Abstract
Background
Achieving negative surgical margins is critical to minimizing the risk of tumor recurrence in patients undergoing breast conservation surgery (BCS) for a breast malignancy. Our objective was to perform a systematic review comparing reexcision rates, sensitivity and specificity of the intraoperative use of the margin assessment techniques of imprint cytology (IC) and frozen section analysis (FSA), against permanent histopathologic section (PS).
Methods
The databases PubMed, Web of Knowledge, Cochrane Library and CINAHL Plus were searched for literature published from 1997 to 2011. Original investigations of patients who underwent BCS for breast cancer that evaluated margin assessment with PS and/or IC or FSA were included. Of 182 titles identified, 41 patient cohorts from 37 articles met inclusion criteria: PS (n = 19), IC (n = 7) and FSA (n = 15). Studies were summarized qualitatively using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort studies and the Strength of Recommendation Taxonomy (SORT) numerical scale for diagnostic studies.
Results
The final reexcision rates after primary BCS were 35 % for PS, 11 % for IC (p = 0.001 vs. PS) and 10 % for FSA (p < 0.0001 vs. PS). For IC, reexcision rates decreased from 26 to 4 % (p = 0.18) and for FSA, reexcision rates decreased from 27 to 6 % (p < 0.0001). The pooled sensitivity of IC and FSA were 72 and 83 %. The pooled specificity of IC and FSA were 97 and 95 %. The average length of each technique was 13 min for IC and 27 min for FSA.
Conclusions
Patients who underwent BCS with intraop-erative IC or FSA to assess negative surgical margins had significantly fewer secondary surgical procedures for excision of their breast malignancies.
Breast conservation surgery (BCS) with radiation has become the preferred method of treatment for patients with Stage I and II breast cancer after the 1990 Consensus Conference on Breast Cancer from the National Institutes of Health.1 To achieve an oncologically and cosmetically successful BCS, performance by the surgeon and an accurate assessment of the surgical margins by the pathologist are critical. Several studies have clearly defined that achieving a negative surgical margin on patients undergoing BCS is imperative.2 Despite this, there is no consensus on what constitutes a negative margin; it varies from cancer not touching the ink in the specimen to 3 cm of normal tissue around the tumor.3 Local recurrence rates increase in patients who have persistent positive surgical margins compared to patients with negative margins.4–7
The best cosmetic results are obtained during the primary BCS procedure. Reexcision of the breast results in a greater volume of resected tissue than with a single primary excision, which ultimately may affect cosmesis. In addition to the added costs of revision surgery, reexcision BCS leads to increased patient anxiety and a higher likelihood of infection.8 Consequently, it is important to identify intra-operative margin assessment methods that would decrease reexcision rates.
Two intraoperative margin assessment methods are frequently reported in the literature: frozen section analysis (FSA) and touch preparation or imprint cytology (IC). FSA is the intraoperative technique that has been most widely used to analyze breast tumor excisions. It consists of freezing and sectioning the sample followed by thawing, fixation and staining. Reports indicate that FSA may cause artifacts in the fatty tissue as a result of the process of freezing and thawing, resulting in loss of tissue. This technique takes approximately 30 min.9 Because of the disadvantages of FSA, IC has been proposed as an alternative intraoperative method. IC is a simple and rapid method where the excised mass is oriented and pressed onto glass slides making an imprint of all 6 margins. Slides are then fixed and stained. The principle is based on the cellular surface characteristics where only malignant cells will adhere to the slides and adipose cells will not. This method has been reported to take only an average of 15 min to provide a diagnosis.2 Variability in the sensitivity of the method has been reported and is related to the size of the tumor and the cytological skills of the pathologist.9 Errors of interpretation are linked to specimen surface irregularity, dryness and presence of atypical cells.10
Currently, there exists no systematic review that addresses the impact of these two intraoperative surgical margin assessment techniques in patients undergoing BCS. The primary objective of this work was to systematically review data published in primary studies of IC and FSA and to compare surgical reexcision rates, sensitivity and specificity of these techniques after library BCS against, permanent histopathologic section (PS).
METHODS
Literature Search
The databases PubMed, Web of Knowledge, Cochrane Library and CINAHL Plus were searched from 1997 to July 1, 2011, using the strategy shown in Fig. 1. The study period from 1997 was chosen, as important cohort studies with intraoperative IC and FSA occurred after 1997. References of included articles were also searched. The overall search strategy included terms for breast cancer (e.g., breast neoplasm, breast cancer), imprint cytology (e.g., Touch prep* cytology, TPC, imprint cytology, intraoperative imprint cytology), frozen section analysis (e.g., frozen section or FS), surgical margin (e.g., surgical margin*, margin assess*, margin evaluat*), conserving surgery (e.g., conserving surger*, lumpectomy, partial mastectom*, segmental mastectom *, quadrantectomy) and reexcision rate (e.g., reexcision rate, reexcision rate) and was limited to peer-reviewed human studies. No language restrictions were applied.
