Abstract
Despite the well-documented relationship between depression and antiretroviral therapy (ART) non-adherence, few studies have identified explanatory pathways through which depression affects adherence. The current study tested lifestyle structure—the degree of organization and routinization of daily activities—as a mediator of this relationship, given previous evidence of lifestyle structure being associated with both depression and ART nonadherence. HIV-infected individuals starting or re-starting ART in the California Collaborative Treatment Group 578 study (n = 199) were assessed over 48 weeks. Adherence was measured using electronic monitoring caps to determine dose timing and doses taken, and viral load was assessed. The mediating role of lifestyle structure was tested using generalized linear mixed-effects modeling and bootstrapping. Lifestyle significantly mediated the relationship between depression and both measures of ART adherence behavior. Interventions that minimize disruptions to lifestyle structure and link adherence to daily activities may be useful for individuals with depression and ART nonadherence.
Keywords: Depression, Antiretroviral therapy, Adherence, Lifestyle structure, Mediation
Introduction
Poor adherence to antiretroviral therapy (ART) is a crucial behavioral factor associated with worse virologic outcomes and mortality among HIV-infected individuals [1, 2]. Although newer regimens are more forgiving [3-5], many individuals continue to demonstrate suboptimal adherence [6], including in particular individuals with depression [7]. Depressive symptoms, either at clinical or sub-clinical levels, are consistently associated with nonadherence [8, 9]. As a consequence, individuals with depressive symptoms and suboptimal adherence are almost six times more likely to die than individuals without depressive symptoms who are adherent [10].
Despite numerous studies demonstrating a relationship between depressive symptoms and ART nonadherence, there is strikingly little research that attempts to explain how depression impacts adherence. A recent meta-analysis and review of studies examining the relationship between depression and ART adherence (n = 35,029; 95 independent samples) noted that none of the included studies examined “how” depression is related to nonadherence [8]. Understanding the mechanisms that drive the relationship between depression and nonadherence may inform intervention efforts that address both symptoms of depression and adherence behaviors [11].
One factor that is associated with both depression and adherence, which may contribute to the understanding of how depression relates to adherence, is the degree of structure and organization in one’s daily life. Previous research examining behavioral patterns of depression have shown that depression interferes with ‘lifestyle structure’—the extent to which activities are organized and routinized in daily life—as evidenced by the observed relationship between depressive symptoms and lower levels of engagement in meaningful and consistent activities [12-19]. Lifestyle structure is also strongly related to medication adherence; numerous lines of research have suggested that one’s patterns of activity engagement are strongly related to ART adherence, and in particular, changes in daily routine and ability to fit a regimen into a daily routine consistently predict ART adherence over and above other factors commonly related to adherence [20-25]. Despite its relevance to both depression and ART adherence, lifestyle structure has not been empirically tested as a potential explanatory mechanism in this relationship between depressive symptoms and ART adherence. The current study examined the role of lifestyle structure in mediating the relationship between depressive symptoms and ART nonadherence in a longitudinal sample of HIV-infected individuals starting or re-starting ART.
Methods
Participants and Procedures
The data for this analysis were drawn from the California Collaborative Treatment Group (CCTG) 578 study, which was a three-arm, randomized controlled trial to evaluate a cognitive behavioral intervention to improve adherence among HIV-infected individuals starting or re-starting ART [26]. Randomization followed a 1:1:1 ratio to either 1) a five-session cognitive-behavioral therapy (CBT) intervention with a two-week placebo practice trial; 2) the five-session CBT intervention without the practice trial; or 3) usual clinical care. Stratification was based upon clinic site and ART status (naïve or experienced). Inclusion criteria included being age 18 years or older, and planning to begin or re-start (after at least two months off treatment) an ART regimen; individuals who had previously been on ART had to report adherence difficulties or indicate that they would benefit from an adherence intervention. Other inclusion criteria included HIV RNA levels at greater than or equal to 3,000 copies/mL, no active substance abuse, and being fluent in English or Spanish. Assessments were administered at baseline (Week 0) and Weeks 4, 12, 24 and 48 after ART initiation, including blood draws. All study participants provided informed consent. All procedures were IRB approved. Procedures were approved by the IRB at each of the five HIV primary care clinics that comprise the CCTG and the RAND Corporation. More details regarding the larger trial can be found in Wagner et al. 2006 [26].
