Abstract
Heliotropium bacciferum is paramount in medicinal perspective and belongs to Boraginaceae family. The crude and numerous fractions of leaves, stem, and roots of the plant were investigated for phytochemical analysis and DPPH radical scavenging activity. Phytochemical analysis of crude and fractions of the plant revealed the presence of alkaloids, saponins, tannins, steroids, terpenoids, flavonoids, glycosides, and phenols. The antioxidant (free radical scavenging) activity of various extracts of the Heliotropium bacciferum was resolute against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical with the avail of UV spectrophotometer at 517 nm. The stock solution (1000 mg/mL) and then several dilutions (50, 100, 150, 200, and 250 mg/mL) of the crude and fractions were prepared. Ascorbic acid was used as a standard. The plant leaves (52.59 ± 0.84 to 90.74 ± 1.00), stem (50.19 ± 0.92 to 89.42 ± 1.10), and roots extracts (49.19 ± 0.52 to 90.01 ± 1.02) divulged magnificent antioxidant activities. For the ascertainment of the fatty acid constituents a gas chromatograph hyphenated to mass spectrometer was used. The essential fatty acids for growth maintenance such as linoleic acid (65.70%), eicosadienoic acid (15.12%), oleic acid (8.72%), and palmitic acid (8.14%) were found in high percentage. The infrared spectra of all extracts of the plant were recorded by IR Prestige-21 FTIR model.
1. Introduction
Medicinal plants and their therapeutic values are extensively used for an array of diseases all over the world. Divergent chemical constituents isolated and characterized from plant species of Boraginaceae family include flavonoids, pyrrolizidine alkaloids, naphthoquinones, phenols, and terpenoids. From different parts of various plants significant pharmacological and biological activities have been reported previously. The biological activities of constituents revealed antitumor, anti-inflammatory, antiviral, antiplatelet, cardiotonic, wound healing, contraceptive, prostaglandin, and wound healing properties [1]. Among foremost health problems, infectious diseases account for 41% of the global disease burden along with noninfectious diseases (43%) and injuries (16%) [2]. A rich source of pyrrolizidine alkaloids is present in Heliotropium bacciferum of family Boraginaceae, some of which have antihyperlipidemic, antitumor, antidiabetic, and antimicrobial properties [3]. Due to the biological activities of the plant antioxidants against reactive oxygen species, such as hydrogen peroxide and superoxide, they have profound significance. Reactive oxygen species (ROS) induce oxidative damage to biomolecules such as carbohydrates, lipids, proteins, and nucleic acids. The oxidative damage causes many diseases such as arteriosclerosis, rheumatoid arthritis, ageing, cancer, and cirrhosis [4]. Because of radiations, chemicals, environmental pollutants, toxins, spicy and deep fried food, and physical stress, free radicals cause change in gene expression, depletion of immune system antioxidants, and abnormal proteins induction. For the production of free radicals in food, living systems, and drugs, oxidation process is one of the most significant routes. Hydroperoxidase and catalase enzymes convert hydroperoxides and hydrogen peroxides to nonradicals and in human body act as natural antioxidants [5]. Several biological mechanisms of polyphenolic substances have been credited to the metal chelating properties or reducing properties of antioxidants [6, 7]. In food nutrition assessment, fatty acids have gained significance in the diagnosis of various diseases and pharmacology [8–10] due to biological importance [11, 12]. In lowering risks of inflammation, heart diseases and, for immunity enhancement, saturated fatty acids either monosaturated or polysaturated have been used [13–18]. For fatty acids determination different analytical techniques have been used which contain spectrophotometric, HPLC [19–21], enzymatic, and gas chromatography (GC) [22, 23]. For the analysis of fatty acids, GC-MS, due to different reasons such as resolution, sensitivity, and speed, was the scheme of choice [24, 25]. The present study was therefore designed to investigate the phytochemical and GC-MS analysis, antioxidant activities, and FTIR spectra of methanol, n-hexane, ethyl acetate, n-butanol, and aqueous extracts of the plant Heliotropium bacciferum.
2. Materials and Methods
2.1. Plant Collection and Identification
Heliotropium bacciferum was collected from district Karak, Khyber Pakhtunkhwa, Pakistan, and then was identified by plant taxonomist in the Department of Plant Sciences, Kohat University of Science and Technology (KUST), Pakistan.
2.2. Extraction and Fractionation
The plant leaves, stem, and roots were shade-dried, crushed, and milled into powder form. The coarse power (500 g) of each part was taken and macerated in methanol for 15 days by the same method as that of Allen Jr. et al. [26]. After maceration, the soluble methanol fraction was filtered and concentrated under vacuum as a consequence of Rotary vacuum evaporator (PLC/MBC (Phy. Std.)/011 Eyela) at 40°C. The crude methanol extract (80 gm) of each part was then suspended in distilled water (500 mL) and partitioned in succession with n-hexane, ethyl acetate, n-butanol, and water.
2.3. Ash Value
The method of Premnath et al. [27] was employed for the determination of ash value of the plant Heliotropium bacciferum. Furnace PLC/MBC/W1/32 was used for the determination of ash value.
2.4. Moisture Value
For the determination of moisture value of the plant, the method of Ashutosh et al. [28] was used. For moisture value determination, Oven PLC/MBC/W1/21 was used.