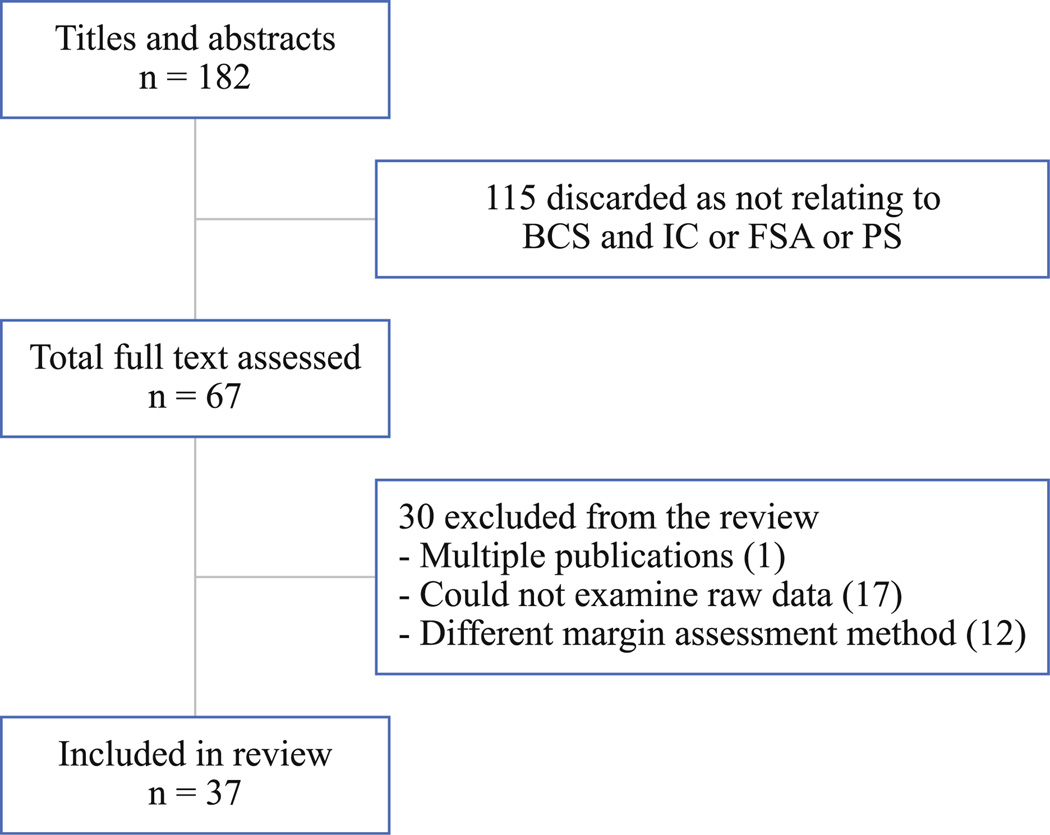
FIG. 1.
Flow diagram of the literature search and study selection. The number of publications does not match the number of patient cohort data sets included in the analysis, which add to 41
Selection Criteria
Articles for inclusion and exclusion were screened by a single reviewer blinding for journal, authors, institution, and country of origin. The inclusion criteria for the overall search targeted articles with the following: (1) individuals with cancer undergoing BCS; (2) surgical margin assessment technique (IC, FSA, PS, or some combination), reexcision rates, and/or specificity and sensitivity values for both intraoperative techniques; (3) study sampling and methods stated and; (4) publication was peer-reviewed. Articles that included sufficient data for cross-tabulation of the results of PS, IC and FSA were included. Articles were excluded if they analyzed or mixed data of lymph nodes, they used other intraoperative margin assessment techniques, or if studies were case reports or meeting abstracts.
Assessment of Methodologic Quality
Articles were extracted and assessed for quality following the same blinding criteria as above. Abstracted data included patient demographics, type of breast cancer, surgical margin assessment techniques, reexcision rates during and after primary BCS, sensitivity and specificity of IC and FSA, false-positive and false-negative cases for IC and FSA, and study quality and type. Quality was assessed by using components of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort studies and the Strength of Recommendation Taxonomy (SORT) numerical scale for diagnostic studies.11,12 To judge quality, information was abstracted on population source, whether the study had institutional approval, statistical methods and publication bias.
Data Synthesis and Statistical Analysis
Data were used on a per-tumor basis rather than per-patient basis to calculate reexcision rates, sensitivity and specificity. Some patients had more than one tumor extracted and analyzed and each tumor was considered as an independent specimen. If sensitivity and specificity data were missing, they were calculated from the available data. To avoid overlapping patient populations, data on recruitment years, data source and geographic location were compared. If there were multiple publications of the same author with the same patient population, only the most comprehensive and relevant study was included. This resulted in the exclusion of one article.13
Nine authors were contacted for unreported secondary information to complete the data tables, and one author replied. From the studies selected, only nine had a SORT/ STROBE score of 2 and the rest had a score of 1, suggesting the findings of this study are reliable.
The final reexcision rates among three groups (FSA, IC and PS) were compared by a general linear model with a t test. The t test was also used to compare the intraoperative reexcision rate and final reexcision rate within IC and FSA pairs during and after primary surgery. Finally, the sensitivity and specificity between FSA and IC groups was compared by t test. All analyses were conducted by SAS software (SAS Institute, Cary, NC).
RESULTS
Our literature search yielded 182 potentially relevant articles, of which 67 were evaluated in full text. Most excluded studies did not report reexcision rates or mixed intraoperative methods. Of the 67 articles, 37 primary studies were eligible for inclusion in the systematic review. The number of publications doesn’t match the number of patient cohort data sets included in the analysis. Four of 37 publications contained two different sets of data within the same publication, resulting in 41 patient cohort data sets for analysis.14–17 Nineteen publications were included for the analysis of permanent section, seven for IC and 15 for FSA.
Description of Studies
The 37 articles included in this systematic review were homogeneous in their primary goal. The articles measured reexcision rates or recurrence rates on patients undergoing BCS either with intraoperative IC, FSA and/or PS. All studies were conducted in medical institution-based surgical and pathology units. Nine studies used prospective cohorts of breast cancer patients and the remaining utilized retrospective cohorts. Most studies analyzed the use of intraoperative FSA and IC in all types of breast cancer. Five studies analyzed data from patients exclusively with invasive carcinoma; three studies analyzed data from patients with in situ carcinoma, and one included phyllodes tumors.
