and the baby died at 10 days of age. Like the first patient, homozygosity for the c.1085dupT was found, but only the father carried the mutation. This raised the possibility that RD in this patient was a result of paternal uniparental isodisomy. This was confirmed using six polymorphic microsatellite markers on chromosome 1 to genotype DNA from the patient and parents. The patient was homozygous for all markers and four of the six markers were informative and paternal in origin (data not shown).
The third patient was born to consanguineous Schmiedeleut Hutterite parents from South Dakota. The baby was delivered prematurely at 34 weeks and was intubated until passing away 24 hours later. The baby was very small, with very thin, taut skin that tore at the hip during delivery. The doctors who treated the mother and patient suspected the child was affected with RD. The fourth patient, who has previously been reported in the literature, was a male Schmiedeleut Hutterite infant from Manitoba also born to consanguineous parents [Reed et al, 1993]. He displayed classical features of RD and died at 32 hours of age. DNA was not available for either Hutterite proband, but upon full sequencing of the coding regions and flanking intronic regions of ZMPSTE24 in the parents of the third patient and sequencing of the region encompassing the c.1085dupT mutation in the parents of the fourth patient, all of the obligate carrier Hutterite parents were found to be heterozygous for the c.1085dupT mutation.
Given that the same c.1085dupT mutation was identified in all Hutterite and OCM families studied, we speculated that this was a single founder mutation accounting for RD in these cases and likely the families originally reported by Lowry. To pursue this, 13 fluorescently labeled microsatellite markers surrounding ZMPSTE24 were used to identify a possible ancestral haplotype. The parents of the Hutterite patients potentially shared a haplotype around ZMPSTE24 spanning 4.32 Mb and 5 microsatellite markers. Homozygosity surrounding ZMPSTE24 in the OCM patients was restricted to only two microsatellite markers on one side of ZMPSTE24. These two homozygous microsatellites were found to be identical to the inferred haplotypes in the obligate carrier parents of both Hutterite patients. This suggests a small, shared haplotype distal to the c.1085dupT mutation of 1.36 Mb (Fig 1).
Figure 1.
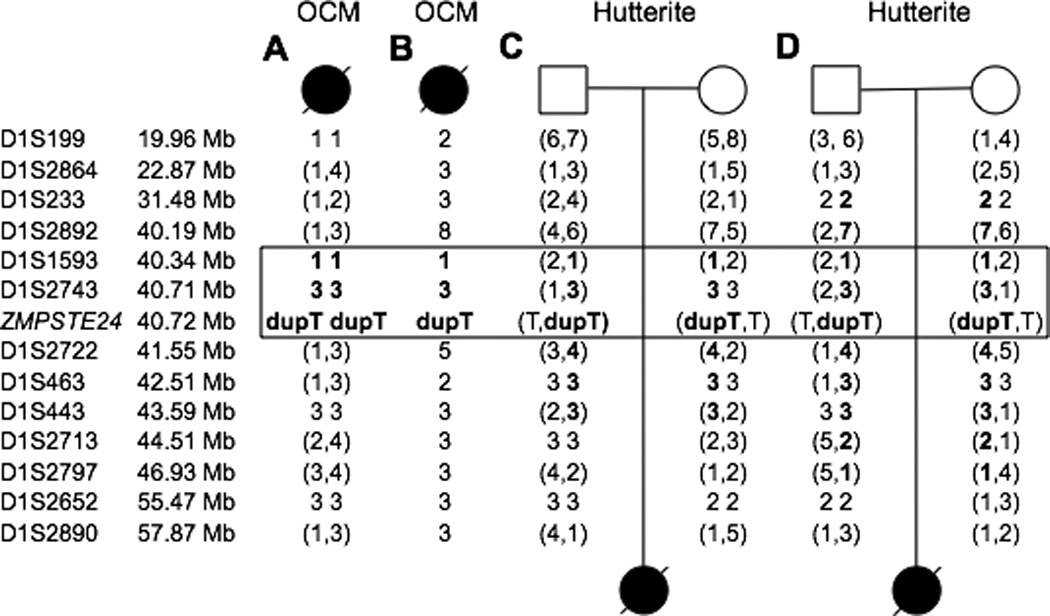
Genotyping reveals a potential shared haplotype surrounding ZMPSTE24. The marker names are indicated on the left, followed by their physical location according to NCBI build 37.1. A) shows the genotypes for Patient 1, B) shows the haplotype for the Patient 2, C) and D) show the genotypes for obligate carrier parents of Patients 3 and 4, respectively. Genotypes in parentheses are phase unknown. Markers in bold indicate either homozygous regions in OCM Patients 1 and 2 or inferred shared haplotypes of the Hutterite parents of Patients 3 and 4. The boxed markers indicate a possible shared haplotype between all patients.