2.5. Extractive Value
The extractive values of all the five (5) extracts of the leaves, stem, and roots of plant Heliotropium bacciferum were determined by the method of Singh et al. [29].
2.6. Preliminary Phytochemical Screening
Qualitative tests were performed on different extracts of leaves, stem, and roots of the plant by employing standard protocols [30–32] for the detection of carbohydrates, saponins, alkaloids, tannins, terpenoids, steroids, flavonoids, and so forth.
2.7. Diphenyl Picryl Hydrazine (DPPH) Radical Scavenging Activity (Antioxidant Activity)
The DPPH radical scavenging activity of the crude and various fractions of leaves, stem, and roots of Heliotropium bacciferum were determined by UV spectrophotometer at 517 nm in opposition to 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. The antioxidant activity was resolved by the procedures described in the past [33] with slight modifications. Stock solution (1000 mg/mL) of extracts of Heliotropium bacciferum was prepared; then dilutions of the crude and fractions (50, 100, 150, 200, and 250 mg/mL) were prepared. As a standard, vitamin C (ascorbic acid) was used. For comparison, dilutions (50, 100, 150, 200, and 250 mg/mL) of ascorbic acid were also prepared. Solution of DPPH (0.003 g/100 mL) was prepared and then this solution was added to each of the five dilutions of the plant extracts. The absorbance was calculated after 30 minutes at 517 nm by spectrophotometer. The increase in the DPPH free radical scavenging activity is attributed to the decline in the absorbance of the DPPH solution. Then the percent radical scavenging activity (% RSA) was calculated by the following formula:
| (1) |
2.8. Fatty Acids Quantification of Heliotropium bacciferum by Gas Chromatography Mass Spectrometry (GC-MS)
2.8.1. Chemicals and Reagents Used
Methanol (10%), boron trifluoride solution (BF3), 0.5 N methanolic sodium hydroxide (NaOH) solution, n-hexane, sodium chloride (NaCl), fatty acid methyl esters (FAMEs), helium gas (99.99%), tridecanoic acid methyl ester, and n-hexane extract of the plant were used.
2.8.2. Preparation of Standards
For the preparation of internal standard, in 1 mL hexane, 13.7 mg tridecanoic acid methyl ester was dissolved. 10 mg of n-hexane extract was diluted in FAMEs mix standard (10 mL) with dichloromethane (CHCl2) for preparation of external standard.
2.8.3. Methodology Used in GC-MS Technique
A gas chromatograph (Shimadzu) hyphenated to mass spectrometer QP 2010 plus (Tokyo, Japan) outfitted with an autoinjector (AOC-20i) and autosampler (AOC-20S) was used. As a carrier gas, helium was used. On a capillary column (TRB-FFAP; Technokroma) having specifications, i.d., 0.35 mm, length, 30 m, thickness, 0.250 μm, all chromatographic separations were performed. Fatty acids (FA) are polar compounds and are not volatile. The sample analyzed must be volatile for gas chromatographic technique. GC-MS procedure was used for fatty acids investigation. Methylation is focal procedure used for the conversion of nonvolatile fatty acids (FA) into volatile fatty acids methyl esters or FAMEs [34].
The standard procedure was used for determination of fatty acid contents [35]. In 25 mg sample, 0.1 mL internal standard and 1.5 mL methanolic NaOH (0.5 N) were added. The solution was heated for 5 minutes on hot plate in boiling water. The sample was then cooled and 10% CH3OH and 2.5 mL BF3 solution were added. Sample solution again was potted and in boiling water on hot plate heated for about 30 minutes. Then cooled and saturated NaCl solution (4 mL) was added to the esterified solution and extracted twice with hexane (1 mL), filtered by 0.45 micrometer (μm) membrane filter and subjected to GC-MS scheme.
2.9. FTIR (Fourier Transform Infrared Spectroscopy) Study of Plant Extracts
IR Prestige-21 (Shimadzu Japan) FTIR model was used with IR Solutions software [36]. The scheme used by Meenambal et al. [37] was carried out for all the plant extracts in dried form by FTIR spectroscopy.
3. Results
3.1. Moisture, Ash, and Extractive Values
The moisture value of the whole plant was 12% and the ash value was 8.67%. The plant extractive values were calculated separately for all the five (5) extracts of leaves, stem, and roots. Methanol extract of leaves, stem, and roots had high percentage of extractive values shown in Table 1.
Table 1.
Moisture, ash, and extractive values of the plant Heliotropium bacciferum.
| Plant parts | Plant extracts | Extractive value (%) ± standard deviations |
Moisture value (%) | Ash value of the whole plant (%) |
|---|---|---|---|---|
| Leaves | Methanol | 32.64 ± 0.02 | 11.36 ± 0.04 | 8.67 ± 0.06 |
| n-Hexane | 14.76 ± 0.03 | |||
| Ethyl acetate | 15.83 ± 0.02 | |||
| n-Butanol | 16.43 ± 0.04 | |||
| Aqueous | 23.79 ± 0.05 | |||
| Stem | Methanol | 18.13 ± 0.05 | ||
| n-Hexane | 12.46 ± 0.01 | |||
| Ethyl acetate | 13.89 ± 0.03 | |||
| n-Butanol | 14.13 ± 0.10 | |||
| Aqueous | 20.10 ± 0.03 | |||
| Roots | Methanol | 13.10 ± 0.08 | ||
| n-Hexane | 10.32 ± 0.03 | |||
| Ethyl acetate | 12.70 ± 0.06 | |||
| n-Butanol | 11.34 ± 0.12 | |||
| Aqueous | 17.16 ± 0.08 |
3.2. Phytochemical Screening
Phytochemical screening of various extracts of the leaves, stem, and roots of plant Heliotropium bacciferum revealed the presence of steroids, tannins, alkaloids, saponins, glycosides, terpenoids, phenols, and flavonoids (Table 2). In all plant extracts alkaloids were present. Except n-hexane fraction, saponins were present in all plant extracts.
