Abstract
TGF-β-induced CD4+Foxp3+ T cells (iTregs) have been identified as important prevention and treatment strategies for cell therapy in autoimmune diseases and other disorders. However, the potential use of iTregs as a treatment modality for acute graft-verse-host disease (GVHD) has not been realized because iTregs may be unstable and less suppressive in this disease. Here we restudied the ability of iTregs to prevent and treat acute GVHD in two different mouse models. Our results showed that so long as an appropriate iTreg-generation protocol is used, these iTregs consistently displayed a potent ability to control acute GVHD development and reduce mortality in the acute GVHD animal models. iTreg infusion markedly suppressed the engraftment of donor CD8+ cells and CD4+ cells, the expression of Granzyme A and B, the cytotoxic effect of donor CD8+ cells and the production of T cell cytokines in acute GVHD. We therefore conclude that so long as the right methods for generating iTreg cells have been employed, iTregs can indeed prevent and even treat acute GVHD.
Introduction
CD4+CD25+Foxp3+ regulatory T cells (Tregs) are crucial in maintaining immune homeostasis and prevention of autoimmune diseases. The numbers and functions of Tregs have been reported to be abnormal in many autoimmune diseases (1, 2). CD4+Foxp3+ Tregs are heterogeneous and can be divided into at least two populations: thymus-derived naturally occurring Tregs (nTregs) (3), and Treg cell subset induced ex vivo with IL-2 and transforming growth factor beta (TGF-β)(iTregs) (4). Both Treg subsets may have different targets or a synergistic role in controlling unnecessary immunological responses (5). Manipulation of Treg cell therapy is providing a promising approach to the treatment of many autoimmune diseases (5–7). Unlike autoimmune diseases, recent studies have reported that use of iTregs was unable to prevent death in murine model acute graft-versus-host disease (GVHD) (8, 9). GVHD is a major complication of allogeneic hematopoietic stem cell transplant (AHSCT) and is associated with significant morbidity and mortality. Current therapies have limited success in controlling acute GVHD, thus, the development of effective preventions and treatments are critical to the continuing success of AHSCT.
The use of nTregs has led to a limited measure of success in the prevention of acute GVHD (10). However, initial frequencies of nTregs are low, and repetitive rounds of expansion are needed for their clinical use. Unfortunately, the phenotype and function of nTregs is usually compromised following their expansion in vitro (11). Conversely, iTregs are available in sufficient numbers and can be induced into antigen-specific Treg subsets. Recent studies have demonstrated iTregs are stable under inflammatory conditions (12, 13), and based on this finding we re-investigated the suitability of iTregs as a treatment for acute GVHD.
In this study, we used two different animal models to examine the role of iTregs in the prevention of acute GVHD development. Our results showed that when properly generated, iTregs have a potent ability to suppress cytokine production, allogeneic cell expansion, cytotoxic activity and are able to prolong survival. To analyze the discrepancy between this finding and previous studies, we demonstrated that the methods employed in the generation of iTregs can lead to different outcomes. Our results strongly suggest that use of iTregs could be still effective in preventing and treating the complications such as acute GVHD in AHSCT.
Materials and Methods
Animals
BALB/c (H-2d), C57BL/6 (H-2b), DBA2 (H-2d), and B6D2F1 (H-2b/d) mice were purchased from Jackson Laboratory (Bar Harbor, ME). C57BL/6 Foxp3 knock-in mice were generously provided by Dr. Talil Chatilla (UCLA). We maintained breeding colonies in our animal facility. Mice were used at age of 8–12 weeks. All experiments using mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees at University of Southern California and Penn State University.
Development of mouse acute-GVHD models
Model one
Acute GVHD was induced by the intravenous injection of 50×106 splenocytes isolated from B6 mice into B6D2F1 mice as previously reported (14). To maintain as much homogeneity of donor cell populations as possible, acute GVHD was induced on the same day using cells processed simultaneously under the same conditions. After 2 weeks, mice were sacrificed and the cells were measured by staining splenocytes with anti–mouse-H2kb and anti-mouse-H2kd antibody (recognizing donor cells) and cell lineage markers, as listed above (14).
Model two
BALB/c hosts were given total body irradiation (TBI; 800 cGy) from a 200-Kv x-ray source. Within 24 hours, BALB/c mice were intravenously injected with 5×106 T cell-depleted BM cells and 2×106 T cells from B6 mice. Mice were kept on antibiotic water (25 μg/ml neomycin/0.3 U/ml polymyxin B; Sigma-Aldrich). The survival of mice was monitored daily and the body weight was measured weekly (8).
Cell isolation and preparation
CD4+ CD62L+ CD25− T cells were isolated from spleen cells of B6 mice or B6 Foxp3 knock-in mice using a naïve CD4+ T cell isolation kit (Miltenyi Biotec). CD4+ CD25+ cells were sorted from the spleen of B6 mice. T cells depleted bone marrow cells and CD3+ T cells were selected by positive selection and negative selection through AutoMACS (Miltenyi Biotec) by staining anti-CD3 PE antibody (Biolegend) and anti-PE beads. CD4+ CD62L+ CD25− cells and CD4+CD25+ nTreg cells were tested with >98% purity before cell culture.
Generation of CD4 iTreg cells ex vivo
Naïve CD4+ CD62+ CD25− T cells derived from B6 or B6 Foxp3 GFP knock-in mice were cultured in 48–well plates and stimulated with anti-mouse-CD3/CD28 beads (1:5) with IL-2 (50 IU/ml), atRA (5 nM) with TGF-β (5 ng/ml) for 3–13 days. In some experiments, naïve CD4+ CD62+ CD25− T cells were cultured in the plate that had been coated with anti-CD3 (10 μg/ml) and CD28 (1 μg/ml) antibodies with IL-2 (50 IU/ml), TGF-β (5 ng/ml) and atRA (5 nM) for 3–13 days. RPMI 1640 medium was supplemented with 100 units/ml penicillin, 100 mg/ml streptomycin, 10 mM HEPES (Invitrogen Life Technologies), and 10% heat-inactivated fetal calf serum (Hyclone) and was used for all cultures. Foxp3 expression was determined by flow cytometry. The suppressive activity of these cells against T cell proliferation was examined using a standard in vitro suppressive assay as previously described (15). Before intravenous injection, the cells were harvested and beads were removed.
Expansion of nTreg cells ex vivo
The CD4+CD25+ or CD4+GFP+(Foxp3+) cells sorted from B6 or B6 Foxp3 knock-in mice (donor) were cultured with anti-mouse CD3/CD28 beads (1:2) and IL-2 200 IU/ml for 7 days and then harvested and the beads were removed. Foxp3 expression levels were examined by flow cytometry before and after expansion. The expanded nTregs were intravenously transferred to acute GVHD mice.
