Abstract
OBJECTIVE
To determine the incremental hospital cost and mortality associated with the development of postoperative acute kidney injury (AKI) and with other associated postoperative complications.
SUMMARY BACKGROUND DATA
Each year 1.5 million patients develop a major complication after surgery. Postoperative AKI is one of the most common postoperative complications and is associated with an increase in hospital mortality and decreased survival for up to 15 years after surgery.
METHODS
In a single-center cohort of 50,314 adult surgical patients undergoing major inpatient surgery we applied risk-adjusted regression models for cost and mortality using postoperative AKI and other complications as the main independent predictors. We defined AKI using consensus RIFLE criteria.
RESULTS
The prevalence of AKI was 39% among 50,314 patients with available serum creatinine. Patients with AKI were more likely to have postoperative complications and had longer lengths of stay in the intensive care unit and the hospital. The risk-adjusted average cost of care for patients undergoing surgery was $42,600 for patients with any AKI compared to $26,700 for patients without AKI. The risk-adjusted 90-day mortality was 6.5% for patients with any AKI compared to 4.4% for patients without AKI. Serious postoperative complications resulted in increased cost of care and mortality for all patients, but the increase was much larger for those patients with any degree of AKI.
CONCLUSIONS
Hospital costs and mortality are strongly associated with postoperative AKI, are correlated with the severity of AKI, and are much higher for patients with other postoperative complications in addition to AKI.
Keywords: Postoperative complications, Acute Kidney Injury, Risk adjustment, Cost, Mortality, Outcomes Assessment
INTRODUCTION
In the United States, where the average American can expect to undergo seven surgical operations during her lifetime, each year at least 150,000 patients die and 1.5 million develop a major complication after surgery.1, 2 Postoperative complications cause a two-fold increase in 30-day mortality and are associated with long-term negative consequences, and thus reducing preventable complications by 20% could save thousands of lives and reduce costs by five billion dollars annually.3-6 Our group has demonstrated that postoperative acute kidney injury (AKI), defined by consensus Risk, Injury, Failure, Loss and End-Stage Renal Disease (RIFLE) criteria based on even small increases in routinely measured serum creatinine (sCr) levels, is not only one of the most common postoperative complications but is also associated with an up to ten fold increase in hospital mortality, decreased survival for up to 15 years after surgery and an increased risk for chronic kidney disease (CKD).7-11
There are few studies addressing the cost effects of AKI and its additive adverse effects on other postoperative complications in terms of cost and mortality.12-16 None of the studies which have documented the hospital costs associated with AKI have used the consensus RIFLE criteria for AKI, and no study has specifically examined the incremental costs associated with AKI as a complication of surgery in the context of other postoperative complications.12, 17 In a large single-center cohort of adult surgical patients with no history of diagnosed chronic kidney disease prior to surgery, we examine the incremental hospital cost and mortality associated with the development of postoperative AKI and compare them to that seen with other common postoperative complications.
METHODS
Data source
Using the University of Florida Integrated Data Repository we have previously assembled a single center cohort of perioperative patients by integrating multiple existing clinical and administrative databases at UF Health.18 The billing database for UF Health, established in 1990, provides detailed information on patient demographics, outcomes, comprehensive hospital charges, hospital characteristics, insurance status and physician identity. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for up to fifty diagnoses and procedures are listed for each admission. We included all patients admitted to the hospital for longer than 24 hours following any type of operative procedure between January 1, 2000 and November 30, 2010. This dataset was integrated with the laboratory, the pharmacy and the blood bank databases and intraoperative database (Centricity Perioperative Management and Anesthesia, General Electric Healthcare, Inc.) to create a comprehensive perioperative database for this cohort. The study was designed and approved by the Institutional Review Board of the University of Florida and the University of Florida Privacy Office.
Participants
We identified patients with age greater or equal to 18 years admitted to the hospital for longer than 24 hours following any type of inpatient operative procedure between January 1, 2000 and November 30, 2010. For patients with multiple surgeries we choose the first procedure. We excluded patients with CKD stage five on admission as identified by the previously validated ICD-9-CM diagnostic and procedure codes (n=1140)19 and those with missing sCr (n=6636). Using primary admission service and primary and secondary procedure codes we classified all surgeries as cardiothoracic surgery, non-cardiac general and vascular surgery, neurologic surgery, specialty surgery (including orthopedic, gynecological, otorhinolaryngology, urology and plastic surgeries) and other surgery (including burn, transplantation and trauma).
Outcomes
We applied the consensus RIFLE definition for AKI using sCr changes only without urine output criteria. RIFLE defines AKI using at least a 50% sCr change from a reference sCr (RsCr) while accounting for patients with documented CKD at admission.20 For all calculations we defined RsCr either as the minimum of the sCr values available within six months prior to admission including the admission day sCr or as the minimum and mean of the sCr values available within seven days prior to admission including the admission day sCr (used for sensitivity analyses).21 Patients with AKI were stratified according to the maximum RIFLE class reached during the hospital admission by comparing the highest sCr during hospitalization with the RsCr. RIFLE-R corresponds to a 50% increase in sCr, RIFLE-I to a doubling in sCr and RIFLE-F to a tripling in sCr compared to RsCr. Patient survival status was determined using hospital discharges and the Social Security Death Index to calculate hospital and 90-day mortality. We defined postoperative complications using previously described criteria (Supplemental digital content (SDC) Table 1).22, 23 We followed the criteria of the Agency for Healthcare Research and Quality for the patient safety indicator “Postoperative Sepsis”.24 Organ failure associated with sepsis was identified by adding ICD-9-CM codes for acute organ dysfunction.25 The exact dates were used to calculate the duration of mechanical ventilation (MV), intensive care unit (ICU), renal replacement therapy (RRT) and hospital stay. We determined need for vasopressor therapy for longer than 24 hours for each patient using administration times and doses from the pharmacy database. All performed procedures and hospital charges were obtained from the billing database. The presence of underlying comorbidities was identified by ICD-9-CM codes based on previously validated criteria.26 We calculated the Charlson-Deyo comorbidity score and grouped patients into score categories of 0, 1-2, 3-4 and ≥5.27 Cost of hospitalization was estimated by applying the ratio of cost-to-charge for urban hospitals in the South Atlantic division to the amount charged for each hospitalization.28 We converted all costs to 2012 dollars using the Consumer Price Index for Medical Services to adjust for inflation over the years.