Table 2.
Phytochemical screening of various extracts of Heliotropium bacciferum.
| Plant parts | Extracts | ALK | SAP | TAN | STE | TER | FLA | GLY | PHE |
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Crude | + | + | + | + | + | + | + | + |
| n-Hexane | + | − | + | + | − | + | − | + | |
| Ethyl acetate | + | + | + | − | + | + | + | + | |
| n-Butanol | + | + | − | − | + | + | − | + | |
| Aqueous | + | + | − | − | + | − | + | − | |
|
| |||||||||
| Stem | Crude | + | + | + | + | + | + | + | + |
| n-Hexane | + | − | + | + | − | − | − | + | |
| Ethyl acetate | + | + | + | − | + | − | + | + | |
| n-Butanol | + | + | − | − | − | + | − | + | |
| Aqueous | + | + | − | − | + | + | − | + | |
|
| |||||||||
| Roots | Crude | + | + | + | + | + | + | + | + |
| n-Hexane | + | − | − | + | − | + | − | − | |
| Ethyl acetate | + | + | + | − | + | + | − | + | |
| n-Butanol | + | + | − | − | − | + | − | + | |
| Aqueous | + | + | − | − | + | − | + | − | |
(+): present; (−): absent; ALK: alkaloids, SAP: saponins, TAN: tannin, STE: steroids, TER: terpenoids, FLA: flavonoids, GLY: glycosides, and PHE: phenols.
3.3. Diphenyl Picryl Hydrazine (DPPH) Radical Scavenging Activity (Antioxidant Activity)
Tables 3, 4, and 5 demonstrate the antioxidant activities of the leaves, stem, and roots of plant Heliotropium bacciferum. Standard “ascorbic acid” exhibited significant DPPH radical scavenging activities. The plant leaves extracts revealed excellent DPPH radical scavenging activities ranging from 52.59 ± 0.84 to 90.74 ± 1.00 at concentrations of 50, 100, 150, 200, and 250 mg/mL, respectively (Figures 1, 2, and 3).
Table 3.
In vitro antioxidant activities of all the extracts of Heliotropium bacciferum (leaves).
| Extracts | Quantity in milligram (mg/mL), mean value ± standard deviation | ||||
|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | |
| Ascorbic acid (standard) | 79.12 ± 0.81 | 86.79 ± 0.33 | 89.84 ± 0.72 | 91.51 ± 0.41 | 93.22 ± 0.58 |
| Crude | 72.57 ± 0.94 | 76.97 ± 0.89 | 78.89 ± 0.59 | 83.63 ± 0.57 | 90.18 ± 0.90 |
| n-Hexane | 67.83 ± 1.02 | 73.47 ± 0.94 | 81.48 ± 0.73 | 87.13 ± 0.87 | 89.19 ± 0.53 |
| Ethyl acetate | 72.57 ± 0.71 | 79.23 ± 0.55 | 84.90 ± 0.76 | 87.58 ± 0.99 | 90.74 ± 1.00 |
| n-Butanol | 70.65 ± 0.34 | 75.95 ± 0.48 | 78.21 ± 0.98 | 80.47 ± 0.70 | 84.31 ± 0.92 |
| Aqueous | 52.59 ± 0.84 | 69.97 ± 0.76 | 70.76 ± 0.42 | 80.02 ± 0.32 | 82.73 ± 0.47 |
Table 4.
In vitro antioxidant activities of all the extracts of Heliotropium bacciferum (stem).
| Extracts | Quantity in milligram (mg/mL), mean value ± standard deviation | ||||
|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | |
| Ascorbic acid (standard) | 79.12 ± 0.81 | 86.79 ± 1.33 | 89.84 ± 0.72 | 91.51 ± 0.41 | 93.22 ± 0.58 |
| Crude | 70.34 ± 0.82 | 74.78 ± 0.73 | 77.72 ± 1.07 | 81.57 ± 0.87 | 88.13 ± 0.49 |
| n-Hexane | 67.83 ± 1.02 | 70.39 ± 0.71 | 76.32 ± 0.63 | 80.17 ± 1.01 | 85.29 ± 0.65 |
| Ethyl acetate | 71.63 ± 1.51 | 74.98 ± 0.95 | 78.90 ± 1.02 | 82.34 ± 0.88 | 89.42 ± 1.10 |
| n-Butanol | 68.53 ± 0.90 | 71.31 ± 1.38 | 77.01 ± 0.98 | 80.98 ± 0.60 | 85.79 ± 1.21 |
| Aqueous | 50.19 ± 0.92 | 64.37 ± 0.62 | 69.06 ± 1.42 | 73.02 ± 0.12 | 78.43 ± 0.70 |
Table 5.