In vivo cytotoxic T cell activity
In vivo cytotoxic activity was determined using CFSE labeled target cells as described previously (16). Briefly, spleen cells from DBA2 mice were stained with 0.5 μM CFSE (CFSElow) and spleen cells from B6 mice were stained with 5 μM CFSE (CFSEhi). B6D2F1 mice were injected i.v. with a 1:1 mixture (1×107 cells each) of both donor cell populations as target cells. CFSE staining density will distinguish injected DBA/2 and B6 cells. 5 hours after cell transfer, the mice were sacrificed and the splenocytes were analyzed by flow cytometry to determine the percentages of CFSElow and CFSEhi cell populations. The mice were tested individually and the absolute number of each target cell population was calculated in each mouse based on the total spleen cell count multiplied by the percentage of CFSE positive cells determined by flow cytometry. The percent specific lysis was determined by the frequency of donor cells. The formula is: % lysis =(%CFSElow in normal F1-% CFSElow in experiments/%CFSElow in normal F1) (17).
Methylation analysis of Foxp3 gene locus
Bead-iTreg and plate-bound-iTreg were induced as described above. Genomic DNA was isolated from purified T cells using the DNeasy tissue kit (Qiagen, Valencia, California, United States) and processed using the EZ DNA Methylation-Gold kit (Zymo Research) according to the manufacturer’s protocol. DNA methylation analysis was performed by bisulphite sequencing as described previously (18). Purified bisulfite-treated DNA was used in bisulfite sequencing PCR. The PCR products were purified and cloned into pMD-18T vector (Takara) and single clones were selected for sequencing. All the sequencing results were analyzed on BDPC DNA methylation analysis platform. The data showed the methylation levels of CpG islands within CNS2 region of Foxp3 gene locus.
Statistical analysis
Data are represented as means ± SEM. Multiple regression and Student’s t test were used for statistical analysis. The differences in mouse survival were analyzed by the Kaplan-Meier log-rank test. P < 0.05 was considered to be significant.
Results
Infusion of iTregs markedly suppressed the engraftment of donor cells and prevented the reduction of host cells in B6-to-B6D2F1 acute GVHD
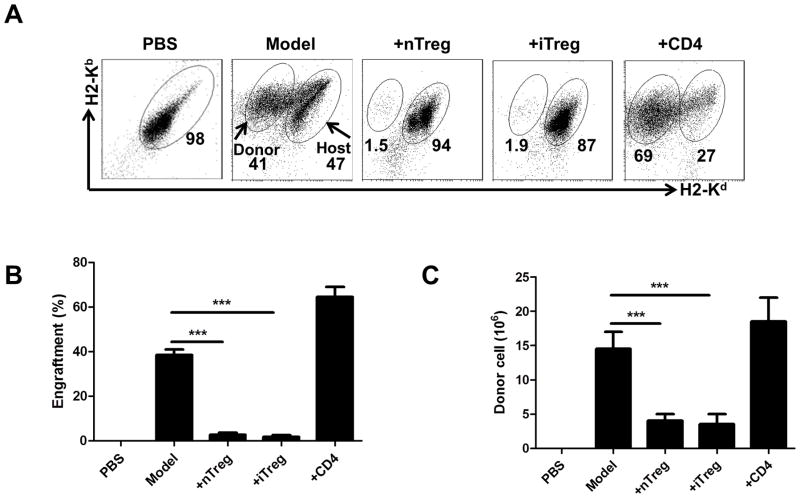
To determine whether the iTregs are able to prevent aGVHD development, 50×106 B6 spleen cells were intravenously injected into immunocompetent B6D2F1 mice. In some groups, 5 ×106 naïve CD4 cells, iTregs or nTregs were co-transferred. After 14 days, mice were sacrificed and the spleen cells were examined by flow cytometry. Donor and host cells can be distinguished by staining H2-Kd and H2-Kb since donor cells are H2-Kb+/d− and host cells are H2-Kb+/d+. Near 50% donor cells can be observed in this aGVHD model 2 weeks after cell transfer that is consistent with a parent (B6)-to-F1 aGVHD model others have previously reported (19) (Fig. 1A). Co-transfer of 5 ×106 CD4 cells significantly increased the donor cell engraftment (Fig. 1A, 1B), suggesting that donor cells promote the development of aGVHD. Interestingly, both iTregs and nTregs markedly prevented the donor cell engraftment. A reduction in numbers of host lymphocytes is one of the characteristics of aGVHD (14). While transfer of B6 spleen cells led to a reduction in numbers of total spleen cells in B6D2F1 mice that provide a standard model control, co-transfer of iTreg or nTregs significantly prevented the reduction of host spleen cells whereas control CD4+ cells did not (Fig. 1C). Thus, both iTregs and nTregs have strongly prevented B6-to-F1 acute GVHD.
FIGURE 1.
Infusion of iTregs markedly suppressed the engraftment of donor cells in model one of acute GVHD. Acute (B6-to-F1) GVHD was induced as described in Material and Methods. After two weeks, mice were sacrificed and donor and host splenic lymphocyte subsets were analyzed by flow cytometry. Splenocytes were stained with H2-Kd and H2-Kb to separate the donor cells from host cells. (A) Representative plots for donor and host cells after 14 days. Relative engraftment (B) and absolute numbers (C) of donor cells are shown for each group. Data are shown as mean ± SEM from 5 independent experiments. Each group in one individual experiment includes 3–4 mice (total n=15–20). *** p <0.001.
