Abstract
Objective
The effects of exercise training on nocturnal blood pressure (BP) dipping status remain unclear. African Americans have the highest prevalence of non-dippers compared to other racial/ethnic populations. In this 6-month study we tested the hypothesis that long-term aerobic exercise training would increase the levels of nocturnal BP dipping in African American non-dippers.
Methods and Results
We recruited African Americans who were non-diabetic, non-smoking and free of cardiovascular (CV) and renal disease. For this analysis, only African Americans with a non-dipping profile, defined as those with absence of a nocturnal decline in systolic or diastolic BP (< 10% of daytime values) which was determined by ambulatory BP monitoring. A pre-post design was employed with baseline and final evaluation including office blood pressure measurement, 24-hour ambulatory blood pressure monitoring, fasted blood sampling, and graded exercise testing. Participants engaged in 6 months of supervised aerobic exercise training (AEXT). Following the AEXT intervention, there were significant increases in systolic BP dipping (baseline: 5.8 ± 3.9% vs. final: 9.4 ± 6.1%, p=0.0055) and pulse pressure dipping (Baseline: −3.1% ± 6.6% vs. final: 5.0% ± 12.8%, p=0.0109). Of the 18 participants with a non-dipping profile at baseline, 8 were non-classified as non-dippers after the AEXT intervention. There were no significant changes in office SBP/DBP following the AEXT intervention.
Conclusions
This study suggests that the non-dipping pattern of ambulatory BP can be improved by chronic AEXT in African American non-dippers and regardless of a change in 24-hr average BP. This finding may be clinically important because of the target organ implication of non-dipping nocturnal BP.
Keywords: dipping status, exercise, nocturnal blood pressure, African American
Introduction
Blood pressure (BP) has a circadian rhythm in which it decreases at night and increases in the morning. Individuals displaying a blunted or absent nocturnal BP reduction, known as non-dipping, have been reported to have increased risk for hypertension, cardiovascular events, and mortality.[1–4] The clinical and prognostic importance of an absence of a normal reduction in BP at night has been demonstrated by the fact that the non-dipper pattern of BP variation is associated with reduced renal function in both normotensive and hypertensive populations.[5–7] Also, nocturnal BP has been considered to be a more important risk factor than office BP since it is not affected by the “white coat effect” and therefore may be a better method of risk stratification for untreated subjects with office hypertension.[8–10] A decrease of 10–20% in night-time BP has been considered to be a normal level of dipping. Lack of nocturnal reduction that is less than 10% compared to the day time BP value has been associated with increased CVD risk factors. [11, 12]
Race is an independent factor that is related to both hypertension and CVD. It is reported that African Americans have the highest prevalence of hypertension.[13, 14] A growing number of Ambulatory Blood Pressure (ABP) studies have reported that African Americans show less nocturnal reduction in BP than Caucasians. [15–18] Given that increased CVD risk is associated with non-dipping, there is a need for efforts toward restoring the nocturnal decline in BP in African Americans.
Aerobic exercise training (AEXT) is one of the non-pharmacologic approaches recommended for hypertension prevention and treatment and evidence has shown AEXT has a substantial effect on the BP control in African Americans.[13–15] The effects of lifestyle behavior changes on nocturnal BP, however, are largely unknown. Despite extensive studies demonstrating health benefits of regular exercise, a relatively low percentage of African Americans participate in routine exercise, which can contribute to hypertension and CVD.[16, 17] Some studies have identified non-dippers as non-responders to exercise compared to dippers with regards to BP management.[18, 19] To our knowledge, however, the effects of AEXT on nocturnal BP in African American non-dippers have not been reported. The purpose of the present study was to investigate the effect of chronic aerobic exercise on the BP dipping status in a selected population of African Americans. It was hypothesized that six months of supervised AEXT would increase the nocturnal BP dipping levels and thereby reduces CVD risk in African American non-dippers.
Methods
Subjects
Subjects were recruited via mailed brochures and local newspaper advertisements. In response to either, subjects were contacted by telephone to assess eligibility. Each subject gave written informed consent following explanation of the study protocol during their first laboratory visit. The protocol was approved by the Temple University Institutional Review Board. The study included African Americans between the ages of 40–63 who were sedentary (regular aerobic exercise <2 days per week), non-smoking, not on lipid-lowering medication, had a clinic BP <160/100mmHg, and had no history of CVD, diabetes, hypercholesterolemia, renal disease or lung disease. Both pre and postmenopausal women were included in the study; all postmenopausal women were not on hormone replacement therapy during the study. Subjects on more than one antihypertensive medication were excluded from the study and subjects using only one antihypertensive medication were tapered off their medication prior to any testing.