In vitro antioxidant activities of all the extracts of Heliotropium bacciferum (roots).
| Extracts | Quantity in milligram (mg/mL), mean value ± standard deviation | ||||
|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | |
| Ascorbic acid (standard) | 79.12 ± 0.81 | 86.79 ± 1.33 | 89.84 ± 0.72 | 91.51 ± 0.41 | 93.22 ± 0.58 |
| Crude | 71.14 ± 0.29 | 75.88 ± 1.03 | 78.82 ± 1.01 | 82.17 ± 0.63 | 88.89 ± 0.39 |
| n-Hexane | 68.13 ± 1.12 | 70.19 ± 1.1 | 76.892 ± 0.13 | 81.17 ± 1.01 | 86.19 ± 0.15 |
| Ethyl acetate | 72.13 ± 1.03 | 75.38 ± 0.81 | 79.10 ± 0.12 | 83.24 ± 0.38 | 90.01 ± 1.02 |
| n-Butanol | 69.13 ± 1.00 | 72.11 ± 1.18 | 78.01 ± 0.12 | 81.28 ± 0.49 | 86.21 ± 1.01 |
| Aqueous | 49.19 ± 0.52 | 63.38 ± 1.62 | 68.16 ± 1.32 | 72.13 ± 0.42 | 77.03 ± 1.30 |
Figure 1.
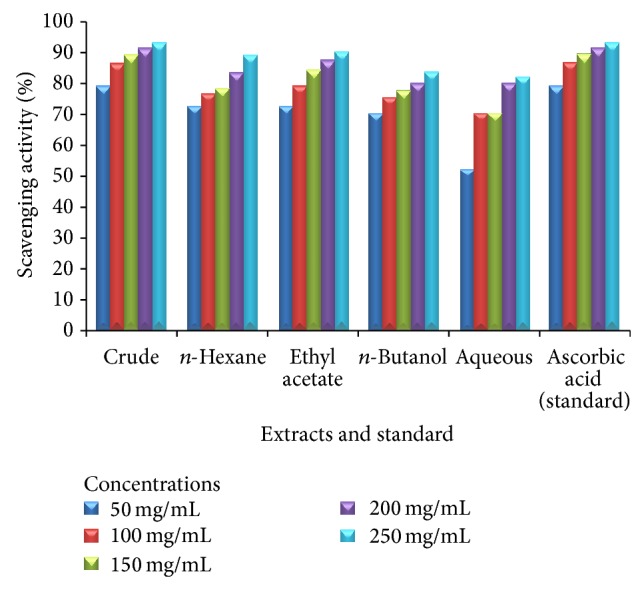
Antioxidant activity of various extracts of the leaves of Heliotropium bacciferum in comparison with the standard ascorbic acid.
Figure 2.
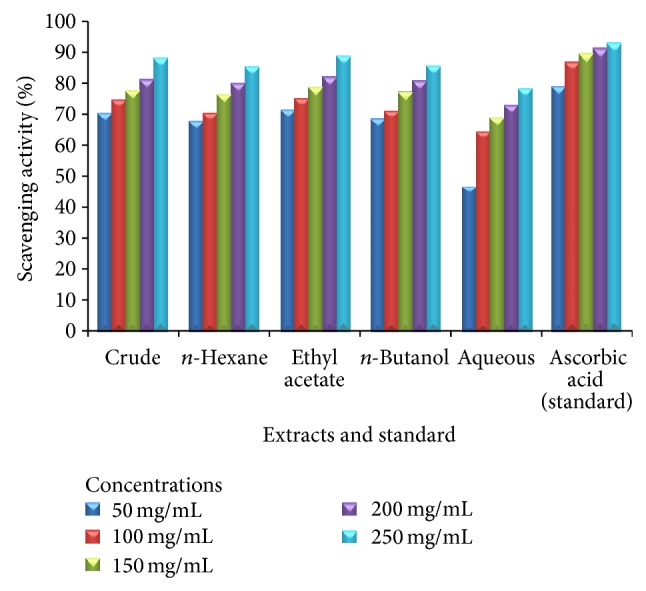
Antioxidant activity of various extracts of the stem of Heliotropium bacciferum in comparison with the standard ascorbic acid.
Figure 3.
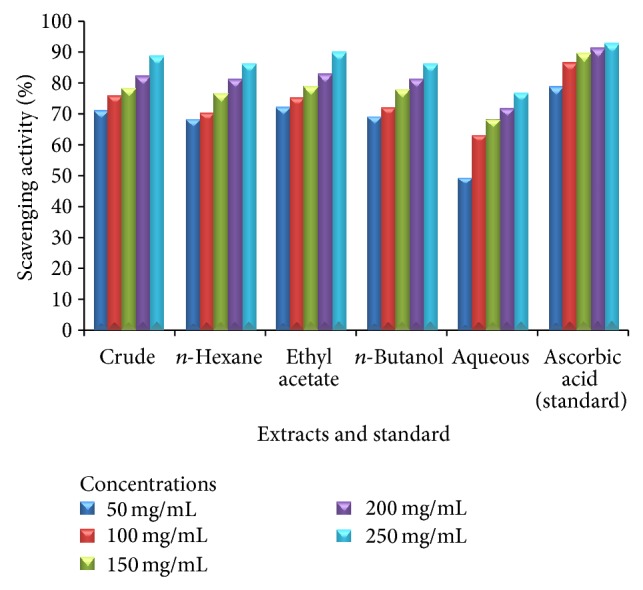
Antioxidant activity of various extracts of the roots of Heliotropium bacciferum in comparison with the standard ascorbic acid.