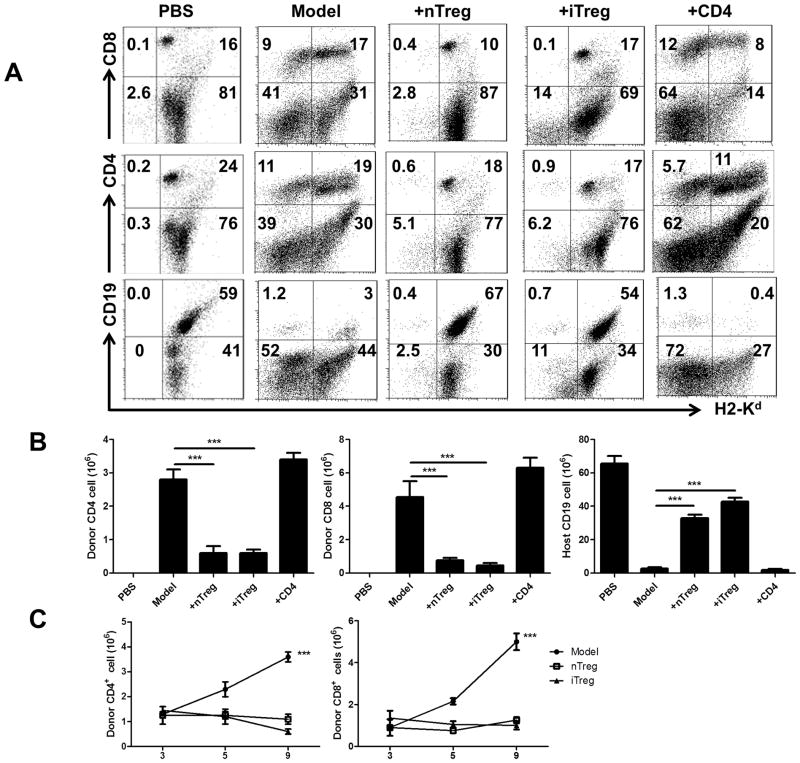
We next examined the cell phenotypes and frequency of donor and host cells in aGVHD and Treg-treatment groups. In B6-to-F1 model, both donor CD4+ and CD8+ cells increased although CD8+ cells increased to a greater degree. It has been known that donor CD8+ cells play a more important role in initiating B6-to-F1 aGVHD (19). Infusion of iTregs or nTregs markedly suppressed the expansion of both donor CD4+ and CD8+ cells (Fig. 2A, 2B). Host B cell killing by activated donor CD8+ cells is another characteristic of B6-to-F1 aGVHD, as shown in Fig. 2A, 2B, the percentages and total numbers of CD19+ B cells in aGVHD were dramatically reduced compared to normal F1 mice. While co-transfer CD4+ cells did not alter host B cell reduction, the co-transfer of iTregs or nTregs almost completely prevented the host B cell decrease. Thus, our data showed that iTregs and nTregs have a potential ability to suppress the expansion of donor CD8 and CD4 cells and prevent cytotoxic effect of donor CD8 cells to host CD19 cells although the cytotoxic effect of CD8 may also need CD4+ cell help.
FIGURE 2.
Infusion of iTregs prevented the expansion of donor CD4 and CD8 cells and the reduction of host cells in model one of acute GVHD. iTreg and nTreg from B6 mice were transferred in acute GVHD phenotype in B6-to-F1. Acute GVHD was induced and mice assessed at 14 days after donor cells transfer as described in Figure 1. (A) Representative plots for donor and host CD4, CD8 and CD19 cells. (B) The frequency of engrafted donor CD4 and CD8 cells and host B cells is shown as total number. Data are from 5 independent experiments. ***P<0.001. (C) 50×106 B6 spleens cells labeled with CFSE were intravenously injected to B6D2F1 mice. In some groups, 5×106 iTregs or nTregs were co-transferred. On day 3, 5 and 9 post transfer, mice were sacrificed and CD4+CFSE+ and CD8+CFSE+ cells in each group were calculated. Data are shown as mean ± SEM from 2 independent experiments. Each group includes 8 mice. *** p <0.001.
Given that donor CD8+ cells play a more important role in the B6-to-F1 aGVHD model, we next focused on the effect of Treg subsets on donor CD8+ cells. Our previous study has demonstrated that iTregs can suppress CD8+ cell proliferation in vitro (20). To validate whether iTregs also can suppress CD8+ immune responses in vivo, we have conducted a suppressive assay in vivo using the aGVHD model. 50×106 B6 CFSE-labeled spleen cells were intravenously injected to B6D2F1 mice. In some groups, 5×106 iTregs or nTregs were co-transferred and total CFSE+ cells are calculated. As shown in Fig. 2C, the total numbers between three groups on day 3 and day 5 after cell transfer were not different, however, donor CD4+ cells, particularly donor CD8+ donor markedly expanded on day 9 after cell transfer. Interestingly, the infusion of either iTregs or nTregs completely prevented the expansion of both donor CD4+ and CD8+ cells (Fig. 2C), indicating both Tregs subsets can suppress effector T cell responses in vivo.
iTregs suppressed cytotoxic role of donor CD8 cells in aGVHD
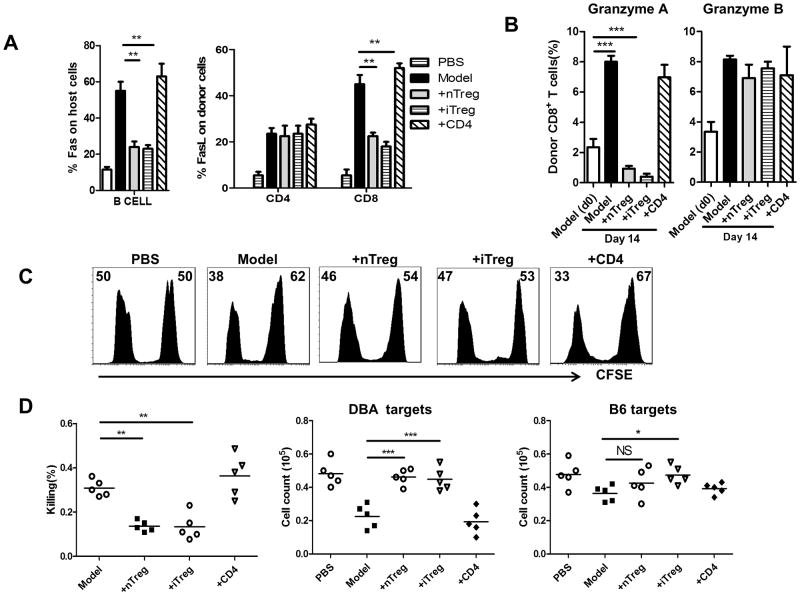
We next sought to determine whether iTregs really suppress the cytotoxic function of CD8+ cells in vivo using aGVHD model. Fas/FasL interaction has been considered to be an important signal pathway in the induction of cell apoptosis in aGVHD via death-inducing signaling complex (21). We examined the levels of Fas and FasL expression in either host or donor cells in aGVHD. We observed that the levels of Fas expression by host B cells was significantly elevated in aGVHD relative to normal F1 mice. Co-transfer of CD4+ cells did not change the Fas expression in host B cells, however, co-transfer of iTregs or nTregs markedly prevented the upregulation of Fas expression in host B cells (Fig. 3A and Fig. 1S). FasL expression on both CD4+ and CD8+ donor cells was elevated following aGVHD induction, and injection of iTregs or nTregs significantly suppressed FasL upregulation on donor CD8+ cells but not CD4+ cells (Fig. 3A and Fig. 1S), suggesting that FasL expression on CD8+ donor cells and Fas expression in host B cells (FasL-Fas signal) are important for the development of aGVHD.