Statistical Analysis
The analytical plan followed the STROBE recommendations for observational cohort studies.29 The Pearson χ2 test or Fisher's exact test was used to test independence between categorical variables. To compare continuous variables with normal distribution we used Student's T-test or analysis of variance, whereas for those not satisfying the normality assumptions we reported medians and 25th and 75th percentiles and compared them using the Kruskal-Wallis test.
We constructed multivariable risk-adjusted models for cost, mortality and length of stay (LOS) using AKI and postoperative complications as the main independent predictors. Each model was additionally adjusted for preoperative covariates including age, gender, ethnicity, primary payer, number of comorbidities, surgery type, emergent surgery status, and weekend admission. We selected explanatory variables based on their significance in a prior univariate analysis and any previously reported association in the literature. Logarithmic transformation of cost and LOS were used to account for the skewness of the distribution. For each postoperative complication we calculated risk-adjusted incremental and average cost and mortality rates with 95% confidence intervals (CI) using generalized linear models 30 and compare them between patients with and without AKI. The exponentiated coefficients associated with each postoperative complication were used to calculate risk-adjusted relative cost and mortality ratios comparing patients with a postoperative complication to patients without the complication. Standard errors were calculated using the smearing estimate to adjust for bias due to transformation of cost to original scale.31 We performed sensitivity analyses by comparing the effect of a) different definitions for RsCr and b) inclusion of patients with missing values for sCr and c) omission of different covariates on model fit for each outcome. All significance tests were two-sided with α<0.05 considered statistically significant. Statistical analyses were performed with SAS (v.9.3, Cary, N.C.).
RESULTS
Baseline characteristics and resource utilization
The overall prevalence of postoperative AKI was 39% (19,644/50,314) among patients who had sCr measurements. Among 6636 patients for whom sCr was not available less than 1% developed postoperative complications and only one death (0.02%) in 90-days follow-up was encountered. The majority of the patients had mild to moderate AKI (RIFLE-R 10,160/19,644 and RIFLE-I 4,855/19,644). Only 24% (4,629/19,644) of all AKI patients reached the RIFLE Failure stage and 9% required RRT (1,744/19,644). Patients with AKI were more likely to be older, male and of African-American ethnicity (Table 1). AKI was more frequent among emergent surgical cases and for those patients admitted on weekends. Patients with Medicare as their primary insurance were significantly more likely to develop AKI (45.1% with AKI vs. 34.6% with no AKI). Patients with no presenting comorbidity (Charlson-Deyo Comorbidity Score 0) were less likely to develop AKI (24.2% with AKI vs. 44.8% with no AKI), while patients with any degree of presenting comorbidity and especially those with documented CKD on admission were more likely to develop AKI.
Table 1.
Clinical characteristics for all patients stratified by the occurrence of acute kidney injury.
| Variables | Overall (n=50314) | No Acute kidney injury (n=30670) | Acute kidney injury (n=19644) |
|---|---|---|---|
| Age (years), Mean (SD) | 55 (17) | 54 (17) | 57 (17)a |
| Female sex, n (%) | 24668 (49.0) | 15536 (50.7) | 9132 (46.5)b |
| African-American ethnicity, n (%) | 6182 (12.3) | 3554 (11.6) | 2628 (13.4)b |
| Rural area residency, n (%) | 16097 (32.1) | 9922 (32.4) | 6175 (31.6)b |
| Annual Income (1000$), median (25th, 75th) | 33 (28, 40) | 34 (28, 40) | 33 (29, 40) |
| Primary Insurance, n (%) | |||
| Medicare | 19467 (38.7) | 10610 (34.6) | 8857 (45.1)b |
| Medicaid | 6517 (13.0) | 3942 (12.9) | 2575 (13.1) |
| Private | 20591 (40.9) | 13527 (44.1) | 7064 (36.0)b |
| No insurance | 3739 (7.4) | 2591 (8.5) | 1148 (5.8)b |
| Weekend admission, n (%) | 6881 (13.7) | 3431 (11.2) | 3450 (17.6)b |
| Weekend discharge, n (%) | 10312 (20.5) | 7082 (23.1) | 3230 (16.4)b |
| Emergency admission, n (%) | 22818 (45.4) | 11331 (36.9) | 11487 (58.5)b |
| Initial admission to medicine service, n (%) | 5664 (11.3) | 2056 (6.7) | 3608 (18.4)b |
| Charlson-Deyo Comorbidity Score, n (%) | |||
| 0 | 18490 (36.8) | 13732 (44.8) | 4758 (24.2)b |
| 1-2 | 19398 (38.6) | 11240 (36.7) | 8158 (41.5)b |
| 3-4 | 7023 (14.0) | 2873 (9.4) | 4150 (21.1)b |
| ≥ 5 | 5403 (10.7) | 2825 (9.2) | 2578 (13.1)b |
| Chronic kidney disease stages 1 to 4 | 4085 (8.1) | 484 (1.6) | 3601 (18.3)b |