Screening
To ensure the eligibility of all qualified subjects, three screening visits were completed before inclusion in the study. Screening visit one consisted of blood sampling and urinalysis following a 12-h overnight fast to assess blood chemistries and renal function. Any individual with total cholesterol >240mg/dL or fasting blood glucose >126mg/dL was excluded from the study. Estimated glomerular filtration rate (eGFR) was calculated using the four-variable modification of diet in renal disease (MDRD) study equation specific to African Americans.[20] Any individual who exhibited evidence of renal disease (eGFR ≤60 mL/min/1.73m2) was excluded from the study. Screening visits two and three required all qualified subjects to undergo a physician-administered physical examination and echocardiogram bicycle stress test to confirm that subjects displayed no evidence of CV, pulmonary or other chronic diseases. In all, 34 African American adults were screened. A total of 18 non-dipping subjects who were qualified based on ambulatory blood pressure, exercise testing, and nocturnal BP patterns participated in the study.
Dietary stabilization
In order to rule out the confounding effect of variations in dietary intake, subjects who met all inclusion criteria after screening underwent dietary stabilization for 6 weeks before testing. Any subject receiving antihypertensive monotherapy (n=3) was tapered off of their medication during this dietary stabilization period. Subjects were instructed by a Registered Dietician on the American Heart Association Dietary Guidelines for Healthy American Adults.[21] This diet consisted of ~55% of total daily calories from carbohydrates, 15% from protein, and <30% from fat, with saturated fat ≤10% of total calories, sodium ≤ 3–4 g/day, and cholesterol intake <300 mg per day. Subjects met with the dietician once per week at which time body weight was recorded for each visit. Subjects were required to remain within 5% of their study entry body weight for the duration of the study in order to minimize the influences of changes in body weight on blood pressure. Compliance to the prescribed diet was monitored by periodic completion of a 3-day food record throughout the study. All subjects remained on this diet until final testing finished.
Office BP measurements
Office BP measurements were made in accordance with JNC 7 guidelines[22] on three separate visits by trained laboratory personnel. BP was measured using an aneroid sphygmomanometer after 5 min of quite rest in a chair with feet on the floor and arm supported at heart level. The appropriate size cuff was determined by upper arm circumference. BP measurements were performed in triplicate, 5 min intervals and the average of the three values was used as the BP for the visit. The mean duration between visits 1 and 2 was 7±1 days. The mean duration between visits 2 and 3 was 8±1 days. The mean systolic BP (SBP) and diastolic BP (DBP) from each visit were used across study visits.
24-H Ambulatory Blood Pressure Monitoring (ABPM)
Subjects underwent 24-h ABPM using a non-invasive monitor (SpaceLabs Medical, Model 90219, Redmond, WA, USA) beginning on the morning of each subject’s typical day, with the exclusion of Friday through Sunday. The BP cuff was fitted to the subject’s non-dominant arm with cuff size determined by upper arm circumference. BP measurements were obtained at 30-min intervals during the day (0600–2200 hours) and 60-min intervals at night (2200–0600 hours). Subjects were instructed not to exercise before or during the monitoring period and to pause momentarily and maintain their body position during each BP measurement. Throughout the duration of the recording period, subjects were required to maintain a diary in which they recorded their activity and mood at the time of each BP measurement. Only recordings of good technical quality (>80% of valid BP measurements) were included in final analyses.