FIGURE 3.
iTregs suppressed cell apoptosis mediated by donor CD8+ cells in aGVHD. (A) 14 days after cell transfer, Fas expression in host B cells and FasL expression in donor T cells from spleens were examined by flow cytometry. (B) Expression of Granzyme A and Granzyme B by donor CD8 T cells from spleen were examined by flow cytometry. Data are shown as mean ± SEM from 5 separate experiments. **P<0.01;***P<0.001. (C) aGVHD induction for each group was described as previously. At day 7 after donor cell transfer, mice were injected i.v. with a 1:1 ratio of CFSElow DBA/2 targets and CFSEhigh B6 targets as described in Material and Methods. After 5 hours, the percentage of surviving B6 and DBA/2 targets was determined by flow cytometry. Data are representative histograms from each group of gated CFSElow/hi target cells and their relative percentages (D) The percentage of anti-host killing relative to parental cells was determined as described in Materials and Methods, and the actual numbers of surviving targets from DBA/2 mice or B6 mice were calculated by cell count for CFSElow and CFSEhi cells. Data are representative or summary of 4 independent experiments. Each group in one experiment includes 3 mice (total n=12). * p <0.05; ** p <0.01; *** p <0.001.
Granzyme A and granzyme B are serine proteases acting in inducing apoptosis in T cells (22), we also examined the expression of granzyme A and granzyme B in donor CD8+ T cells. As shown in Fig. 3B and Fig. 2S, the expression of granzyme A and granzyme B on donor CD8+ cells gradually increased (from 1% to 8% for granzyme A and 4% to 10% for granzyme B from day 0 to day14 after cell transfer). Both Treg subsets treatment can completely prevent the upregulation of granzyme A but not granzyme B on donor CD8+ cells.
We then developed an in vivo CTL experiment to validate whether Treg subsets can suppress cytotoxic effects of CD8+ cells in the aGVHD model. For these experiments, B6-to-F1 aGVHD was induced and Treg treatment was employed as described above, followed 7 days later by co-transfer of 10×106 B6 splenocytes labeled with CFSEhigh and 10×106 DBA2 splenocytes labeled with CFSElow to aGVHD mice that had been treated with or without Treg subsets. Anti-host CTL activity can be analyzed and calculated with the frequency of CFSE-labeled DBA2 (representing host cells) 5 hours after cell transfer (17, 19). As shown in Fig. 3C, injection of CFSE+ cells to normal F1 mice resulted in 50% CFSEhi to 50% CFSElow cell populations, however, when both B6 and DBA2 cells were transferred to aGVHD mice, only 38% of DBA2 cells can be observed, indicating that the donor CD8+ cells have selectively killed DBA2 cells in 5 hours. Treatment with Tregs but not control CD4+ cells markedly prevented the reduction and killing of CFSE+ DBA2 cells (Fig. 3D). Thus, our data have demonstrated that that iTregs and nTregs inhibit anti-host CTL activity in vivo.
iTregs are stable in aGVHD in vivo
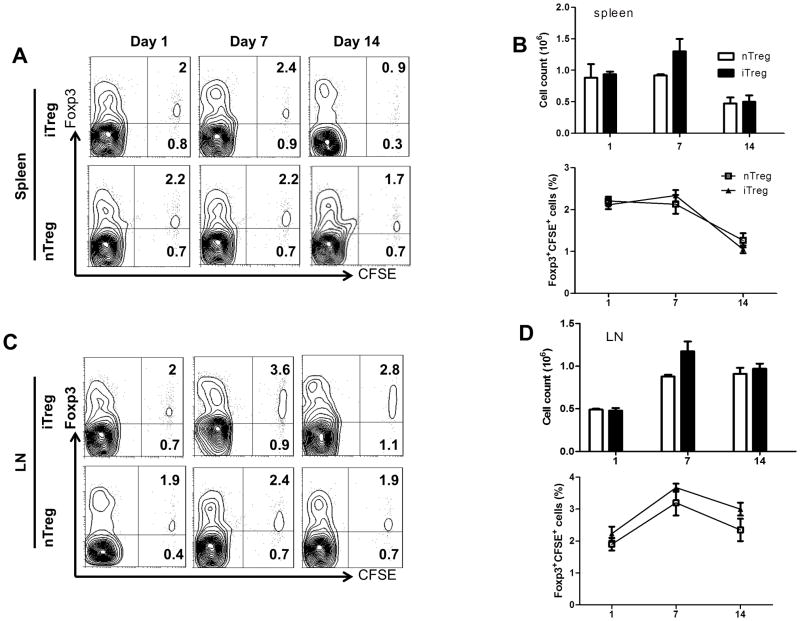
Stability of Tregs has become a critical concern that not only compromises Treg function but could even result in the development of severe autoimmune diseases (23–25). While we and others found that iTreg cells are stable under inflammatory conditions (13, 24), others have reported that iTregs can exhibit some instability when transferred in aGVHD (8). We used a head-to-head comparison to determine the stability of both Treg subsets in B6-to F1 aGVHD model. To this purpose, both iTregs and nTregs from B6 donor mice were labeled with CFSE and transferred to aGVHD mice. Foxp3 expression was fairly stable on both Treg subsets in the draining lymph nodes on day 7 and 14 after cell transfer. As the recipient mouse (F1) is immunocompetent, we did not note evident cell cycle of donor cells before three weeks after cell transfer but some levels of cell cycle can be observed after one month (Fig. 3S). We previously have reported that donor cells had experienced cell proliferation after two months of cell transfer in immunocompletent mice in a heart transplantation model (16). Although Foxp3 expression and total cell numbers of Treg subsets in the spleens were slightly diminished over time, however, no differences were observed between nTregs and iTregs (Fig. 4). Thus, we believe that both Treg subsets are stable at least during the early stages of aGVHD.
FIGURE 4.
iTregs are stable in aGVHD in vivo. CD4+CD25+ nTregs were sorted from the thymus of B6 mice and expanded in vitro with coated beads and IL-2 for 5 days. iTregs were induced as above and CD25+ cells were sorted by FACS. 2×106 nTregs and iTregs from B6 were stained with CFSE and then adoptively transferred to B6-to-F1 aGVHD model. At day 1, 7 and 14, mice were sacrificed and splenocytes were examined by flow cytometry. (A) Representative contour plots of CFSE+Foxp3+ cells in splenocytes at each time point for iTregs and nTregs after transplantation. (B) Absolute and relative number of CD4+Foxp3+ T cells after transplantation. (C) Representative contour plots of CFSE+Foxp3+ cells in LN at each time point for iTregs and nTregs after cell transfer. (D) Absolute and relative numbers of CD4+Foxp3+ T cells after cell transfer. Data are shown as mean ± SEM from 3 independent experiments. Each group in one experiment includes 4 mice (total n=12).