The studies were conducted in the United States (n = 21), Canada (n = 1), Brazil (n = 2), Europe (n = 9), Turkey (n = 1), Asia (n = 4), and Australia (n = 1). Sample sizes ranged from 44 to 2,770 for PS studies (Table 1), from 12 to 1,193 for IC studies (Table 2) and from 54 to 1,016 for FSA studies (Table 3) with a median of 351, 328 and 259, respectively.
TABLE 1.
Characteristics of permanent section studies
| Study | Year | Country | Carcinoma type | BCS | Age range (y) |
n | Reexcision ratea | LOE | Study type |
|---|---|---|---|---|---|---|---|---|---|
| Arora et al.24 | 2007 | USA | ADH, DCIS | Lumpectomy | 42–87 | 44 | 55.0 % (24) | 1 | Retrospective cohort |
| Aziz et al.5 | 2006 | Canada | IC, DCIS | Lumpectomy | 55b | 1,172 | 13.0 % (152) | 1 | Retrospective cohort |
| Cabioglu et al.14 | 2007 | USA | IC, DCIS | Wide local excision, segmental mastectom |
27–95 | 261 | 44.0% (116) | 1 | Retrospective cohort |
| Camp et al.15 (personal communication) | 2005 | USA | IC, DCIS | Lumpectomy | 26–78 | 78 | 33.3 % (26) | 1 | Retrospective cohort |
| Huston et al.25 | 2006 | USA | DCIS, LCIS, IDC, ILC |
Lumpectomy | 30–91 | 171 | 29.2 % (50) | 2 | Retrospective cohort |
| Landercasper et al.26 | 2010 | USA | Breast cancer | Lumpectomy | – | 568 | 19.0 % (108) | 1 | Retrospective cohort hospital report |
| Loibl et al.16 | 2006 | Germany | ILC, IDC | Lumpectomy | 26–78 | 248 | 27.0 % (67) | 1 | Retrospective cohort RCT–GEPARDUO |
| McCahill et al.27 | 2010 | USA | DCIS, IDC, ILC | Partial mastectom | – | 712 | 13.0 % (95) | 2 | Retrospective cohort |
| Menes et al.28 | 2005 | USA | DCIS, ILC, IDC | Lumpectomy | 27–87 | 459 | 49.9 % (229) | 1 | Retrospective cohort Prospective database |
| Miller et al.29 | 2004 | USA | DCIS, ILC, IDC | BCS | 38–64 | 143 | 18.2 % (26) | 1 | Retrospective cohort |
| Moorthy et al.30 | 2004 | UK | IDC, ILC | Lumpectomy, wide local excision, quadrantectomy |
47–73 | 505 | 27.1 % (137) | 1 | Prospective cohort |
| Mullenix et al.31 | 2004 | USA | IDC, ILC | BCS | 48–73 | 150 | 51.0 % (90) | 1 | Retrospective cohort, prospective database |
| Ooi et al.32 | 2003 | Australia | DCIS, ILC, IDC, IC, medullary |
BCS | 28–80 | 742 | 3.9 % (29) | 2 | Retrospective cohort |
| O’Sullivan et al.33 | 2007 | USA | IDC, DCIS, ILC | Lumpectomy | 20–91 | 2,770 | 59.6 % (1,651) | 1 | Retrospective cohort, prospective database |
| Perez34 | 2003 | USA | ILC, IDC | Quadrantectomy, wide local excision, local excision |
– | 1,347 | 52.3 % (704) | 1 | Retrospective cohort |
| Ramanah et al.35 | 2008 | France | ILC, IDC | Lumpectomy, wide local excision |
44–68 | 206 | 41.0 % (84) | 1 | Retrospective cohort |
| Sanchez et al.36 | 2010 | USA | IDC, ILC, DCIS, atypia |
BCS | 22–88 | 351 | 34.0% (118) | 2 | Retrospective cohort |
| van den Broek et al.37 | 2007 | Netherlands | ILC | BCS | – | 416 | 14.4 % (60) | 2 | Retrospective cohort |
| Vicini et al.38 | 2001 | USA | DCIS | Excisional biopsy | – | 146 | 64.0 % (95) | 2 | Retrospective cohort |
| Results | 351b | 35 % (±3 %)c |
BCS breast conservation surgery, LOE level of evidence (SORT), ADH atypical ductal hyperplasia, IC invasive carcinoma, DCIS ductal carcinoma-in-situ, LCIS lobular carcinoma-in-situ, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma
After first BCS
Sample median
Pooled estimates
TABLE 2.