Assessment Measures
Demographics
Participants self-reported their age, gender, race/ethnicity, employment status (coded yes/no for employed vs. unemployed), and housing stability (coded yes/no for stable housing arrangement).
Medication adherence
ART adherence was measured using electronic monitoring caps (electronic drug exposure monitor eDEM caps; AARDEX Ltx., Zug, Switzerland). Data from the caps were used to calculate both the percentage of prescribed doses taken (“dose taking”) and the percentage of prescribed doses taken within the specified time window (±2 h for twice-a-day regimens and ±3 h for once-a-day regimens) for the antiretroviral monitored by the cap (“dose timing”). Adherence to only one antiretroviral pill in the participant’s ART regimen was monitored by the cap; this was the medication with the most daily doses or the medication considered to be the “base” of the regimen. Dose taking calculations were adjusted in line with self-reported occasions in which instructions related to the use of the eDEM cap were not followed (i.e., multiple doses were removed at once, doses were dispensed from another bottle, or the cap was removed without taking a dose) [27, 28]. Adherence was not monitored by the electronic caps continuously; rather, only for the first 4 weeks of ART and then again during the 2 weeks prior to each subsequent assessment.
HIV viral load
Blood draws were completed at all adherence assessment time points to obtain a measure of HIV RNA level as a biological indicator of adherence. Assays were processed at a central laboratory (Quest Diagnostic Laboratories, San Clemente, California, USA) using Roche Amplicore ultrasensitive version 1.5, limit of detection 50 copies/mL. Percentage of individuals with undetectable viral load (<50 copies/mL) also was calculated.
Baseline HIV clinical characteristics
ART naivety (i.e., whether a participant had ever been on ART prior to the current trial) was determined via the primary care provider’s assessment of clinical history at baseline. CD4 cell count at baseline was assessed via blood draw.
Depressive symptoms
Depressive symptoms were measured using the depression subscale of the Brief Symptom Inventory [29], which has previously been validated and used with medical populations [30]. It includes six symptoms (e.g., feeling blue; feeling hopeless about the future). Respondents indicate how much they were bothered or distressed by each symptom during the past week on a 5-point Likert type scale (0 = not at all; 4 = extremely). The raw depression score is the mean of the six items. Standardized t-scores can be calculated for ease of interpretation (i.e., comparing to a mean in the general population of 50, S.D. = 10, and clinical cut-off of 63) [29, 30]. Internal consistency in the current sample was good (at baseline: α = 0.86).
Lifestyle structure
Lifestyle structure was measured using a previously validated 9-item self-report measure of the degree of structure and organization in one’s life [23, 31]. Example items include “my days consist of doing the same things at the same times” and “I get up at nearly the same time each day”. Responses are rated on a 5-point Likert scale (1 = never; 5 = always); a mean score was calculated. Higher scores represent greater structure in one’s daily life. Internal consistency in the current sample ranged from α = 0.70–0.77 across assessments.
Statistical Analyses
The primary aim was to test the longitudinal mediational role of lifestyle structure in the relationship between depressive symptoms and ART adherence. Three measures of ART adherence were considered in separate models: dose taking, dose timing, and viral load (as a biological indicator of adherence). Study arm was controlled for in all analyses. Analyses were conducted using generalized linear mixed-effects modeling in SPSS 21.
A sequential process mediation model [32] was used (i.e., the predictor variable was assessed temporally before the mediator, and the mediator was assessed temporally before the DV). The temporal sequencing of these variables has been suggested to be a necessary requirement for testing mediation [33]. For each model, the predictor variable was time invariant (depressive symptoms at baseline), the mediator (lifestyle) was time varying (i.e., baseline through week 12), and each of the DVs were time varying (i.e., weeks 12–48). In sequentially stacking these variables, this model addresses temporal ordering and allows for testing of mediation [33].