iTregs suppressed cytokine production by donor cells in aGVHD in vivo
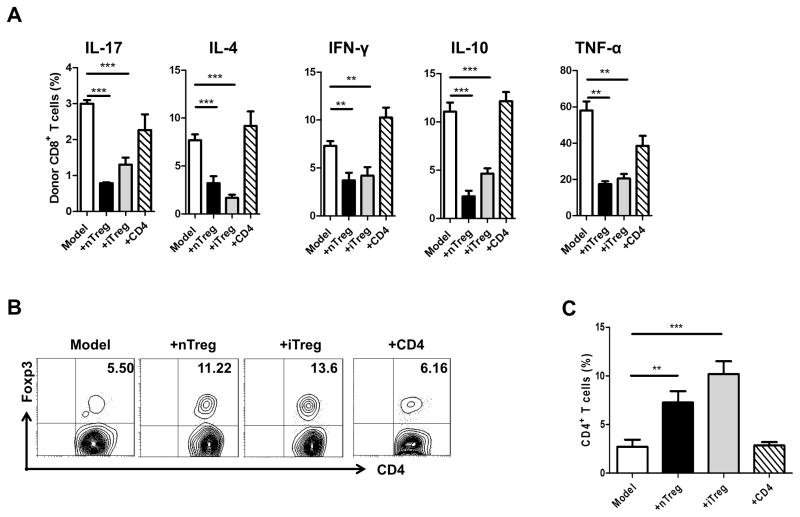
Previous study has suggested that cytokine production by donor cells may contribute to the pathogenesis of aGVHD. To determine whether iTregs suppress aGVHD through controlling cytokine production by donor CD8+ cells, we have investigated the mechanisms underlying the decrease in severity of aGVHD. Injection of iTregs into mice with aGVHD significantly reduced the percentages of donor CD8+ cells secreting the pro-inflammatory cytokines such as IFN-γ, IL-17, IL-4, TNF-α, and IL-10 (Fig. 5A). Other Th2 cytokines, such as IL-5 and IL-13 were undetectable in B6-to-F1 aGVHD model and iTreg treatment did not alter their levels (data not shown).
FIGURE 5.
Infusion of iTreg from B6 mice inhibited cytokine expression of aGVHD and increased the expression of Foxp3 in host cells. (A) Expression of cytokines, including IL-17, IL-4, IFN-γ, IL-6, IL-10, TNFα on Donor CD8+ cells were determined by flow cytometry. Bars show the mean ±SEM of 5 mice per group from 1 of 2 independent experiments.* <0.05,** <0.01, ***<0.001, for this figure and all subsequent figures. (B) Induction of aGVHD was as described previously, iTregs were stained with CFSE before transfer, representative contour plots gated on CFSE− cells of CD4+Foxp3+ expression were determined by flow cytometry. (C) CD4+CFSE− Foxp3+ cells are shown as total number in CD4 T cells. Data are from 3 independent experiments. Each group in one experiment includes 4 mice (total n=12). * p <0.05; ** p <0.01; *** p <0.001.
iTreg and nTreg treatment induces an increased host CD4+Foxp3+ cell population and proper function of these cells may be important for aGVHD protection
Since previous studies have demonstrated that the effect of iTreg treatment is associated with the induction of new generation of Foxp3+ Tregs in the host in chronic GVHD (26), we sought to test whether Foxp3+ Tregs change in the host is also involved in the protective effect of Treg treatment in aGVHD. We examined the levels of host cell Foxp3+ expression following treatment with each donor subset. CFSE labeled iTregs or nTregs were co-transferred to aGVHD and mice were sacrificed 14 days later. Co-transfer of donor CD4+ T cells slightly decreased the percentage of CD4+ host cells expressing Foxp3 (Fig. 5B, 5C). nTreg treatment doubled the frequency of host CD4+ cells expressing Foxp3, but interestingly, iTreg treatment tripled this frequency (Fig. 5B, 5C). It is possible that Treg treatment can induce or expand host CD4+Foxp3+ cells, thus indirectly protecting the development of aGVHD.
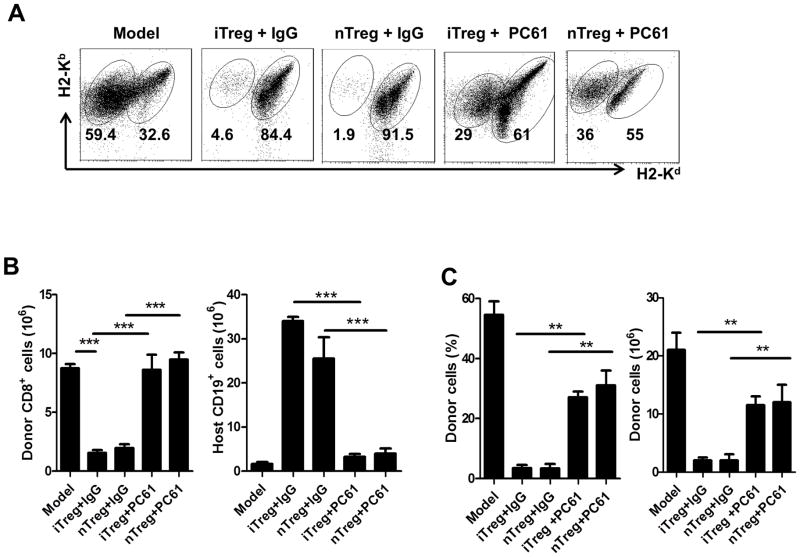
To determine whether Tregs are important for the protection of aGVHD development, we have administrated monoclonal antibody against CD25 (CD25MoAb or PC61) in experimental animals. This approach can abolish the function of Tregs (27, 28). Mice were injected intraperitoneally (IP) with PC61 (anti-CD25 monoclonal antibody; 250 μg/mouse) or control IgG. On the same day, 50×106 B6 cells were injected intravenously into F1 mice with 1 ×106 CD4 cells, iTregs or nTregs. After 14 days, the mice were sacrificed and the spleen cells were examined by flow cytometry. The protective effect of both nTregs and iTregs on aGVHD was substantially abrogated following treatment with PC61. The Treg-mediated suppression on donor CD8+ cells, protection of host B cells and total host cells were significantly abolished under these conditions (Fig. 6).
FIGURE 6.