Characteristics of imprint cytology studies
| Study | Year | Country | Carcinoma type |
BCS | Age range |
n | Intraoperative reexcision ratea |
Reexcision rate finalb |
Sensitivity | Specificity | Time (min) |
False- positive cases |
False-negative cases |
LOE | Study type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barros et al.39 |
2004 | Brazil | IC | Quadrantectomy | 28–79 | 102 | 37 % (38) | 0 % (0) | – | – | 15–20 | 13: Atypical cells present |
10: Focal for DCIS (6), IC (3) and presence of carcinoma at margin in a single lymphatic space (1) |
2 | Prospective cohort |
| Cox et al40 |
1997 | USA | DCIS | Lumpectomy | 30–82 | 104 | – | 0 % (1) | 99 % | 98 % | 2 | 1: Focal for DCIS | 1 | Prospective database |
|
| D’Haulluin et al.41,c |
2009 | France | IDC, DCIS, ILC, LCIS |
Lumpectomy | 29–88 | 396 | 38.63 % (153) |
12.6 % (50) |
88.6 % | 92.2 % | 7–17 | 25: ILC (10.2 %), IDC histological grade 3 (5.6 %), fibricystic mastopathy lesions |
9: Contact with 1 margin (4), 2 mm away from margin (5). DCIS, size ≥30 mm, histological grade of 1 or 2. |
1 | Retrospective cohort |
| Klimberg et al42 |
1998 | USA | DCIS, ILC | Lumpectomy | 22–86 | 428 | 1.4 % (6) | 0 % (0) | 96.4 % | 100% | 2–3 | None | 3: Focal for DCIS (2), LCIS (1). Thought to be missed by sampling error. |
1 | Prospective cohort |
| Valdes et al.17,d |
2007 | USA | ILC | Lumpectomy | 12 | 33 % (4) | 8.3 % | 98.3 % | 15 | 1: Atypical lobular hyperplasia |
8: Focal for ILC (7) and ILC plus LCIS (1) |
2 | Prospective cohort |
||
| Valdes et al.17,d |
2007 | USA | IDC | Lumpectomy | – | 61 | – | 23 % (14) | 65.7 % | 95.5 % | 15 | 4.10 % | 3.3 %, DCIS | 2 | Prospective cohort |
| Weinberg et al.10 |
2004 | USA | DCIS, ILC, IDC |
Lumpectomy | – | 1,193 | – | 6.2 % (74) | – | – | 15 | – | – | 1 | Retrospective cohort |
| Results | 104e | 26% (±21 %)f |
11 % (±4 %)f |
72 % (±38 %)f |
97 % (±3 %)f |
13 |
BCS breast conservation surgery, LOE level of evidence (SORT), IC invasive carcinoma, DCIS ductal carcinoma-in-situ, LCIS lobular carcinoma-in-situ, IDC invasive ductal carcinoma, ILC Invasive lobular carcinoma
During first BCS
After first BCS
Cross-publication
Publication used twice in analysis because of different study cohorts in same publication
Sample median
Pooled estimates
TABLE 3.
Characteristics of frozen section analysis studies
| Study | Year | Country | Carcinoma type | BCS | Age range (y) | n | Intraoperative reexcision ratea |
|
|---|---|---|---|---|---|---|---|---|
| Cabioglu et al.14 | 2007 | USA | IC, DCIS | Wide local excision, segmental mastectom |
27–95 | 110 | 20.0 % (22) | |
| Camp et al.15 (personal communication) | 2005 | USA | IC, DCIS | Lumpectomy | 26–78 | 189 | 27.0 % (51) | |
| Cendan et al.43 | 2005 | USA | DCIS, LCIS, ILC, IDC, MC | BCS | 48–71 | 97 | 44.0 % (43) | |
| Chen et al.44 | 2005 | Taiwan | PT | Local and wide excision | 11–73 | 113 | ||
| Dener et al.45 | 2009 | Turkey | IC | Lumpectomy | 18–94 | 186 | 16.0 % (30) | |
| Fukamachi et al.19 | 2010 | Japan | IDC, DCIS, ILC, MC, AC | Wide excision, quadrantectomy |
32–87 | 122 | 27.0 % (33) | |
| Ikeda et al.46 | 1997 | Japan | DCIS, IC | Quadrantectomy | 33–66 | 54 | 37 % (20) | |
| Loibl et al.16 | 2006 | Germany | ILC, IDC | Lumpectomy | 25–78 | 240 | – | |
| Munhoz et al.20 | 2009 | Brazil | DCIS, ILC, IDC | Lumpectomy | 23–71 | 218 | 28.8 % (63) | |
| Olson et al.47 | 2007 | USA | IC, DCIS | Lumpectomy | 27–89 | 290 | 24.0 % (70) | |
| Park et al.48 | 2011 | Korea | ILC, IDC, MC, TC, PC, medullary | BCS | 20–78 | 705 | – | |
| Riedl et al.21 | 2009 | Austria | DCIS, ILC, IDC | Lumpectomy | 24–92* | 1,016 | – | |
| Rusby et al.18 | 2008 | UK | DCIS, ILC, IDC, MC, TC, PC | Partial mastectom | 40–59 | 115 | 33.0 % (38) | |
| Weber et al.49 | 1997 | USA | DCIS | Lumpectomy | – | 140 | 15 % (21) | |
| Weber et al.50 | 2008 | Switzerland | IC, DCIS, ADH | Lumpectomy | 34–86 | 80 | – | |
| Results | 163c | 27 % (±9 %)d | ||||||
| Reexcision rate finalb | Sensitivity | Specificity | Time (min) | False-positive cases |
False-negative cases | LOE | Study type | |
| 8.8 % (10) | 91.7 % | 77.8 % | – | – | 1 | Retrospective cohort | ||
| 5.8 % (11) | – | – | 13 | – | – | 1 | Retrospective cohort | |
| 19.6 % (19)c | 58.1 % | 100.0 % | 13 | None | 22: DCIS (20), LCIS (2) | 1 | Retrospective cohort | |
| 41.6 % (47) | – | – | – | – | 2 | Retrospective cohort | ||
| 0 % (0) | 100.0 % | 100.0 % | None | None | 1 | Retrospective cohort | ||
| 9.8 % (12) | 78.6 % | 100.0 % | 53# | None | – | 1 | Retrospective cohort | |
| 0 % (0) | 94% | 90% | 4: Atypical | 1 | 1 | Retrospective cohort | ||
| 13.3 % (32) | – | – | – | – | 1 | Retrospective cohort RCT-GEPARDUO | ||
| 5.5 % (12) | – | – | – | 12: IDC (7), IDC plus DCIS (2), DCIS (2). Younger age and larger tumors. |
1 | Retrospective cohort | ||
| 5.5 % (16) | 73.1 % | 99.6 % | 27 | 17 | 1 | Retrospective cohort | ||
| 13.5 % (95) | – | – | – | 28: Focal intraductal carcinoma | 1 | Retrospective cohort | ||
| 9 % (91) | – | – | 30 | – | 91: IDC (88), ILC (3); Small lessions, microcalcifications, neoadjuvant therapy |
1 | Retrospective cohort | |
| 7 % (8) | 83.0 % | 97.0 % | 8: Atypia, sclerosing adenosis (3); in situ or IC (4) |
9: Sampling error, small volume of tissue |
1 | Retrospective cohort, prospective database | ||
| 0 % (1) | 91 % | 100% | None | 3: DCIS (2), LCIS (1) |
1 | Retrospective cohort | ||
| 12.5 % (10) | 80.0 % | 87.5 % | – | – | 1 | Retrospective cohort | ||
| 10 % (±6 %)d | 83 % (±13 %)d | 95 % (±8 %)d | 27.25c | |||||
BCS breast conservation surgery, LOE level of evidence (SORT), IC invasive carcinoma, MC mucinous carcinoma, AC apocrine carcinoma, TC tubular carcinoma, PC papillary carcinoma, DCIS ductal carcinoma-in-situ, LCIS lobular carcinoma-in-situ, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, PT phyllodes tumors, ADH atypical ductal hyperplasia
During first BCS
After first BCS
Sample median
Pooled estimates
Personal communication
Permanent sections and frozen sections were stained with hematoxylin and eosin in all but one study in which methylene blue was used.18 The majority of IC studies used the Diff-Quik (Baxter Diagnostics, McGaw Park, IL), rapid Papanicolaou and hematoxylin and eosin techniques. The surgical margin results of IC and FSA were categorized as positive, suspicious/close or negative in all studies. Six articles reported the time taken to perform intraoperative IC (mean, 13 min; Table 2) and four articles reported the duration of FSA (mean, 27 min; Table 3). One FSA study reported a mean of 53 min as they performed a total circumference sampling of the specimen which required more slides to analyze.19 From the IC and FSA analysis publications, cross-tabulation of results against permanent section was either reported or could be inferred from the data provided.