3.4. Fatty Acids Quantification of Heliotropium bacciferum by Gas Chromatography Mass Spectrometry (GC-MS)
Table 6 viewing the names of fatty acids, area of relevant peaks, relative percentage compositions, times of the analysis, and retention time (R. time) was obtained from gas chromatography mass spectrometry (GC-MS) analysis. The percentage concentration and areas are the mean of the 3 measurements shown in Table 6. Figure 5 shows the obtained GC-MS chromatogram of the n-hexane extract of the plant Heliotropium bacciferum with regularly labeled signals detected by GC-MS detector (Analytes). In the sample under investigation, the saturated and the unsaturated fatty acids were found (Figure 4).
Table 6.
Quantitative results of fatty acids of Heliotropium bacciferum by GC-MS analysis.
| S. number | Name | R. timeα | Area* | Percentage* | Std. Dev.β |
|---|---|---|---|---|---|
| 1 | C18:2c; linoleic acid | 21.361 | 95520 | 65.70 | 0.004 |
| 2 | C20:2; eicosadienoic acid | 21.739 | 23034 | 15.12 | 0.002 |
| 3 | C18:1c; oleic acid | 20.155 | 12574 | 8.72 | 0.007 |
| 4 | C16:0; palmitic acid | 14.618 | 51990 | 8.14 | 0.005 |
| 5 | C18:0; stearic acid | 19.628 | 9500 | 1.74 | 0.003 |
| 6 | C18:1; elaidic acid | 20.392 | 638 | 0.58 | 0.002 |
| 7 | C14:0; myristic acid | 10.955 | 1242 | 0.20 | 0.005 |
αRetention time, *average of three (3) measurements, and βstandard deviation of the three measurements.
Figure 5.
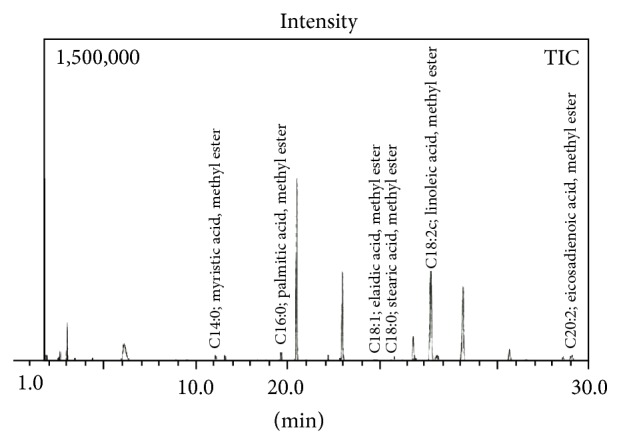
GC-MS chromatogram of the plant n-hexane extract with labeled signals detected by GC-MS detector (Analytes).
Figure 4.
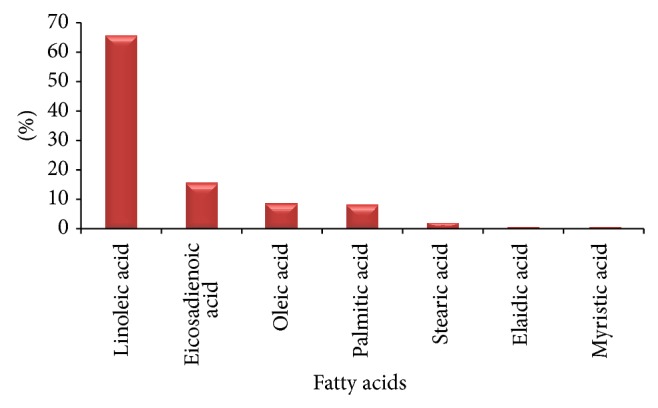
Quantitative analysis of fatty acids of Heliotropium bacciferum by GC-MS analysis.
3.5. FTIR (Fourier Transform Infrared) Spectroscopy
The infrared spectra of various extracts of the plant were recorded by IR Prestige-21 Fourier transform infrared spectroscopy (FTIR) and run under Infrared region of 400–4000 cm−1 range. From absorption spectra, the vibrational assignments, wave number (cm−1), and intensities of dominant peaks were recorded. The dominant IR peaks (see Figures 6–10 in the Supplementary Material available online at http://dx.doi.org/10.1155/2014/829076) of the plant signify the presence of different compounds such as aldehydes, alcohols, amides, ketones, ethers, and carboxylic acids. The more intense bands occurring at 2924 cm−1, 2998 cm−1, 2854 cm−1, 2853 cm−1, 1724 cm−1, 1489 cm−1, and 1230 cm−1 corresponding to the stretching or bending vibrations of O–H or N–H or C–H, C=O and C–Cl or C–S, respectively, signify the existence of amino acids, nitrates, alkenes, ethers, organic-halogen compounds, and carbohydrates.
4. Discussion
Plants containing steroids and flavonoid present in fruits and vegetables reduce the risk of atherosclerosis, which is build-up of fatty deposits in the artery walls [38]. Phenols and flavonoids in olive act as antioxidant, anticancer, antimicrobial, and antibacterial agents [39]. For compound identification, FTIR spectroscopy was used and run between the ranges of 400 and 4000 cm−1 under IR region. The peaks revealed that the plant has compounds such as amides, alcohol, aldehyde, ethers, ketone, and carboxylic acid [40].