| Surgery type | |||
| Cardiothoracic Surgery | 6755 (13.4) | 2747 (9.0) | 4008 (20.4)b |
| Non-Cardiac General and Vascular Surgery | 10741 (21.4) | 6349 (20.7) | 4392 (22.4) b |
| Neurologic Surgery | 8385 (16.7) | 5895 (19.2) | 2490 (12.7) b |
| Specialty Surgeryc | 15229 (30.3) | 11467 (37.4) | 3762 (19.2) b |
| Other Surgeryd | 9204 (18.3) | 4212 (13.7) | 4992 (25.4) b |
| Postoperative complications, n (%) | |||
| Acute kidney injury | 19644 (39.0) | ||
| Pulmonary complications and/or mechanical ventilation | 15526 (30.9) | 4932 (16.1) | 10594 (53.9)a |
| Cardiovascular complications and/or need for vasopressors | 11786 (23.4) | 3738 (12.2) | 8048 (41.0)a |
| Procedural complications | 4099 (8.2) | 1567 (5.1) | 2532 (12.9) |
| Neurological complications and/or delirium | 4037 (8.0) | 1570 (5.1) | 3467 (12.6)a |
| Mechanical wound complications and surgical infections | 2390 (4.8) | 876 (2.9) | 1514 (7.7)a |
| Severe sepsis | 2302 (4.6) | 65 (0.2) | 2237 (11.4)a |
| Gastrointestinal complications | 1595 (3.2) | 619 (2.1) | 976 (5.0)a |
| Venous thromboembolism | 1448 (2.9) | 308 (1.0) | 1140 (5.8)a |
P<0.05 using Kruskal-Wallis to compare to no acute kidney injury group.
P<0.05 using Pearson χ2 test or Fisher's exact test to compare to no acute kidney injury group.
Other surgeries include trauma, burn and transplant surgeries.
Specialty surgeries include orthopedic, gynecologic, urologic, plastic and otorhinolaryngology surgeries.
Only 19% (3,723/19,644) of AKI patients did not develop other postoperative complications as compared to 50% (15,426/30,670) of patients without AKI. Patients with AKI were more likely to develop any of the postoperative complications compared to those with no AKI (Table 1). Median lengths of stay in both the ICU (6 days vs. 2 days) and hospital (12 days vs. 5 days) were longer for patients who developed AKI compared to those patients with no AKI. Patients who developed AKI were almost twice as likely to be admitted to an ICU at some point during their hospitalizations compared to patients with no AKI (74.1% vs. 38.1%). Patients with AKI were much more likely to have spent any time on mechanical ventilation (47.7% vs. 11.4%), they spent more total days on the ventilator (5 days vs. 2 days), and they were much more likely to suffer prolonged mechanical ventilation (defined as ≥ 2 weeks, 16.3% vs. 1.6%) compared to patients with no AKI. The resource utilization in ICU including use of invasive monitoring, blood products and vasopressors was significantly higher among AKI patients. Patients who developed AKI were significantly less likely to be discharged to home (65.8% vs. 83.4%) compared to patients who did not develop AKI (SDC Table 2 and 3).
AKI and Hospital Cost
Unadjusted median hospital costs for patients who did and did not develop AKI were $38,000 and $14,000 respectively (SDC Table 2). After adjusting for other explanatory variables, patients with any AKI still had hospitals costs that were 159% of the costs for patients without AKI (Table 2). Patients with RIFLE-R AKI had costs that were 44% higher, those with RIFLE-I AKI had costs that were 88% higher, and patients with RIFLE-F AKI had costs that were over twice as high as the costs for patients without AKI. These relative cost increases were associated with a risk-adjusted additional cost of $15,800 for patients with any AKI compared to patients with no AKI. Patients with RIFLE-R AKI had an additional cost of $10,700, those with RIFLE-I AKI had an additional cost of $21,400, and patients with RIFLE-F AKI had an additional cost of $38,200 compared to patients with no AKI. The risk adjusted average cost of care for patients undergoing surgery was $26,700 for patients with no AKI and $42,600 for patients with any AKI.
Table 2.
Risk-adjusted change in cost associated with acute kidney injury.
| Risk-adjusted Relative Cost Ratio (95% CI) | Risk-Adjusted Incremental Cost per Patient ($1000) Mean (95% CI) | Risk-Adjusted Average Cost per Patient ($1000) Mean (95% CI) | |
|---|---|---|---|
| Patients with no acute kidney injury | 1 (Reference) | 0 (Reference) | 26.7 (26.5, 27.0) |
| Patients with any acute kidney injury | 1.59 (1.57, 1.61)a | 15.8 (15.4, 16.3)a | 42.6 (42.2, 42.9)a |
| Acute kidney injury, Stage RIFLE-R | 1.44 (1.42, 1.46)a | 10.7 (10.3, 11.1)a | 35.1 (34.7, 35.5)a |
| Acute kidney injury, Stage RIFLE-I | 1.88 (1.84, 1.91)a | 21.4 (20.7, 22.2)a | 45.8 (45.1, 46.6)a |
| Acute kidney injury, Stage RIFLE-F | 2.57 (2.51, 2.63)a | 38.2 (37.0, 39.5)a | 62.6 (61.4, 63.8)a |
Abbreviations. CI, confidence interval; RIFLE, Risk, Injury, Failure, Loss, and End-stage kidney criteria; R, Risk; I, Injury; F, Failure.