Reexcision Rates
The total number of tumors analyzed across the studies of permanent section was 10,489 with a mean of 542. For IC, a total of 2,296 tumors were analyzed with an average of 300 specimens. Finally, 3,621 tumors were analyzed in FSA publications and 259 tumors were studied on average.
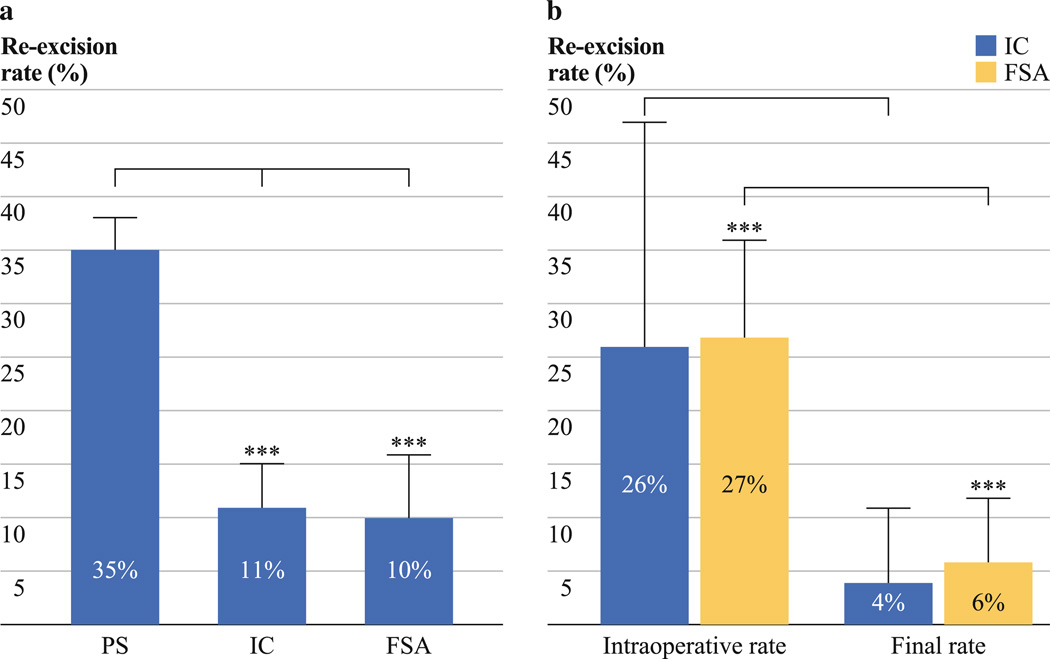
By means of the general linear model, we found significant differences in final reexcision rates among the three groups with an overall p-value of <0.0001. Subsequently, we performed a detailed examination with the t test. The final reexcision rates of FSA (10 ± 6 %) was significantly lower than PS (35 ± 3 %) (p < 0.0001). The final reexcision rate of IC (11 ± 4 %) was also significantly lower than PS (35 ± 3 %) (p = 0.001) (Fig. 2a). The final reexcision rates for FSA versus IC were not significantly different (p = 0.92).
FIG. 2.
Graphs of reexcision rates of permanent section, IC and FSA. a Pooled estimates of final reexcision rates after primary BCS. PS (n = 19), IC (n = 7) and FSA (n = 15). IC versus PS (p = 0.001) and FSA versus PS (p<0.0001). b Pooled estimates of intraoperative reexcision rates during primary BCS versus final reexcision rates after primary BCS when using intraoperative IC and FSA as surgical margin assessment techniques. IC (n = 3) and FSA (n = 10). IC reexcision rates decreased from 26 to 4 % (p = 0.18) and FSA reexcision rates decreased from 27 to 6 % (p<0.0001)
To further analyze the effects of intraoperative IC and FSA during primary BCS, we compared intraoperative reexcision rates and postoperative reexcision rates within FSA and IC. For FSA, the intraoperative reexcision rate of 27 ± 9 % was significantly reduced to a final rate of 6 ± 6 % (p < 0.0001). Similarly, the intraoperative reex-cision rate for IC (26 ± 21 %) was reduced to a final reexcision rate of 4 ± 7 %. However, it was not significantly different (p = 0.18) as a result of the variation in the IC group (Fig. 2b). We found that the intraoperative re-excision rate for FSA was not statistically different from the IC group (p = 0.86).