Participants were treated as random effects, with random slopes and intercepts, while all remaining variables were fixed effects. In doing so, each participant was allowed to have a unique initial score and growth trajectory. Further, the scaled identity covariance structure was chosen based on the best goodness-of-fit (as evaluated by the Akaike Information Criterion), compared to competing covariance structures. The mediator and all DVs—dose taking, dose timing, and viral load (VL)—were assessed for normality. Each adherence DV was highly skewed; therefore, viral load values were log10 transformed, and to properly model the non-normally distributed ART adherence/VL data, a gamma regression was employed (i.e., a gamma distribution with a log link for testing paths b, c, and c’). Lifestyle structure was normally distributed, and a linear model was utilized (for testing path a).
Study arm and housing stability were statistically controlled in all analyses. Additional potential covariates were also considered, including age, gender, race/ethnicity, employment status, number of ART doses prescribed per day, baseline CD4 count, and ART naivety prior to the study. However, only housing stability was significantly related to the DVs and included as a potential covariate. Yet, it is worth noting that even when other potential covariates were retained, the parameter estimates in the mediation model were not significantly altered. In path b, baseline depressive symptoms and the first assessment of ART adherence were also controlled.
If paths a and b were both significant, mediation was tested using a Monte Carlo simulator to test the statistical significance of the mediating, or indirect effect (a*b) [32]. The indirect effect, a*b, is mathematically equivalent to c minus c’, and as such, represents the quantification of a mediated effect [34]. The Monte Carlo method is a form of bootstrapping, in which random draws from the distributions of the a and b paths are simulated and the product of these values is computed, which are used to estimate a 95 % confidence interval (CI) around the observed value of a*b. The indirect effect is interpreted as “statistically significant” if 0 is not contained between the lower and upper bounds of the CI [35, 36].
Bootstrapping methods are generally preferred over traditional methods of studying mediation (i.e., the causal steps approach and the product of coefficients approach; [37]), particularly for small samples, because there are no assumptions about the shape of the sampling distribution of the indirect effect [38]. Additionally, the causal steps approach requires a significant c path. However, current statistical thinking recommends that a significant c path may be superfluous and not required to test for mediation, and that only paths a and b are required to be significant to test for mediation [37, 39]. Given these limitations and in order to increase our power in the current analyses, we utilized bootstrapping methods to test the indirect effect.
Results
A sample of 241 individuals was screened for study eligibility and 230 were enrolled and randomized to one of the three study arms. Of the 230 randomized, 199 started ART and constituted the sample for this analysis. There were 135 participants on ART at week 48 (see [26] for more details regarding the number of participants at each assessment wave).
Demographic and Clinical Characteristics
The sample was 20 % female, 30 % Caucasian, 14 % African American, 49 % Latino(a), and 2 % Asian-Pacific Islander; 35 % were employed, 17 % had a college degree, and 28 % reported having unstable housing accommodations (see [26] for additional demographic characteristics). Mean depression score at baseline was 0.95 (S.D. = 0.85), which indicates elevated depressive symptoms (i.e., normed standardized t score [30] of 74.94 in comparison to the mean in the general population of 50 and BSI clinical cut-off of 63) [29]. Regarding baseline HIV clinical characteristics, 30 % were HIV antiretroviral naïve and 86 % had CD4 cell count <350 cells/μl. Depending on assessment wave, 60–66 % of the sample was taking a PI-based ART regimen, and 72–76 % were on a twice-a-day regimen (vs. 24–27 % on once-a-day). Mean dose taking adherence during the assessment period was 87.9 % (SD = 18.9) for dose taking and 78.2 % (SD = 24.5) for dose timing. Percentage of the sample with an undetectable viral load (<50 copies/mL) was 10 % at the start of the assessment period and 17 % at week 48. Across time, bivariate correlations revealed that viral load was significantly related to both dose timing adherence (r = −0.16, p = 0.004) and dose taking adherence (r = −0.18, p = 0.001). Similarly, point-biserial bivariate correlations revealed that viral load suppression (<50 copies/mL) was significantly related to both dose timing adherence (r = 0.11 p = 0.04) and dose taking adherence (r = 0.14, p = 0.01). Dose timing and dose taking were also highly correlated (r = 0.81, p < 0.0001).