The risk-adjusted relative cost ratios were derived using generalized linear models adjusted for age, gender, ethnicity, primary payer, Charlson Comorbidity Index, surgery type, emergent surgery status, weekend admission, estimated GFR (glomerular filtration rate), and all postoperative complications.
P<0.001 using generalized linear models to compare to no acute kidney injury group.
Serious postoperative complications resulted in increased cost of care across all patients, but the increase was much larger for those patients with any degree of AKI (Table 3). For patients with no AKI the risk-adjusted incremental cost increase for postoperative complications ranged from $1,900 for wound complications to $11,000 for pulmonary complications. This resulted in a risk-adjusted average cost for patients with no AKI which ranged from $20,300 for patients with wound complications to $29,000 for patients with severe sepsis. Patients with any degree of AKI had risk-adjusted incremental cost increases ranging from $12,100 for neurological complications to $47,700 for patients who developed severe sepsis. This resulted in a risk-adjusted average cost for patients with any AKI which ranged from $74,300 for patients with neurological complications to $103,200 for patients with severe sepsis.
Table 3.
Risk-adjusted incremental cost associated with postoperative complications stratified by occurrence of acute kidney injury.
| Patients with no acute kidney injury (n=30670) | Patients with acute kidney injury (n=19644) | |||||
|---|---|---|---|---|---|---|
| Risk-adjusted Relative Cost Ratio (95% CI) | Risk-Adjusted Incremental Cost ($1000) Mean (95% CI) | Risk-Adjusted Average Cost ($1000) Mean (95% CI) | Risk-adjusted Relative Cost Ratio (95% CI) | Risk-Adjusted Incremental Cost ($1000) Mean (95% CI) | Risk-Adjusted Average Cost ($1000) Mean (95% CI) | |
| Patients with no other complications | 1 (Reference) | 0 (Reference) | 14.6 (14.5, 14.8) | 1 (Reference) | 0 (Reference) | 26.1 (25.3, 26.8)a |
| Pulmonary complications and/or mechanical ventilation | 1.74 (1.70, 1.77) | 11.0 (10.5, 11.5) | 27.4 (27.0, 27.8) | 1.91 (1.87,1.95)a | 36.2 (35.0, 37.3)a | 76.1 (75.2, 77.0)a |
| Cardiovascular complications and/or need for vasopressors | 1.33 (1.30, 1.37) | 5.2 (4.7, 5.6) | 23.3 (22.9, 23.7) | 1.56 (1.53, 1.60)a | 27.5 (26.2, 28.9)a | 76.4 (75.4, 77.5) a |
| Procedural complications | 1.24 (1.20, 1.28) | 3.4 (2.9, 4.0) | 21.5 (20.9, 22.0) | 1.25 (1.22, 1.29) | 15.5 (13.6, 17.5)a | 77.2 (75.3, 79.0)a |
| Neurological complications and/or delirium | 1.26 (1.22, 1.31) | 3.8 (3.2, 4.4) | 21.4 (20.8, 22.0) | 1.19 (1.16, 1.23)a | 12.1 (10.1, 14.0)a | 74.3 (72.5, 76.2)a |
| Mechanical wound complications and surgical infections | 1.14 (1.09, 1.19) | 1.9 (1.2, 2.6) | 20.3 (19.6, 21.0) | 1.42 (1.37, 1.47)a | 25.7 (22.8, 28.5)a | 87.1 (84.3, 89.9)a |
| Severe sepsis | 1.64 (1.15, 2.33) | 8.8 (0.8, 16.7) | 29.0 (25.3, 32.7) | 1.86 (1.81, 1.92) | 47.7 (44.9, 50.6)a | 103.2 (100.5, 105.9)a |
| Gastrointestinal complications | 1.30 (1.24, 1.36) | 4.1 (3.3, 4.9) | 22.0 (21.1, 22.9) | 1.22 (1.17, 1.27)a | 13.7 (10.6, 16.8)a | 77.2 (74.1, 80.2)a |
| Venous thromboembolism | 1.49 (1.38, 1.60) | 6.7 (5.2, 8.2) | 25.3 (23.8, 26.8) | 1.45 (1.40, 1.51) | 28.0 (24.7, 31.3)a | 89.7 (86.5, 92.9)a |
Abbreviations. CI, confidence interval.
The risk-adjusted relative cost ratios were derived using generalized linear models adjusted for age, gender, ethnicity, primary payer, Charlson Comorbidity Index, surgery type, emergent surgery status, weekend admission, estimated GFR (glomerular filtration rate), and all postoperative complications.
P<0.05 using t-test to compare to no acute kidney injury group.
AKI and Mortality
Patients who developed postoperative AKI had significantly higher unadjusted hospital mortality (8.8% vs. 0.6%) and 90-day mortality (11.3% vs. 2.2%) compared to patients with no AKI (SDC Table 2). After adjusting for other explanatory variables, patients with any AKI had an increased relative risk for 90 day and hospital mortality (1.65 and 2.38) compared to patients without AKI (Table 4). The relative risk for 90 day and hospital mortality increased with increasing RIFLE AKI stage (1.30 and 1.71 for RIFLE-R AKI, 1.88 and 2.33 for RIFLE-I AKI, and 3.49 and 5.53 for RIFLE-F AKI). These increased relative risks resulted in increased adjusted 90-day mortality (6.5% vs. 4.4%) and increased adjusted hospital mortality (4.2% vs. 2.1%) for patients with any AKI compared to patients with no AKI.
Table 4.