The c.1085dupT is a common mutation resulting in RD [Navarro et al., 2005], that has been seen in populations outside of the Hutterites and OCMs. In fact, it accounts for nearly 75% of the causative RD mutations in ZMPSTE24 in the literature. Furthermore, the mononucleotide sequence in which the duplication occurs may be unstable [Navarro et al., 2005], suggesting that this may be a recurrent mutation due to a mutational hotspot. Although it is possible that the c.1085dupT mutation arose independently in the Hutterites and OCMs, the two shared microsatellite markers directly adjacent to ZMPSTE24 suggest the presence of a small ancestral haplotype associated with the c.1085dupT mutation in these populations. To date, we have been unable to study any obligate carrier relatives of the original kindreds reported by Lowry and colleagues, however we speculate given the widespread nature of this mutation in OCM and Hutterite patients across North America that this is likely the founder mutation for RD shared by these two populations.
We have identified this mutation in two Schmiedeleut Hutterite patients, whereas Lowry et al. reported one Dariusleut and one Lehrerleut Hutterite patient. Additionally, we have studied a cohort of ~1200 Schmiedeleut Hutterites from South Dakota and have determined a high carrier frequency of this mutation at approximately 1/21 (data not shown). Thus, it appears likely that this mutation predates the separation of the Hutterites into the three current essentially endogamous leuts in 1874, lending support to Lowry’s hypothesis that the mutation was introduced into the Hutterites in 1783 when some Mennonites joined the Hutterite brethren. This idea is further strengthened by the greater size of the inferred Hutterite haplotype, with three additional markers distal to the c.1085dupT mutation, as compared to the smaller OCM haplotype. In addition, the c.1085dupT mutation has been seen in patients from the general population with either Dutch or German background [Moulson et al., 2005]; the same ancestry as OCM individuals, suggesting that the c.1085dupT mutation is an ancestral European mutation.
This work further underscores that the OCM and OOM are distinct genetic isolates. While it has not always been explicitly stated in previous publications, we speculate that the c.54dupT mutation is causative of RD in the OOM population. Recently, Miner reported [2010] that the c.54dupT mutation identified in their original Mennonite patients was found in Pennsylvania kindreds and their relatives in Alberta. Both OOM and OCM individuals reside in the province of Alberta, and we speculate that this extended family was likely OOM. Identification of the population-specific mutation causing RD will allow for rapid molecular genetic or prenatal diagnosis in future cases of RD in Hutterite, OCM and OOM families. Of course, it remains possible that other mutations will be identified in these populations in the future.
Finally, this data highlights the importance of considering uniparental disomy as a mechanism for a child manifesting an autosomal recessive disorder when only one parent carries a mutation, facilitating accurate genetic counseling [Baskin et al., 2010]. This is particularly relevant in cases like the second patient when only one parent descends from a genetically isolated population with a high carrier frequency of a certain genetic disorder.
ACKNOWLEDGMENTS
We would like to acknowledge Dr Brian Lowry for his leadership in establishing genetic services in Southern Alberta and whose attention to clinical phenotyping has allowed for ongoing molecular discoveries that have enhanced the care of affected children and their families. We would also like to thank Dr Erik Puffenberger for helpful discussions. CL is supported by the CIHR Training Program in Genetics, Child Development and Health at the University of Calgary.