Sensitivity and Specificity
Five IC studies and nine FSA studies were used to analyze pooled intraoperative sensitivity and specificity. The sensitivity of FSA (83 ± 13 %) versus IC (72 ± 38 %) was not significantly different (p = 0.53). Similarly, the specificity of FSA (95 ± 8 %) versus IC (97 ± 3 %) was not significantly different (p = 0.58). In IC studies, the occurrence of false-positive cases was primarily the result of the observation of atypical cells with characteristics of invasive components. False-negative cases were due to patients presenting with ductal carci-noma-in-situ, then invasive lobular carcinoma and lobular carcinoma-in-situ (Table 2). With the intraoperative use of FSA, false-positive cases were linked to atypical cells and sclerosing adenosis. Conversely, false-negative cases were due to all types of malignancy (Table 3). Overall, there were more false-negative cases when using intraoperative FSA in comparison with IC. In one study of FSA, false-negative findings correlated with younger patients and with larger tumors.20 In other FSA publications, a correlation was found between false negative cases and patients with small volume of lesions, microcalcifications and neoadju-vant therapy.18,21
DISCUSSION
The intraoperative use of margin assessment techniques in breast cancer patients undergoing BCS has proven to be sufficiently rapid to be used in a clinically relevant time period during the original surgical procedure. In addition, this approach can efficiently reduce but not eliminate the need for additional surgeries to attain negative margins in these patient populations. Both FSA and IC had a final pooled reexcision rate of approximately 10 %, which is meaningful for surgical outcomes. During surgery, IC took less than 15 min and FSA took less than 30 min to perform. The sensitivity and specificity for IC was found to be comparable to FSA. However, there was a greater degree of variation present in the sensitivity of IC among studies. Overall, for IC and FSA, most false-negative cases were observed in tumors diagnosed with in-situ disease. In addition, false-negative cases for FSA occurred on specimens from invasive ductal carcinoma. These false-negative cases could be related to sampling errors, size of tumor, size of lesions and the nonpalpable state of the tumor.
Results of this systematic review are limited by the quality of the reporting of the studies analyzed. Both prospective and retrospective patient cohorts were reviewed; however, no randomized clinical trial data were available on this topic. On the basis of SORT and STROBE criteria, the quality of the studies selected was high; even though it is difficult to make comparisons when there are variations in the IC method, histopathological staining techniques and FSA specimen sectioning. The experience of the patholo-gists with each technique, especially cytopathological proficiency, was seldom mentioned and bias was unlikely to occur because most patients had the intraoperative and histological tests performed as part of a protocol.
Increased utilization of intraoperative IC and FSA is likely to lead to declines in reexcision surgery, surgical costs, patient anxiety and surgical complications and increases in cosmetic results, patient safety and patient satisfaction. Indeed, Uecker et al., have demonstrated the cost savings associated with intraoperative assessment of surgical margins through reduced surgical reoperations from two hospitals in Austin, TX.22 In the hospital that performed routine PS, 60 % of cases required reoperation with average costs of $22,013 per patient. Whereas in the hospital that performed intraoperative assessment, 24 % of cases needed reoperation with average costs of $15,341. Likewise, a recent cost-effectiveness analysis of FSA by Osborn et al. compared patients who underwent lumpec-tomy with and without intraoperative margin assessment.23 Patients who had lumpectomy without FSA, underwent a reoperation 15–50 % of the time, while patients who had FSA executed during BCS, had a reoperation 3 % of the time and the costs to provider and payor were significantly reduced.
The results of this review suggest that it is worthwhile for clinicians to consider adopting the use of IC and FSA if their current reexcision rates exceed 10 %. The use of IC and FSA as intraoperative margin assessment tools requires a multidisciplinary team effort among the surgeons, pathologists and radiologists. The presence of an on-site pathologist would be a requirement for performance of these procedures and may not be available at institutions where the pathology is performed at a separate location or there is limited experience with cytopathologic outcomes. In considering FSA and IC, the multidisciplinary breast team should evaluate their current reexcision rates and if in the 10 % rate range because of current practices of specimen imaging or use of intraoperative ultrasound, a change in practice may not be indicated. As new technology such as those using radiofrequency ablation and intraoperative optical imaging are refined, their performance against FSA and IC should be considered on the basis of this systematic review.
ACKNOWLEDGMENT
K.E. is supported by National Institutes of Health Grant 5R01CA114462-02 under Dr. Patricia Keely and by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) Grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS) Grant 9U54TR000021.