Risk-adjusted change in mortality associated with acute kidney injury.
| 90 day mortality | Hospital mortality | |||
|---|---|---|---|---|
| Risk-adjusted relative mortality ratio (95% CI) | Risk-adjusted mean % (95% CI) | Risk-adjusted relative mortality ratio (95% CI) | Risk-adjusted mean % (95% CI) | |
| Patients with no acute kidney injury | 1 (Reference) | 4.4 (4.0, 4.7) | 1 (Reference) | 2.1 (1.8, 2.4) |
| Patients with any acute kidney injury | 1.65 (1.48, 1.84)a | 6.5 (6.2, 6.8)a | 2.38 (2.00, 2.84)a | 4.2 (4.0, 4.4)a |
| Acute kidney injury, Stage RIFLE-R | 1.30 (1.15, 1.47)a | 4.8 (4.5, 5.2)a | 1.71 (1.41, 2.08)a | 2.8 (2.5, 3.1)a |
| Acute kidney injury, Stage RIFLE-I | 1.88 (1.64, 2.16)a | 6.5 (6.0, 7.0)a | 2.33 (1.90, 2.84)a | 3.6 (3.2, 3.9)a |
| Acute kidney injury, Stage RIFLE-F | 3.49 (3.01, 4.05)a | 10.5 (9.7, 11.3)a | 5.53 (4.52, 6.77)a | 6.8 (6.3, 7.3)a |
Abbreviations. CI, confidence interval.
The risk-adjusted relative mortality ratios and mean percentages were derived using generalized linear models adjusted for age, gender, ethnicity, primary payer, Charlson Comorbidity Index, surgery type, emergent surgery status, weekend admission, estimated GFR (glomerular filtration rate), and all postoperative complications.
P-value < 0.001 using generalized linear models to compare to no acute kidney injury group.
Serious postoperative complications resulted in increased mortality rates across all patients, and the increase was much larger for patients with any degree of AKI (Table 5). For patients with no AKI the risk-adjusted 90-day mortality associated with postoperative complications ranged from 1.0% for mechanical wound complications and surgical infections to 6.1% severe sepsis. For patients with AKI the risk-adjusted 90-day mortality associated with postoperative complications ranged from 9.7% for patients with mechanical wound complications and surgical infections to 21.2% for patients with severe sepsis. Risk-adjusted hospital mortality was less than 90-day mortality for all patients but followed a similar pattern.
Table 5.
Risk-adjusted incremental mortality associated with postoperative complications stratified by occurrence of acute kidney injury.
| Patients with no acute kidney injury (n=30670) | Patients with acute kidney injury (n=19644) | |||||
|---|---|---|---|---|---|---|
| Postoperative Complications | n (% of no AKI patients) | Risk-Adjusted 90-day mortality % (95%CI) | Risk-Adjusted hospital mortality % (95%CI) | n (% of AKI patients) | Risk-Adjusted 90-day mortality % (95%CI) | Risk-Adjusted hospital mortality % (95%CI) |
| Patients with no other complications | 15426 (50.3) | 1.6 (1.4, 1.9) | <0.001 (<0.001, <0.001) | 3723 (19.0) | 3.5 (2.8, 4.1) a | 0.3 (0.1, 0.4)a |
| Pulmonary complications and/or mechanical ventilation | 4932 (16.1) | 3.1 (2.7, 3.5) | 1.4 (1.2, 1.7) | 10594 (53.9) | 13.2 (12.6, 13.8)a | 10.4 (10.0, 10.9)a |
| Cardiovascular complications and/or need for vasopressors | 3738 (12.2) | 3.4 (2.9, 3.9) | 1.4 (1.1, 1.6) | 8048 (41.0) | 16.0 (15.2, 16.8)a | 12.6 (12.0, 13.3)a |
| Procedural complications | 1567 (5.1) | 2.5 (1.8, 3.2) | 0.9 (0.6, 1.3) | 2532 (12.9) | 13.4 (12.3, 14.5)a | 10.9 (9.9, 11.8)a |
| Neurological complications and/or delirium | 1570 (5.1) | 4.4 (3.6, 5.1) | 1.5 (1.1, 1.8) | 2467 (12.6) | 13.9 (12.9, 15.0)a | 10.2 (9.32, 11.0)a |
| Mechanical wound complications and surgical infections | 876 (2.9) | 1.0 (0.4, 1.6) | 0.2 (0, 0.5) | 1514 (7.7) | 9.7 (8.5, 10.9)a | 8.8 (7.7, 9.8)a |
| Severe sepsis | 65 (0.2) | 6.1 (2.7, 9.6) | 1.9 (0.8, 3.0) | 2237 (11.4) | 21.2 (19.7, 22.6)a | 17.2 (16.1, 18.3)a |
| Gastrointestinal complications | 619 (2.0) | 1.1 (0.3, 1.9) | 0.3 (0, 0.7) | 976 (5.0) | 10.3 (8.6, 12.0)a | 8.4 (6.9, 9.9)a |
| Venous thromboembolism | 308 (1.0) | 2.7 (1.4, 3.9) | 0.4 (0, 0.8) | 1140 (5.8) | 11.3 (10.0, 12.7)a | 8.0 (7.0, 9.0)a |
Abbreviations. CI, confidence interval.
The risk-adjusted relative mortality rates were derived using generalized linear models adjusted for age, gender, ethnicity, primary payer, Charlson Comorbidity Index, surgery type, emergent surgery status, and weekend admission, estimated GFR (glomerular filtration rate), and all postoperative complications.
P<0.05 using t-test to compare to no acute kidney injury group.