Mediation Results: Dose Taking Adherence
Elevated depressive symptoms at baseline significantly predicted lowered lifestyle structure across time, γ = −0.16, SE = 0.04, 95 % CI [−0.25, −0.08], t(525) = −3.7, p < 0.0001 (path a). In turn, poor lifestyle structure predicted lower dose taking adherence across time, γ = 0.05, SE = 0.02, 95 % CI [0.006, 0.09], t(332) = 2.2, p = 0.03 (path b). Neither path c (relationship between depressive symptoms and adherence), γ = −0.001, SE = 0.02, 95 % CI [−0.03, 0.03], t(332) = −0.08, p = 0.94, nor path c’ were significant (γ = −0.02, SE = 0.02, 95 % CI [−0.06, 0.02], t(351) = −1.05, p = 0.30). However, there was a significant effect for the mediational path via calculation of the indirect effect, or a*b (γ = −0.008, 95 % CI [−0.02, −0.001]).
Mediation Results: Dose Timing Adherence
Elevated depressive symptoms at baseline significantly predicted lowered lifestyle structure across time (path a), γ = −0.16, SE = 0.04, 95 % CI [−0.25, −0.08], t(525) = −3.7, p < 0.0001. In turn, poor lifestyle structure predicted lower dose timing adherence across time (path b), γ = 0.08, SE = 0.03, 95 % CI [0.02, 0.13], t(329) = 2.8, p = 0.005. The relationship between depressive symptoms and adherence (path c) also emerged as significant, with elevated depressive symptoms at baseline predicting poorer adherence across time, γ = −0.06, SE = 0.03, 95 % CI [−0.11, −0.003], t(349) = −2.1, p = 0.04. Path c’ was non-significant (γ = −0.01, SE = 0.02, 95 % CI [−0.05, 0.03], t(329) = −0.51, p = 0.61) and there was a significant effect for the mediational path via calculation of the indirect effect, a*b (γ = −0.013, 95 % CI [−0.026, −0.003]). See Fig. 1 for a depiction of both models (dose taking and dose timing).
Fig. 1.
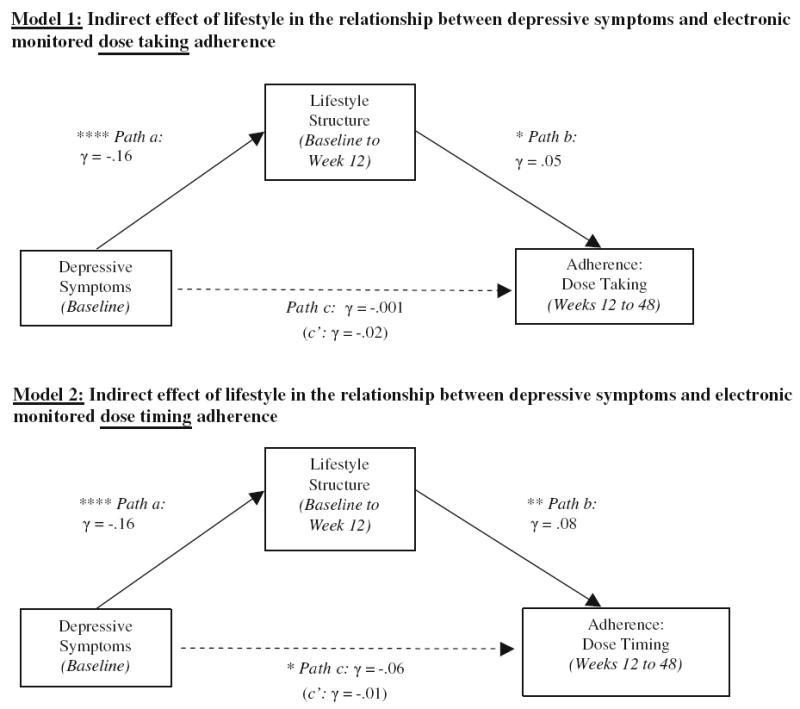
Models testing indirect effect of lifestyle structure in the relationship between depressive symptoms and two measures of electronically-monitored ART adherence. Note * = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001. The indirect effect (a*b) was significant in both models
Mediation Results: Viral Load
Elevated depressive symptoms at baseline significantly predicted lowered lifestyle structure across time (path a), γ = −0.16, SE = 0.04, 95 % CI [−0.25, −0.08], t(525) = −3.7, p < 0.0001. However, poor lifestyle structure did not significantly predict viral load across time (path b), γ = −0.04, SE = 0.05, 95 % CI [−0.13, 0.05], t(353) = −0.9, p = 0.37. Given that path b was non-significant, a significant mediational pathway (i.e., a*b, indirect effect) was not possible to be tested.