Many herbs and plant species have been reported to possess DPPH radical scavenging activity. The plant Heliotropium bacciferum revealed significant DPPH radical scavenging activity. Other plants of genus Heliotropium also showed antioxidant activity. Plant aqueous fraction was primarily active. It has an EC50 value of 20.51 μg/mL. Modak isolated three (3) flavonoids, 3-O-methylgalangin, 7-O-methyleriodictiol, and naringenin from the plant Heliotropium taltalense. The isolated flavonoids exhibited DPPH radical scavenging activity which recommends that Heliotropium bacciferum may possess flavonoids accountable for radical scavenging activity [41]. Phenolic compounds, for example, flavonoids, are of fastidious interest because of their antioxidant activity through oxygen radicals scavenging and peroxidation inhibition. Antioxidants that scavenge free radicals have a key role in inflammatory disorders, cancer, aging, and cardiovascular diseases [42]. Many antioxidant activities are due to the presence of coumarin lignans, flavonoids, flavones, anthocyanin, isocatechins, isoflavones, and catechins [43]. Heliotrine alkaloid demonstrated temporary hypotension perse in dogs and extensively condensed the nicotine induced vasopressor spasmogenic responses [44].
Drugs formulations on the basis of antioxidants are mostly used for the treatment and for the prevention of different diseases, such as Alzheimer's disease, stroke, cancer, diabetes, and atherosclerosis [45]. Some bacterial fatty acid profiles vary in composition according to external stimuli (temperature, pH, nitrogen source, salinity, etc.) [46]. In order to use specific fatty acid biomarkers to interpret environmental community structure, microorganisms should be examined for fatty acid patterns and their variation under different conditions. Taylor and Parkes showed that fatty acid profiles in some sulphate-reducing bacteria can be influenced by carbon source; however, in all cases major fatty acid biomarkers were identifiable [47]. Linoleic acid was found in highest percentage (65.70 ± 0.004%) in Heliotropium bacciferum followed by eicosadienoic acid (15.12 ± 0.002%), oleic acid (8.72 ± 0.007%), palmitic acid (8.14 ± 0.005%), stearic acid (1.74 ± 0.003%), elaidic acid (0.58 ± 0.002%), and myristic acid (0.20 ± 0.005%), respectively. In food nutrition evaluation, fatty acids have immense biological importance. In pharmacology and disease diagnosing, fatty acid also has key significance [48]. The unsaturated (monounsaturated or polyunsaturated) fatty acids are frequently used for declining heart disease risks, inflammation and increasing the immunity [14, 49].
Supplementary Material
The supplementary materials: reveal the FTIR spectra of various plant extracts, which signify the presence of diverse compounds such as aldehydes, alcohols, amides, ketones etc.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Sharma R. A., Singh B., Singh D., Chandrawat P. Ethnomedicinal, pharmacological properties and chemistry of some medicinal plants of Boraginaceae in India. Journal of Medicinal Plants Research. 2009;3(13):1153–1175. [Google Scholar]
- 2.Noumedem J. A. K., Mihasan M., Lacmata S. T., Stefan M., Kuiate J. R., Kuete V. Antibacterial activities of the methanol extracts of ten Cameroonian vegetables against Gram-negative multidrug-resistant bacteria. BMC Complementary and Alternative Medicine. 2013;13, articl 26 doi: 10.1186/1472-6882-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murugesh K., Yeligar V., Dash D. K., Sengupta P., Maiti B. C., Maity T. K. Antidiabetic, antioxidant and antihyperlipidemic status of Heliotropium zeylanicum extract on streptozotocin-induced diabetes in rats. Biological and Pharmaceutical Bulletin. 2006;29(11):2202–2205. doi: 10.1248/bpb.29.2202. [DOI] [PubMed] [Google Scholar]
- 4.Ebadi M. S. Phaemacodynamic Basis of Herbal Medicine. 2nd 2006. [Google Scholar]
- 5.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? The Lancet. 1994;344(8924):721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- 6.Soobrattee M. A., Neergheen V. S., Luximon-Ramma A., Aruoma O. I., Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutation Research—Fundamental and Molecular Mechanisms of Mutagenesis. 2005;579(1-2):200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Williams R. J., Spencer J. P. E., Rive-Evans C. Flavonoids and isofavones (Phytoestrogens): absorption, metabolism and bioactivity. Free Radical Biology and Medicine. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Tomaino R. M., Parker J. D., Larick D. K. Analysis of free fatty acids in whey products by solid-phase microextraction. Journal of Agricultural and Food Chemistry. 2001;49(8):3993–3998. doi: 10.1021/jf001503r. [DOI] [PubMed] [Google Scholar]
- 9.Martin C. A., Carapelli R., Visantainer J. V., Matsushita M., de Souza N. E. Trans fatty acid content of Brazilian biscuits. Food Chemistry. 2005;93(3):445–448. doi: 10.1016/j.foodchem.2004.10.022. [DOI] [Google Scholar]