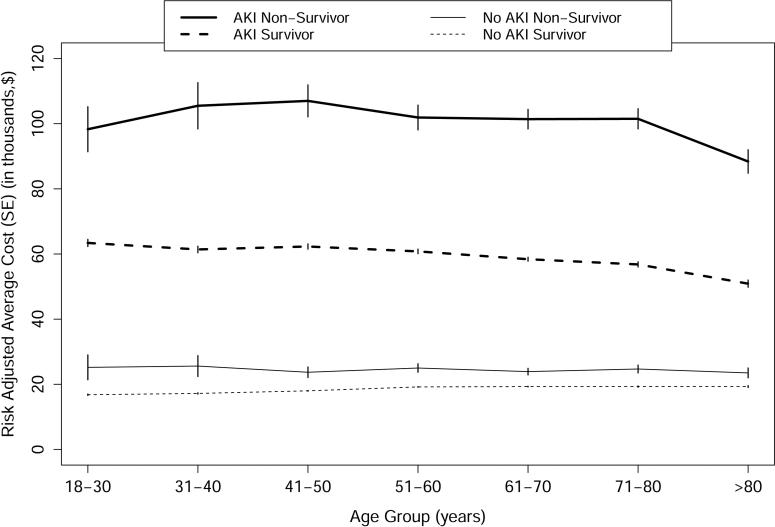
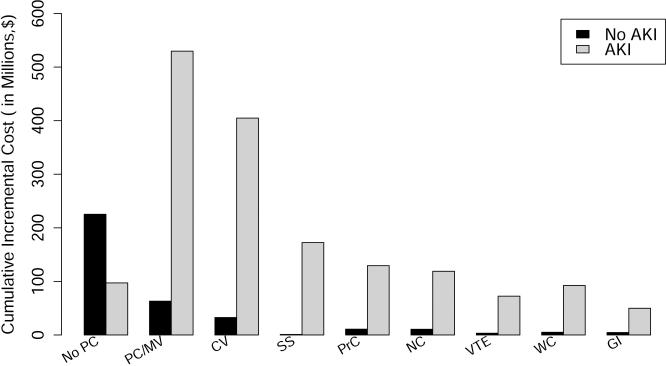
We examined adjusted mean length of stay and costs for patients with and without AKI, who did and did not survive past 90 days after surgery, and stratified the results based on patient age by decade and need for renal replacement therapy (Table 6 and Figure 1A). At all ages the median costs were much highest for patients with AKI who died, followed by patients with AKI who survived, followed by patients with no AKI both survivors and those who died. The trend was for lower adjusted mean costs with advancing age. The AKI was associated with 10 days longer hospital stay in non-survivors and 5.5 days among survivors even after adjusting for all other covariates. The risk-adjusted increase in length of stay was proportional to AKI severity and for all AKI stages was higher among non-survivors. Among non-survivors, RIFLE-F patients with RRT compared to those without RRT had incremental cost of $22,000 without concomitant increase in risk-adjusted LOS. We also examined the adjusted cumulative incremental cost of individual postoperative complications, stratified by AKI (Figure 1B). While all complications were associated with higher costs, pulmonary and cardiovascular complications were associated with much higher costs for those patients with AKI compared to those with no AKI.
Table 6.
Risk-adjusted incremental change in length of stay and cost associated with acute kidney injury for patients stratified by 90-day survival.
| Non-survivors (n=2896) | Survivors (n=47418) | |||
|---|---|---|---|---|
| Risk-Adjusted Incremental Length of Stay per Patient Mean (95% CI) | Risk-Adjusted Incremental Cost per Patient ($1000) Mean (95% CI) | Risk-Adjusted Incremental Length of Stay per Patient Mean (95% CI) | Risk-Adjusted Incremental Cost per Patient ($1000) Mean (95% CI) | |
| Patients with no acute kidney injury | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Patients with any acute kidney injury | 9.9 (8.9, 10.9) a | 37.4 (33.7, 41.1)a | 5.5 (5.3, 5.6) a | 14.7 (14.3, 15.1)a |
| Acute kidney injury, Stage RIFLE-R | 4.8 (3.8, 5.8)a | 17.9 (14.6, 21.3)a | 3.9 (3.8, 4.1) a | 10.3 (9.8, 10.7)a |
| Acute kidney injury, Stage RIFLE-I | 9.7 (8.4, 11.1)a | 34.0 (29.7, 38.2)a | 7.6 (7.4, 7.9) a | 20.9 (20.1, 21.7)a |
| Acute kidney injury, Stage RIFLE-F with no RRT | 17.7 (15.8, 19.6)a | 58.5 (52.8, 64.2)a | 12.1 (11.6, 12.6)a | 34.6 (33.2, 36.1)a |
| Acute kidney injury, Stage RIFLE-F with RRT | 16.5 (14.3, 18.6)a | 81.0 (73.3, 88.7)a | 14.4 (13.5, 15.2) a | 42.6 (40.2, 45.0)a |
Abbreviations. CI, confidence interval; RRT, renal replacement therapy.
The risk-adjusted relative mortality ratios and mean percentages were derived using generalized linear models adjusted for age, gender, ethnicity, primary payer, Charlson Comorbidity Index, surgery type, emergent surgery status, weekend admission, estimated GFR (glomerular filtration rate) and all postoperative complications.
P-value < 0.001 using generalized linear models to compare to no acute kidney injury group.
Figure 1.