Exercise training program
A submaximal graded exercise test was performed to determine subject’s cardiovascular fitness and to develop individualized exercise prescriptions for the AEXT intervention. A modified Bruce protocol submaximal treadmill exercise test was performed with continuous measurement of breath by-breath gas sampling oxygen consumption (VO2) using a calibrated metabolic cart (Vmax Encore, SensorMedics, Yorba Linda, CA, USA). ECG was continuously monitored, and the treadmill test was terminated when the subject reached 75–80% of their predicted heart rate reserve. A standard regression formula using data collected by indirect calorimetry (VO2 averaged over each 60-second period) and ECG (minute heart rates) was used to predict VO2max, a measure of CV fitness, as recommended by the American College of SportsMedicine Guidelines for Exercise Testing and Prescription. Subjects then engaged in a 24-week AEXT intervention under direct supervision of lab personnel 3×/week, beginning with 20 minutes of exercise/session at 50% of VO2max. Training duration was then increased by 5 minutes each week until 40 minutes of exercise at 50% of VO2max was reached. Training intensity was then increased by 5% each week until 65% of VO2max was achieved. At week 8, subjects reached the desired exercise duration and intensity of 40 minutes at 65% of VO2max, which they maintained as their prescription for the remainder of the study. Exercise modes included treadmill walking/jogging, stair stepping, stationary cycling, rowing ergometry, arm ergometry, and elliptical cross-training. To monitor exercise intensity, subjects were instructed on how to use heart rate monitors. Study personnel recorded subjects’ exercise mode, heart rate, and duration in printed logs every 10 minutes to ensure adherence to the prescribed exercise training program. Heart rate was recorded every 10 minutes. At week 12, subjects completed a second submaximal treadmill exercise test as a basis for adjustment of their exercise prescription to account for changes in CV fitness. The gradual progression of training duration and intensity was used in order to avoid excessive fatigue and musculoskeletal complaints, thereby maximizing adherence.
Blood sample outcome measures
In the morning after a 12-hour overnight fast, blood samples were drawn into EDTA tubes. Samples were centrifuged at 3000 rpm for 20 minutes at 4 °C, after which plasma samples were sent to Quest Diagnostics Inc. as per the standard instructions for the measurements of blood glucose, total cholesterol, triglycerides and hs-CRP.
Statistical analysis
Data are expressed as means ± s.e.m. The distribution of all variables was examined using the Shapiro-Wilk test of normality. ANOVA was used to compare subject demographics between men and pre- and post-menopause women. Variables that were not normally distributed (total cholesterol and triglycerides) were log transformed for statistical analyses, but true physiological values are reported throughout the paper for ease of interpretation. Pre-AEXT and post-AEXT values were compared using the paired t-test. Simple linear regression was used to calculate relationships between the variables. P-values <0.05 were considered statistically significant for all analyses. Statistical analyses were performed using SPSS version 21.0 (SPSS, Chicago, IL, USA).
Results
Subjects
This population was predominately middle-aged (52.9 ± 6.0 years) and women made up 94% of the total. The majority of the population was obese (55.6%); 27.8% were overweight and 16.6% had a normal weight. Twenty-two percent (22.2%) of the subjects were using one antihypertensive medication for BP control before entering the study. One third of the subjects were diagnosed as having pre-diabetes and the remainder had normal plasma glucose levels classification. Demographics of the subjects are summarized in Table 1.
Table 1.
Demographics and biomedical values in Baseline and final stages
| Variables | N | ||||
|---|---|---|---|---|---|
| Age (years) | N=18 | 52.9 ± 6.0 | |||
| Sex (%women) | N=18 | 94.40% | |||
| Menopause (%) | N=8 | 50% | |||
| Antihypertensive medication (%) | N=3 | 22% | |||
| Hormone replacement therapy (%) | N=9 | 6% | |||
|
Baseline
|
Final
|
p-value | |||
| Body mass index (kg/m2) | N=18 | 31.56 ± 6.7 | N=17 | 31.32 ± 6.94 | 0.7660 |
| Body fat% | N=18 | 42.97 ± 9.11 | N=16 | 41.04 ± 8.94 | 0.8921 |
| Blood glucose (mg/dL) | N=18 | 94.33 ± 10.57 | N=16 | 88.56 ± 9.86 | 0.0010 |
| Serum creatinine (μmol/L) | N=18 | 0.86 ± 0.12 | N=16 | 0.80 ± 0.13 | 0.0165 |
| eGFR MDRD (mL/min/1.73m2) | N=18 | 93.23 ± 16.32 | N=16 | 100.65 ± 23.95 | 0.0338 |
| hs-CRP (mg/L) | N=18 | 4.93 ± 5.03 | N=16 | 2.34 ± 2.09 | 0.0025 |
| Total cholesterol (mg/dL) | N=18 | 194.17 ± 30.03 | N=17 | 198.35 ± 31.62 | 0.5580 |
| Triglycerides (mg/dL) | N=18 | 81.83 ± 31.35 | N=17 | 74.18 ± 20.41 | 0.1474 |
| VO2max(ml/kg/min) | N=18 | 23.77 ± 4.54 | N=18 | 26.07 ± 6.73 | 0.0364 |
Values are reported as means ± standard deviations.