- 10.Calder P. C. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;79(3–5):101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Wallace F. A., Neely S. J., Miles E. A., Calder P. C. Dietary fats affect macrophage-mediated cytotoxicity towards tumour cells. Immunology and Cell Biology. 2000;78(1):40–48. doi: 10.1046/j.1440-1711.2000.00867.x. [DOI] [PubMed] [Google Scholar]
- 12.Cherif S., Frikha F., Gargouri Y., Miled N. Fatty acid composition of green crab (Carcinus mediterraneus) from the Tunisian mediterranean coasts. Food Chemistry. 2008;111(4):930–933. doi: 10.1016/j.foodchem.2008.05.007. [DOI] [Google Scholar]
- 13.Calder P. C. Dietary fatty acids and the immune system. Lipids. 1999;34(6):S137–S140. doi: 10.1007/BF02562264. [DOI] [PubMed] [Google Scholar]
- 14.Hamberg M., Hamberg G. 15(R)-hydroxylinoleic acid, an oxylipin from oat seeds. Phytochemistry. 1996;42(3):729–732. doi: 10.1016/0031-9422(96)00059-3. [DOI] [Google Scholar]
- 15.Hargrove R. L., Etherton T. D., Pearson T. A., Harrison E. H., Kris-Etherton P. M. Low fat and high monounsaturated fat diets decrease human low density lipoprotein oxidative susceptibility in vitro . Journal of Nutrition. 2001;131(6):1758–1763. doi: 10.1093/jn/131.6.1758. [DOI] [PubMed] [Google Scholar]
- 16.Yaqoob P. Monounsaturated fatty acids and immune function. European Journal of Clinical Nutrition. 2002;56(supplement 3):S9–S13. doi: 10.1038/sj.ejcn.1601477. [DOI] [PubMed] [Google Scholar]
- 17.Villa B., Calabresi L., Chiesa G., Risè P., Galli C., Sirtori C. R. Omega-3 fatty acid ethyl esters increase heart rate variability in patients with coronary disease. Pharmacological Research. 2002;45(6):475–478. doi: 10.1006/phrs.2002.0989. [DOI] [PubMed] [Google Scholar]
- 18.Siscovick D. S., Raghunathan T. E., King I., Weinmann S., Wicklund K. G., Albright J., Bovbjerg V., Arbogast P., Smith H., Kushi L. H., Cobb L. A., Copass M. K., Psaty B. M., Lemaitre R., Retzlaff B., Childs M., Knopp R. H. Dioxins and dioxin-like compounds in the food supply. Journal of the American Medical Association. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J., Li S. P., Yang F. Q., Li P., Wang Y. T. Simultaneous determination of saponins and fatty acids in Ziziphus jujuba (Suanzaoren) by high performance liquid chromatography-evaporative light scattering detection and pressurized liquid extraction. Journal of Chromatography A. 2006;1108(2):188–194. doi: 10.1016/j.chroma.2005.12.104. [DOI] [PubMed] [Google Scholar]
- 20.Romanowicz L., Galewska Z., Gogiel T., Jaworski S., Sobolewski K. Fatty acid composition of triacylglycerols from Wharton's jelly determined by high-performance liquid chromatography. Journal of Biochemical and Biophysical Methods. 2008;70(6):973–977. doi: 10.1016/j.jbbm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Yue X.-F., Zhang Y.-N., Zhang J., Zhang Z.-Q. Free fatty acids profile analysis of alcohol extract of Aconitum taipeicum Hand.-Mazz. with gas chromatography-mass spectrometry. Analytical Methods. 2010;2(6):668–672. doi: 10.1039/b9ay00307j. [DOI] [Google Scholar]
- 22.Rosenfeld J. M. Application of analytical derivatizations to the quantitative and qualitative determination of fatty acids. Analytica Chimica Acta. 2002;465(1-2):93–100. doi: 10.1016/S0003-2670(02)00467-1. [DOI] [Google Scholar]
- 23.Shantha N. C., Napolitano G. E. Gas chromatography of fatty acids. Journal of Chromatography. 1992;624(1-2):37–51. doi: 10.1016/0021-9673(92)85673-H. [DOI] [PubMed] [Google Scholar]
- 24.Destaillats F., Cruz-Hernandez C. Fast analysis by gas-liquid chromatography: perspective on the resolution of complex fatty acid compositions. Journal of Chromatography A. 2007;1169(1-2):175–178. doi: 10.1016/j.chroma.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 25.Yi L., He J., Liang Y., Yuan D., Gao H., Zhou H. Simultaneously quantitative measurement of comprehensive profiles of esterified and non-esterified fatty acid in plasma of type 2 diabetic patients. Chemistry and Physics of Lipids. 2007;150(2):204–216. doi: 10.1016/j.chemphyslip.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Allen L. V., Jr., Popovich N. G., Ansel H. C. Ansel’s Pharmaceutical Dosage forms and Drug Delivery Systems. 8th. Baltimore, Md, USA: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 27.Premnath D., Priya J. V., Ebilin Shabthika E., Patric Gomez M. Antifungal and anti bacterial activities of chemical constituents from Heliotropium indicum Linn. plant. Drug Invention Today. 2012;4(11):564–568. [Google Scholar]
- 28.Ashutosh M., Kumar P. D., Ranjan M. M., et al. Phytochemical screening of ichnocarpus frutescens plant parts. International Journal of Pharmacognosy and Phytochemical Research. 2009;1(1):5–7. [Google Scholar]