A. Risk-adjusted average cost for patients stratified by 90 day survival, occurrence of acute kidney injury and age. Abbreviations: AKI, Acute kidney injury; SE, standard error. Figure 1.B. Risk-adjusted cumulative incremental cost of postoperative complications stratified by occurrence of acute kidney injury. Abbreviations: AKI, Acute kidney injury; No PC, No postoperative complications; PC/MV, Pulmonary complications and/or mechanical ventilation; CV, Cardiovascular complications and/or need for vasopressors; SS, Severe sepsis; NC, Neurological complications and/or delirium; VTE, Venous thromboembolism; WC, Mechanical wound complications and surgical infections; GI, Gastrointestinal complications.
The sensitivity analyses examining the effect of two methods of assigning the RsCr value demonstrated no significant change in model fit for each outcome. Similarly, inclusion of patients with missing values for sCr and omission of different covariates did not affect any of the reported outcomes (data not shown).
DISCUSSION
Acute kidney injury is a common postoperative complication, whose pervasiveness has been more appreciated recently with the adoption of standardized reporting of minimal injury by the consensus definitions of the RIFLE criteria. Multiple recent studies have shown that patients who have AKI tend to have higher short and long term mortality rates than those patients without AKI.7-11 While few studies have examined the costs associated with AKI, one landmark study of AKI diagnosed in the hospital, published shortly before the adoption of the RIFLE criteria, estimated that one episode of hospital-based AKI resulted in nearly $7500 in excess costs and that hospital-based AKI resulted in annual expenditures which exceed $10 billion.16 In a large single-center cohort of adult surgical patients we examined the incremental hospital cost and mortality associated with the development of postoperative acute kidney injury and with other associated postoperative complications. Using risk-adjusted models for cost and mortality, with postoperative complications as the main independent predictors, we show that postoperative AKI is associated with significant and independent increases in hospital cost and both hospital and 90 day mortality, and that those increases are larger with more severe AKI. We also show that patients with any AKI and other common postoperative complications have much higher hospital costs and a much higher risk of both in-hospital and 90 day mortality compared to patients with those complications but without AKI.
This study adds to the growing body of work showing an association between even small changes in serum creatinine after surgery and worse outcomes. Observational studies cannot provide a causal link between AKI and associated outcomes, especially for a condition like AKI which is often associated with other critical illnesses and multiorgan dysfunction.32 However AKI is no longer viewed as an indicator of overall severity of illness, but rather as an important complication with significant independent effects on outcomes such that “patients die because of AKI rather than with AKI”.33 Physiologic derangements in AKI, including decreased free water clearance, volume overload,34, 35 acid-base disorders and electrolyte abnormalities interfere with the normal functioning of many bodily processes including the recovery of kidney function. AKI is characterized by local and systemic inflammatory reactions, where locally produced cytokines mediate AKI and concomitantly exert distant organ injury through their systemic release from the renal tubular cells.36, 37 Patients with AKI not only have an increased prevalence of infection but inadequate antimicrobial dosing plays an important role in the morbidity of AKI.33, 38
Previous work has shown that postoperative complications are more important than preoperative risks and intraoperative factors in determining short and long term survival after surgery.39 By demonstrating the significant interaction between AKI and other postoperative complications in the costs and mortality after surgery we aim to raise the awareness of the individual provider of the importance of screening to identify surgical patients who are at risk for postoperative AKI, and of the need for appropriate perioperative and postoperative management of those patients to minimize their chance for AKI. The observed tremendous increase in cost and mortality resulting from the concomitant occurrence of AKI and other postoperative complications should provide impetus to the systemic efforts to find more effective ways to prevent postoperative AKI and eventually to treat it once it occurs. The development of clinical quality improvement tools to incorporate widespread adoption of recent guidelines40 and further research into the early risk stratification of postoperative acute kidney injury are warranted.
This study has some limitations. The retrospective nature of this study design makes selection bias one potential concern. The large and representative sampling of our database likely minimizes the effect of selection bias. It is possible that, despite our attempts to adjust for all possible confounders, we may not have measured some variables that contribute to the size of the associations that we demonstrate. In particular the use of the Charlson-Deyo comorbidity score may not capture the severity of patient comorbidities. As with any administrative database there is the potential for incorrect reporting of procedure and cost codes. Our cohort included patients undergoing inpatient surgery with at least 24 hour admission thus likely represents population with more comorbidities and more high-risk surgeries and may not be applicable to the patients undergoing outpatient or same-day inpatient surgeries. Although sCr was not available for 10% of the cohort the sensitivity analyses including these patients in regression models demonstrated no effect on reported results. We estimated hospital cost from hospital charges which depends upon accurate coding and accurate capture in the billing database. Inaccurate or uncaptured costs in the database could result in true costs that are higher than those that we report, while overcoding could result in true costs lower than those that we report. Our cost analysis does not reflect other costs to society related to AKI, such as lost wages for the patient, disability compensation, and the opportunity costs to the hospital associated with the provision of care for the patient with AKI.
Despite these limitations, our study shows that hospital costs and mortality are strongly associated with postoperative AKI, are correlated with the severity of AKI, and are much higher for patients with other postoperative complications in addition to AKI. Given the financial and human costs associated with postoperative AKI, there is a critical need for strategies to better identify those patients at risk for postoperative AKI, to better manage those patients towards preventing AKI, and finally to treat AKI once it occurs.
Supplementary Material
Acknowledgment
We want to thank Gigi Lipori, Christine Bono and Yue Du for assistance with data retrieval.
Source of Funding: AB is supported by Award Number K23GM087709 from the National Institutes of Health, I Heermann Anesthesia Foundation, Inc. and has received grants from Astute Medical, Inc. AK was supported by the University of Florida University Scholars fellowship.
Footnotes
Conflicts of Interest: The preliminary report from this research was presented as a poster presentation at the 42nd Annual Meeting of the Society of Critical Care Medicine.