Effects of AEXT
The effects of AEXT on clinical characteristics are presented in Table 1. There was a significant increase in predicted VO2max following AEXT. The AEXT intervention also elicited a significant increase in eGFR. Fasting glucose and circulating levels of hs-CRP showed significant decreases following AEXT. There were no significant changes in total cholesterol, triglyceride levels, and BMI.
AEXT did not change 24-H average BP
As Table 2 shows, six months of AEXT did not result in significant changes in office systolic or diastolic BP. There were also no significant changes in 24 hr average, daytime and nighttime SBP and DBP before and after six months of AEXT.
Table 2.
Ambulatory blood pressure monitoring values
| Baseline (N=18) | Final (N=18) | p-value | |
|---|---|---|---|
| Office SBP (mmHg) | 125.3 ± 15.3 | 123.7 ± 15.4 | 0.4157 |
| Office DBP (mmHg) | 78.7 ± 8.6 | 78.0 ± 8.4 | 0.5685 |
| 24 hrPP (mmHg) | 50.4 ± 7.7 | 51.6 ± 9.6 | 0.4386 |
| Daytime PP (mmHg) | 50.2 ± 8.0 | 51.8 ± 10.3 | 0.1803 |
| Night-time PP (mmHg) | 51.4 ± 7.0 | 50.4 ± 9.3 | 0.2567 |
| 24 hrMAP (mmHg) | 94.9 ± 11.5 | 95.4 ± 11.3 | 0.5290 |
| Daytime MAP (mmHg) | 96.6 ± 11.7 | 97.4 ± 11.9 | 0.4182 |
| Night-time MAP (mmHg) | 88.2 ± 11.5 | 86.0 ± 9.4 | 0.1151 |
| 24 hrHR (beats/min) | 76.9 ± 8.6 | 74.8 ± 10.8 | 0.1584 |
| Daytime HR (beats/min) | 78.8 ± 8.7 | 76.5 ± 11.6 | 0.1596 |
| Night-time HR (beats/min) | 69.2 ± 10.2 | 66.9 ± 10.3 | 0.2686 |
| 24-hr SBP (mmHg) | 127.6 ± 14.5 | 128.9 ± 14.7 | 0.4597 |
| Daytime SBP (mmHg) | 129.1 ± 14.9 | 131.0 ± 15.6 | 0.3292 |
| Night-time SBP (mmHg) | 121.5 ± 13.2 | 118.3 ± 12.6 | 0.0708 |
| 24-hr DBP (mmHg) | 77.2 ± 10.9 | 77.4 ± 10.1 | 0.7312 |
| Daytime DBP (mmHg) | 79.0 ± 11.0 | 79.5 ± 10.5 | 0.5410 |
| Night-time DBP (mmHg) | 70.1 ± 11.3 | 68.2 ± 8.8 | 0.2493 |
| Night-time decrease in SBP (%) | 5.8% ± 3.9% | 9.4% ± 6.0% | 0.0055 |
| Night-time decrease in DBP (%) | 11.2% ± 8.2% | 14.0% ± 5.3% | 0.2474 |
| Night-time decrease in HR (%) | 12.4% ± 7.4% | 12.5% ± 6.4% | 0.9424 |
| Night-time decrease in PP (%) | −3.1% ± 6.6% | 5.0% ± 12.8% | 0.0109 |
| Night-time decrease in MAP (%) | 8.7% ± 6.4% | 11.5% ± 5.1% | 0.0995 |
Values are reported as means ± standard deviations. SBP, systolic blood pressure; DBP, diastolic BP; PP, pulse pressure; MAP, mean arterial pressure; HR, heart rate. Day time: 6:00 AM to 10: 00 PM; Night time: 10:00 PM to 6: 00 AM.