Discussion
The current study is one of the first efforts to identify mediators of the relationship between depressive symptoms and ART nonadherence among individuals living with HIV. Given its previously established relationship to both depression and ART adherence [23], the current study focused on lifestyle structure—the degree of structure and organization in one’s daily life—as an explanatory factor in the relationship between depressive symptoms and three longitudinally assessed indicators of ART adherence. Assessments were conducted sequentially to allow for testing of mediation.
Our data demonstrated a significant mediating effect of lifestyle structure in the relationship between depressive symptoms and both behavioral measures of adherence (dose taking and dose timing). These findings suggest that the degree of structure to one’s daily activities may be an important path through which depression disrupts adherence behavior and furthers our understanding of how depression affects ART adherence. By pinpointing specific processes through which depression influences adherence, targeted strategies may be developed with aims of improving the efficacy and parsimony of existing interventions. Current findings suggest that efforts to improve both depression and adherence should incorporate a consideration of the structure to one’s daily life activities. Existing empirically supported interventions that address increasing structure and regularity of activities, such as behavioral activation (BA), which aims to reduce depressive symptoms by scheduling regular enjoyable and meaningful activities (e.g. [40]), may be particularly suited for this purpose. Indeed, existing treatments that integrate adherence interventions and BA techniques for depressed HIV-positive clients (e.g., [28, 41, 42]) promote increasing structure and regularity of daily routines, and these interventions have shown improvements in both depression and adherence. Furthermore, many ART adherence counseling interventions, including the intervention administered in this study, incorporate exercises that help clients tailor their ART regimens to their daily routine by linking the timing of doses to specific routinized activities, and building structure or routinization of activities when needed [26, 43]. Our findings suggest that intervention research should examine lifestyle structure as a mechanism that may explain the effects of the intervention on both depression and adherence outcomes.
In contrast to other studies (e.g., [9, 44]) our data do not support a relationship between depression and dose taking adherence; however, depression was significantly related to dose timing, suggesting that depression interferes with one’s ability to take medications on time, but not whether the dose is actually taken. This adds further credence to the need for more nuanced investigations of the relationship between depression and adherence, with measurement strategies that go beyond global depression or distress to include measures of symptom type (i.e., cognitive vs. somatic) and severity [9], and adherence measures other than the convention mean or dichotomized dose taking adherence. Also, our findings suggest that other factors—such as disruptions in daily life structure—may be what drive the relationship between depression and adherence, not just the presence of depressive symptoms. It is also noteworthy that these findings remained significant over and above housing stability—a factor that could have an extremely important role in these relationships. Given the robustness of the mediating effect in both models, it is somewhat surprising that there was in fact a significant relationship between depressive symptoms and dose timing (path c; Model 2).
Given that lifestyle structure was not associated with viral load, we could not test the mediation model with viral load as the outcome. The lack of relationship between lifestyle structure and viral load may be the case for a few reasons. First, the relationship between lifestyle structure and viral load is likely mediated by behavioral adherence, which will be important to investigate in future research with larger samples that are powered to test a larger model with multiple mediating pathways. Additionally, 70 % of the sample had previously been on ART, and as such may have been more likely to have drug resistance upon initiating ART in this study, which may have affected these individuals’ biological response to ART. Finally, given that a low percentage of our sample had viral load suppression, there may not have been sufficient variability in rates of viral load suppression to be powered to detect changes in viral load suppression over time.
Strengths of the study include longitudinal assessments over 48 weeks, objective measurement of adherence and biological outcomes, as well as use of multilevel modeling techniques to test for mediation. Although multilevel modeling increased our power to test study aims, a larger sample size is important for future work. Further, although our analyses are an improvement over cross-sectional designs in that we can address temporal precedence more accurately, only experimental designs that manipulate the mediator—lifestyle structure—can speak to causality. Finally, we relied on self-report methods for depression and lifestyle, rather than more objective diagnostic interviews and direct observation.
