Abstract
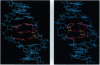
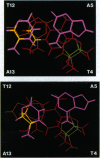
We have used 1H NMR spectroscopy to determine the structural changes induced in the DNA oligomer d(5'-GCGTACGC-3')2 upon conversion of the 4'-hydroxy-methyl-4,5',8-trimethylpsoralen-DNA furan-side monoadduct (MAf) to the interstrand cross-link (XL). The MAf is a photochemical intermediate on the path to interstrand XL and has the psoralen intercalated into the helix. The local DNA structure is distorted in both adducts, but it returns to normal within three base pairs. The formation of XL requires displacement of the psoralen toward the initially unmodified strand, accompanied by a change in the hybridization of the thymine C-5 and C-6 carbons and a change in the local helix twist. The MAf is intercalated in the helix. There is no significant bend in the helix axis of either the MAf or XL. There are significant changes in the local helix dynamics upon photoadduct formation that may be recognized by cellular DNA repair enzyme systems. We hypothesize that the repair enzymes target lesions by detecting the conformational flexibility of the sugar-phosphate backbone induced by DNA-damaging agents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beamer L. J., Pabo C. O. Refined 1.8 A crystal structure of the lambda repressor-operator complex. J Mol Biol. 1992 Sep 5;227(1):177–196. doi: 10.1016/0022-2836(92)90690-l. [DOI] [PubMed] [Google Scholar]
- Botuyan M. V., Keire D. A., Kroen C., Gorenstein D. G. 31P nuclear magnetic resonance spectra and dissociation constants of lac repressor headpiece.duplex operator complexes: the importance of phosphate backbone flexibility in protein.DNA recognition. Biochemistry. 1993 Jul 13;32(27):6863–6874. doi: 10.1021/bi00078a009. [DOI] [PubMed] [Google Scholar]
- Cheng S., Sancar A., Hearst J. E. RecA-dependent incision of psoralen-crosslinked DNA by (A)BC excinuclease. Nucleic Acids Res. 1991 Feb 11;19(3):657–663. doi: 10.1093/nar/19.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaret J. P., Brunie S., Ballini J. P., Vigny P. Geometry of intercalation of psoralens in DNA approached by molecular mechanics. Photochem Photobiol. 1989 Jul;50(1):7–21. doi: 10.1111/j.1751-1097.1989.tb04124.x. [DOI] [PubMed] [Google Scholar]
- Gochin M., Zon G., James T. L. Two-dimensional COSY and two-dimensional NOE spectroscopy of d(AC)4.d(GT)4: extraction of structural constraints. Biochemistry. 1990 Dec 25;29(51):11161–11171. doi: 10.1021/bi00503a003. [DOI] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Johnston B. H., Johnson M. A., Moore C. B., Hearst J. E. Psoralen-DNA photoreaction: controlled production of mono- and diadducts with nanosecond ultraviolet laser pulses. Science. 1977 Aug 26;197(4306):906–908. doi: 10.1126/science.887929. [DOI] [PubMed] [Google Scholar]
- Jones B. K., Yeung A. T. DNA base composition determines the specificity of UvrABC endonuclease incision of a psoralen cross-link. J Biol Chem. 1990 Feb 25;265(6):3489–3496. [PubMed] [Google Scholar]
- Kanne D., Straub K., Rapoport H., Hearst J. E. Psoralen-deoxyribonucleic acid photoreaction. Characterization of the monoaddition products from 8-methoxypsoralen and 4,5'8-trimethylpsoralen. Biochemistry. 1982 Mar 2;21(5):861–871. doi: 10.1021/bi00534a008. [DOI] [PubMed] [Google Scholar]
- Karslake C., Botuyan M. V., Gorenstein D. G. 31P NMR spectra of oligodeoxyribonucleotide duplex lac operator-repressor headpiece complexes: importance of phosphate ester backbone flexibility in protein-DNA recognition. Biochemistry. 1992 Feb 18;31(6):1849–1858. doi: 10.1021/bi00121a038. [DOI] [PubMed] [Google Scholar]