AEXT increased BP dipping levels and changed the non-dipping pattern
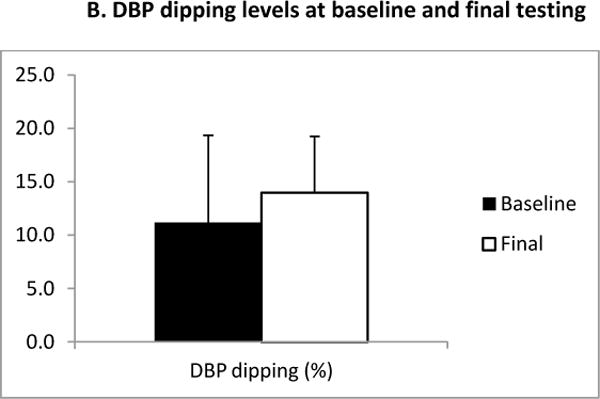
The six month AEXT intervention elicited significant increases in dipping levels of systolic blood pressure (SBP) (Figure 1. A, baseline: 5.8 ± 3.9% versus final: 9.4 ± 6.05%, p=0.006). 8 of the 18 (44.4%) subjects changed from a SBP non-dipper to a SBP dipper pattern. One third of the subjects remained as non-dipper but increased their levels of dipping. Even though the differences were not statistically significant in regards to dipping levels of diastolic blood pressure (DBP) (Figure 1. A2) (baseline: 11.2 ± 8.2 % versus final: 14.0 ± 5.3%), greater dipping levels of DBP were observed after six months of AEXT. At final testing, the number of DBP non-dipper decreased from 4 (44.4%) to 2 (22.2%). Figure 2 summarizes the average hourly SBP dipping levels for the study group at both baseline and final measurements at night time.
Figure 1.
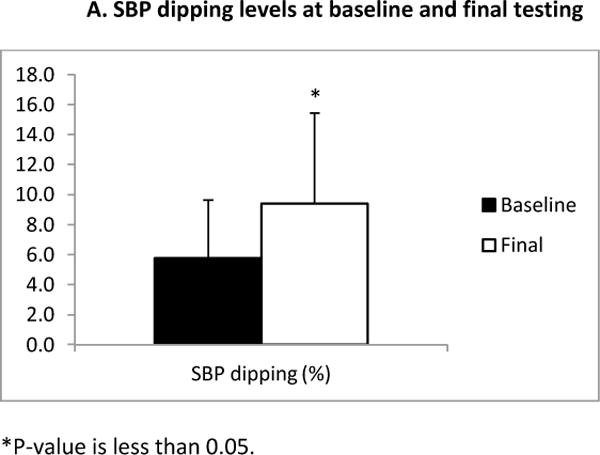
A: Systolic blood pressure dipping levels at baseline and final testing. The black bar represents the measurements at baseline, and the white bar represents the measurements at final test. Bars are expressed as mean ± SEM. *Denotes significantly different (p≤0.05).
B: Diastolic blood pressure dipping levels at baseline and final testing. The black bar represents the measurements at baseline, and the white bar represents the measurements at final test. Bars are expressed as mean ± SEM.
Figure 2.
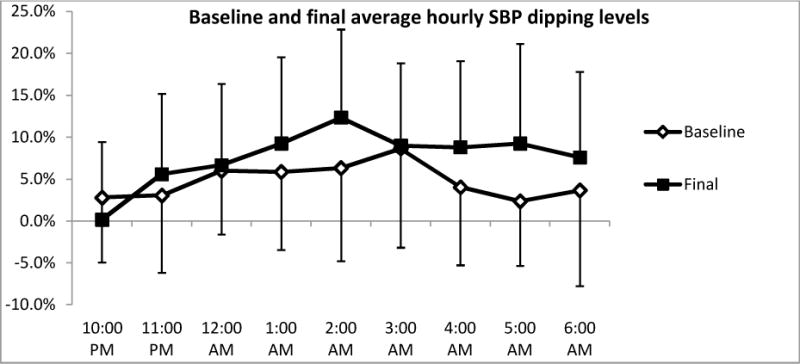
Night time (10: 00 PM to 6: 00 AM) hourly average systolic blood pressure at baseline and final testing. Open line represents the measurements at baseline, and the solid line represents the measurements at final test. Bars are expressed as mean ± SEM.