In conclusion, findings provide evidence for lifestyle structure as an important factor to consider in understanding the relationship between depression and ART adherence behavior. Intervention components that aim to minimize disruptions in lifestyle and daily structure, increase routinization of daily activities and link dose taking to these routines may serve to improve both adherence and depression. Further research is needed to better our understanding of the underlying mechanisms that drive the relationship between depressive symptoms and adherence, including the role of daily structure and routines. We hope these findings provide a useful starting point for this line of work.
Acknowledgments
This study and Dr. Wagner were supported by R01MH61695 and the University Wide AIDS research program at the University of California, center Grant numbers CC99-SD003 and CC02-SD-003. Dr. Magidson’s work on this manuscript was supported by R36DA034513 (PI: Magidson) and T32MH093310 (PIs: Henderson and Fricchione). Dr. Blashill was supported by K23MH096647 (PI: Blashill), and Dr. Safren was supported by K24MH094214 (PI: Safren).
Contributor Information
Jessica F. Magidson, Email: jmagidson@partners.org, Department of Psychiatry, Massachusetts General Hospital/Harvard Medical School, One Bowdoin Square, 7th Floor, Boston, MA 02114, USA.
Aaron J. Blashill, Department of Psychiatry, Massachusetts General Hospital/Harvard Medical School, One Bowdoin Square, 7th Floor, Boston, MA 02114, USA
Steven A. Safren, Department of Psychiatry, Massachusetts General Hospital/Harvard Medical School, One Bowdoin Square, 7th Floor, Boston, MA 02114, USA
Glenn J. Wagner, RAND Corporation, Santa Monica, CA, USA
References
- 1.Genberg BL, Wilson IB, Bangsberg DR, et al. Patterns of anti-retroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS. 2012;26:1415–23. doi: 10.1097/QAD.0b013e328354bed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de García Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30:105–10. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45:372–9. doi: 10.1345/aph.1P587. [DOI] [PubMed] [Google Scholar]
- 4.Martin M, Del Cacho E, Codina C, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: A prospective cohort study. AIDS Res Hum Retrovir. 2008;24:1263–8. doi: 10.1089/aid.2008.0141. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR. Less than 95 % adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–41. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 6.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, Wilson IB. Incident depression symptoms are associated with poorer HAART adherence: a longitudinal analysis from the Nutrition for Healthy Living study. J Acquir Immune Defic Syndr. 2010;53:266–72. doi: 10.1097/QAI.0b013e3181b720e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58:181–7. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner GJ, Goggin K, Remien RH, et al. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med. 2011;42:352–60. doi: 10.1007/s12160-011-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima VD, Geller J, Bangsberg DR, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21:1175–83. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 11.Blashill A, Gordon J, Mimiaga M. HIV/AIDS and depression. In: Richards S, O’Hara M, editors. The Oxford handbook of depression and comorbidity. New York: Oxford University Press; 2014. [Google Scholar]
- 12.Carvalho JP, Gawrysiak MJ, Hellmuth JC, et al. The reward probability index: design and validation of a scale measuring access to environmental reward. Behav Ther. 2011;42:249–62. doi: 10.1016/j.beth.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hopko DR, Lejuez CW, Ruggiero KJ, Eifert GH. Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clin Psychol Rev. 2003;23:699–717. doi: 10.1016/s0272-7358(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 14.Hopko DR, Mullane CM. Exploring the relation of depression and overt behavior with daily diaries. Behav Res Ther. 2008;46:1085–9. doi: 10.1016/j.brat.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Kanter JW, Rusch LC, Busch AM, Sedivy SK. Validation of the behavioral activation for depression scale (BADS) in a community sample with elevated depressive symptoms. J Psychopathol Behav Assess. 2009;31:36–42. [Google Scholar]