- Lavery R., Sklenar H. Defining the structure of irregular nucleic acids: conventions and principles. J Biomol Struct Dyn. 1989 Feb;6(4):655–667. doi: 10.1080/07391102.1989.10507728. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Sancar A. A new mechanism for repairing oxidative damage to DNA: (A)BC excinuclease removes AP sites and thymine glycols from DNA. Biochemistry. 1989 Oct 3;28(20):7979–7984. doi: 10.1021/bi00446a002. [DOI] [PubMed] [Google Scholar]
- O'Handley S. F., Sanford D. G., Xu R., Lester C. C., Hingerty B. E., Broyde S., Krugh T. R. Structural characterization of an N-acetyl-2-aminofluorene (AAF) modified DNA oligomer by NMR, energy minimization, and molecular dynamics. Biochemistry. 1993 Mar 16;32(10):2481–2497. doi: 10.1021/bi00061a005. [DOI] [PubMed] [Google Scholar]
- Pearlman D. A., Holbrook S. R., Pirkle D. H., Kim S. H. Molecular models for DNA damaged by photoreaction. Science. 1985 Mar 15;227(4692):1304–1308. doi: 10.1126/science.3975615. [DOI] [PubMed] [Google Scholar]
- Pu W. T., Kahn R., Munn M. M., Rupp W. D. UvrABC incision of N-methylmitomycin A-DNA monoadducts and cross-links. J Biol Chem. 1989 Dec 5;264(34):20697–20704. [PubMed] [Google Scholar]
- Rinkel L. J., Altona C. Conformational analysis of the deoxyribofuranose ring in DNA by means of sums of proton-proton coupling constants: a graphical method. J Biomol Struct Dyn. 1987 Feb;4(4):621–649. doi: 10.1080/07391102.1987.10507665. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Schmitz U., Sethson I., Egan W. M., James T. L. Solution structure of a DNA octamer containing the Pribnow box via restrained molecular dynamics simulation with distance and torsion angle constraints derived from two-dimensional nuclear magnetic resonance spectral fitting. J Mol Biol. 1992 Sep 20;227(2):510–531. doi: 10.1016/0022-2836(92)90904-x. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Griffith J., Gamper H., Hearst J. E. Evidence for structural deformation of the DNA helix by a psoralen diadduct but not by a monoadduct. Nucleic Acids Res. 1988 Sep 26;16(18):8945–8952. doi: 10.1093/nar/16.18.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek F. M., Munn M. M., Rupp W. D., Howard-Flanders P. In vitro repair of psoralen-DNA cross-links by RecA, UvrABC, and the 5'-exonuclease of DNA polymerase I. J Biol Chem. 1989 Apr 25;264(12):6755–6765. [PubMed] [Google Scholar]
- Spielmann H. P., Sastry S. S., Hearst J. E. Methods for the large-scale synthesis of psoralen furan-side monoadducts and diadducts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4514–4518. doi: 10.1073/pnas.89.10.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Analysis of sequential steps of nucleotide excision repair in Escherichia coli using synthetic substrates containing single psoralen adducts. J Biol Chem. 1988 Nov 15;263(32):16553–16560. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigny P., Blais J., Ibanez V., Geacintov N. E. A flow linear dichroism study of the orientation of 4',5'-psoralen-DNA photoadducts. Photochem Photobiol. 1987 May;45(5):601–607. doi: 10.1111/j.1751-1097.1987.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Wiesehahn G., Hearst J. E. DNA unwinding induced by photoaddition of psoralen derivatives and determination of dark-binding equilibrium constants by gel electrophoresis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2703–2707. doi: 10.1073/pnas.75.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. L., Patel D. J. Solution structure of the luzopeptin-DNA complex. Biochemistry. 1991 Apr 23;30(16):4026–4041. doi: 10.1021/bi00230a030. [DOI] [PubMed] [Google Scholar]