Dipping status of heart rate (HR), mean arterial pressure (MAP) and pulse pressure (PP) were also examined both at baseline and following AEXT (Table 2). No significant differences were found in HR or MAP between the baseline and final measurements. After 6-months of AEXT, dipping status of PP increased significantly, suggesting that this risk factor for CVD was lowered.
Discussion
In this study, we selected a group of 18 African American subjects with non-dipping status to complete a six month AEXT intervention. The current findings are the first, to our knowledge, to show that AEXT improves the levels of blood pressure dipping in this high-risk population of African Americans with non-dipping status. In particular, we found that AEXT significantly increased the nocturnal reduction of systolic BP and pulse pressure. Moreover, ~44% of the individuals who were classified as non-dippers at study entry were classified as dippers following AEXT.
AEXT was chosen for this study because it is considered to be effective in BP control in subjects with hypertension or at high risk for hypertension.[23] Furthermore, the understanding of chronic aerobic exercise adaptations allows for more efficacious study into possible variations of exercise prescriptions for non-responsive groups such as non-dippers.
The effect of AEXT on ambulatory BP has not been fully determined, especially in high risk populations such as African Americans and non-dippers. Several studies have reported inconsistent results on the effects of exercise to BP dipping status.[24–26] In the present study, at baseline, subjects had blunted nocturnal reduction of BP as demonstrated by average levels of dipping of 5.8 ± 3.9%. After six months of AEXT, the results demonstrated a beneficial effect of chronic exercise towards nocturnal BP dipping by increasing the level to 9.4 ± 6.0%. The results provide further evidence that increased daytime physical activity is a factor that contributes to greater nocturnal BP dipping.[12, 27]
There was no significant change in 24-hr HR average or MAP after six month of AEXT. According to a previous study, blunted heart rate dipping independently predicts cardiovascular mortality.[28] Heart rate is less dependent than BP on physical activity and is therefore less confounded by daily activities involving exercise or movement.[29, 30] It may explain why there was no significant change in 24-hr HR after chronic AEXT. In addition, MAP is determined by the product of cardiac output and systemic vascular resistance, which was found to be strongly associated with younger but not older subjects.[31] The average age of the present study group is 52.9 ± 6.0 years, and it might be a reason for the unchanged MAP after chronic AEXT.
In the present study, no changes were observed in office or 24-hr average ambulatory BP or office BP after six-month AEXT. This finding is consistent with previous studies which showed that AEXT sometimes failed to reduce 24-hr average ambulatory BP in non-dipping hypertensives.[18, 19] We wanted to determine if the lack of change in 24-hr average ambulatory BP or office BP was due to our modest sample size. Therefore, we retrospectively calculated the effect sizes for the differences between baseline and final office BP. Based on these effect sizes, in order to observe a significant difference between baseline and final office SBP, the sample size needed to be 433. For the difference in office DBP, the sample size needed to be 292. Thus, we are confident that the lack of change in BP was not due to the small sample size.
The AEXT intervention improved many of the abnormalities associated with greater cardiovascular risk: reduced eGFR, elevated hs-CRP and high blood glucose. Given the increased cardiovascular risk in African Americans, an intervention that has the potential to improve abnormalities associated with cardiovascular risk is promising, particularly among older individuals with elevated BP who are more likely to have hypertension and CVD.
The Ohasama study examined 1542 subjects from a general population aged 40 and above, and found that each 5% decrease in nocturnal BP dipping was associated with an 18% increase in the risk of cardiovascular mortality.[12] In the present study, no significant correlations were found between the increase in nocturnal BP reduction and the changes in body weight, eGFR, hs-CRP, serum total cholesterol and triglyceride levels after six months of AEXT. The results of the present study demonstrate that the improvement in levels of BP dipping status elicited by AEXT were independent of these changes, which is in agreement with an earlier study that reported increased physical activity levels were independent predictors of the magnitude of nocturnal dip in BP.[32] This suggests that the increased dipping level elicited by AEXT may be beneficial as an adjunct to cardiovascular health in this subject population.