Foxp3+ Treg function is important for the protection of aGVHD. Host mice were injected intraperitoneally (IP) with PC61 (anti-CD25 monoclonal antibody; 250g/mouse) or control IgG while cells were transferred. The induction of aGVHD and iTreg was as described previously. After 14 days, mice were sacrificed and donor cells and host cells were enumerated by flow cytometry. (A) Representative plots for donor and host cells 14 days after cells transfer. (B) Engraftment of donor CD8 cells and host B cells were shown as total numbers. (C) Relative and absolute numbers of engraftment for donor cells were shown in each group. Data are shown as mean ± SEM from 2 independent experiments. Each group in one experiment includes 4 mice (total n=8). ** p <0.01; *** p <0.001.
iTregs reduced mortality and prevented weight loss in the B6→BALB/c model
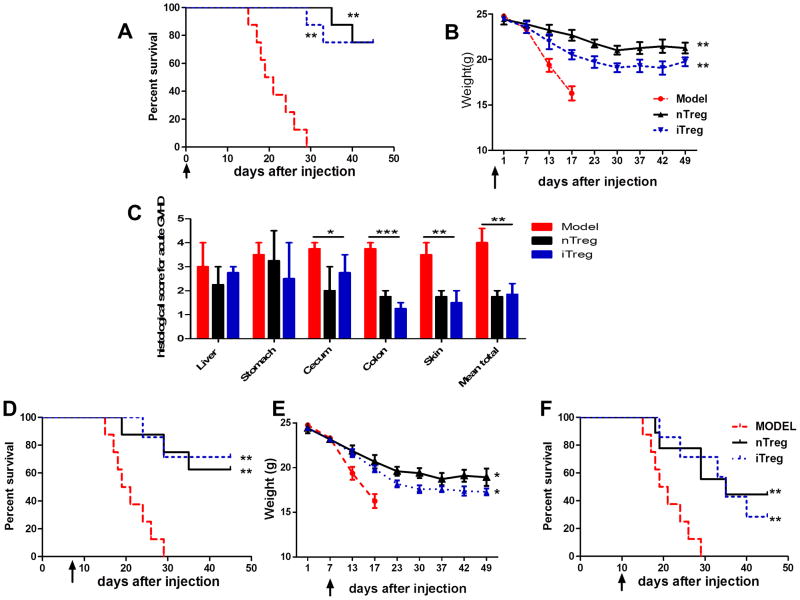
We next used a different (B6-to-BALB/c) aGVHD model to further validate whether iTregs are consistently efficacious in acute GVHD. Mortality and weight loss are two common parameters in the evaluation of this aGVHD (29). Others have found that iTregs induced from B6 donor mice were ineffective in protection from mortality using this model of aGVHD. Lethally irradiated (800 cGy) BALB/c recipients were transplanted with B6 donor derived 5×106 T-cell-depleted BM (TCD-BM) plus 2×106 MACS-purified T cells alone, or together with 1 ×106 nTregs or iTregs isolated or induced from B6 mice. Recipients of nTregs and iTregs showed a significantly improved survival compared with the recipients of T cells alone (Figure 7A, 7B). The pathological examination showed the significantly lower scores for inflammation in different organs such as stomach, cecum, colon and skin in both iTreg and nTreg treatment compared to disease group (Fig. 7C).
FIGURE 7.
iTregs reduced the death and prevented the weight losing in B6- to-BALB/c aGVHD. Irradiated BALB/c mice were transplanted with T cell-depleted BM cells and CD3+ splenic T cells alone (BM only) or with/without 1 ×106 CD4+Foxp3+ iTregs or nTregs from B6 mice. (A) Mice survival while Treg subsets were transferred on day 0. (B) Mice weight while Treg subsets were transferred on day 0. (C) Histological examination in indicated organs at day 21 post cell transfer. Data are the mean ± SEM of three mice in each group. (D and E) Mice survival and weight while Treg subsets were transferred on day 7 after spleen cells transfer. (F) Mice survival while Treg subsets were transferred on day 10 after spleen cells transfer. Data are shown as the mean ± SEM and the cumulative results from four independent experiments (total n=8 in each group). * p <0.05; ** p <0.01; *** p <0.001.
We further evaluated the possibility that the iTregs and nTregs could be effective for treatment of B6-to-BALB/c aGVHD. iTregs and nTregs were injected 7 days or 10 days after BM and T cell transplantation (Fig. 7D, 7E). As in the other model, iTregs and nTregs significantly prolonged mice survival after disease onset. Not surprisingly, the treatment was more effective when Tregs were given on day 7 rather than on day 10. Taken together, our results suggest that both iTregs and nTregs possess the capability of inducing peripheral tolerance in mouse acute GVHD model.
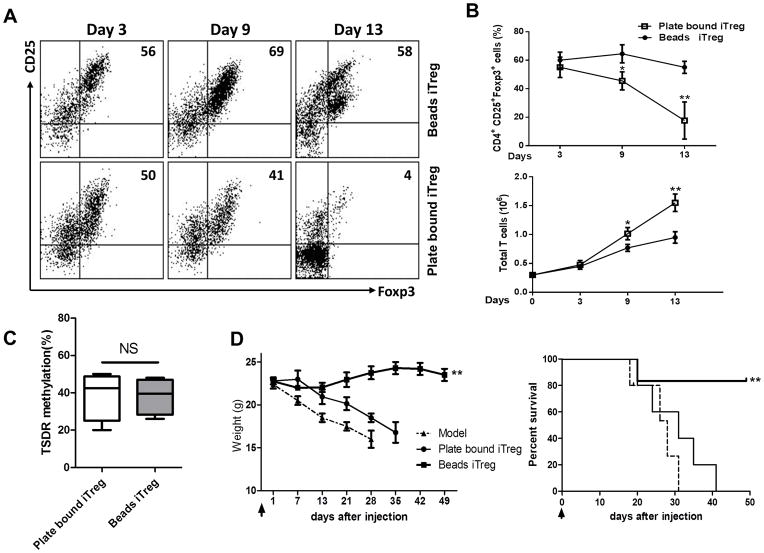
To evaluate the differences observed in iTreg treatment of aGVHD between other groups and the current study, we analyzed the methods used in the other studies and compared them to our own. Notably, we found a significant difference in the method used in the generation of iTregs. Whereas others (9, 18) have used plate-bound anti-CD3 antibody to stimulate iTreg differentiation, we instead used the beads that had been pre-coated with anti-CD3 and anti-CD3/CD28 antibodies. First, we examined Foxp3 expression of iTreg subsets generated using each of the two methods. While iTregs generated using plate-bound antibodies and coated-beads showed similar expression of Foxp3 at day 3, the expression of Foxp3 differentiated with plate-bound was significantly decreased by day 7 and markedly lost by day 13. Conversely, iTregs induced with coated-beads showed much greater Foxp3 stability during day 9 to day 13 (Figure 8A, 8B). It came as no surprise that the frequency of Foxp3− T cells was much higher in iTreg populations generated on plate-bound antibodies as compared to those generated with coated-beads (Fig. 8B).