- 16.Lewinsohn PM. A behavioral approach to depression. In: Fried-man RJ, Katz MM, editors. The psychology of depression: contemporary theory and research. Oxford: John Wiley & Sons; 1974. p. 318. [Google Scholar]
- 17.Lewinsohn PM, Graf M. Pleasant activities and depression. J Consult Clin Psychol. 1973;41:261–8. doi: 10.1037/h0035142. [DOI] [PubMed] [Google Scholar]
- 18.Ferster C. A functional analysis of depression. Am Psychol. 1973;28:857–70. doi: 10.1037/h0035605. [DOI] [PubMed] [Google Scholar]
- 19.MacPhillamy C, Lewinsohn PM. The pleasant events schedule. Eugene: University of Oregon; 1971. [Google Scholar]
- 20.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 21.Gifford AL, Bormann JE, Shively MJ, et al. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23:386–95. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KJ. Barriers to and facilitators of HIV-positive patients’ adherence to antiretroviral treatment regimens. AIDS Patient Care STDs. 2000;14:155–68. doi: 10.1089/108729100317948. [DOI] [PubMed] [Google Scholar]
- 23.Wagner GJ, Ryan GW. Relationship between routinization of daily behaviors and medication adherence in HIV-positive drug users. AIDS Patient Care STDs. 2004;18:385–93. doi: 10.1089/1087291041518238. [DOI] [PubMed] [Google Scholar]
- 24.Wenger N, Gifford A, Liu H, et al. Patient characteristics and attitudes associated with antiretroviral adherence. Presented at the 6th Conference on Retroviruses and Opportunistic Infections; 1999; Chicago. [Google Scholar]
- 25.Ryan GW, Wagner GJ. Pill taking “routinization”: a critical factor to understanding episodic medication adherence. AIDS Care. 2003;15:795–806. doi: 10.1080/09540120310001618649. [DOI] [PubMed] [Google Scholar]
- 26.Wagner GJ, Kanouse DE, Golinelli D, et al. Cognitive-behavioral intervention to enhance adherence to antiretroviral therapy: a randomized controlled trial (CCTG 578) AIDS. 2006;20:1295–302. doi: 10.1097/01.aids.0000232238.28415.d2. [DOI] [PubMed] [Google Scholar]
- 27.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–66. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 28.Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J Consult Clin Psychol. 2012;80:404–15. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derogatis LR. BSI 18, Brief symptom inventory 18: administration scoring and procedures manual. Iowa: NCS Pearson Incorporated; 2001. [Google Scholar]
- 30.Tchekmedyian NS, Kallich J, McDermott A, Fayers P, Erder MH. The relationship between psychological distress and cancer-related fatigue. Cancer. 2003;98:198–203. doi: 10.1002/cncr.11463. [DOI] [PubMed] [Google Scholar]
- 31.Wagner GJ, Remien RH, Carballo-Diéguez A, Dolezal C. Correlates of adherence to combination antiretroviral therapy among members of HIV-positive mixed status couples. AIDS Care. 2002;14:105–9. doi: 10.1080/09540120220097973. [DOI] [PubMed] [Google Scholar]
- 32.Selig JP, Preacher KJ. Mediation models for longitudinal data in developmental research. Res Hum Dev. 2009;6:144–64. [Google Scholar]
- 33.Kraemer H, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27:S101–8. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKinnon D. Introduction to statistical mediation analysis. Routledge: CRC Press; 2007. [Google Scholar]
- 35.Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: new procedures and recommendations. Psychol Methods. 2006;11:142–63. doi: 10.1037/1082-989X.11.2.142. [DOI] [PubMed] [Google Scholar]
- 36.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivar Behav Res. 2004;39:99. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–45. [PubMed] [Google Scholar]
- 38.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 39.Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76:408–20. [Google Scholar]
- 40.Lejuez CW, Hopko DR, Acierno R, Daughters SB, Pagoto SL. Ten year revision of the brief behavioral activation treatment for depression: revised treatment manual. Behav Modif. 2011;35:111–61. doi: 10.1177/0145445510390929. [DOI] [PubMed] [Google Scholar]
- 41.Daughters SB, Magidson JF, Schuster RM, Safren SA. ACT HEALTHY: a combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cogn Behav Pr. 2010;17:309–21. doi: 10.1016/j.cbpra.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28:1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39:1151–62. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 44.Paterson DL, Swindells S. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]