REFERENCES
- 1.National Institutes of Health Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [Google Scholar]
- 2.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 3.Sabel MS. Surgical considerations in early-stage breast cancer: lessons learned and future directions. Semin Radiat Oncol. 2011;21:10–19. doi: 10.1016/j.semradonc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Vapiwala N, Harris E, Huang WT, et al. Long-term outcome for mammographically detected ductal carcinoma in situ managed with breast conservation treatment: prognostic significance of reexcision. Cancer J. 2006;12:25–32. doi: 10.1097/00130404-200601000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Aziz D, Rawlinson E, Narod SA, et al. The role of reexcision for positive margins in optimizing local disease control after breast conserving surgery for cancer. Breast J. 2006;12:331–337. doi: 10.1111/j.1075-122X.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 6.Swanson G, Rynearson K, Symmonds R. Significance of margins of excision on breast cancer recurrence. Am J Clin Oncol. 2002;25:438–441. doi: 10.1097/00000421-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Tartter PI, Kaplan J, Bleiweiss I, et al. Lumpectomy margins, reexcision and local recurrence of breast cancer. Am J Surg. 2000;179:81–85. doi: 10.1016/s0002-9610(00)00272-5. [DOI] [PubMed] [Google Scholar]
- 8.Wazer DE, DePetrillo T, Schmidt-Ullrich R, et al. Factors infu-encing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992;10:356–363. doi: 10.1200/JCO.1992.10.3.356. [DOI] [PubMed] [Google Scholar]
- 9.Laucirica R. Intraoperative assessment of the breast—guidelines and potential pitfalls. Arch Pathol Lab Med. 2005;129:1565–1574. doi: 10.5858/2005-129-1565-IAOTBG. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg E, Cox C, Dupont E, et al. Local recurrence in lump-ectomy patients after imprint cytology margin evaluation. Am J Surg. 2004;188:349–354. doi: 10.1016/j.amjsurg.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Ebell MH, Siwek J, Weiss BD, et al. Strength of Recommendation Taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548–556. [PubMed] [Google Scholar]
- 13.D’Halluin F, Tas P, Coue O, et al. Role of intraoperative imprint cytology for evaluation of surgical margins in breast cancer: a prospective controlled study about 400 lumpectomy. Virchows Arch. 2008;452:S24–S25. [Google Scholar]
- 14.Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007;14:1458–1471. doi: 10.1245/s10434-006-9236-0. [DOI] [PubMed] [Google Scholar]
- 15.Camp ER, McAuliffe PF, Gilroy JS, et al. Minimizing local recurrence after breast conserving therapy using intraoperative shaved margins to determine pathologic tumor clearance. J Am Coll Surg. 2005;201:855–861. doi: 10.1016/j.jamcollsurg.2005.06.274. [DOI] [PubMed] [Google Scholar]
- 16.Loibl S, von Minckwitz G, Raab G, et al. Surgical procedures after neoadjuvant chemotherapy in operable breast cancer: results of the GEPARDUO trial. Ann Surg Oncol. 2006;13:1434–1442. doi: 10.1245/s10434-006-9011-2. [DOI] [PubMed] [Google Scholar]
- 17.Valdes EK, Boolbol SK, Ali I, Feldman SM, Cohen JM. Intra-operative touch preparation cytology for margin assessment in breast-conservation surgery: does it work for lobular carcinoma? Ann Surg Oncol. 2007;14:2940–2945. doi: 10.1245/s10434-007-9364-1. [DOI] [PubMed] [Google Scholar]
- 18.Rusby JE, Paramanathan N, Laws SA, Rainsbury RM. Immediate latissimus dorsi miniflap volume replacement for partial mastectom: use of intra-operative frozen sections to confirm negative margins. Am J Surg. 2008;196:512–518. doi: 10.1016/j.amjsurg.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Fukamachi K, Ishida T, Usami S, et al. Total-circumference intraoperative frozen section analysis reduces margin-positive rate in breast-conservation surgery. Jpn J Clin Oncol. 2010;40:513–520. doi: 10.1093/jjco/hyq006. [DOI] [PubMed] [Google Scholar]
- 20.Munhoz AM, Montag E, Arruda E, et al. Immediate reconstruction following breast-conserving surgery: management of the positive surgical margins and influence on secondary reconstruction. Breast. 2009;18:47–54. doi: 10.1016/j.breast.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Riedl O, Fitzal F, Mader N, et al. Intraoperative frozen section analysis for breast-conserving therapy in 1016 patients with breast cancer. Eur J Surg Oncol. 2009;35:264–270. doi: 10.1016/j.ejso.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Uecker JM, Bui EH, Foulkrod KH, Sabra JP. Intraoperative assessment of breast cancer specimens decreases cost and number of reoperations. Am Surg. 2011;77:342–344. [PubMed] [Google Scholar]
- 23.Osborn JB, Keeney GL, Jakub JW, Degnim AC, Boughey JC. Cost-effectiveness analysis of routine frozen-section analysis of breast margins compared with reoperation for positive margins. Ann Surg Oncol. 2011;18:3204–3209. doi: 10.1245/s10434-011-1956-0. [DOI] [PubMed] [Google Scholar]
- 24.Arora S, Menes TS, Moung C, Nagi C, Bleiweiss I, Jaffer S. Atypical ductal hyperplasia at margin of breast biopsy—is re-excision indicated? Ann Surg Oncol. 2008;15:843–847. doi: 10.1245/s10434-007-9681-4. [DOI] [PubMed] [Google Scholar]
- 25.Huston TL, Pigalarga R, Osborne MP, Tousimis E. The influence of additional surgical margins on the total specimen volume excised and the reoperative rate after breast-conserving surgery. Am J Surg. 2006;192:509–512. doi: 10.1016/j.amjsurg.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Landercasper J, Ellis RL, Mathiason MA, et al. A community breast center report card determined by participation in the National Quality Measures for Breast Centers program. Breast J. 2010;16:472–480. doi: 10.1111/j.1524-4741.2010.00970.x. [DOI] [PubMed] [Google Scholar]