FIGURE 8.
iTregs induced with coating-beads showed greater stability in vitro and superior efficacy effect in vivo than iTregs induced with plate-bound stimulation. iTregs were cultured from naïve CD4+ CD62+ CD25− T cells with anti-CD3/CD28 beads (1:5), IL-2 (50 IU/ml), TGF-β (5 ng/ml) and atRA (5 nM) for 3–13 days. For control iTregs, naïve CD4+ CD62+ CD25− T cells were cultured in the plate that had been coated with anti-CD3 (10 mg/ml) and anti-CD28 (1 mg/ml) antibodies with IL-2 (50 IU/ml), TGF-β (5 ng/ml) and atRA (5 nM) for 3–13 days. Induction of aGVHDs was described as previously. iTregs produced by two methods were derived from B6 mice. Data in vivo were representative and summary of three independent experiments, each with at least 8 recipients in each group. (A) Representative plots gated on CD4+ cells for CD25+Foxp3+ expression of beads-iTreg and plate-bound-iTreg on days indicated in vitro. (B) The percentages of CD25+Foxp3+ cells and the absolute numbers at each time points. Data were mean± SEM of 5 independent experiments. ** p <0.01; *** p <0.001. (C) TSDR methylations were analyzed by bisulphite sequencing of genomic DNA isolated from ex vivo–Beads iTreg and plate bound iTreg from Foxp3 GFP mice. Percentages indicate average methylation within the TSDR. Data were mean± SEM of 5 independent experiments. (D) Both Treg subsets and B6 spleen cells were co-transferred to BALB/c mice, mice survival and weight were monitored (total n=12 in each group). ** p <0.01.
As previous studies have revealed that DNA methylation of CpG islands in Foxp3 gene locus affects the Foxp3 stability (18), we also detected the DNA methylation level of CpG in Foxp3 gene locus in iTreg subsets generated with two different protocols. We have studied methylation of CpG in the conserved non-coding DNA sequence 2 (CNS2) elements at the Foxp3 locus since maintenance of the active state of the Foxp3 locus depends on recruitment of Foxp3–Runx1–Cbf-β complexes to CNS2 (30). As shown in Fig. 8C, both iTreg subsets displayed around 40% methylated CpG in CNS2 of Foxp3 gene locus, and there was no significant difference between beads-iTregs and plate-bound-iTregs that is similar with previous reports showing CpG methylation does not compromise iTreg phenotype and function (31, 32). This result suggests that DNA methylation status cannot explain the difference of Foxp3 stability on both iTreg subsets.
We then directly compared the effects of each iTreg population in the prevention of B6-to BALB/c aGVHD. Lethally irradiated (800 cGy) BALB/c recipients were transplanted with 5×106 T-cell-depleted BM (TCD-BM) plus 2×106 MACS-purified T cells alone, or together with 1 ×106 iTregs generated with plate-bound antibodies or beads from B6 mice. BALB/c recipients receiving bead-iTregs showed greatly improved survival and morbidity compared with recipients receiving plate-bound iTregs. Thus, methods used in the induction of iTregs appear critical for the generation of cells that are effective for the prevention of aGVHD.
Discussion
iTregs induced with IL-2 and TGF-β have proved to have a protective efficacy in many kinds of disease models, including collagen induced arthritis (33), colitis (34), EAE (35), allergy (36) and transplantation (16). However it was recently reported that CD4+ iTregs are not stable in vivo and failed in preventing acute GVHD in the B6-to-BALB/c model (8, 9). To determine why iTregs can suppress autoimmune diseases but not acute GVHD, we re-investigated the protective effect of iTregs on acute GVHD using two different animal models.
Unexpectedly, we observed that transfer of iTregs can markedly prevent the development of acute GVHD in a manner similar to that demonstrated for nTreg cell transfer. Two different aGVHD models were used to exclude the possibility that our finding was model-specific. Our results also suggest that the protective effect of iTregs is not dependent on immune status since the iTregs appear to work under both immune-competent and immune-compromised conditions.
The protective effects provided by iTregs in aGHVD were readily apparent. Firstly, iTreg treatment suppressed the production of inflammatory cytokines by donor cells. Because inflammatory cytokine such as IFN-γ and IL-17 play important pathogenic roles, iTregs may inhibit aGVHD through the suppression of these highly pro-inflammatory cytokines. Secondly, iTregs both inhibited the proliferation of donor CD4 and CD8 cells and reduced the cytotoxicity of donor CD8 cells to host CD19 cells. CD8+ cell-mediated anti-host CTL is a major mechanism by which host B cells are killed in aGVHD. Additionally, iTregs reduced the expression of Fas on B cells and FasL on CD8 cells, thereby reducing a critical pro-apoptotic signal pathway (37). Thirdly, iTreg treatment increased the percentage of host cells expressing Foxp3. That these cells are important in the protection afforded in this model is highlighted by Treg depletion studies in which the protective effect of transferred cells abolished when antibodies depleting CD25+ cells are used. Our finding is consistent with previous reports demonstrating Tregs can induce long-term immune tolerance through “infectious tolerance” (16, 38–40). Recently, we demonstrated that tolerant dendritic cells are critical to the long-term protective effect of iTregs (26).
The stability of Tregs has become a big issue for their functional activity. nTregs retain a measure of plasticity, allowing them to be converted to various classes of Th cells when they encounter inflammatory conditions (23, 24, 41–44), raising the possibility that nTregs are more useful in preventing rather than curing inflammatory diseases (41, 45). nTregs have been recognized for their ability to successfully prevent aGVHD (32), however, lack of antigen-specificity and low cell frequencies limits their clinical use.
Although sufficient numbers of antigen-specific iTregs can successfully be induced, the stability of these cells in aGVHD is a concern. To determine the stability and functionality of iTregs in aGVHD, we have re-examined the possibility that iTregs can be used in the prevention of aGVHD. Surprisingly, we found that iTregs are indeed stable both in vitro and in vivo. Importantly, adoptive transfer of iTregs can markedly prevent the development of aGVHD in two distinct animal models. We even observed that iTregs we induced can treat established aGVHD.
We further investigated the differences between iTregs generated in this study and those used in previous works. We demonstrated that stimulation with beads coated with anti-CD3 and CD28 antibodies in the presence of IL-2, TGF-β and atRA resulted in an optimal TCR signal that sustains Foxp3 expression on CD4+ T cells. Conversely, iTregs stimulated with plate-blond anti-CD3 had an appropriate level of Foxp3 in early stages of development, but expression of this key transcription factor was decreased and even lost over time. We also found that there is no difference of the DNA methylation level of CpG in CNS2 islands of Foxp3 gene locus between beads-iTregs and plate-bound-iTreg, strongly suggesting that the different strengths of TCR signals can lead to the different outcomes when iTregs are used in the treatment of aGVHD. Another potential difference is that while others used total CD4+ cells as precursor cells for iTregs differentiation (9), we used naïve CD4+ cells. It is possible that the mixture of memory CD4+ cells in precursor cells will interfere with the iTreg differentiation and adversely affect their stability (46).