REFERENCES
- 1.Lee PH, Gawande AA. The number of surgical procedures in an American lifetime in 3 states. J Am Coll Surg. 2008;207:S75–S75. [Google Scholar]
- 2.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. The Lancet. 372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Weeks WB, Karia RJ, et al. Who pays for poor surgical quality? Building a business case for quality improvement. J Am Coll Surg. 2006;202:933–7. doi: 10.1016/j.jamcollsurg.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Gust C, Dimick JB, et al. Hospital quality and the cost of inpatient surgery in the United States. Ann Surg. 2012;255:1–5. doi: 10.1097/SLA.0b013e3182402c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall BL, Hamilton BH, Richards K, et al. Does Surgical Quality Improve in the American College of Surgeons National Surgical Quality Improvement Program: An Evaluation of All Participating Hospitals. Ann Surg. 2009;250:363–376. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan-Sarrazin M, Bayman L, Rosenthal G, et al. The business case for the reduction of surgical complications in VA hospitals. Surgery. 2011;149:474–483. doi: 10.1016/j.surg.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Bihorac A, Delano MJ, Schold JD, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252:158–65. doi: 10.1097/SLA.0b013e3181deb6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 9.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–8. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbertson BH, Roughton S, Jenkinson D, et al. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–33. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 12.Dasta JF, Kane-Gill SL, Durtschi AJ, et al. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–4. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 13.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–7. doi: 10.1016/j.jamcollsurg.2004.05.276. [DOI] [PubMed] [Google Scholar]
- 14.Dimick JB, Pronovost PJ, Cowan JA, et al. Complications and costs after high-risk surgery: where should we focus quality improvement initiatives? J Am Coll Surg. 2003;196:671–8. doi: 10.1016/S1072-7515(03)00122-4. [DOI] [PubMed] [Google Scholar]
- 15.Vaughan-Sarrazin MS, Bayman L, Cullen JJ. Costs of postoperative sepsis: the business case for quality improvement to reduce postoperative sepsis in veterans affairs hospitals. Arch Surg. 2011;146:944–51. doi: 10.1001/archsurg.2011.78. [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 17.White LE, Chaudhary R, Moore LJ, et al. Surgical Sepsis and Organ Crosstalk: The Role of the Kidney. J Surg Res. 2011;167:306–315. doi: 10.1016/j.jss.2010.11.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bihorac A, Brennan M, Ozrazgat Baslanti T, et al. National Surgical Quality Improvement Program Underestimates the Risk Associated with Mild and Moderate Postoperative Acute Kidney Injury. Crit Care Med. 2013;41:2570–83. doi: 10.1097/CCM.0b013e31829860fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald R, Waikar SS, Liangos O, et al. Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. J Vasc Surg. 2006;43:460–466. doi: 10.1016/j.jvs.2005.11.053. discussion 466. [DOI] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siew ED, Ikizler T, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. J Am Soc Nephrol. 2012;7:712–720. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPar DJ, Bhamidipati CM, Mery CM, et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–50. doi: 10.1097/SLA.0b013e3181e8fd75. discussion 550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guller U, Hervey S, Purves H, et al. Laparoscopic versus open appendectomy: outcomes comparison based on a large administrative database. Ann Surg. 2004;239:43–52. doi: 10.1097/01.sla.0000103071.35986.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality [April 9, 2012];Patient safety indicators: technical specifications. Ver. 4.2 2010. Available at: http://www.qualityindicators.ahrq.gov.
- 25.Dombrovskiy VY, Martin AA, Sunderram J, et al. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med. 2005;33:2555–62. doi: 10.1097/01.ccm.0000186748.64438.7b. [DOI] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity Measures for Use with Administrative Data. Medical Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality [2013];Healthcare Cost and Utilization Project 2008 National Statistics. Journal [serial online] 2008 Available from: Agency for Healthcare Research and Quality, Rockville, MD. [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 30.Pasta DJ. Estimating Standard Errors for CLASS Variables in Generalized Linear Models Using PROC IML. SAS Users Group International Proceedings. 2003;28:364–28. [Google Scholar]
- 31.Duan N. Smearing Estimate: A Nonparametric Retransformation Method. J Am Stat Assoc. 1983;78:605–610. [Google Scholar]
- 32.White LE, Hassoun HT, Bihorac A, et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg. 2013;75:432–8. doi: 10.1097/TA.0b013e31829de6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoste EA, De Corte W. Clinical consequences of acute kidney injury. Contributions to Nephrology. 2011;174:56–64. doi: 10.1159/000329236. [DOI] [PubMed] [Google Scholar]
- 34.Grams ME, Estrella MM, Coresh J, et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966–73. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–7. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 36.Grigoryev DN, Liu M, Hassoun HT, et al. The local and systemic inflammatory transcriptome after acute kidney injury. Journal of the American Society of Nephrology. 2008;19:547–58. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bihorac A, Baslanti TO, Cuenca AG, et al. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg. 2013;74:1005–13. doi: 10.1097/TA.0b013e31828586ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bihorac A, Efron PA, Ang D, et al. Acute kidney injury is associated with nosocomial infections and surgical site infections after trauma. Surgical Infections. 2011;12:S017. [Google Scholar]
- 39.Khuri SFMD, Henderson WGP, DePalma RGMD, et al. Determinants of Long-Term Survival After Major Surgery and the Adverse Effect of Postoperative Complications. Ann Surg. 2005;242:326–343. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group Clinical Practice Guideline for Acute Kidney Injury: AKI Definition. Kidney Int Suppl. 2012;2:19–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.