REFERENCES
- Baskin B, Geraghty M, Ray PN. Paternal isodisomy of chromosome 2 as a cause of long chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency. Am J Med Genet Part A. 2010;152A:1808–1811. doi: 10.1002/ajmg.a.33462. [DOI] [PubMed] [Google Scholar]
- Boycott KM, Parboosingh JS, Chodirker BN, Lowry RB, McLeod DR, Morris J, Greenberg CR, Chudley AE, Bernier FP, Midgley J, Moller LB, Innes AM. Clinical genetics and the Hutterite population: a review of Mendelian disorders. Am J Med Genet Part A. 2008;146A:1088–1098. doi: 10.1002/ajmg.a.32245. [DOI] [PubMed] [Google Scholar]
- Hostetler JA. History and relevance of the Hutterite population for genetic studies. Am J Med Genet. 1985;22:453–462. doi: 10.1002/ajmg.1320220303. [DOI] [PubMed] [Google Scholar]
- Li C. Homozygosity for the common mutation c.1085dupT in the ZMPSTE24 gene in a Mennonite baby with restrictive dermopathy and placenta abruption. Am J Med Genet Part A. 2010a;152A:262–263. doi: 10.1002/ajmg.a.33163. [DOI] [PubMed] [Google Scholar]
- Li C. Response to restrictive dermopathy and ZMPSTE24 mutations in Mennonites: Evidence for allelic heterogeneity. Am J Med Genet Part A. 2010b;152A:2142. doi: 10.1002/ajmg.a.33503. [DOI] [PubMed] [Google Scholar]
- Lowry RB, Machin GA, Morgan K, Mayock D, Marx L. Congenital contractures, edema, hyperkeratosis, and intrauterine growth retardation: a fatal syndrome in Hutterite and Mennonite kindreds. Am J Med Genet. 1985;22:531–543. doi: 10.1002/ajmg.1320220311. [DOI] [PubMed] [Google Scholar]
- Miner JH. Restrictive dermopathy and ZMPSTE24 mutations in Mennonites: Evidence for allelic heterogeneity. Am J Med Genet Part A. 2010;152A:2140–2141. doi: 10.1002/ajmg.a.33503. [DOI] [PubMed] [Google Scholar]
- Morais P, Magina S, Ribeiro Mdo C, Rodrigues M, Lopes JM, Thanh Hle T, Wehnert M, Guimarães H. Restrictive dermopathy--a lethal congenital laminopathy. Case report and review of the literature. Eur J Pediatr. 2009;168:1007–1012. doi: 10.1007/s00431-008-0868-x. [DOI] [PubMed] [Google Scholar]
- Moulson CL, Go G, Gardner JM, van der Wal AC, Smitt JH, van Hagen JM, Miner JH. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol. 2005;125:913–919. doi: 10.1111/j.0022-202X.2005.23846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro CL, Cadiñanos J, De Sandre-Giovannoli A, Bernard R, Courrier S, Boccaccio I, Boyer A, Kleijer WJ, Wagner A, Giuliano F, Beemer FA, Freije JM, Cau P, Hennekam RC, López-Otín C, Badens C, Lévy N. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum Mol Genet. 2005;14:1503–1513. doi: 10.1093/hmg/ddi159. [DOI] [PubMed] [Google Scholar]
- Orton NC, Innes AM, Chudley AE, Bech-Hansen NT. Unique disease heritage of the Dutch-German Mennonite population. Am J Med Genet Part A. 2008;146:1072–1087. doi: 10.1002/ajmg.a.32061. [DOI] [PubMed] [Google Scholar]
- Puffenberger EG. Genetic heritage of the Old Order Mennonites of southeastern Pennsylvania. Am J Med Genet C Semin Med Genet. 2003;121C:18–31. doi: 10.1002/ajmg.c.20003. [DOI] [PubMed] [Google Scholar]
- Reed MH, Chudley AE, Kroeker M, Wilmot DM. Restrictive dermopathy. Pediatr Radiol. 1993;23:617–619. doi: 10.1007/BF02014983. [DOI] [PubMed] [Google Scholar]
- Toriello HV, Higgins JV, Waterman DF. Autosomal-recessive aplasia cutis congenita--report of two affected sibs. Am J Med Genet. 1983;15:153–156. doi: 10.1002/ajmg.1320150122. [DOI] [PubMed] [Google Scholar]