Our findings in this study present a new understanding of induced regulatory T cells that can effectively suppress cytokine production and CTL activity against host B cells, may sustain their suppression via the induction of host Foxp3+ T cells in vivo and prevent the development of experimental acute GVHD. We have defined the protocols which are useful for the induction of iTregs in vitro to gain sufficient numbers of iTreg for cellular therapy to aGVHD and autoimmune diseases. iTregs provide the special advantages on clinical setting, however, the protocol to develop iTregs is important. Our study has ruled out that coated-bead pathway is superior to any other methods most currently used for iTreg differentiation and thus have greatly advanced Treg therapy on autoimmune diseases and the prevention of organ transplantation rejection. Further investigations are needed to explore underlying TCR signal mechanisms for the development of iTregs.
Acknowledgments
This work was supported in part by grants from the NIH AR059103, AI084359, the ACR WOR Fund, the Arthritis Foundation, the Wright Foundation, the SFSP (PKJ2009-Y41) and the NSFC (81100270).
Abbreviations used in this article
- aGVHD
acute GVHD
- BM
bone marrow
- Foxp3
forkhead box P3
- iTregs
induced regulatory T cell
- nTregs
natural regulatory T cell
- Treg
regulatory T cell
- RA
retinoic acid
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Nochy D, Debré P, Piette JC, Gorochov G. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 4.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 5.Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr1047. Epub 2011 Dec 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 8.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, Huehn J, Ganser A, Forster R, Prinz I. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. European Journal of Immunology. 2009;39:3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Tey SK, Koyama M, Kuns RD, Olver SD, Lineburg KE, Lor M, Teal BE, Raffelt NC, Raju J, Leveque L, Markey KA, Varelias A, Clouston AD, Lane SW, MacDonald KP, Hill GR. Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. Journal of immunology. 2013;191:5291–5303. doi: 10.4049/jimmunol.1301181. [DOI] [PubMed] [Google Scholar]
- 10.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 12.Kong N, Lan Q, Chen M, Wang J, Shi W, Horwitz DA, Quesniaux V, Ryffel B, Liu Z, Brand D, Zou H, Zheng SG. Antigen-specific transforming growth factor β-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthritis Rheum. 2012;64:2548–2558. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor RA, Leech MD, Suffner J, Hämmerling GJ, Anderton SM. Myelin-reactive, TGF-β-induced regulatory T cells can be programmed to develop Th1-like effector function but remain less proinflammatory than myelin-reactive Th1 effectors and can suppress pathogenic T cell clonal expansion in vivo. J Immunol. 2010;185:7235–7243. doi: 10.4049/jimmunol.1001551. [DOI] [PubMed] [Google Scholar]
- 14.Via CSSS, Shearer GM. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987;139:1840–1849. [PubMed] [Google Scholar]
- 15.Zhou X, Wang J, Shi W, Brand DD, Liu Z, Fan H, Zheng SG. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J Mol Cell Biol. 2010;2:164–169. doi: 10.1093/jmcb/mjq007. doi:110.1093/jmcb/mjq1007. Epub 2010 Apr 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng SG, Meng L, Wang JH, Watanabe M, Barr ML, Cramer DV, Gray JD, Horwitz DA. Transfer of regulatory T cells generated ex vivo modifies graft rejection through induction of tolerogenic CD4+CD25+ cells in the recipient. Int Immunol. 2006;18:279–289. doi: 10.1093/intimm/dxh368. [DOI] [PubMed] [Google Scholar]
- 17.Puliaev R, Puliaeva I, Welniak LA, Ryan AE, Haas M, Murphy WJ, Via CS. CTL-promoting effects of CD40 stimulation outweigh B cell-stimulatory effects resulting in B cell elimination and disease improvement in a murine model of lupus. J Immunol. 2008;181:47–61. doi: 10.4049/jimmunol.181.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soloviova K, Puliaiev M, Foster A, Via CS. The parent-into-F1 murine model in the study of lupus-like autoimmunity and CD8 cytotoxic T lymphocyte function. Methods Mol Biol. 2012;900:253–270. doi: 10.1007/978-1-60761-720-4_12. [DOI] [PubMed] [Google Scholar]
- 20.Zheng SG, Wang JH, Koss MN, Quismorio F, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 21.Waring P, Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77:312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 22.Susanto O, Stewart SE, Voskoboinik I, Brasacchio D, Hagn M, Ellis S, Asquith S, Sedelies KA, Bird PI, Waterhouse NJ, Trapani JA. Mouse granzyme A induces a novel death with writhing morphology that is mechanistically distinct from granzyme B-induced apoptosis. Cell Death Differ. 2013;20:1183–1193. doi: 10.1038/cdd.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 24.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-Hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3(+) T cells into TH17 cells in autoimmune arthritis. Nat Med. 2013 doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 26.Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, Brand D, Ramalingam R, Kiela PR, Horwitz DA, Liu Z, Zheng SG. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol. 2012;4:409–419. doi: 10.1093/jmcb/mjs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montero E, Nussbaum G, Kaye JF, Perez R, Lage A, Ben-Nun A, Cohen IR. Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. J Autoimmun. 2004;23:1–7. doi: 10.1016/j.jaut.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. Journal of immunology. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y, Ma S, Zhang Y, Wang Y, Cheng Q, Wu Y, Jin Y, Zheng D, Wu D, Liu H. IL-1beta and TLR4 Signaling Are Involved in the Aggravated Murine Acute Graft-versus-Host Disease Caused by Delayed Bortezomib Administration. Journal of immunology. 2013 doi: 10.4049/jimmunol.1203428. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, Xia Z, Wang J, Han Y, Shi W, Quesniaux V, Ryffel B, Brand D, Li B, Liu Z, Zheng SG. All-trans retinoic acid promotes TGF-β-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS One. 2011;6:e24590. doi: 10.1371/journal.pone.0024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong N, Lan Q, Chen M, Wang J, Shi W, Horwitz DA, Quesniaux V, Ryffel B, Liu Z, Brand D, Zou H, Zheng SG. Antigen-specific transforming growth factor β-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthritis & Rheumatism. 2012;64:2548–2558. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hang L, Blum AM, Setiawan T, Urban JP, Jr, Stoyanoff KM, Weinstock JV. Heligmosomoides polygyrus bakeri Infection Activates Colonic Foxp3+ T Cells Enhancing Their Capacity To Prevent Colitis. Journal of immunology. 2013;191:1927–1934. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. Journal of immunology. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su W, Fan H, Chen M, Wang J, Brand D, He X, Quesniaux V, Ryffel B, Zhu L, Liang D, Zheng SG. Induced CD4+ forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-β1. J Allergy Clin Immunol. 2012;130:444–452. e447. doi: 10.1016/j.jaci.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Silva KL, Melo LM, Perosso J, Oliveira BB, Santos PS, de Eugenio FR, Lima VM. CD95 (FAS) and CD178 (FASL) induce the apoptosis of CD4+ and CD8+ cells isolated from the peripheral blood and spleen of dogs naturally infected with Leishmania spp. Vet Parasitol. 2013;197:470–476. doi: 10.1016/j.vetpar.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 39.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 45.Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181:8209–8213. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]