- 27.McCahill LE, Single R, Ratliff J, Sheehey-Jones J, Gray A, James T. Local recurrence after partial mastectom: relation to initial surgical margins. Am J Surg. 2011;201:374–378. doi: 10.1016/j.amjsurg.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Menes TS, Tartter PI, Bleiweiss I, Godbold JH, Estabrook A, Smith SR. The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann Surg Oncol. 2005;12:881–885. doi: 10.1245/ASO.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Miller AR, Brandao G, Prihoda TJ, Hill C, Cruz AB, Yeh IT. Positive margins following surgical resection of breast carcinoma: analysis of pathologic correlates. J Surg Oncol. 2004;86:134–140. doi: 10.1002/jso.20059. [DOI] [PubMed] [Google Scholar]
- 30.Moorthy K, Asopa V, Wiggins E, Callam M. Is the reexcision rate higher if breast conservation surgery is performed by surgical trainees? Am J Surg. 2004;188:45–48. doi: 10.1016/j.amjsurg.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 31.Mullenix PS, Cuadrado DG, Steele SR, et al. Secondary operations are frequently required to complete the surgical phase of therapy in the era of breast conservation and sentinel lymph node biopsy. Am J Surg. 2004;187:643–646. doi: 10.1016/j.amjsurg.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Ooi CWL, Serpell JW, Rodger A. Tumour involvement of the re-excision specimen following clear local excision of breast cancer with positive margins. ANZ J Surg. 2003;73:979–982. doi: 10.1046/j.1445-2197.2003.t01-9-.x. [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan MJ, Li T, Freedman G, Morrow M. The effect of multiple reexcisions on the risk of local recurrence after breast conserving surgery. Ann Surg Oncol. 2007;14:3133–3140. doi: 10.1245/s10434-007-9523-4. [DOI] [PubMed] [Google Scholar]
- 34.Perez CA. Conservation therapy in T1-T2 breast cancer: past, current issues, and future challenges and opportunities. Cancer J. 2003;9:442–453. doi: 10.1097/00130404-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Ramanah R, Pivot X, Sautiere JL, Maillet R, Riethmuller D. Predictors of re-excision for positive or close margins in breast-conservation therapy for pT1 tumors. Am J Surg. 2008;195:770–774. doi: 10.1016/j.amjsurg.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez C, Brem RF, McSwain AR, Rapelyea JA, Torrente J, Teal CB. Factors associated with re-excision in patients with early-stage breast cancer treated with breast conservation therapy. Am Surg. 2010;76:331–334. [PubMed] [Google Scholar]
- 37.van den Broek N, van der Sangen MJ, van de Poll-Franse LV, van Beek MW, Nieuwenhuijzen GA, Voogd AC. Margin status and the risk of local recurrence after breast-conserving treatment of lobular breast cancer. Breast Cancer Res Treat. 2007;105:63–68. doi: 10.1007/s10549-006-9431-5. [DOI] [PubMed] [Google Scholar]
- 38.Vicini FA, Kestin LL, Goldstein NS, Baglan KL, Pettinga JE, Martinez AA. Relationship between excision volume, margin status, and tumor size with the development of local recurrence in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Surg Oncol. 2001;76:245–254. doi: 10.1002/jso.1041. [DOI] [PubMed] [Google Scholar]
- 39.Barros AC, Pinotti M, Teixeira LC, Ricci MD, Pinotti JA. Outcome analysis of patients with early infiltrating breast carcinoma treated by surgery with intraoperative evaluation of surgical margins. Tumori. 2004;90:592–595. doi: 10.1177/030089160409000610. [DOI] [PubMed] [Google Scholar]
- 40.Cox CE, Hyacinthe M, Gonzalez RJ, et al. Cytologic evaluation of lumpectomy margins in patients with ductal carcinoma in situ: clinical outcome. Ann Surg Oncol. 1997;4:644–649. doi: 10.1007/BF02303749. [DOI] [PubMed] [Google Scholar]
- 41.D’Halluin F, Tas P, Rouquette S, et al. Intra-operative touch preparation cytology following lumpectomy for breast cancer: a series of 400 procedures. Breast. 2009;18:248–253. doi: 10.1016/j.breast.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Klimberg VS, Westbrook KC, Korourian S. Use of touch preps for diagnosis and evaluation of surgical margins in breast cancer. Ann Surg Oncol. 1998;5:220–226. doi: 10.1007/BF02303776. [DOI] [PubMed] [Google Scholar]
- 43.Cendan JC, Coco D, Copeland EM3rd. Accuracy of intraoper-ative frozen-section analysis of breast cancer lumpectomy-bed margins. J Am Coll Surg. 2005;201:194–198. doi: 10.1016/j.jamcollsurg.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Chen WH, Cheng SP, Tzen CY, et al. Surgical treatment of phyllodes tumors of the breast: retrospective review of 172 cases. J Surg Oncol. 2005;91:185–194. doi: 10.1002/jso.20334. [DOI] [PubMed] [Google Scholar]
- 45.Dener C, Inan A, Sen M, Demirci S. Interoperative frozen section for margin assessment in breast conserving energy. Scand J Surg. 2009;98:34–40. doi: 10.1177/145749690909800107. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda T, Enomoto K, Wada K, et al. Frozen-section-guided breast-conserving surgery: implications of diagnosis by frozen section as a guide to determining the extent of resection. Surg Today. 1997;27:207–212. doi: 10.1007/BF00941646. [DOI] [PubMed] [Google Scholar]
- 47.Olson TP, Harter J, Mun˜oz A, Mahvi DM, Breslin T. Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol. 2007;14:2953–2960. doi: 10.1245/s10434-007-9437-1. [DOI] [PubMed] [Google Scholar]
- 48.Park S, Park HS, Kim SI, Koo JS, Park BW, Lee KS. The impact of a focally positive resection margin on the local control in patients treated with breast-conserving therapy. Jpn J Clin Oncol. 2011;41:600–608. doi: 10.1093/jjco/hyr018. [DOI] [PubMed] [Google Scholar]
- 49.Weber S, Storm FK, Stitt J, Mahvi DM. The role of frozen section analysis of margins during breast conservation surgery. Cancer J Sci Am. 1997;3:273–277. [PubMed] [Google Scholar]
- 50.Weber WP, Engelberger S, Viehl CT, et al. Accuracy of frozen section analysis versus specimen radiography during breast-conserving surgery for nonpalpable lesions. World J Surg. 2008;32:2599–2606. doi: 10.1007/s00268-008-9757-8. [DOI] [PubMed] [Google Scholar]