There are some limitations to the present study. First, there was no non-dipper control group for the exercise training. Since the subjects we recruited were individuals with higher cardiovascular risk factors, and some of them used anti-hypertension medications, we could not taper the medication without adding any interventions. Second, the relatively small sample size is also a limiting factor. The study population consisted of subjects who were sedentary and apparently healthy African Americans without diabetes and with clinical BP < 160/100 mmHg, and who were able to finish six months AEXT. Therefore this is a highly selective population. Third, the reproducibility of ABPM is controversial because of the large influence of daily activity on BP.[33, 34] However, there are several studies that have shown highly reproducible results of both short-term and long-term ABPM recordings.[6, 35] Still, further repetition of the ABPM sessions over a longer period of time may have provided an even more accurate BP profile of subjects.
In conclusion, long-term aerobic exercise training proved effective among African American with non-dipping status, resulting in improved nocturnal reduction of SBP and PP. More research is needed to determine the optimal intensity of aerobic training resulting in reductions in 24-hour BP as well as the nocturnal reduction in BP. Given the high prevalence of non-dipping in African Americans, AEXT may be useful as a treatment modality in the management of BP levels among African Americans.
Acknowledgments
This research was supported by NIH/NHLBI Grant RO1 [HL085497] to Michael D. Brown.
Footnotes
No conflicts of interest.
References
- 1.Verdecchia P, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24(6):793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 2.Brotman DJ, et al. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens. 2008;21(1):92–7. doi: 10.1038/ajh.2007.7. [DOI] [PubMed] [Google Scholar]
- 3.Ayala DE, et al. Circadian pattern of ambulatory blood pressure in hypertensive patients with and without type 2 diabetes. Chronobiol Int. 2013;30(1–2):99–115. doi: 10.3109/07420528.2012.701489. [DOI] [PubMed] [Google Scholar]
- 4.International Society for, C et al. 2013 ambulatory blood pressure monitoring recommendations for the diagnosis of adult hypertension, assessment of cardiovascular and other hypertension-associated risk, and attainment of therapeutic goals. Chronobiol Int. 2013;30(3):355–410. doi: 10.3109/07420528.2013.750490. [DOI] [PubMed] [Google Scholar]
- 5.Ahmet Soylu MY, Duzenli Mehmet Akif, Tokac Mehmet, Ozdemir Kurtulus, Gok Hasan. Relation Between Abnormalities in Circadian Blood Pressure Rhythm and Target Organ Damage in Normotensives. Circulation Journal. 2009;73:899–903. doi: 10.1253/circj.cj-08-0946. [DOI] [PubMed] [Google Scholar]
- 6.Cesare Cuspidi GM, Sampieri Lorena, Fusi Veronica, Severgnini Barbara, Michev Iassen, Salerno Maurizio, Magrini Fabio, Zanchetti Alberto. Target organ damage and non-dipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. Journal of Hypertension. 2001;19:1539–1545. doi: 10.1097/00004872-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia P, et al. Blood Pressure Monitoring. Task force III: Target-organ damage, morbidity and mortality. Blood Press Monit. 1999;4(6):303–17. doi: 10.1097/00126097-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Espinosa R, et al. Can blood pressure measurements taken in the physician’s office avoid the ‘white coat’ bias? Blood Press Monit. 2011;16(5):231–7. doi: 10.1097/MBP.0b013e32834b45d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa J, et al. Age and the difference between awake ambulatory blood pressure and office blood pressure: a meta-analysis. Blood Press Monit. 2011;16(4):159–67. doi: 10.1097/MBP.0b013e328346d603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdecchia P. Prognostic value of ambulatory blood pressure : current evidence and clinical implicationss. Hypertension. 2000;35(3):844–51. doi: 10.1161/01.hyp.35.3.844. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien E. Dipping comes of age: the importance of nocturnal blood pressure. Hypertension. 2009;53(3):446–7. doi: 10.1161/HYPERTENSIONAHA.108.127571. [DOI] [PubMed] [Google Scholar]