- 29.Singh S., Khatoon S., Singh H., Behera S. K., Khare P. B., Rawat A. K. S. A report on pharmacognostical evaluation of four Adiantum species, Pteridophyta, for their authentication and quality control. Revista Brasileira de Farmacognosia. 2013;23(2):207–216. doi: 10.1590/S0102-695X2013005000023. [DOI] [Google Scholar]
- 30.Kayani S.-A., Masood A., Achakzai A. K. K., Anbreen S. Distribution of secondary metabolites in plants of Quetta-Balochistan. Pakistan Journal of Botany. 2007;39(4):1173–1179. [Google Scholar]
- 31.Khan A. M., Qureshi R. A., Ullah F., Gilani S. A., Nosheen A., Sahreen S., Laghari M. K., Laghari M. Y., Hussain I., Murad W. Phytochemical analysis of selected medicinal plants of Margalla hills and surroundings. Journal of Medicinal Plant Research. 2011;5(25):6017–6023. doi: 10.5897/JMPR11.869. [DOI] [Google Scholar]
- 32.Ayoola G. A., Coker H., Adesegun S. A., et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research. 2008;7(3):1019–1024. [Google Scholar]
- 33.Brand-Williams W., Cuvelier M. E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 34.Dron J., Linke R., Rosenberg E., Schreiner M. Trimethylsulfonium hydroxide as derivatization reagent for the chemical investigation of drying oils in works of art by gas chromatography. Journal of Chromatography A. 2004;1047(1):111–116. doi: 10.1016/j.chroma.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Harborn J. B. Phytochemical Method. 17th. chapter 41. 2000. (AOAC 991.39). [Google Scholar]
- 36.Collee J. G., Marr W. Mackie & McCartney Practical Medical Microbiology. 14th. New York, NY, USA: Charchill Living Stone; 1996. Specimen collection, culture containers and media; pp. 95–111. [Google Scholar]
- 37.Meenambal M., Pughalendy K., Vasantharaja C., et al. Phytochemical information from FTIR and GC-MS studies of methol extract of Delonix elat leaves. International Journal of Chemical and Analytical Science. 2012;3(6):1446–1448. [Google Scholar]
- 38.Goldberg A. C., Ostlund R. E., Jr., Bateman J. H., Schimmoeller L., McPherson T. B., Spilburg C. A. Effect of plant stanol tablets on low-density lipoprotein cholesterol lowering in patients on statin drugs. The American Journal of Cardiology. 2006;97(3):376–379. doi: 10.1016/j.amjcard.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 39.Dembitsky V. M. Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids. 2005;40(9):869–900. doi: 10.1007/s11745-005-1449-2.L9814 [DOI] [PubMed] [Google Scholar]
- 40.Ishaq M. S., Hussain M. M., Afridi M. S., et al. In vitro phytochemical, antibacterial, and antifungal activities of leaf, stem, and root extracts of Adiantum capillus veneris . The Scientific World Journal. 2014;2014 doi: 10.1155/2014/269793.269793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modak B., Salina M., Rodilla J., Torres R. Study of the chemical composition of the resinous exudate isolated from heliotropium sclerocarpum and evaluation of the antioxidant properties of the phenolic compounds and the resin. Molecules. 2009;14(11):4625–4633. doi: 10.3390/molecules14114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cioffi G., D'Auria M., Braca A., Mendez J., Castillo A., Morelli I., de Simone F., de Tommasi N. Antioxidant and free-radical scavenging activity of constituents of the leaves of Tachigalia paniculata . Journal of Natural Products. 2002;65(11):1526–1529. doi: 10.1021/np0200764. [DOI] [PubMed] [Google Scholar]
- 43.Aqil F., Ahmad I., Mehmood Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turkish Journal of Biology. 2006;30(3):177–183. [Google Scholar]
- 44.Pandey V. B., Singh J. P., Rao Y. V., Acharya S. B. Isolation and pharmacological action of heliotrine, the major alkaloid of Heliotropium indicum seeds. Planta Medica. 1982;45(4):229–233. doi: 10.1055/s-2007-971378. [DOI] [PubMed] [Google Scholar]
- 45.Devasagayam T. P., Tilak J. C., Boloor K. K., Sane K. S., Ghaskadbi S. S., Lele R. D. Free radicals and antioxidants in human health: current status and future prospects. Journal of Association of Physicians of India. 2004;52:794–804. [PubMed] [Google Scholar]
- 46.Lechevalier P. M. Lipids in bacterial taxonomy—a taxonomist’s view. Critical Reviews in Microbiology. 1976;7:109–210. doi: 10.3109/10408417709102311. [DOI] [PubMed] [Google Scholar]
- 47.Taylor J., Parkes R. J. Identifying different populations of sulphate-reducing bacteria within marine sediment systems, using fatty acid biomarkers. Journal of General Microbiology. 1985;131:631–642. [Google Scholar]
- 48.Stoddart L. A., Smith N. J., Milligan G. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacological Reviews. 2008;60(4):405–417. doi: 10.1124/pr.108.00802. [DOI] [PubMed] [Google Scholar]
- 49.Calder P. C. Dietary fatty acids and the immune system. Lipids. 1999;34(1, supplement):S137–S140. doi: 10.1007/BF02562264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary materials: reveal the FTIR spectra of various plant extracts, which signify the presence of diverse compounds such as aldehydes, alcohols, amides, ketones etc.