- 12.Ohkubo T, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10(11):1201–7. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 13.Staffileno BA, et al. Blood pressure responses to lifestyle physical activity among young, hypertension-prone African-American women. J Cardiovasc Nurs. 2007;22(2):107–17. doi: 10.1097/00005082-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard TR, et al. Importance of aerobic fitness in cardiovascular risks in sedentary overweight and obese African-American women. Nurs Res. 2007;56(6):407–15. doi: 10.1097/01.NNR.0000299851.67676.34. [DOI] [PubMed] [Google Scholar]
- 15.Duey WJ, et al. Effects of exercise training on aerobic fitness in African-American females. Ethn Dis. 1998;8(3):306–11. [PubMed] [Google Scholar]
- 16.Carter-Parker K, Edwards KA, McCleary-Jones V. Correlates of physical activity and the theory of planned behavior between African American women who are physically active and those who are not. ABNF J. 2012;23(3):51–8. [PubMed] [Google Scholar]
- 17.Johnston LD, Delva J, O’Malley PM. Sports participation and physical education in American secondary schools: current levels and racial/ethnic and socioeconomic disparities. Am J Prev Med. 2007;33(4 Suppl):S195–208. doi: 10.1016/j.amepre.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Nami R, et al. Aerobic exercise training fails to reduce blood pressure in nondipper-type hypertension. Am J Hypertens. 2000;13(6 Pt 1):593–600. doi: 10.1016/s0895-7061(99)00265-4. [DOI] [PubMed] [Google Scholar]
- 19.Di Raimondo D, et al. Aerobic physical activity based on fast walking does not alter blood pressure values in non-dipper essential hypertensives. Int Angiol. 2012;31(2):142–9. [PubMed] [Google Scholar]
- 20.Levey AS, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Krauss RM, et al. Dietary guidelines for healthy American adults. A statement for health professionals from the Nutrition Committee, American Heart Association. Circulation. 1996;94(7):1795–800. doi: 10.1161/01.cir.94.7.1795. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury A, Lip GY. Exercise and hypertension. J Hum Hypertens. 2005;19(8):585–7. doi: 10.1038/sj.jhh.1001851. [DOI] [PubMed] [Google Scholar]
- 24.Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens. 2013;31(4):639–48. doi: 10.1097/HJH.0b013e32835ca964. [DOI] [PubMed] [Google Scholar]
- 25.Ciolac EG, et al. Acute aerobic exercise reduces 24-h ambulatory blood pressure levels in long-term-treated hypertensive patients. Clinics (Sao Paulo) 2008;63(6):753–8. doi: 10.1590/S1807-59322008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciolac EG, et al. Acute effects of continuous and interval aerobic exercise on 24-h ambulatory blood pressure in long-term treated hypertensive patients. Int J Cardiol. 2009;133(3):381–7. doi: 10.1016/j.ijcard.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Sherwood A, et al. Nighttime blood pressure dipping in postmenopausal women with coronary heart disease. Am J Hypertens. 2012;25(10):1077–82. doi: 10.1038/ajh.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Dov IZ, et al. Blunted heart rate dip during sleep and all-cause mortality. Arch Intern Med. 2007;167(19):2116–21. doi: 10.1001/archinte.167.19.2116. [DOI] [PubMed] [Google Scholar]
- 29.Verdecchia P, et al. Adverse prognostic value of a blunted circadian rhythm of heart rate in essential hypertension. J Hypertens. 1998;16(9):1335–43. doi: 10.1097/00004872-199816090-00015. [DOI] [PubMed] [Google Scholar]
- 30.Sternberg H, et al. Altered circadian rhythm of blood pressure in shift workers. J Hum Hypertens. 1995;9(5):349–53. [PubMed] [Google Scholar]
- 31.Sesso HD, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in Men. Hypertension. 2000;36(5):801–7. doi: 10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 32.Leary AC, et al. Physical activity level is an independent predictor of the diurnal variation in blood pressure. Journal of Hypertension. 2000;18(4):405–410. doi: 10.1097/00004872-200018040-00008. [DOI] [PubMed] [Google Scholar]
- 33.Dimsdale JE, Heeren MM. How reliable is nighttime blood pressure dipping? Am J Hypertens. 1998;11(5):606–9. doi: 10.1016/s0895-7061(98)00033-8. [DOI] [PubMed] [Google Scholar]
- 34.Dimsdale JE, et al. Reliability of nocturnal blood pressure dipping. Blood Press Monit. 2000;5(4):217–21. doi: 10.1097/00126097-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Cuspidi C, et al. Short-term reproducibility of a non-dipping pattern in type 2 diabetic hypertensive patients. J Hypertens. 2006;24(4):647–53. doi: 10.1097/01.hjh.0000217846.65089.19. [DOI] [PubMed] [Google Scholar]


